Abstract
Sex differences in neural development are established via a number of cellular processes (i.e., migration, death and survival). One critical factor identified is the neonatal rise in testosterone (T) which activates gene transcription via androgen (AR) and, after aromatization to estradiol, estrogen receptors (ERα and β). Recent evidence shows that AR and ERs interact with histone modifying enzymes. Post-translational modifications of histones, including acetylation and methylation, are involved in transcriptional regulation during normal development. Therefore, we hypothesized that acetylation and/or methylation of histone H3 may underlie sexual differentiation, at least in some regions of the brain. We measured levels of acetylated (H3K9/14Ac) and trimethylated (H3K9Me3) H3 in whole neonatal mouse brains and in three regions: preoptic area + hypothalamus, amygdala and cortex + hippocampus (CTX/HIP). Sex differences in H3K9/14Ac and H3K9Me3 (males > females) were noted in the CTX/HIP on embryonic day 18, the day of birth, and six days later. To determine if T mediates these changes in H3 modifications, pregnant dams received vehicle or T for the final four days of gestation; pup brains were collected at birth. Methylation of H3 was sexually dimorphic despite hormone treatment. In contrast, H3 acetylation in the CTX/HIP of females from T-treated dams rose to levels equivalent to males. Thus, H3 modifications are sexually dimorphic in the developing mouse CTX/HIP and acetylation, but not methylation, is masculinized in females by T in utero. This is the first demonstration that histone modification is associated with neural sexual differentiation.
Keywords: chromatin, epigenetics, development, sex differences, learning and memory, autism
Introduction
Epigenetic mechanisms, i.e., DNA methylation and histone modification, are important for normal development including imprinting, X-inactivation, cell differentiation and transcription.1,2 The status of transcription (activation and silencing) is associated with specific types of modifications at particular residues of histone tails.3 Environmental variables, including stress, maternal behavior, environmental disruptors and nutrition affect neural development and also influence sexual differentiation of the neonatal brain.4–6 Epigenetic regulation provides a novel mechanism by which these environmental factors modify neural development and/or adult function.7–9 For example, pup licking by rat dams in the first week of life persistently determines glucocorticoid receptor (GR) expression in the hippocampus of pups by altering DNA methylation and histone acetylation at the GR promoter.7 More globally, an X-linked gene product, MeCP2, recruits histone deacetylases (HDACs) to silence gene expression, is transiently sexually dimorphic in the neonatal brain, and its disruption depresses juvenile play behavior in males.10,11
Neural sexual differentiation occurs during late embryonic and early postnatal development concurrent with increased testosterone (T) production by the neonatal testes. Testosterones affects transcription via androgen receptors (AR) or, after it is aromatized, via estrogen receptors (ERα and β).12 Steroid receptors access DNA in complexes with co-activators and co-repressors, several of which have enzymatic activities for histone modification.13–15 For example, CBP/p300 and LSD1, which are AR co-activators, possess histone acetyltransferase and demethylase activities, respectively.16,17 In rodent brain, ERα, ERβ and AR are abundant in the brain,18,19 particularly in the amygdala and hypothalamus, which contain structural sex differences and are important for expression of sexually dimorphic behaviors.12 The hippocampus and cortex regulate cognitive behaviors, many of which are sexually dimorphic.20,21 Sex differences in steroid receptors and features of neuronal morphology have been reported in the cortex, but the mechanisms that underlie these dimorphisms in these areas are understudied.22–24
Here we tested the novel hypothesis that histone modifications are sexually dimorphic in the developing mouse brain. In the CTX/HIP, we noted a sex difference (males > females) in levels of H3K9/14Ac (associated with gene activation) and H3K9Me3 (associated with gene silencing) as well as a developmental shift whereby the sex difference in acetylation began earlier and ended sooner than the dimorphism in methylation. In female brains, prenatal T-treatment masculinized H3K9/14Ac, but did not affect H3K9Me3 levels. Taken together, we show that H3 histone modification is sexually dimorphic in some areas of the neonatal brain, and prenatal T interacts with H3 acetylation to reverse this dimorphism.
Results
No sex differences in embryonic whole brains
No sex differences were noted in whole brains for any of the three embryonic ages (E12, E16 and E18). Levels of H3K9/14Ac, H3K9Me3 and H4 did not vary by sex at any age (Table 1). When levels (H3K9/14Ac/H4 and H3K9Me3/H4) in males were expressed as the percentage of the levels in females, again, no sex differences were noted.
Table 1.
Whole brain H3 acetylation (H3K9/14Ac) and trimethylation (H3K9Me3) during embryonic development
| H3K9/14Ac | H3K9Me3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Sex | N | acH3 | H4 | % | 3mH3 | H4 | % | |
| E12 | M | 10 | 700.30 ± 30.21 | 56.07 ± 8.79 | 101.34 ± 8.38 | 657.19 ± 64.44 | 49.38 ± 6.59 | 101.86 ± 6.34 |
| F | 11 | 612.82 ± 28.05 | 48.12 ± 4.88 | 100.00 ± 5.77 | 675.00 ± 53.58 | 42.24 ± 5.67 | 100.00 ± 9.94 | |
| E16 | M | 14 | 663.03 ± 69.12 | 57.09 ± 8.78 | 110.94 ± 7.65 | 606.32 ± 52.00 | 67.97 ± 7.66 | 109.71 ± 11.23 |
| F | 14 | 526.14 ± 45.86 | 51.95 ± 8.13 | 100.00 ± 3.45 | 627.63 ± 88.07 | 67.00 ± 6.40 | 100.00 ± 2.76 | |
| E18 | M | 14 | 882.34 ± 59.35 | 60.14 ± 6.91 | 111.37 ± 7.42 | 764.20 ± 27.90 | 58.17 ± 8.32 | 104.07 ± 8.13 |
| F | 17 | 839.35 ± 31.54 | 68.06 ± 5.44 | 100.00 ± 2.92 | 678.13 ± 32.08 | 66.73 ± 6.40 | 100.00 ± 3.16 | |
Intensities of acetylated and trimethylated H3 and total H4 measured by densitometry were expressed as mean ± SEM. Levels of H3K9/14Ac and H3K9Me3 were expressed as ratios normalized to total H4 and then set as the percentage relative to the females of the same ages (as 100%). E, embryonic day; M, males; F, females.
Sex differences in acetylation and methylation in the neonatal CTX/HIP
Cortex + hippocampi dissected from neonatal mouse brains on E18, PN0 and PN6 revealed sex differences that varied over development. On E18 [t(13) = 2.56, p < 0.025] and PN0 [t(12) = 3.28, p < 0.008] male CXT/HIP contained higher levels of H3 acetylation than females, but no sex differences were noted in PN6 tissues [t(12) = 1.09; Fig. 2]. Interestingly a sex effect was noted for H3K9Me3 both in PN0 [t(12) = 2.97, p < 0.01] and PN6 CTX/HIP [t(12) = 2.44, p < 0.03], but not at the earliest time point [E18; t(13) = 0.57; Fig. 2]. In all cases males had higher levels of H3K9Me3 than did females (Fig. 2, Table 2). These temporal changes in global H3 modifications suggest that hyperacetylation begins prior to and ends earlier than hypermethylation of H3 in male CTX/HIP during the critical period for neural sexual differentiation.
Figure 2.
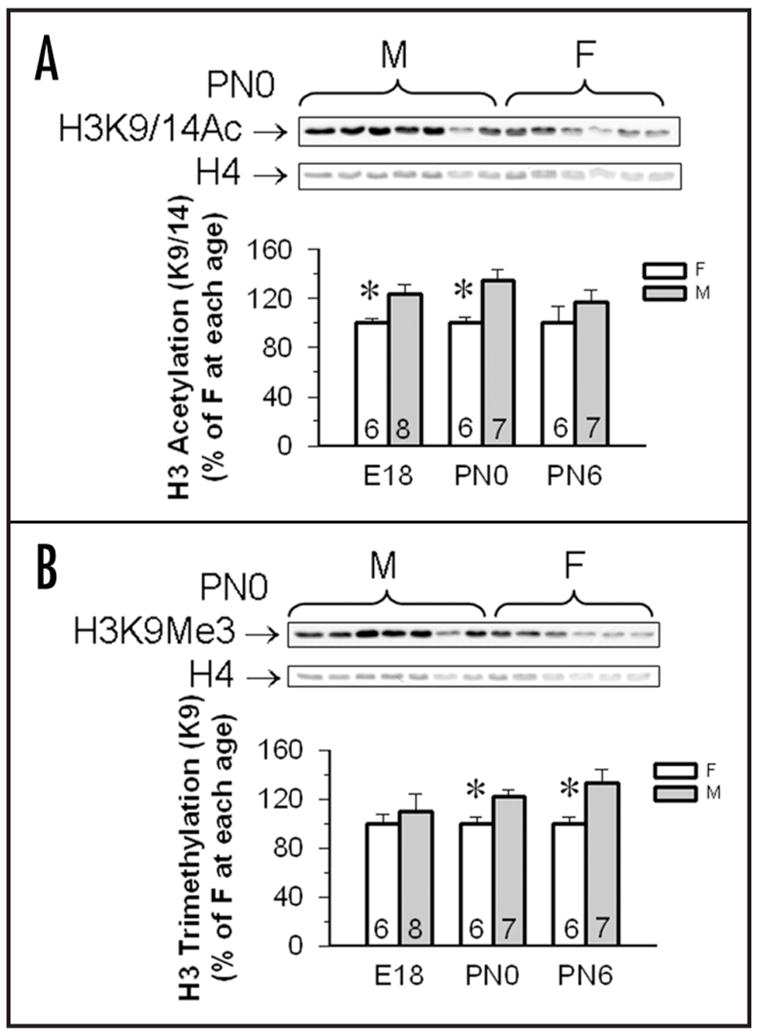
H3K9/14Ac and H3K9Me3 in the cortex/hippocampus of male and female mouse embryos and pups. (A) Top shows a gel and set of representative immunoblot from PN0 males and females. The top row is a western blot showing H3K9/14Ac bands and the bottom row is Coomassie for total H4 from the same individuals. In the graph below we present mean optical densities (±SEM) of H3K9/14Ac in the cortex/hippocampus of male and female embryos and pups were normalized to levels of H4 and expressed as the percentage of females (as 100%). (B) Top shows a representative immunoblot from PN0 males and females. The top row is a western blot showing H3K9Me3 bands and the bottom row is Coomassie for total H4 from the same individuals. Below are mean optical densities (±SEM) of H3K9Me3 in the cortex/hippocampus of male and female embryos and pups were normalized to levels of H4, and expressed as the percentage of females (as 100%). *Females have significantly less protein than males at these time points, p < 0.05. Sample sizes are show in the base of each histogram. E18 = embryonic day 18, PN0 = day of birth, PN6 = postnatal day 6, M = male, F = female.
Table 2.
H3 acetylation and trimethylation in the combined cortex and hippocampus during early development
| H3K9/14Ac | H3K9Me3 | |||||
|---|---|---|---|---|---|---|
| Sex | N | acH3 | H4 | 3mH3 | H4 | |
| E18 | M | 8 | 756.87 ± 50.61 | 25.83 ± 1.99 | 775.53 ± 78.01 | 24.17 ± 2.42 |
| F | 6 | 726.07 ± 87.03 | 28.48 ± 2.58 | 781.13 ± 91.06 | 25.03 ± 3.12 | |
| PN0 | M | 7 | 1083.58 ± 83.52 | 50.58 ± 1.86 | 1365.99 ± 85.75 | 49.63 ± 2.88 |
| F | 6 | 798.40 ± 62.11 | 50.38 ± 3.23 | 984.56 ± 84.00 | 43.48 ± 3.22 | |
| PN6 | M | 7 | 1149.91 ± 146.00 | 13.96 ± 1.57 | 1268.64 ± 150.41 | 10.46 ± 1.36 |
| F | 6 | 966.42 ± 191.93 | 13.57 ± 1.45 | 1117.72 ± 148.42 | 11.95 ± 1.43 | |
Intensities of acetylated and trimethylated H3 and total H4 measured by densitometry of histone western blots were expressed as mean ± SEM. E, embryonic day; PN, postnatal day; PN0, the day of birth; M, males; F, females.
Lack of sex differences in histone modification in the preoptic/hypothalamus or AMY
Preoptic/hypothalamic and AMY tissues dissected from mouse brains collected on E18, PN0 and PN6 did not reveal any sex differences in levels of H3K9/14Ac, H3K9Me3, or H4 (Tables 3 and 4). In addition, when levels (H3K9/14Ac/H4 and H3K9Me3/H4) in males were expressed as the percentage of the levels in females, again, no sex differences were noted (Tables 3 and 4).
Table 3.
H3 acetylation and trimethylation in the combined preoptic area and hypothalamus during early development
| H3K9/14Ac | H3K9Me3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Sex | N | acH3 | H4 | % | 3mH3 | H4 | % | |
| E18 | M | 9 | 695.90 ± 121.03 | 38.83 ± 5.12 | 82.15 ± 8.66 | 684.00 ± 119.31 | 43.95 ± 8.64 | 104.70 ± 9.32 |
| F | 10 | 773.74 ± 87.66 | 35.89 ± 4.33 | 100.00 ± 4.22 | 596.03 ± 89.66 | 38.23 ± 6.71 | 100.00 ± 6.23 | |
| PN0 | M | 8 | 600.31 ± 77.38 | 31.05 ± 5.80 | 127.67 ± 19.26 | 469.97 ± 51.83 | 32.25 ± 4.66 | 105.98 ± 9.00 |
| F | 6 | 619.66 ± 34.82 | 40.24 ± 6.43 | 100.00 ± 2.82 | 547.18 ± 23.00 | 41.11 ± 3.65 | 100.00 ± 2.72 | |
| PN6 | M | 10 | 539.59 ± 51.80 | 19.96 ± 1.82 | 107.60 ± 6.88 | 626.78 ± 70.18 | 19.63 ± 3.71 | 110.97 ± 9.05 |
| F | 7 | 660.02 ± 110.49 | 27.44 ± 4.19 | 100.00 ± 9.42 | 738.99 ± 76.98 | 28.72 ± 5.89 | 100.00 ± 8.09 | |
Intensities of acetylated and trimethylated H3 and total H4 measured by densitometry of histone western blots were expressed as mean ± SEM. Levels of H3K9/14Ac and H3K9Me3 were expressed as ratios normalized to total H4 and then set as the percentage relative to the females of the same ages (as 100%). E, embryonic day; PN, postnatal day; PN0, the day of birth; M, males; F, females.
Table 4.
H3 acetylation and trimethylation in the amygdala during early development
| H3K9/14Ac | H3K9Me3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Sex | N | acH3 | H4 | % | 3mH3 | H4 | % | |
| E18 | M | 6 | 750.40 ± 106.01 | 33.14 ± 8.12 | 86.06 ± 16.23 | 722.62 ± 147.12 | 30.04 ± 7.26 | 109.65 ± 6.79 |
| F | 7 | 842.47 ± 84.36 | 31.09 ± 6.25 | 100.00 ± 7.64 | 728.99 ± 83.54 | 33.30 ± 5.09 | 100.00 ± 7.08 | |
| PN0 | M | 7 | 514.92 ± 131.39 | 23.49 ± 3.50 | 102.72 ± 29.28 | 579.36 ± 108.59 | 18.87 ± 3.13 | 94.57 ± 22.96 |
| F | 6 | 725.97 ± 74.17 | 32.24 ± 1.89 | 100.00 ± 6.97 | 800. 58 ± 100.40 | 22.05 ± 2.11 | 100.00 ± 4.54 | |
| PN6 | M | 7 | 809.16 ± 115.93 | 1.25 ± 0.36 | 190.10 ± 95.61 | 738. 01 ± 134.94 | 2.94 ± 0.36 | 93.24 ± 7.50 |
| F | 6 | 848.02 ± 98.02 | 1.43 ± 0.33 | 100.00 ± 23.67 | 805.49 ± 50.90 | 3.28 ± 0.49 | 100.00 ± 9.69 | |
Intensities of acetylated and trimethylated H3 and total H4 measured by densitometry of histone western blots were expressed as mean ± SEM. Levels of H3K9/14Ac and H3K9Me3 were expressed as ratios normalized to total H4 and then set as the percentage relative to the females of the same ages (as 100%). E, embryonic day; PN, postnatal day; PN0, the day of birth; M, males; F, females.
Prenatal testosterone affects acetylation but not methylation in the neonatal female CTX/HIP
Histone modifications in CTX/HIP tissues collected from PN0 pups were sexually dimorphic (males > females), moreover, T exposure masculinized female levels of H3K9/14Ac, but did not affect H3K9Me3 (Fig. 3). Total levels of H3K9/14Ac, H3K9Me3 and H4 did not vary by sex of the neonate and/or hormone treatment (Table 5). When levels of H3K9/14Ac/H4 and H3K9Me3/H4 were expressed as a percentage of the vehicle treated females, we noted significant effects of sex [F(1,35) = 5.76; p < 0.02 and F(1,34) = 7.56; p < 0.01 respectively] for each modification. No independent effects of hormone treatment were noted [F(1,35) = 1.35 for H3K9/14Ac and F(1,34) = 2.26 for H3k9Me3], nor was an interaction detected for the H3K9Me3 data [F(1,34) = 0.56]. However, an interaction between sex of the neonate and hormone treatment was found for the H3K9/14Ac modification [F(1,35) = 4.02; p < 0.05]. The interaction was caused by the control female group which had significantly lower levels of H3K9/14Ac as compared to any of the other groups (p < 0.05).
Figure 3.
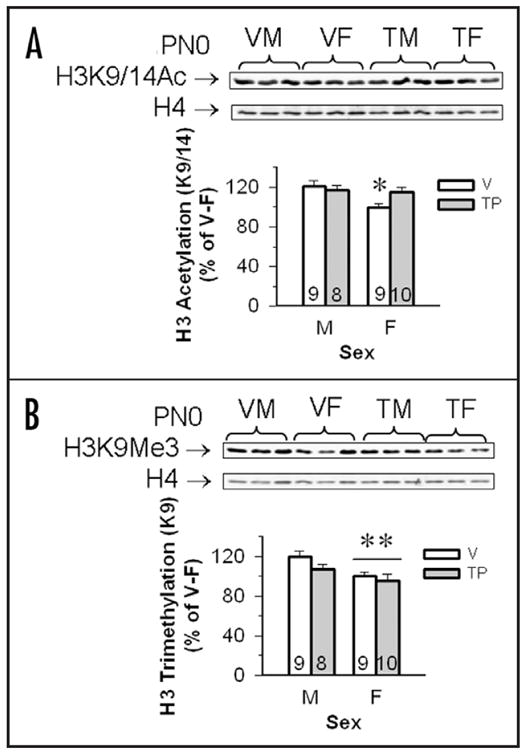
Testosterone propionate (TP) increased H3K9/14Ac, but not H3K9Me3 in the cortex/hippocampus of female mouse pups. (A) Top shows a set of representative immunoblot from PN0 males and females. The western blots on the top row show H3K9/14Ac bands and the bottom row is total H4 from the same individuals. Mean optical densities (±SEM) of H3K9/14Ac in the cortex/hippocampus of vehicle (V) and TP-treated male (M) and female (F) neonates were normalized to levels of H4, and expressed as the percentage of V-treated F controls (100%). *Females whose dams received vehicle during gestation had significantly lower levels of H3K9/14Ac as compared with the other three groups (p < 0.05). (B) Top shows a representative immunoblot from PN0 males and females. The western blots on the top row show H3K9Me3 bands and the bottom row is total H4 from the same individuals. Mean optical densities (±SEM) of 3mH3 in the cortex/hippocampus of V and TP-treated M and F neonates were normalized to levels of H4, and expressed as the percentage of V-treated females (as 100%). **Females have significantly less H3K9Me3 than males, p < 0.05. Sample sizes are shown in each histogram.
Table 5.
Effects of testosterone propionate (TP) during the end of gestation on levels of acetylated and trimethylated H3 in the cortex/hippocampus of PN0 mice
| H3K9/14Ac | H3K9Me3 | |||||
|---|---|---|---|---|---|---|
| Sex | N | acH3 | H4 | 3mH3 | H4 | |
| V | M | 9 | 886.85 ± 91.33 | 474.06 ± 38.72 | 811.82 ± 54.50 | 439.17 ± 41.82 |
| F | 9 | 866.98 ± 119.39 | 540.41 ± 44.54 | 759.78 ± 109.64 | 439.02 ± 51.61 | |
| TP | M | 8 | 845.16 ± 67.38 | 478.51 ± 33.92 | 734.44 ± 73.91 | 436.27 ± 28.45 |
| F | 10 | 853.55 ± 90.97 | 469.33 ± 39.86 | 812.12 ± 108.51 | 508.23 ± 45.80 | |
Intensities of acetylated and trimethylated H3 and total H4 measured by densitometry of histone western blots were expressed as mean ± SEM. M, males; F, females; V, vehicle given to pregnant dam on gestational days 16–19; TP, testosterone propionate given to pregnant dams on gestational days 16–19. All pups were collected on the day of birth.
Discussion
We report, for the first time, that H3K9/14Ac and H3K9Me3; two epigenetic marks that are nearly mutually exclusive and respectively are typically associated with increased and decreased gene transcription, are sexually dimorphic in the developing CTX/HIP. It is worth noting that the sex differences we found are on the order of 30% which, given the heterogeneity of brain tissue, is remarkable and could represent up to 30% of the epigenome. Our CTX/HIP dissections included many subregions, but the major areas, the hippocampus, cortex and the dentate gyrus, have well documented functions in cognition, many of which are sexually dimorphic and modified by steroid hormones.20,28,29 Moreover, sex differences documented in CTX/HIP include differences in cell numbers, thickness of cortical layers, numbers of spines and electrophysiological properties.24,30 Interestingly, the neonatal CTX/HIP contains steroid receptors and steroid synthesizing enzymes,13,23,31,32 and neural structures in CTX/HIP can be modified by not only steroid hormones,20,28,29 but also the environment.9,33,34
Our studies also revealed a developmental timeline in the CTX/HIP such that the sex difference in H3K9/14Ac was detected on embryonic day 18 and again on the day of birth; however six days later, the same trend was present but failed to reach significance. On the other hand, H3K9Me3 was only sexually dimorphic after birth. If we take the most parsimonious view of the functions of histone acetylation and methylation perhaps transcription in the male CXT/HIP is elevated due to increase acetylation (relative to the female brain) prior to birth, followed by a period of decreased transcription, caused by enhanced methylation, again relative to females. In the male mouse neonatal T exhibits two peaks in plasma, one during the final three days of gestation (E17–19) and the second surge is directly after birth.35–37 Interestingly, our T treatment (on gestational days 16–19) only masculinized H3K9/14Ac levels in neonatal females, suggesting that H3 acetylation might be primarily mediated by the prenatal rise in T. In contrast, H3K9Me3 is dimorphic after birth but male-like levels of H3K9Me3 cannot be mimicked in females by prenatal T treatment. This does not rule out the possibility that H3K9Me3 might be regulated by the second T surge coincident with parturition. If this is the case, T treatment during late gestation was not timed correctly to masculinize H3K9Me3 in females. Our findings suggest that perhaps there are at least two critical periods for histone modification in the CTX/HIP.
The prenatal and neonatal rise in T activates AR and/or ERs (after aromatization to estradiol) to control the development of sexual dimorphisms in brain structures and behaviors.12,38 Ligand-bound AR and ERs regulate target gene transcription via interactions with a variety of coactivators and corepressors.16,39 Many of them contain enzymatic activity for histone modification and/or interact with histone modifying enzymes. The best studied is CBP/p300, a histone acetyltransferase and general coactivator for AR and ERs.40 In vitro studies indicate that liganded steroid receptors are present together with CBP, acetylated histones, and RNA polymerase, on the promoters of active AR- and ER-target genes, suggesting that histone acetylation might play an important role in upregulation of steroid receptor-mediated gene expression.41–43 Levels of CBP in the rat hypothalamus are higher in male neonates than in females, and knockdown of CBP in the hypothalamus of males by anti-sense oligonucleotides causes behavioral feminization, suggesting that CBP might be critical for the control of sexual differentiation in that region.44 Unfortunately other brain regions were not included in that study, but it is possible that CBP mediates the effects we report in the CTX/HIP.
Steroid receptors also interact with the enzymes responsible for histone methylation which regulate gene transcription. In particular the H3K9 methylation is associated with transcriptional repression.26 The H3K9 methyltransferase, G9a, functions as either a coactivator or corepressor with steroid receptors.45 A new member of the JmjC containing demethylase family, s-JMJD1C, is found in adult male rat brain in AR-rich regions such as the anterior hypothalamus and baso-medial amygdala but also is detected in the cerebellum, cortex and hippocampus.46 Castration results in reduced expression of immuno-positive s-JMJD1C in cells in the cortex and amygdala. Yet, despite the presence of these steroid cofactors, in our study H3K9Me3 was unaffected by prenatal T manipulation.
While the early neonatal hormonal environment is the factor we know the most about, some behavioral sex differences can be attributed to sex chromosome complement47–49 and dimorphisms in the density of vasopressin fibers in the lateral septum depends in part on XX versus XY sex chromosome compliment.47,50,51 Interestingly, neural tube defects in p53-null mice are more severe in XX than in XY individuals regardless of gonadal sex.52 Several X- and Y-chromosome genes are involved in histone modification, such as the H3 demethylases Jarid1C and Jarid1D.53 Recent data show that Jarid1C is present in mouse CTX/HIP, and is expressed in a sexually dimorphic pattern in brain which maps to sex chromosome complement (XX > XY), not to gonadal sex.54 This gene escapes X-inactivation and may be one of the genes that modify trimethylation on H3. Also well known is the X-chromosome gene, MeCP2, which is active during development and classically is thought of as a gene silencer that binds to methylated cytosine residues on DNA and recruits binding proteins including Sin3A and histone deactylases.55 Two time points in neonatal rat brains were examined for sex differences in MeCP2.10 Unfortunately neither the cortex nor hippocampus was included in the analysis, but in amygdala relative MeCP2 mRNA and protein levels were higher in female pups as compared to males on the day after birth (PN1).
Given that the classic sex differences in brain structure reside in the amygdala, preoptic area and hypothalamus, it is surprising that histone modifications were only sexually dimorphic in the CTX/HIP. The explanation could be as simple as a missing time point, the heterogeneity of cell types in these regions may mask important variation within subpopulations, or subtle gene specific, rather than global, changes are occurring. Our data in whole brains did not reveal sex differences in either H3K9/14Ac or H3K9Me3. Yet, in subdivided tissue regions sex differences in H3K9/14Ac were clear at E18. Considering that the sexually dimorphic cell populations in the amygdala, preoptic area and hypothalamus only represent a very small fraction of the cells that reside in these areas, it is likely that a finer dissection of these regions will yield significant sex differences in histone modifications. Alternatively it may be that the CTX/HIP is particularly sensitive to epigenetic modification. Much of the work conducted on epigenetics and behavior has focused on the hippocampus and involves learning and memory.8,9,56 Synaptic plasticity is regulated by epigenetic mechanisms and is essential for learning and memory in adults.57–59 Perhaps the confluence of experience, hormones and sex differences make these regions unique and especially susceptible to epigenetic mechanisms.59,60
Accumulating evidence implicates that histone modifications are also involved in many aspects of brain development including; neurogenesis, synapse formation, cell differentiation and cell migration.61–64 Several genes (i.e., MECP2, FMR1, ATRX and JARID1C) implicated in neurobehavioral disease by human patient data as well as mouse models validate the importance of acetylation and methylation for normal neuronal development.53,65–68 One striking aspect of neurobehavioral diseases is their sexually dimorphic incidence rates; i.e., autism spectrum disorders (males > females), attention deficit/hyperactivity disorder (males > females) and Rhett syndrome (females > males).69 To date there is no satisfactory explanation of why these disorders are so biased toward one sex or the other. Perhaps the confluence of genes and hormones acting during selected times in development make the CTX/HIP particularly susceptible to errors in transcription regulated by histone modifications thereby loading disorders to one sex over the other. We propose that sex differences in histone modification are involved in development of normal cognitive function and disruptions of these processes may underlie some neurodevelopmental behavioral diseases.
Materials and Methods
Animals
All mice used in this study were in a C57BL/6J background strain. Mice were housed in constant conditions under a 12:12 photoperiod (lights on at 0600 h). Food (Harlan Teklad Mouse/Rat Sterilizable Diet #7012) and water were provided ad libitum. All of the experimental procedures were approved by University of Virginia Animal Use and Care Committee and were performed according to the ALAC guidelines.
Experiment 1: characterization of H3 acetylation and methylation in embryonic brain
Adult female mice were paired with fertile males and checked each morning for the presence of mating plugs. The day the plug was found was designated as embryonic day 0 (E0). On E12, E16 and E18, embryos were quickly removed from deeply anesthetized pregnant dams between 11:00 and 14:00 h and kept in Dulbecco’s phosphate-buffered saline on ice. Whole brains were removed and immediately frozen at −80°C until processed for histone extraction and immunoblotting. Other tissues from these embryos were collected for sex determination. Sample sizes for each group are detailed in Table 1 (10–17 per group).
Experiment 2: quantification of H3 acetylation and methylation in specific neural regions
On E18, the day of birth (PN0), and 6 days after birth (PN6), brains were removed and rapidly dissected under a dissecting microscope into specific regions including: the preoptic area and hypothalamus areas, amygdala (AMY) and cortex along with the hippocampus (CTX/HIP). The tissues were later processed for histone extraction and immunoblotting for H3K9/14Ac and H3K9Me3. Sample sizes for each group are detailed in Tables 2–4 and Figure 2 (between 6–10 per group).
Experiment 3: effect of testosterone propionate (TP) on H3 acetylation and methylation in the CTX/HIP
Timed pregnant female mice were similarly prepared as described in Experiment 1. Starting on E16, females received daily sc injections of vehicle (0.05 ml sesame oil) or testosterone propionate (TP, 2 μg). On the day of birth (PN0), within 2 hours after birth, pups were collected and brains were dissected as described in Experiment 2. Tissues from the CTX/HIP were stored at −80°C till processing for histone extraction and immunoblotting. Sample sizes for each group are detailed in Table 5 and Figure 3 (between 8–10 per group).
Genotyping
Genomic DNA was extracted from tails and limbs using Sigma REDExtract-N-AmpTM Tissue PCR Kit as described in the manufacturer’s protocol (Sigma, St. Louis, MO). For sex determination, DNA was amplified by PCR for the YMT2/B sequence (a member of the Ssty family present on the long arm of Y chromosome).25 Our methods have been described (Gatewood et al., 2006).
Histone extraction
Mouse brain tissues were first homogenized in cold modified RIPA buffer (0.05 M Tris, 0.9% NaCl, 5 mM EDTA, pH = 7.4) with protease inhibitor cocktail (Sigma), PMSF (1 mM) and DTT (1 mM). Using sterile syringes, brain tissues were homogenized by drawing and ejecting 5–10 times through the needles of different sizes (from 20G to 27G), followed by centrifugation at 8,000 g for 20 min at 4°C. The pellets containing nuclei were resuspended with 0.4 N sulfuric acid and continuously mixed for 1 h at 4°C. The samples were centrifuged at 10,000 g for 10 min at 4°C, the supernatant was separated, and trichloroacetic acid (18 μl per 200 μl sample) added. After precipitation overnight, the pellets were centrifuged at 16,100 g for 10 min at 4°C, followed by washing with 0.1% HCl-acetone and acetone. Pellets were dried using a Speed-Vac and later reconstituted with RIPA buffer (0.05 M Tris, 0.9% NaCl, 5 mM EDTA, 1% NP-40 and 0.25% sodium deoxycholate, pH = 7.4) with protease inhibitor cocktail, PMSF, and DTT. The lysate protein concentrations for each sample were determined by BCA (bicinchoninic acid) Protein Assays (Pierce Chemical Co., Rockford, IL).
Immunoblotting
Samples (5 μg protein each) were subjected to electrophoresis on either 14 or 16% polyacrylamide-SDS gels and transferred to nitrocellulose. The membranes were blocked in Tris-buffered saline with 0.1% Tween containing 10% milk at 4°C overnight. After blocking, blots were rinsed with TBST and incubated with the primary antibodies against acetylated (K9/14 H3; Millipore-Upstate in Temecula, CA #06–599; 1:5,000) or trimethylated (K9; Millipore-Upstate #07–523; 1:5,000) histone H3 for 1 h at room temperature. We selected H3K9/14Ac and H3K9Me3 because these modification are associated with changes in gene transcription.26,27 After rinsing, blots were incubated for 1 h in a horseradish peroxidase (HRP)-conjugated donkey anti- rabbit IgG secondary antibody (1: 10,000; Amersham Pharmacia Biotech, Arlington Heights, IL), followed by detection on X-ray film (Kodak X-OMAT, Kodak Co., Rochester, NY) with SuperSignal® West Pico Chemiluminescent Substrate (Pierce Chemical Co.,). In experiment 3, the same blots were re-probed with the antibody against total H4 (Millipore-Upstate, #07–108).
Coomassie staining
After transferring, the gels were fixed in a fixative with 25% isopropanol and 10% acetic acid. Gels were stained with 10% acetic acid containing 0.01% BioRad R-250 Coomassie Brilliant Blue R-250 (Hercules, CA) and destained with 10% acetic acid. The intensity of total H4 on individual films and total histone on gels were measured by densitometry and analyzed with ImageQuant (Molecular Dynamics, Inc., Sunnyvale, CA).
Validation
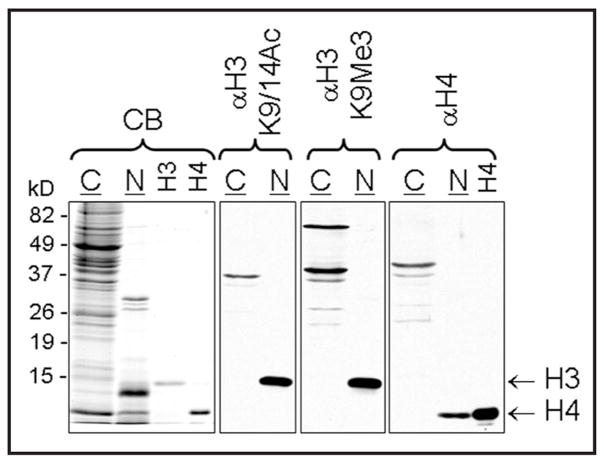
Whole brain was collected from an intact male C57BL/6J mouse, homogenized and the nuclei were isolated and separated from the cytosol, followed by histone extraction. On an untransferred gel separated histone proteins, including both H3 and H4, were visualized below the size of 15 kD in the nuclear extract by Coomassie blue staining (Fig. 1). On western blots, modified H3 and total H4 were detected only in nuclear, but not cytosolic extracts (Fig. 1).
Figure 1.
Representative immunoblots showing detection of H3K9/14Ac, H3K9Me3 and H4 in the nuclear (N) but not in the cytosolic (C) extract of the mouse whole brain. Nuclear (5 μg) and cytosol lysates (25 μg) were separated on polyacrylamide-SDS gels, transferred to nitrocellulose membranes, and immunoblotted for H3K9/14Ac and H3K9Me3 as well as total H4 as described in the Materials and Methods section. In the left panel, nuclear and cytosolic proteins and recombinant histone H3 and H4 were stained with Coomassie Blue (CB). In the western blot on the right the H4 was from the same recombinant source.
Statistical analyses
To normalize each individual sample, the densities of H3K9/14Ac and H3K9Me3 were normalized with the band density of H4 (stained by Coomassie Blue in experiments 1 and 2) or detected by western blot (experiment 3). All the samples from each experiment could not be run on the same gel. Therefore, to reduce inter-gel variation, the normalized histone levels in individual samples were standardized to the mean level of females in the same developmental stage (experiment 1) and (experiment 2) or the female control group (experiment 3) and each sample was expressed as a percentage of the female (set as 100%). Unfortunately, one limitation of this design is that it does not permit the direct comparison of tissues from different developmental stages since we did not run samples from all ages on all gels. In experiments 1 and 2, raw data for H3K9/14Ac, H3K9Me3, and H4 were quantified along with mean relative levels of modified histone. All data were analyzed by Student’s t-tests with sex as the single factor. In experiment 3 raw data for total H3K9/14Ac, H3K9Me3, H4 and the mean relative levels of each histone modification were analyzed by two-way ANOVAs where the two factors were sex and hormone treatment; this analysis was accompanied by Bonferroni’s paired comparisons where appropriate.
Acknowledgments
We thank Dr. Jin Ho Park for editorial comments, and Ms. Aileen Wills for technical assistance. This work was funded by NIH grants R01 NS055218 and MH057759.
Abbreviations
- T
testosterone
- ERα
estrogen receptor α
- ERβ
estrogen receptor β
- H3K9/14Ac
acetylated histone 3 residues K9 and 14
- H3K9Me3
trimethylated histone 3 residue K9
- CTX/HIP
cortex and hippocampus
- GR
glucocorticoid receptor
- HDACS
histone deacetylases
- AR
androgen receptor
- E
embryonic day
- PN
postnatal day
- TP
testosterone proprionate
References
- 1.Cheung WL, Briggs SD, Allis CD. Acetylation and chromosomal functions. Curr Opin Cell Biol. 2000;12:326–33. doi: 10.1016/s0955-0674(00)00096-x. [DOI] [PubMed] [Google Scholar]
- 2.Fukuda S, Taga T. Cell fate determination regulated by a transcriptional signal network in the developing mouse brain. Anat Sci Int. 2005;80:12–8. doi: 10.1111/j.1447-073x.2005.00097.x. [DOI] [PubMed] [Google Scholar]
- 3.Li B, Carey M, Workman JL. The Role of Chromatin during Transcription. Cell. 2007;128:707–19. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Moore CL, Wong L, Daum MC, Leclair OU. Mother-infant interactions in two strains of rats: implications for dissociating mechanism and function of a maternal pattern. Dev Psychobiol. 1997;30:301–12. [PubMed] [Google Scholar]
- 5.Ward IL, Ward OB, Affuso JD, Long WD, French JA, Hendricks SE. Fetal testosterone surge: specific modulations induced in male rats by maternal stress and/or alcohol consumption. Horm Behav. 2003;43:531–9. doi: 10.1016/s0018-506x(03)00061-8. [DOI] [PubMed] [Google Scholar]
- 6.Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol. 2006;28:111–8. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7:103–23. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nature Reviews Neuroscience. 2005;6:108–18. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- 9.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–82. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 10.Kurian JR, Forbes-Lorman RM, Auger AP. Sex difference in mecp2 expression during a critical period of rat brain development. Epigenetics. 2007;2:173–8. doi: 10.4161/epi.2.3.4841. [DOI] [PubMed] [Google Scholar]
- 11.Kurian JR, Bychowski ME, Forbes-Lorman RM, Auger CJ, Auger AP. Mecp2 organizes juvenile social behavior in a sex-specific manner. J Neurosci. 2008;28:7137–42. doi: 10.1523/JNEUROSCI.1345-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19:323–62. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- 13.Wilson ME, Westberry JM, Prewitt AK. Dynamic regulation of estrogen receptor-alpha gene expression in the brain: a role for promoter methylation? Front Neuroendocrinol. 2008;29:375–85. doi: 10.1016/j.yfrne.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKenna NJ, O’Malley BW. Nuclear receptors, coregulators, ligands and selective receptor modulators: making sense of the patchwork quilt. Ann N Y Acad Sci. 2001;949:3–5. doi: 10.1111/j.1749-6632.2001.tb03997.x. [DOI] [PubMed] [Google Scholar]
- 15.Metzger E, Wissmann M, Yin N, Muller JM, Schneider R, Peters AHFM, et al. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437:436–9. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- 16.Baek SH, Ohgi KA, Nelson CA, Welsbie D, Chen C, Sawyers CL, et al. Ligand-specific allosteric regulation of coactivator functions of androgen receptor in prostate cancer cells. Proc Natl Acad Sci USA. 2006;103:3100–5. doi: 10.1073/pnas.0510842103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahl P, Gullotti L, Heukamp LC, Wolf S, Friedrichs N, Vorreuther R, et al. Androgen receptor coactivators lysine-specific histone demethylase 1 and four and a half LIM domain protein 2 predict risk of prostate cancer recurrence. Cancer Res. 2006;66:11341–7. doi: 10.1158/0008-5472.CAN-06-1570. [DOI] [PubMed] [Google Scholar]
- 18.Shah NM, Pisapia DJ, Maniatais S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43:313–9. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behavioral Neuroscience. 2003;117:1283–91. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- 21.Rizk-Jackson AM, et al. Effects of sex on object recognition and spatial navigation in humans. Behav Brain Res. 2006;173:181–90. doi: 10.1016/j.bbr.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 22.Juraska JM. Sex differences in “cognitive” regions of the rat brain. Psychoneuroendocrinology. 1991;16:105–9. doi: 10.1016/0306-4530(91)90073-3. [DOI] [PubMed] [Google Scholar]
- 23.Nunez JL, Huppenbauer CB, Macbee MD, Juraska JM, DonCarlos LL. Androgen receptor expression in the developing male and female rat visual and prefrontal cortex. J Neurobiol. 2003;56:293–302. doi: 10.1002/neu.10236. [DOI] [PubMed] [Google Scholar]
- 24.Markham JA, Mckian KP, Stroup TS, Juraska JM. Sexually dimorphic aging of dendritic morphology in CA1 of hippocampus. Hippocampus. 2005;15:97–103. doi: 10.1002/hipo.20034. [DOI] [PubMed] [Google Scholar]
- 25.Turner JM, Mahadevaiah SK, Benavente R, Offenberg HH, Heyting C, Burgoyne PS. Analysis of male meiotic “sex body” proteins during XY female meiosis provides new insights into their functions. Chromosoma. 2000;109:426–32. doi: 10.1007/s004120000097. [DOI] [PubMed] [Google Scholar]
- 26.Daniel JA, Pray-Grant MG, Grant PA. Effector proteins for methylated histones: an expanding family. Cell Cycle. 2005;4:919–26. doi: 10.4161/cc.4.7.1824. [DOI] [PubMed] [Google Scholar]
- 27.Bernstein BE, Kamal M, Lindblad-Toh K, Bekiranov S, Bailey DK, Huebert DJ, et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005;120:169–81. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Imwalle DB, Bateman HL, Wills A, Honda S, Harada N, Rissman EF. Impairment of spatial learning by estradiol treatment in female mice is attenuated by estradiol exposure during development. Horm Behav. 2006;50:693–8. doi: 10.1016/j.yhbeh.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Fugger HN, Cunningham SG, Rissman EF, Foster TC. Sex differences in the activational effect of ERalpha on spatial learning. Horm Behav. 1998;34:163–70. doi: 10.1006/hbeh.1998.1475. [DOI] [PubMed] [Google Scholar]
- 30.Juraska JM. Neural plasticity and the development of sex differences. Annu Rev Sex Res. 1998;9:20–38. [PubMed] [Google Scholar]
- 31.Mitev YA, et al. Gender differences in the regulation of 3 alpha-hydroxysteroid dehydrogenase in rat brain and sensitivity to neurosteroid-mediated stress protection. Neuroscience. 2003;120:541–9. doi: 10.1016/s0306-4522(03)00287-2. [DOI] [PubMed] [Google Scholar]
- 32.Lustig RH, Hua P, Wilson MC, Federoff HJ. Ontogeny, sex dimorphism and neonatal sex hormone determination of synapse-associated messenger RNAs in rat brain. Brain Res Mol Brain Res. 1993;20:101–10. doi: 10.1016/0169-328x(93)90114-5. [DOI] [PubMed] [Google Scholar]
- 33.Mora F, Segovia G, del Arco A. Aging, plasticity and environmental enrichment: structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res Rev. 2007;55:78–88. doi: 10.1016/j.brainresrev.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Del Arco A, Segovia G, Garrido P, de Blas M, Mora F. Stress, prefrontal cortex and environmental enrichment: studies on dopamine and acetylcholine release and working memory performance in rats. Behav Brain Res. 2007;176:267–73. doi: 10.1016/j.bbr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 35.vom Saal FS, Bronson FH. Sexual characteristics of adult female mice are correlated with their blood testosterone levels during prenatal development. Science. 1980;208:597–9. doi: 10.1126/science.7367881. [DOI] [PubMed] [Google Scholar]
- 36.Roffi J, Chami F, Corbier P, Edwards DA. Testicular hormones during the first few hours after birth augment the tendency of adult male rats to mount receptive females. Physiol Behav. 1987;39:625–8. doi: 10.1016/0031-9384(87)90163-6. [DOI] [PubMed] [Google Scholar]
- 37.Corbier P, Edwards DA, Roffi J. The neonatal testosterone surge: a comparative study. Arch Int Physiol Biochim Biophys. 1992;100:127–31. doi: 10.3109/13813459209035274. [DOI] [PubMed] [Google Scholar]
- 38.Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19:469–98. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- 39.Lonard DM, O’Malley BW. Gene transcription: two worlds merged. Nature. 2008;452:946–7. doi: 10.1038/452946a. [DOI] [PubMed] [Google Scholar]
- 40.Chan HM, La Thangue NB. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J Cell Sci. 2001;114:2363–73. doi: 10.1242/jcs.114.13.2363. [DOI] [PubMed] [Google Scholar]
- 41.Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9:601–10. doi: 10.1016/s1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- 42.Metivier R, Reid G, Gannon F. Transcription in four dimensions: nuclear receptor-directed initiation of gene expression. EMBO Rep. 2006;7:161–7. doi: 10.1038/sj.embor.7400626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metivier R, Penot G, Hubner MR, Reid G, Brand H, Kos M, et al. Estrogen receptor-alpha directs ordered, cyclical and combinatorial recruitment of cofactors on a natural target promoter. Cell. 2003;115:751–63. doi: 10.1016/s0092-8674(03)00934-6. [DOI] [PubMed] [Google Scholar]
- 44.Auger AP, Perrot-Sinal TS, Auger CJ, Ekas LA, Tetel MJ, McCarthy MM. Expression of the nuclear receptor coactivator, cAMP response element-binding protein, is sexually dimorphic and modulates sexual differentiation of neonatal rat brain. Endocrinol. 2002;143:3009–16. doi: 10.1210/endo.143.8.8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee DY, Northrop JP, Kuo MH, Stallcup MR. Histone H3 lysine 9 methyltransferase G9a is a transcriptional coactivator for nuclear receptors. J Biol Chem. 2006;281:8476–85. doi: 10.1074/jbc.M511093200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolf SS, Patchev VK, Obendorf M. A novel variant of the putative demethylase gene, s-JMJD1C, is a coactivator of the AR. Arch Biochem Biophys. 2007;460:56–66. doi: 10.1016/j.abb.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 47.Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, et al. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26:2335–42. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gioiosa L, Chen X, Watkins R, Umeda EA, Arnold AP. Sex chromosome complement affects nociception and analgesia in newborn mice. J Pain. 2008;9:962–9. doi: 10.1016/j.jpain.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McPhie-Lalmansingh AA, Tejada LD, Weaver JL, Rissman EF. Sex chromosome complement affects social interactions in mice. Horm Behav. 2008;54:565–70. doi: 10.1016/j.yhbeh.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, et al. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9:220–6. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- 51.De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, et al. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–14. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen X, Watkins R, Delot E, Reliene R, Schiestl RH, Burgoyne PS, et al. Sex difference in neural tube defects in p53-null mice is caused by differences in the complement of X not Y genes. Dev Neurobiol. 2008;68:265–73. doi: 10.1002/dneu.20581. [DOI] [PubMed] [Google Scholar]
- 53.Iwase S, Lan F, Bayliss P, de la Torre-Ubieta L, Huarte M, Qi HH, et al. The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell. 2007;128:1077–88. doi: 10.1016/j.cell.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 54.Xu J, Deng X, Disteche CM. Sex-specific expression of the X-linked histone demethylase gene Jarid1c in brain. PLoS ONE. 2008;3:2553. doi: 10.1371/journal.pone.0002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet. 2000;9:2395–402. doi: 10.1093/hmg/9.16.2395. [DOI] [PubMed] [Google Scholar]
- 56.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–25. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 57.Wu JI, Lessard J, Olave IA, Qui Z, Ghosh A, Graef IA, et al. Regulation of dendritic development by neuron-specific chromatin remodeling complexes. Neuron. 2007;56:94–108. doi: 10.1016/j.neuron.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 58.Taniura H, Sng JC, Yoneda Y. Histone modifications in the brain. Neurochem Int. 2007;51:85–91. doi: 10.1016/j.neuint.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 59.Levenson JM, Roth TL, Lubin FD, Miller CA, Huang IC, Desai P, et al. Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem. 2006;281:15763–73. doi: 10.1074/jbc.M511767200. [DOI] [PubMed] [Google Scholar]
- 60.Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–59. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 61.Hsieh J, Gage FH. Chromatin remodeling in neural development and plasticity. Curr Opin Cell Biol. 2005;17:664–71. doi: 10.1016/j.ceb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Kishi N, Macklis JD. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol Cell Neurosci. 2004;27:306–21. doi: 10.1016/j.mcn.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 63.Lin W, Zhang Z, Srajer G, Chen YC, Huang M, Phan HM, et al. Proper expression of the Gcn5 histone acetyltransferase is required for neural tube closure in mouse embryos. Dev Dyn. 2008;237:928–40. doi: 10.1002/dvdy.21479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang SK, Cha SH, Jeon HG. Curcumin-induced histone hypoacetylation enhances caspase-3-dependent glioma cell death and neurogenesis of neural progenitor cells. Stem Cells Dev. 2006;15:165–74. doi: 10.1089/scd.2006.15.165. [DOI] [PubMed] [Google Scholar]
- 65.Hagerman RJ. Lessons from fragile X regarding neurobiology, autism and neurodegeneration. J Dev Behav Pediatr. 2006;27:63–74. doi: 10.1097/00004703-200602000-00012. [DOI] [PubMed] [Google Scholar]
- 66.Shibayama A, Cook EH, Feng J, Glanzmann C, Yan J, Craddock N, et al. MECP2 structural and 3′-UTR variants in schizophrenia, autism and other psychiatric diseases: a possible association with autism. Am J Med Genet B Neuropsychiatr Genet. 2004;128:50–3. doi: 10.1002/ajmg.b.30016. [DOI] [PubMed] [Google Scholar]
- 67.Berube NG, Jagla M, Smeenk C, De Repentigny Y, Kothary R, Picketts DJ. Neurodevelopmental defects resulting from ATRX overexpression in transgenic mice. Hum Mol Genet. 2002;11:253–61. doi: 10.1093/hmg/11.3.253. [DOI] [PubMed] [Google Scholar]
- 68.Weaving LS, Christodoulou J, Williamson SL, Friend KL, McKenzie OL, Archer H, et al. Mutations of CDKL5 cause a severe neurodevelopmental disorder with infantile spasms and mental retardation. Am J Hum Genet. 2004;75:1079–93. doi: 10.1086/426462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dean SL, McCarthy MM. Steroids, sex and the cerebellar cortex: implications for human disease. Cerebellum. 2008;7:38–47. doi: 10.1007/s12311-008-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]