Abstract
Geoffrey Wingfield Harris’ demonstration of hypothalamic hormones regulating pituitary function led to their structural identification and therapeutic utilization in a wide spectrum of diseases. Amongst these, Gonadotropin Releasing Hormone (GnRH) and its analogs are widely employed in modulating gonadotropin and sex steroid secretion to treat infertility, precocious puberty and many hormone-dependent diseases including endometriosis, uterine fibroids and prostatic cancer. While these effects are all mediated via modulation of the pituitary gonadotrope GnRH receptor and the Gq signaling pathway, it has become increasingly apparent that GnRH regulates many extrapituitary cells in the nervous system and periphery. This review focuses on two such examples, namely GnRH analog effects on reproductive behaviors and GnRH analog effects on the inhibition of cancer cell growth. For both effects the relative activities of a range of GnRH analogs is distinctly different from their effects on the pituitary gonadotrope and different signaling pathways are utilized. As there is only a single functional GnRH receptor type in man we have proposed that the GnRH receptor can assume different conformations which have different selectivity for GnRH analogs and intracellular signaling proteins complexes. This ligand-induced selective-signaling recruits certain pathways while by-passing others and has implications in developing more selective GnRH analogs for highly specific therapeutic intervention.
Keywords: GnRH, GnRH receptor, Ligand-induced selective-signaling, Reproduction, Antiproliferation, GPCR, Signal tracking, Cancer
1. Introduction
This article arises from the Geoffrey Wingfield Harris Memorial Lecture delivered at the International Neuroendocrinology Congress held in Pittsburgh in 2006 entitled “GnRH: A Peptide for all Seasons”. It is dedicated to three neuroendocrinology colleagues who recently passed away. Sam Yen died on 26 December 2006 after a life-time of major research contributions to gynecological endocrinology including many of the earliest studies on GnRH effects in women. Sam (Don) McCann pioneered the quest to discover the hypothalamic factor regulating gonadotropins and Richard (Dick) Peter who elucidated the neuroendocrine control of gonadotropins in fish. Both died early this year.
Following the pioneering discoveries of the magnocellular neurosecretory neurons by Ernst and Berta Schaller, Geoffrey Harris (Fig. 1) conducted a series of seminal studies in the 1940s that demonstrated that a capillary portal vessel system which connected the hypothalamic region of the central nervous system with the pituitary (see review [46]). He demonstrated this by means of a series of exquisite experiments including the injection of India ink into the capillary network (Fig. 2). He went on to propose that this portal system conducted humoral factors from the brain to the pituitary to regulate hormone secretion. In support of this hypothesis, Harris further demonstrated that disruption of these portal vessels gave rise to a decline in reproductive activity in ferrets exposed to stimulatory photoperiods. There was considerable opposition to Harris’ conclusions by Solly Zuckerman’s group doing similar experiments and it is testimony to Harris’ experimental prowess and his tenacity that his proposal endured and the concept of hypothalamic hormone regulation of pituitary function was spawned. The quest to determine the structure of these releasing factors was spearheaded by the groups of McCann, Schally and Guillemin. In 1971, after a monumental purification effort from millions of pig brains, Schallyfirst announced the structure of the decapeptide GnRH [5,80,125] (Fig. 3). This discovery, together with that of the other hypothalamic hormones, was a major breakthrough in neuroendocrinology and has culminated in the legacy of extensive clinical applications of hypothalamic peptide analogs which impinge on almost all fields of medicine. GnRH analogs, themselves, are extensively used as interventive therapies in a wide spectrum of pathologies which is reffected in annual sales of about $2 billion. Chronic pharmacological administration of GnRH agonists induces gonadotrope desensitization and an inhibition of gonadotropin secretion resulting in an inhibition of gonadal sex steroid hormones and gametogenesis. This is a slow process requiring one to two weeks. In contrast, GnRH antagonists have an immediate inhibition of gonadotropins; the rate of decline equating that of their half life. Therapeutic applications in hormone-dependent diseases include prostatic, breast and ovarian cancer, endometriosis, uterine fibroids, precocious puberty, polycystic ovarian syndrome, and acute intermittent porphyria [7,16,24,25,33,37,92,99]. Natural GnRH administered in hourly pulses (to mimic hypothalamic secretion) is effective in treating hypogonadotropic hypogonadism (e.g. in anorexia nervosa and Kallman’s syndrome) and in cryptorchidism. GnRH agonists and antagonists are also very extensively used to inhibit endogenous gonadotropin to allow regulated add-back for the induction of ovulation in vitro fertilization (IVF) treatment of infertility. GnRH antagonists also hold promise as a new generation of male and female contraceptives with add-back gonadal steroids [4,38,93,108].
Fig. 1.

Geoffrey Wingfield Harris.
Fig. 2.

Harris’ original demonstration of the capillary network (injected with India ink) connecting the median eminence (P.T., P.V) with the pars distalis (P.D.) of the pituitary.
Fig. 3.
Primary amino acid sequences of naturally occurring decapeptide gonadotropin-releasing hormone (GnRH) structural variants spanning approximately 600 million years of evolution. The boxed regions show the conserved amino- and carboxyl-terminal residues which are involved in receptor binding and activation. The GnRHs are named according to the species in which they were first discovered and they may be represented in more than one species. For example, mammalian GnRH is widely conserved in amphibians and primitive bony fish, and chicken GnRH II is present in most vertebrate species, including man. An octopus GnRH and an additional Ciona GnRH comprising 12 and 16 amino acids, respectively, but retaining the conserved amino- and carboxyl-terminal domains are not shown (Reproduced from [9]).
While the primary function of GnRH is to regulate gonadotropins, it became apparent during the ensuing 30 years since its discovery that the hormone and its receptors are also expressed, and have actions, in many other tissues. These include various regions of the central and peripheral nervous system, in most reproductive tissues, in many non-reproductive tissues, in immune cells and also in many cancer cells [21,48].
Although the Geoffrey Harris lecture painted a broad canvas covering a wide spectrum of GnRH research, some of these areas (such as intracellular signaling, GnRH receptors and GnRH analogs) have been the subject of extensive and recent review [39,58,83,86,87,95,104,105,126,135] and will not be reiterated in detail in this article. We would instead focus on three extra-pituitary features of GnRH action. The first is the effects of GnRHs in stimulating reproductive behaviors. The second relates to the direct inhibition of cancer cells by GnRHs and analogs. For both of these the pharmacology of the effects of GnRH agonists and antagonists is distinctly different from their effects on pituitary gonadotropin secretion. The third arises from this phenomenon and encompasses the concept of ligand-induced selective-signaling (LiSS) by GnRH analogs which we developed to explain the differing pharmacology in different cellular environments [81,89,95]. In support of this we present evidence of the existence of different conformations of the GnRH receptor which give rise to selective binding of GnRH analogs and differential intracellular signaling. In order to understand these phenomena it is necessary to first briefly review the structures and molecular functioning of GnRHs and GnRH receptors.
2. Diversity of GnRH structures
Following the structural elucidation of the mammalian GnRH, a number of studies on hypothalamic extracts from various vertebrates claimed that their GnRHs were identical to the mammalian structure. However, this conclusion was based on low-resolution gel filtration separation of hypothalamic extracts and the use of antisera of poorly defined specificity. In view of the structural diversity of the related oxytocin and vasopressin family of peptides we considered that this conservation of GnRH structures was unlikely. Utilizing a range of sequence-specific antisera against mammalian GnRH and ion exchange chromatography, we demonstrated that a series of different structures of GnRH were present in different vertebrates [64,65]. This set the scene for the isolation of the first novel GnRH from chicken hypothalamus (chicken I GnRH) which differed by a single substitution of glutamine for arginine in position 8 (Fig. 3) [66,67]. Thirteen structural variants of GnRH have now been identified from all the vertebrate classes, nine from protochordates and one from a species of octopus, see Refs. [2,9,95,129]. These diverse structures represent “experiments of nature” over some 600 million years and provide insight into conserved and functionally important residues. It is apparent that the length of the peptide and the amino- and carboxyl-terminal domains are highly conserved and that these features are important for receptor binding and activation. Furthermore, the achiral amino acid, glycine, is conserved in position 6 in all the jawed vertebrate GnRHs (Fig. 3) and facilitates a folded βII′-type turn conformation such that the amino- and carboxyl-termini are closely apposed when bound to the receptor. Interestingly, in jawless fish species (lamprey), and in the protochordates (sea squirt species) a chiral amino acid is present in position 6 thus hindering the adoption of the folded conformation and suggesting that, in these species, the peptides bind to the receptor in a more extended conformation (see below). In vertebrates, the GnRH sequence is represented as a single copy within a gene and the active peptide is cleaved from the precursor protein by processing enzymes [90,100,127]. In contrast, several GnRH sequences are encoded in tandem within single genes in protochordates [2].
3. Three-dimensional structure of GnRH I
The notion that in the jawed vertebrates glycine in position 6 allows folding of the GnRH to bind its receptor was supported by the demonstration that protochordate GnRHs with the chiral amino acid in position 6 had very poor activity at vertebrate GnRH receptors but this activity could be enhanced more than 10-fold by substitution with the achiral glycine in position 6 (Fig. 4) [9]. In contrast, protochordate GnRH receptors from sea squirts (tunicates) did not distinguish between the GnRHs with different amino acid residues in this position suggesting that the peptide is able to interact with the receptor binding sites in a more extended form and that evolution of receptors requiring interaction with GnRH in the folded conformation only arose after the evolution of jawed vertebrates. Structural analysis of the GnRHs using a combination of ion-mobility mass spectrometry and molecular modeling revealed a close correlation between the propensity for the formation of the βII′-type turn conformation (e.g. mammalian GnRH and a Gly6-substituted sea squirt GnRH) and higher binding affinity at vertebrate receptors [9] (Fig. 5). These findings indicated that the substitution of glycine for a chiral amino acid during evolution facilitates a more constrained conformation of GnRH for receptor binding and that this subtle single amino acid substitution in a site remote from the ligand functional domains has marked effects on its structure and activity.
Fig. 4.
Competitive binding of mammalian, Ciona and position six substituted Ciona I GnRHs to human, Ciona A and chicken GnRH receptors transiently transfected in COS-7 cells. (a,b) ■, mammalian GnRH; ▲, Ciona I GnRH; △, Ciona II GnRH; ○, Ciona III GnRH. (c,d) ■, mammalian GnRH; ▲, Ciona I GnRH; ●, [Gly6]Ciona I GnRH; □, [D-Ala6]Ciona I GnRH (Reproduced from [9]).
Fig. 5.

Ribbon representations of peptide structures. The peptide backbone of: (a) Ciona I GnRH, (b) [Gly6]Ciona I GnRH, and (c) mammalian GnRH are shown. The Ciona I peptides contain protonated His2 and mammalian GnRH has Arg8 and His2 both protonated. Each is a snapshot from dynamics performed with the Born–Solvent model, with the amino- and carboxyl-terminal residues represented by CPK structure. The distance between the alpha carbons of the terminal residues in each snapshot is marked. The [D-Ala6]Ciona I GnRH structure was similar to [Gly6]Ciona I GnRH (not shown) (Reproduced from [9]).
In the absence of a crystal structure of GnRH, indirect studies utilizing fluorescence spectroscopy [97,130], region specific antisera [91], conformational memory approaches [45] and NMR [17,77] support the above conclusion that mammalian GnRH I adopts the folded structure. A three-dimensional structure of GnRH based on a recent NMR report (PDB code: 1YY1) is shown in Fig. 6. We have utilized this three-dimensional structure when interrogating the binding of GnRH to its cognate receptor (see below).
Fig. 6.

The NMR structure of GnRH I (PDB Code: 1YY1).
4. GnRH receptor structure and function
The first cloning of the mammalian GnRH receptor was accomplished by Tsustmi et al. [141] utilizing RNA from the LβT2 mouse gonadotrope cell line. Subsequently, a large number of GnRH receptors have been cloned from many species in all the major vertebrate classes as well as from modern representatives of protochordate progenitors of the vertebrates (see review [95]). In addition, GnRH receptor orthologs have been cloned from a mollusc [122] and an insect [50] and even from the primitive nematode, Caenorhabditis elegans [95]. However, it is intriguing that the cognate ligand for these receptors is not GnRH and that GnRH evolved later as a ligand for the receptor. In Drosophila, the cognate ligand is a nonapeptide called adipokinetic hormone [134].
The GnRH receptors are classical G protein-coupled receptors (GPCRs) with seven transmembrane domains (TMs) connected by extracellular loops (ECLs) and intracellular loops (ICLs). They are members of the Class A or Group 1 rhodopsin family of GPCRs and carry the hallmarks of conserved amino acid residues of the rhodopsin superfamily (see Fig. 7). The human and all mammalian pituitary GnRH receptors (designated type I GnRH receptors) [95] are characterized by the unique absence amongst GPCRs of a carboxyl-terminal tail. In all other GPCRs and the GnRH receptors from non-mammalian vertebrates and the protochordates, there is a carboxyl-terminal tail which is the target for homologous and heterologous phosphorylation which results in the docking of β-arrestin. This event results in a rapid desensitization of the receptor through uncoupling to G proteins and also mediates receptor internalization (see below).
Fig. 7.
Two-dimensional representation of the human GnRH receptor. The 7-TM domains (boxed) are connected by 3 ECLs and 3 ICLs. Ligand binding residues (red) and residues thought to be important in receptor structure or binding pocket configuration (green) are shown. These include disulfide bond formation and glycosylation sites. Residues involved in receptor activation are shown in blue. Residues in squares are ones highly conserved throughout the rhodopsin-like family of GPCRs and designated as N.50 using the nomenclature of Ballesteros and Weinstein. Residues involved in coupling to Gproteins are shown in orange. Putative protein kinase C (PKC) and protein kinase A (PKA) phosphorylation sites are indicated. The intermolecular interactions between GnRH I residues and the receptor are indicated with red lines (Modified from [95]).
A number of highly conserved amino acid residues are present in all members of the rhodopsin family, particularly in the 7-TM domains, and almost all GPCRs can activate G-proteins, supporting the concept that GPCRs share a common protein folding and activation mechanism. However, the human and other mammalian GnRH receptors have some unusual features in addition to the absence of a carboxyl-terminal tail such as a reciprocal exchange of the conserved Asp2.50-Asn7.49 pair in TM 2 and TM 7 (Fig. 7). We demonstrated that mutation of Asn87(2.50) to Asp abolished receptor function, but a second mutation of Asp318(7.49) to Asn in the mouse GnRH receptor, recreating the arrangement found in other GPCRs, restored ligand binding [146]. This restoration of ligand binding by reciprocal mutation demonstrates that the side-chains of the two residues in TMs 2 and 7 have complementary roles in maintaining the structure of the receptor and occupy the same microenvironment within the receptor helical bundle. Subsequently, the crystal structure of bovine rhodopsin revealed that these residues are capable of interacting through a water molecule [110] thus validating the GnRH receptor models. Interestingly, this exchange prevents the human GnRH receptor coupling to phospholipase D by the small G-proteins ARF and RhoA, implying receptor conformation-dependent signaling selectivity [98].
We have built a homology model of the human GnRH receptor in the inactive state using the high-resolution crystal structure of bovine rhodopsin in the dark state (1U19) as a template with the “MODELLER” module within DS Modeling (version 1.6, Accelrys, San Diego, CA, USA). The use of the rhodopsin crystal structure to comparatively model the GnRH receptor has been extensively validated by our laboratory and independent groups using site-directed mutagenesis and peptide and non-peptide ligand docking [10,53,132]. All known GnRH receptor sequences from a wide range of species were aligned with bovine rhodopsin using “ALIGN2D” within the MODELLER program. This was followed by minor manual adjustments ensuring that the most highly conserved residues, motifs, and the disulfide bridge Cys114–Cys196 were located at the same place as in the rhodopsin structure. The experimentally identified disulfide bond between Cys14, in the N-terminal domain and Cys200 in the second extracellular loop (ECL 2) [27] was also incorporated into the model as an additional constraint of the receptor structure. The MODELLER-generated models with the highest values of the 3D-profile score were selected for refinement according to the experimentally identified receptor inter- and intra-molecular interactions. The model was then subjected to energy-minimization and molecular dynamics (MD) simulations by means of the CHARMM program. Harmonic constraints of 2.5 kcal/mol/Å2 on the receptor backbone atoms were applied to allow small conformational changes without loss of the overall topology. The model reveals the experimentally identified hydrogen bonds between Asn53(1.50)-Asn87(2.50)- Asp319(7.49), between Asp98(2.61)-Lys121(3.32), and between Asp138(3.49)-Arg139(3.50) (Fig. 8). This model has provided insights into the potential intramolecular contacts between the seven-TM domains of the human GnRH receptor which are involved in receptor folding, tertiary structural configuration and receptor activation. Using site-directed mutagenesis guided by molecular modeling, we have identified a number of receptor intramolecular interactions (Fig. 8) which modulate receptor conformational states accounting for ligand binding selectivity and signaling efficacy. Mutations of certain residues in the intracellular segments of TM domains to Ala (Met132(3.43), Met227(5.54), Phe272(6.40), Phe276(6.44) and Ile322(7.52)) of the human GnRH receptor allosterically increased ligand binding affinity for GnRH II, but had little effect on GnRH I binding affinity [75]. We propose that these residues form part of the receptor intramolecular allosteric network (Fig. 8) which transfer to specific ligand structural elements. Disruption of the intramolecular interactions by mutation of the network residues alters the receptor conformational state, changing ligand binding and signaling selectivity. This aspect is considered in greater depth later in the article.
Fig. 8.
A model of the 7-TM domains of the human GnRH receptor. The model shows the experimentally identified receptor intramolecular H-bond interactions: Asn53-Asn87-Asp319; Asp98-Lys121, Asp138-Arg139, and the hydrophobic interactions: Met132-Phe272-Ile322 (and the surrounding residues Met227 and Phe276).
The crystal structure of a photoactivated deprotonated intermediate of rhodopsin reveals that transformation of rhodopsin from the ground state to the intermediate state involves only minor changes in the structure, in contrast to earlier predictions of major structural rearrangements [124]. This indicates that a relatively subtle change can generate different receptor conformational states. We have built an active human GnRH receptor model based on this crystal structure (PDB code: 2I37) using a similar method to that described above. A βII′-type turn conformation of GnRH I (derived from an NMR structure; PDB code: 1YY1) and of GnRH II were docked into the model according to the experimentally identified GnRH binding sites (Fig. 9). GnRH ligand docking requires a large displacement of ECL 2 and the disulfide bonded N-terminal domain. The ligand–receptor complex was then optimized by energy-minimization and MD simulations of 150 ps using a similar setup as described for the oxytocin receptor [35] with harmonic restraints (2.5 kcal/mol/Å2) on the receptor backbone atoms except for ECL 2 and the N-terminal domain. The molecular docking shows the intermolecular interactions between GnRH I and the receptor: pGlu1 interacts with Asn212, His2 with Lys121/Asp98, Tyr5 with Tyr290, Arg8 with Asp302 and Pro9-Gly10NH2 with Trp101/Asn102 (Fig. 9). Interestingly, when GnRH II is docked into the receptor model using the same intermolecular interactions (His2 with Lys121, Pro9-Gly10NH2 with Trp101 and Asn102, and His5 with Tyr290), Tyr8 of GnRH II (unlike Arg8 of GnRH I) faces away from Asp302 and towards ECL 2. We therefore propose that the different intramolecular interactions between Arg8 of GnRH I and Asp302 of ECL 3, and Tyr8 of GnRH II with residues in ECL 2 play important roles in stabilizing different receptor active conformations with differing signaling capability (see later).
Fig. 9.
Molecular docking of GnRH I and GnRH II. A βII′ conformation of GnRH I derived from the NMR structure (1YY1) and GnRH II were docked into the receptor model according to the experimentally identified and putative intermolecular interactions between GnRH and the receptor. (a) GnRH I docking shows that pGlu1 interacts with Asn212, His2 with Lys121/Asp98, Tyr5 with Tyr290 and Arg8 with Asp302 and Pro9Gly10NH2 with Trp101/Asn102. (b) GnRH II docking shows the same contacts with the exception that the side-chain of Tyr8 of GnRH II does not interact with Asp302 and instead faces away from Asp302 towards ECL 2.
5. Absence of rapid desensitization and ligand-induced internalization of mammalian GnRH receptors
Although chronic administration of GnRH agonists gives rise to a diminution in secretion of biologically active gonadotropins and consequent decline in gonadal activity and sex steroid hormone production, this is a slow pharmacological process which takes days to weeks and is distinctly different from the classical rapid desensitization (min) and internalization of receptors which occurs with the majority of GPCRs. Indeed, it is evident, that the unique absence of a cytoplasmic carboxyl-terminal tail conveys a resistance of the GnRH receptor to rapid desensitization and ligand-induced internalization as there is no recruitment of β-arrestin [26,82,95,143]. Consequently, the receptor experiences prolonged activation and the elimination of the cytoplasmic carboxyl-terminal tail during evolution may have arisen in order to allow increased duration of gonadotropin secretion required for oocyte stimulation and ovulation. This lack of desensitization has consequences in the ability of chronic GnRH exposure to inhibit proliferation and induce apoptosis in tumor cells expressing the GnRH receptor. Inhibition of gonadotropin by GnRH agonist treatment is frequently attributed to GnRH receptor “down-regulation” and loosely as “desensitization”. However, it is evident that this is incorrect as gonadotropin α-subunit remains massively elevated even after years of treatment with GnRH agonist in prostate cancer patients. Thus, GnRH receptors and intracellular machinery are continuing to respond to GnRH agonist but biologically active mature gonadotropins are no longer produced.
The internalization pathways utilized by GnRH receptors differ between receptor sub-types. The lack of a carboxyl- terminal domain in the mammalian type I GnRH receptors probably accounts for their β-arrestin-independency for internalization [143]. This region has been extensively reported to mediate GPCR interactions with β-arrestin [36]. Murine GnRH receptors undergo a slow rate of internalization in αT3-1 and LβT2 cells, and the internalization of human type I GnRH receptor expressed in αT4 pituitary cells proceeds at a slow rate and is β-arrestin- independent [51]. This contrasts with the Xenopus type I GnRH receptor, which possesses a carboxyl-terminal tail and internalizes more rapidly in a β-arrestin-dependent manner when expressed in αT4 cells [51]. It appears that mammalian and non-mammalian GnRH receptors can both be targeted for clathrin-mediated internalization, regardless of their β-arrestin-dependence or independence. The catfish and chicken GnRH receptors both exhibit rapid internalization kinetics and were shown to be dependent on their carboxyl-terminal tail domains for this process [12,13,112]. The chicken GnRH receptor internalized 125I-[His5,D-Tyr6]GnRH at an initial rate of 11.3% of surface receptor per minute to a maximal level of approximately 75%, compared with only 0.71% per minute and maximal level of 25% for the human GnRH receptor in the same cellular background. To determine whether the presence of the cytoplasmic carboxyl-terminal tail was responsible for the more rapid internalization of the chicken GnRH receptor, the tail was truncated at Ser337. Internalization of 125I-[His5, D-Tyr6]GnRH by the Ser337- stop-chicken GnRH receptor was much slower than the wild-type chicken receptor, and displayed similar internalization kinetics to the human GnRH receptor, with a rate of 0.55% per minute and a maximal level of approximately 25% [112]. A threonine-doublet located at the distal end of the chicken GnRH receptor cytoplasmic tail was shown to be critical, as was a membrane proximal cysteine residue [114]. The chicken GnRH receptor was shown to preferentially undergo rapid agonist-induced internalization in a dynamin- and caveolae-dependent (but arrestin-independent) manner, and palmitoylation of the membrane proximal cysteine residue may serve to target the chicken GnRH receptor to caveolae microdomains for signaling and internalization [114]. The internalization pathways of the three bullfrog GnRH receptor subtypes have been partially characterized [1]. The bullfrog type I GnRH receptor was shown to internalize via a β-arrestin and dynamin-dependent pathway, whereas the bullfrog type II and III GnRH receptors internalize via a pathway that is β-arrestin-independent, but dynamin-dependent, similar to the pathway utilized by the chicken GnRH receptor [1,114]. Unlike all the mammalian type I GnRH receptors, the cloned marmoset type II GnRH receptor [94] has a carboxyl-terminal tail, and a study identified a serine-doublet in the carboxyl-terminal tail as critical for internalization of the type II GnRH receptor and suggested that these residues undergo phosphorylation by GRKs. However, neither of these residues, nor the carboxyl-terminal tail, was shown to be required for β-arrestin-dependent internalization [123]. The above studies thus suggest that the carboxyl-terminal tail of the non-mammalian GnRH receptors and mammalian type II GnRH receptors plays a pivotal role in their function and sub-cellular trafficking to divergent internalization pathways. While there is clearly divergence in the internalization pathways utilized by the tailed GnRH receptors, the sequence motifs and structural elements within the cytoplasmic carboxyl-terminal domain that determine which internalization pathway will be utilized have yet to be properly elucidated.
By measuring the trafficking of radioactive GnRH agonists, the above studies concluded that mammalian type I GnRH receptors undergo slow ligand-dependent internalization. However, by the direct measurement of mammalian type I GnRH receptor trafficking both in the presence and absence of unlabeled GnRH agonist we have measured low basal levels of constitutive agonist-independent internalization [115]. Stimulation with GnRH agonist did not significantly enhance the level of mammalian type I receptor internalization above the basal level, in contrast to mammalian receptor chimeras with cytoplasmic tails, or the TRH receptor [115]. These data suggest that as a result of the deletion of the cytoplasmic carboxyl-terminal tail during evolution the mammalian type I GnRH receptors was directed at avoiding internalization and desensitization for prolonged activation of the pituitary receptor [26,82,115]. Recent biophysical evidence for type I GnRH receptor self-association and localization to low-density membrane microdomains highlights the complexities of GnRH receptor multi-protein scaffolding and trafficking processes [11,52,106,107]. Although agonists and antagonists both slow type I GnRH receptor lateral diffusion, only agonists induce the formation of large multi-protein complexes [52]. These events are apparently not related to receptor internalization which is identical for antagonists and agonists [115]. The biophysical approaches used by Clay and colleagues [11,52,106,107] may provide the means for investigating ligand-induced selective signaling at the GnRH receptor in different cell types following the binding of different GnRH analogs (see below) [89,95].
6. Most vertebrates have several GnRH forms and several cognate GnRH receptors serving diverse functions
Elucidation of the sequence of GnRHs and GnRH receptors from a wide range of vertebrates revealed that multiple forms of the ligands and multiple forms of cognate receptors exist in the majority of vertebrates. Phylogenetic analysis of the gene sequences further revealed that there are three families of ligands and receptors [95,100]. In teleost fish and amphibians it is apparent that there are three GnRHs and three cognate receptors which have a wide tissue distribution indicating that they have been co-opted as regulators of diverse functions. In the central nervous system of teleost fish, the three GnRHs are discretely distributed. GnRH I is localized in the preoptic area and appears to be a major regulator of pituitary function. GnRH II is localized in the mid-brain and is thought to be a regulator of reproductive behavior, GnRH III is localized in the terminal nerve area of the forebrain and appears to regulate olfactory systems. Indeed, there is apparently a coordinated activation of these three GnRH systems in some species such as the salmon to regulate several aspects of reproduction. When salmon begin their migration from the ocean up rivers to their spawning grounds, it appears that the GnRH I neurons become activated to stimulate pituitary gonadotropins, GnRH II neurons become activated to stimulate reproductive behavior and GnRH III neurons are activated to sensitize the olfactory system to allow detection of chemicals for navigation to spawning streams (Swanson et al., personal communication).
GnRH I is highly variable in structure amongst vertebrates while the GnRH II structure is totally conserved in jawed vertebrates. The GnRH III structure is also conserved in teleosts. GnRHs are known to regulate pituitary hormones other than gonadotropins in teleost fish and it is intriguing that distinct GnRH receptor subtypes are discretely localized to gonadotropes, somatotropes and lactotropes [111]. An example of the co-localization of the teleost type IA and type III receptors with gonadotropes and somatotropes, respectively, is shown in Fig. 10. GnRH and the receptors are also expressed in the gonads of teleost fish where they affect gametogenesis and steroidogenesis. In addition to actions in the central nervous system, pituitary and reproductive tissues, it is apparent that GnRHs and their receptors have also been recruited during evolution to regulate the peripheral nervous system. In the bullfrog, the type II GnRH receptor is expressed in the sympathetic ganglion and GnRH II is a potent regulator of M currents (inward rectifying K+ channels) which causes a partial depolarization and sensitization of neurons [14,55,57,140].
Fig. 10.
Coronal sections of adult fish pituitary immunofluorescence (A) gonadotropin-releasing hormone receptor (GnRH-R) type IA labeled with Texas Red, (B) luteinizing hormone (LH) β-subunit labeled with fluorescein-isothiocyanate (FITC) and (C) colocalization of GnRH-R type IA and LH β-subunit is seen as yellow fluorescence using a dual-band filter. Sagittal sections of adult pituitary immunofluorescence, (D) GnRH-R type III labeled with Texas Red Texas Red, (E) growth hormone (GH)-containing cells labeled with FITC and (F) colocalization of GnRH-R type III and GH cells seen as yellow fluorescence using a dual-band filter. Scale bar: (A–C) 200 μm; (D–F) 200 μm (Reproduced from [111]).
In reptile, bird and mammalian species only two GnRHs (GnRH I and GnRH II) and two receptors ( type I and type II) are present. This suggests that the third form of GnRH and the third receptor were eliminated from the genome during the transition from amphibia to reptiles. An alternative, but probably less cogent possibility is that there was an additional gene duplication of GnRH and the GnRH receptor in teleosts and amphibia subsequent to the evolution of the reptiles, to give rise to the third ligand and receptor.
7. Potential functions of GnRH in mammals
Hypotheses suggesting multiple physiological roles for GnRH in mammals have been proposed, sometimes by extrapolation, from the comparative tissue distribution of GnRH peptides or their receptors in fish, amphibian, avian and mammalian species [71,78,118,121,133,144]. However, the extent to which the biological roles of GnRH in mammals, compared to other vertebrates, have been modified, superseded or abandoned during evolution is not yet fully understood. Nevertheless, theories concerning the function of GnRH in neuronal, neuroendocrine and reproductive tissues during mammalian development have been considered [56,72,120]. The functions for GnRH in juvenile to adult reproductive maturation and in the maintenance of adult reproductive tissues via autocrine-paracrine mechanisms are both common themes [15,41,68]. The manner in which local GnRH action modulates the physiology and patho-physiology of reproductive tissues is a focus of investigation [23,48,139]. More specialized roles in blastocyst implantation, physiological changes occurring during pregnancy, including modulation of the cellular immune system, and influences on reproductive and parental behavior are also regarded as possibilities in certain mammalian species [15,19,22,40,59,61]. Many mechanistic questions concerning the precise involvement of GnRH in such diverse processes remain unanswered. For example, we do not fully understand the roles of GnRH in the mammalian central nervous system. Some detailed mapping and functional analyses of GnRH I and GnRH II neurons have been performed in mammals [28,29,131,145]. However, the action of GnRH on GnRH neurons or their neighboring cells has not been characterized in great depth, although several model cell lines, derived from mouse hypothalamic GnRH neurons, have provided the foundations for further functional studies [84,119]. More recently, the role of the metastin/kisspeptin receptor (gpr54) in influencing GnRH neuron function has been elucidated [30,85,117,138], but how mammalian GnRH I and GnRH II and the type I or type II GnRH receptors integrate into the complexity of neuronal function, in both males and females, still requires further elaboration.
8. Silencing of GnRHs and GnRH receptors in mammals
GnRH I and GnRH II and the two types of GnRH receptor (type I and type II) are present in the majority of mammalian species studied. Comparative genomics shows that the genes encoding mammalian GnRH ligand precursors and receptors exhibit a remarkable degree of syntenic conservation, exhibited by the retention of particular and characteristic flanking genes at the relevant chromosomal loci [100]. Furthermore, analysis of DNA sequence data and functional studies indicate that there has been sporadic silencing or deletion of GnRH II and/or the type II receptor in various mammals. Thus, GnRH I and GnRH II and the type I and type II GnRH receptors are expressed in some primates such as the marmoset [94] and green monkey while the type II GnRH receptor has been silenced in man and the chimpanzee by stop codons and frame shifts [101]. From a molecular genetics point of view, it is possible that, during evolution, the mammalian type II GnRH receptor gene came under functional pressure by close apposition of 5′ and 3′ anking genes (potentially leading to interference with transcription) and subsequent mutations led to gene silencing. The GnRH II peptide precursor also appears to have been inactivated in the chimpanzee by a premature stop codon. In contrast, both forms of GnRH and receptors may be functional in the pig while both GnRH II and the type II receptor have been silenced through stop codons, deletions and substitutions in the sheep and cow [102]. The 3.7 kb genomic DNA segment lying between the conserved pex11b and RBM8 genes on mouse chromosome 3 does not contain a type II GnRH receptor gene (see Ensembl database, www.ensembl.org) and a similar situation is apparent for the 5.2-kb pex11b–RBM8 inter-genic region on rat chromosome 2. Bioinformatics analyses suggest that the type II GnRH receptor gene and the GnRH-II precursor gene have been deleted from the genomes of laboratory mouse and rat strains. This is not the case for all small mammalian species. For instance, a gene encoding a type II GnRH receptor is retained in the genome of insectivores, such as the common shrew (Sorex araneus) and the musk shrew (Suncus murinus) (Ensembl database and K. Morgan, unpublished data), known to express a functional GnRHII peptide hormone. These observations coupled with other data suggest that the GnRH I/type I receptor system could substitute for the GnRH II/type II receptor system in some species. Interestingly, GnRH I and the type I GnRH receptor are never silenced, only the type II system. This is understandable as the type I GnRH receptor binds GnRH I and GnRH II well, while the type II GnRH receptor is highly selective for GnRH II and binds GnRH I with very low affinity [86]. Thus the type I GnRH receptor can substitute as a mediator of GnRH II actions but the type II GnRH receptor cannot mediate GnRH I actions.
9. GnRH II as a regulator of reproductive behavior
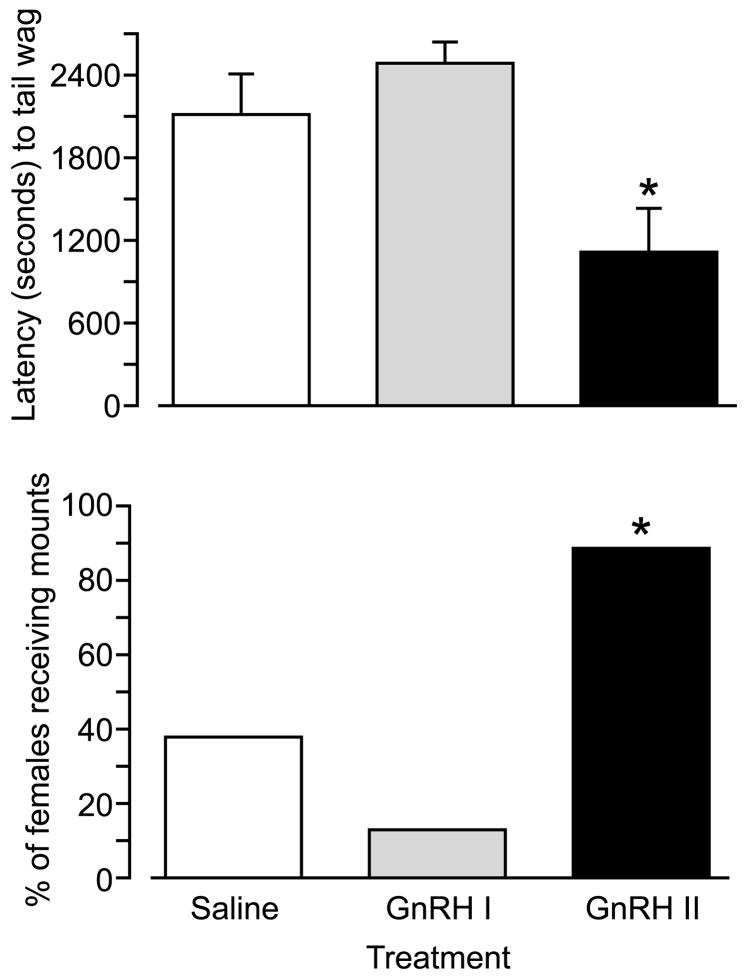
GnRH I and GnRH analogs as well as fragments of GnRH I were shown to stimulate lordosis in female rats many years ago [103]. Subsequently, GnRH II was shown to stimulate courting behavior in ring doves [20] and induces female solicitation of males in song sparrows [78]. GnRH also stimulates spawning behavior in the gold-fish [142]. Another study demonstrated stimulation of male behavior by injecting GnRH II into female green iguanas [116]. In the green anole [3] GnRH also stimulates reproductive behavior. These findings encouraged further exploration of GnRH actions on reproductive behavior in other mammalian species. Introcerebroventricular administration of GnRH II was shown to stimulate sexual behavior in female musk shrews, while GnRH I was inactive (Fig. 11) [137]. Moreover, an antagonist at the type I GnRH receptor which is an agonist at the type II GnRH receptor (antagonist 135-18 stimulates reproductive behavior in female musk shrews suggesting that the effect is mediated by the type II receptor [60]. This conclusion is further supported by the demonstration that antide which is an antagonist at the type I receptor but is inactive at type II receptors, had no effect on GnRH II stimulation of behavior. Interestingly, these effects could only be demonstrated after food restriction and GnRH II itself was found to inhibit feeding behavior in musk shrews [59,60]. Thus, it appears that under normal conditions when animals are satiated the endogenous GnRH II system is fully activating reproductive behavior and exogenous GnRH II is not required. In food restrained animals GnRH II in the midbrain appears to be decreased such that exogenous GnRH II administration can re-instate reproductive behavior. These researchers also showed that GnRH II decreased short-term feed intake. There appears, therefore, to be an interesting dual effect of GnRH II in that at the time that it stimulates reproductive behavior it also inhibits feeding. Thus, GnRH II can play a pivotal role in integrating nutritional status and reproduction which is crucial for survival. This participation of the GnRH system in the relationship between nutrition and reproduction appears to have ancient evolutionary origins as we have found that a GnRH receptor ortholog is expressed in the neuroscrecretory motor neurone in C. elegans which integrates feeding and reproduction (Swanson et al., unpublished).
Fig. 11.
Effects of icv administered GnRH I and GnRH II on latency to tail wag and frequency of mount reception in female musk shrews. *Significant difference (p < 0.05) from other treatments (Modified from [137]).
These studies suggested that GnRH II may also be effective in primates. Studies on the effect of GnRH II on proceptive female behavior in ovariectomised and estrogen replaced female marmosets [8] indicated that intracerebral infusion of GnRH II at 1 and 10 μg doses stimulated proceptive behaviors in the absence of estrogen replacement (Fig. 12). In the presence of estrogen replacement, the peptide was found to be less effective. Thus, similar to the effect of feeding in the musk shrew estrogen may be activating the GnRH II system and exogenous GnRH II is less effective. A model encapsulating these proposals is outlined in Fig. 13. As in the musk shrew experiment, we also utilized a GnRH antagonist which inhibits the type I GnRH receptor but acts as an agonist at the type II GnRH marmoset receptor. The antagonist 135-18 at 1 μg icv was able to Significantly stimulate proceptive behavior in the female marmoset in the absence of estrogen but was ineffective in the presence of estrogen. Another antagonist 135-25 which has similar agonistic properties to 135-18 at the type II GnRH receptor [8] was also able to stimulate proceptive behaviors in the female marmosets at a dose of 1 μg in the absence of estrogen replacement. In contrast, GnRH I at a dose of 1 μg was ineffective at stimulating proceptive reproductive behaviors. Thus, the pharmacology of GnRH analog effects on reproductive behavior is distinctly different from effects on stimulating gonadotropin. For the marmoset this difference is due to the involvement of two distinct receptors (type I and type II) which have been cloned and shown to exhibit different pharmacology. The musk shrew appears to be utilizing a similar system as both receptors appear to be present (Morgan et al., in preparation). In contrast, the aberrant pharmacology for GnRH analog effects on behavior in the rat described many years ago [103] cannot be the result of utilizing a type II GnRH receptor as it has been deleted from the rat genome [100]. It therefore appears that a different pharmacology can be manifested at the type I receptor due to the intracellular context (see LiSS below).
Fig. 12.
Effect of icv administration of GnRH I, GnRH II and GnRH antagonist 135-18 on proceptive behaviors in female marmosets. GnRH II and antagonist 135-18 were Significantly different from vehicle control (Data derived from [8]).
Fig. 13.

A proposed model of the role of GnRH II neurons in mediating reproductive behaviors in female mammals.
10. Direct inhibition of proliferation and stimulation of apoptosis in cancer cells by GnRH analogs
GnRH agonists are extensively used in the treatment of sex hormone-dependent cancers. Chronic administration of these agonists induces the desensitization of pituitary gonadotropes resulting in a decrease in sex steroid hormone secretion and amelioration of the disease. However, a plethora of studies also points to direct inhibitory effect of GnRH analogs in cancer cells.
Responses of post-menopausal women with breast cancer to GnRH analogs [49] suggested that GnRH analogs may also have direct antiproliferative effects which are independent of their actions in decreasing sex steroid hormones. The presence of GnRH receptors in breast cancer tissue [31] and the demonstration of antiproliferative actions of GnRH analogs in breast cancer cell lines [32,47,96] supported this interpretation. Antiproliferative effects of GnRH analogs and the expression of GnRH receptors have now been demonstrated in a number of cell lines of reproductive tract tumors, including prostate, uterine and ovarian cancers, and also in non-reproductive tract tumors [21,43,47,70,73,79]. In contrast to GnRH actions at the gonadotrope, which are mediated through the Gαq protein, these antiproliferative and apoptotic effects on tumor cells are thought to be mediated via the Gαi protein [42,54,81], focal adhesion complexes involving c-Src, and the JNK and P38 stress-activated kinases [42,54,70,73,81]. Other mechanisms which have been implicated in the antiproliferative effects, include the down-regulation of growth factor actions by decreased expression of growth factors and their receptors and activation of phosphotyrosine phosphatase [42], and the inhibition of Akt and the 60S acidic ribosomal phosphoproteins which modulate cell survival and protein synthesis [18,62,63,69,74,136]. We have demonstrated that the effects of a series of GnRH analogs on antiproliferation and apoptosis in cancer cell lines is very different from their effects on gonadotropin secretion [81]. We have dubbed the phenomenon “ligand-induced selective-signaling” (LiSS) [81,89,95] (Fig. 14). Thus GnRH II [88,113] is more potent than GnRH I in the inhibition of cell growth while GnRH II is less potent than GnRH I in stimulating the Gαq pathway for gonadotrophin secretion. An extreme difference is the pure antagonism of antagonist 135-25 at Gαq while it acts as a full agonist in inhibiting cell growth (Fig. 15). A number of antagonists of gonadotrope GnRH receptors act as GnRH agonists in the inhibition of tumor cell lines [32,34,44,81,128].
Fig. 14.
Schematic of the concept of multiple active states (conformers) of a single GnRH receptor (R1–R4) that are selective for different agonist analogs (L1–L4). The different active receptor conformers are selectively coupled to different signaling complexes (SC1–SC4) that give rise to different effects in cells. Similar to Kenakin’s “agonist trafficking of receptor signals” we envisage that a specific agonist (e.g. L1) will selectively bind and stabilize the active receptor conformer (R1), which will activate signaling complex (SC1). In the presence of L1 and the absence of other agonists, more of R1 will be stabilized and more SC1 is recruited and activated (shown in bold black). It also follows that the presence of more protein complexes comprising SC1 (e.g. a specific cell type, or prior homologous or heterologous receptor activation resulting in phosphorylation etc.) will stabilize more of active receptor conformer R1and increased binding of R1-selective agonist L1 (Modified from [89]).
Fig. 15.
Effects of GnRH I, GnRH II and GnRH antagonist on stimulation of Gαq (inositol phosphate production) (blue) and inhibition of proliferation (red) in a novel cell line of HEK 293 cells stably transfected with the rat GnRH receptor. Similar pharmacology is observed in human cancer cells expressing the GnRH receptor.
Research on the structure–activity relationship (SAR) of GnRH analogs has focused on the ability of ligands to modulate Gαq activation which stimulates gonadotropin secretion. Since antiproliferative and apoptotic effects are mediated by different signaling pathways from those stimulating gonadotropins, we have undertaken a SAR study on the inhibition of cell number by GnRH analogs using a model system of HEK 293 cells expressing either the rat or the human GnRH receptor. Since GnRH II is more potent than GnRH I, the three different amino acids in GnRH II (His5, Trp7 and Tyr8) were systematically incorporated into synthetic GnRH I peptides and the relative antiproliferative and inositol phosphate production (Gαq) compared. Tyr5 substitution by His5resulted in increased affinity, which correlated with higher potencies for both of the responses studied. Trp7 substitution for Leu7 into GnRH I did not Significantly modify binding affinity and inositol phosphate (IP) production (Gαq) but increased potency 10- to 20-fold for the inhibition of cell growth. The substitution of Arg8 in GnRH I by Tyr8 was the single change that resulted in the most selective inhibitor of cell growth. At the rat receptor, [Tyr8]GnRH I was >30-fold less potent in IP generation but 4-fold more potent in inhibiting cell growth, compared to GnRH I. Although this analog has a very low potency for IP production, it is still more potent than GnRH I in inhibiting cell growth and is thus the most selective analog for inhibiting cell growth. Analogs with double substitutions [His5,Trp7]GnRH I and [His5,Tyr8]GnRH I, displayed properties approximating the product of the single substitutions.
In Fig. 16 the ratio of IP and inhibition of cell growth shows that analogs with Tyr8 are generally the most selective for cell growth inhibition. We have examined the possible explanation for the enhanced selective inhibition of cell growth with the substitution of Arg8 by Tyr8 in GnRH I. It appears that the substitution of Arg8 with Tyr8 enables the ligand and receptor to adopt new conformations that better stabilize the receptor in the active state that mediates the antiproliferative/apoptotic effect. Our studies on the docking of GnRH I and GnRH II with the refined rhodopsin homology model of GnRH receptor [75] reveal that Arg8 satisfactorily interacts with Asp302, but when GnRH II is docked to the receptor, Tyr8 faces away from Asp302 and appears to make contacts with residues in ECL 2 thus stabilizing a different receptor conformation (see earlier section on GnRH receptor and Fig. 9 therein). Hence, the findings that substitution of Arg8 with Tyr8 give rise to a decrease in IP generation but increase in the inhibition of cell growth suggests that Arg8 of GnRH I stabilizes a receptor conformation with preferential Gαq coupling, while its substitution with Tyr favors a receptor conformation associated with signaling to proapoptotic and antiproliferative pathways. These findings further support the LiSS concept. This is exemplified by the natural GnRH I and GnRH II ligands, which show inverted potency ratios for the stimulation of inositol phosphate synthesis and inhibition of cell growth and this has been further refined in the [Tyr8]GnRH I analog. These studies together with those on GnRH antagonists reveal four distinct classes of analogs (Table 1) with the potential for more selective actions as therapeutics.
Fig. 16.
Relative effects of GnRH I analogs substituted with GnRH II residues (His5, Trp7 and Tyr8) on inositol phosphate production (IP) and antiproliferative activity (PA) in HEK 293 cells stably expressing the rat GnRH receptor. Note that Tyr8 substitution produces analogs with greater antiproliferative selectivity. Data represent the mean ratios derived from three or more experiments. The analogs with Tyr8 had Significantly higher ratios than GnRH I.
Table 1.
Classes of GnRH modulators for cancer therapy
| Classical agonist | Novel agonist | Classical antagonist | Novel antagonist | |
|---|---|---|---|---|
| Steroid inhibition (Gq) | Yes | Slight | Yes | Yes |
| Direct inhibition (Gi) | Yes | Yes | Slight | Yes |
| Castration | Yes | No | Yes | Yes |
| Flare | Yes | No | No | No |
| Delayed onset | Yes | No | No | No |
11. Differential GnRH receptor conformations dictate different ligand-selectivity and different signaling
Since GnRH II was more potent than GnRH I in the inhibition of cancer cells but less potent in stimulating inositol phosphate production, which mediates gonadotropin secretion in the pituitary, we proposed that the two ligands are able to preferentially stabilize different conformations of the type I GnRH receptor which give rise to differential signaling output (Fig. 14). This LiSS concept predicts that GnRH I and GnRH II make common and different contacts with the type I GnRH receptor which stabilize different receptor conformations and which transduce different intracellular signaling. Molecular support for this concept requires a detailed delineation of the binding sites for GnRH I and GnRH II as well as the identification of the intramolecular receptor interactions which stabilize the receptor in different conformations which are differentially selective for ligands and intracellular signaling. These elements were described in the section on GnRH receptor structure and function. GnRH I and GnRH II were found to utilize common receptor interacting sites for positions 1, 2, 3, 5, 9 and 10 but employ different sites for interactions with the amino acids in position 8 (Arg and Tyr) (Fig. 9).
Intramolecular interactions in the TMs which control receptor conformational states and consequent ligand binding selectivity and signaling efficacy were identified as (Met132(3.43), Met227(5.54), Phe272(6.40), Phe276(6.44) and Ile322(7.52)) (Fig. 8). Mutation of these residues increased ligand binding affinity for GnRH II, but had little effect on GnRH I binding affinity [75] (Fig. 17). We then examined the role of the three amino acids which differ in the two endogenous ligands and found that Tyr8 in GnRH II plays a dominant role for the increased affinity of the receptor mutants for GnRH II. We propose that creation of a high affinity binding site for GnRH II accompanies receptor conformational changes, i.e. “induced fit” or “conformational selection”, mainly determined by the intermolecular interactions between Tyr8 and the receptor contact residues, which can be facilitated by disruption of particular sets of receptor-stabilizing intramolecular interactions. On the other hand, Arg8 of GnRH I interacted with Asp302 of the receptor to stabilize a different conformation. Thus these mutations result in a change in ligand selectivity which closely parallels our observations on the inhibition of cell growth. We therefore predict that these mutant receptors will be more effective in inhibiting cell growth as they stabilize the receptor conformations which are selective for GnRH II and [Tyr8]GnRH.
Fig. 17.
Competition binding of GnRH I and GnRH II in human wild-type and Ala mutant receptors of Met132 and Phe272. (a) GnRH I binding; (b) GnRH II binding. ●, wild type; ▼, M132A;■, F272A (Reproduced from [75]). Note the large increase in affinity for GnRH II but not GnRH I when residue M132 or F272 remote from the binding site is mutated to Ala suggesting the generation of a different receptor conformation with different ligand selectivity.
The findings suggest that the human type I GnRH receptor is evolutionarily configured for high affinity binding of GnRH I, but has a lower affinity for GnRH II and GnRHs from other species which differ from GnRH I at position 8. These natural GnRHs in which Arg8 is substituted by a neutral amino acid are partial agonists in the activation of inositol phosphate generation but potent antiproliferative agents (our unpublished data). Mutation of Cys279(6.47) to Ala or Tyr (a mutation causing infertility in man) and Asn315(7.45) to Ala increases ligand binding affinity for GnRHs from other species and converts them to full agonists [76]. Recent studies have shown that partial agonists stabilize a receptor active conformation differing from that of full agonists, with different signaling capability [6,109]. Taken together these findings suggest that a complex receptor intramolecular interaction network evolved during evolution for selective binding of ligands and G protein activation. These highly conserved amino acids apparently form part of an allosteric network which constrains the receptor in inactive states. Substitutions of residues within this allosteric network can result in subtle receptor conformational changes which alter receptor binding selectivity for ligands and G proteins and might constitute one of the mechanisms for the evolution of ligand binding and signaling selectivity and diversity of GPCR function. GnRH ligand-induction of new receptor intra- and intermolecular interactions appears to be a fundamental component for GnRH receptor activation. The delineation of the structural elements for ligand and receptor conformational selection could have implications for the development of novel ligands that selectively activate certain signaling pathways, with improved therapeutic application [76].
12. Conclusion and future perspectives
Geoffrey Harris’ discovery of the hypothalamic-hypophyseal portal system and the subsequent identification of the humoral factors regulating pituitary hormone secretion have stimulated the development of new diagnostic tools and therapeutic interventions. GnRH analogs, in particular, have found extensive application in reproductive tract pathologies, in assisted conception technologies and potentially in new contraceptive systems with reduced side-effects. The discovery that GnRHs also regulate sexual behavior and have direct effects on normal and neoplastic reproductive tissues holds the promise of novel therapeutic applications. The discovery that GnRH analogs can be developed to specifically recruit certain signaling pathways while by-passing others offers the opportunity of improving selectivity for conventional and novel applications, with reduced deleterious effects.
References
- 1.Acharjee S, Maiti K, Soh JM, Im WB, Seong JY, Kwon HB. Differential desensitization and internalization of three different bullfrog gonadotropin-releasing hormone receptors. Mol Cell. 2002;14:101–107. [PubMed] [Google Scholar]
- 2.Adams BA, Tello JA, Erchegyi J. Six novel gonadotropinreleasing hormones are encoded as triplets on each of two genes in the protochordate, Ciona intestinalis. Endocrinology. 2003;144:1907–1919. doi: 10.1210/en.2002-0216. [DOI] [PubMed] [Google Scholar]
- 3.Alderete MR, Tokarz RR, Crews D. Luteinizing hormonereleasing hormone induction of female sexual receptivity in the lizard, Anolis carolinensis. Neuroendocrinology. 1980;30:200–205. doi: 10.1159/000123001. [DOI] [PubMed] [Google Scholar]
- 4.Anderson RA, Baird DT. Male contraception. Endocr Rev. 2002;23:735–762. doi: 10.1210/er.2002-0002. [DOI] [PubMed] [Google Scholar]
- 5.Baba Y, Matsuo H, Schally AV. Structure of the porcine LH- and FSH-releasing hormone. II. Confirmation of the proposed structure by conventional sequential analyses. Biochem Biophys Res Commun. 1971;44:459–463. doi: 10.1016/0006-291x(71)90623-1. [DOI] [PubMed] [Google Scholar]
- 6.Baneres JL, Mesnier D, Martin A, Joubert L, Dumuis A, Bockaert J. Molecular characterization of a purified 5-HT4 receptor: a structural basis for drug efficacy. J Biol Chem. 2005;280:20253–20260. doi: 10.1074/jbc.M412009200. [DOI] [PubMed] [Google Scholar]
- 7.Barbieri RL. Clinical applications of GnRH and its analogues. Trends Endocrinol Metab. 1992;3:30–34. doi: 10.1016/1043-2760(92)90089-j. [DOI] [PubMed] [Google Scholar]
- 8.Barnett DK, Bunnell TM, Millar RP, Abbot DH. Gonadotropin- releasing hormone II stimulates female sexual behavior in marmoset monkeys. Endocrinol Metab Clin North Am. 2005;147:615–623. doi: 10.1210/en.2005-0662. [DOI] [PubMed] [Google Scholar]
- 9.Barran PE, Roeske RW, Pawson AJ, Sellar R, Bowers MT, Morgan K, Lu ZL, Tsuda M, Kusakabe T. Evolution of constrained GnRH ligand conformation and receptor selectivity. J Biol Chem. 2005;280:38569–38575. doi: 10.1074/jbc.M503086200. [DOI] [PubMed] [Google Scholar]
- 10.Betz SF, Reinhart GJ, Lio FM, Chen C, Struthers RS. Overlapping, nonidentical binding sites of different classes of nonpeptide antagonists for the human gonadotropin-releasing hormone receptor. J Med Chem. 2006;49:637–647. doi: 10.1021/jm0506928. [DOI] [PubMed] [Google Scholar]
- 11.Bliss SP, Navratil AM, Breed M, Skinner DC, Clay CM, Roberson MS. Signaling complexes associated with the type I gonadotropin- releasing hormone (GnRH) receptor: colocalization of extracellularly regulated kinase 2 and GnRH receptor within membrane rafts. Mol Endocrinol. 2007;21:538–549. doi: 10.1210/me.2006-0289. [DOI] [PubMed] [Google Scholar]
- 12.Blomenrohr M, Bogerd J, Leurs R, Schulz RW, Tensen CP, Zandbergen MA, Goos HJ. Differences in structure-function relations between nonmammalian and mammalian gonadotropinreleasing hormone receptors. Biochem Biophys Res Commun. 1997;238:517–522. doi: 10.1006/bbrc.1997.7331. [DOI] [PubMed] [Google Scholar]
- 13.Blomenrohr M, Heding A, Sellar R, Leurs R, Bogerd J, Eidne KA, Willars GB. Pivotal role for the cytoplasmic carboxylterminal tail of a nonmammalian gonadotropin-releasing hormone receptor in cell surface expression, ligand binding, and receptor phosphorylation and internalization. Mol Pharmacol. 1999;56:1229–1237. doi: 10.1124/mol.56.6.1229. [DOI] [PubMed] [Google Scholar]
- 14.Bosma MM, Bernheim L, Leibowitz MD, Pfaffinger PJ, Hille B. Modulation of M current in frog sympathetic ganglion cells. The Rockefeller University Press; 1990. pp. 43–59. [PubMed] [Google Scholar]
- 15.Casan EM, Raga F, Bonilla-Musoles F, Polan ML. Human oviductal gonadotropin-releasing hormone: possible implications in fertilization, early embryonic development, and implantation. J Clin Endocrinol Metab. 2000;85:1377–1381. doi: 10.1210/jcem.85.4.6503. [DOI] [PubMed] [Google Scholar]
- 16.Casper RF. Clinical uses of gonadotropin-releasing hormone analogues. Can Med Assoc J. 1991;144:153–158. [PMC free article] [PubMed] [Google Scholar]
- 17.Chary KV, Srivastava S, Hosur RV, Roy DB, Govil G. Molecular conformation of gonadoliberin using two-dimensional NMR spectroscopy. Eur J Biochem. 1986;158:323–332. doi: 10.1111/j.1432-1033.1986.tb09754.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen A, Kaganovsky E, Rahimipour S, Ben-Aroya N, Okon E, Koch T. Two forms of gonadotropin-releasing hormone (GnRH) are expressed in human breast tissue and overexpressed in breast cancer: a putative mechanism for the antiproliferative effect of GnRH by down-regulation of acidic ribosomal phosphoproteins P1 and P2. Cancer Res. 2002;62:1036–1044. [PubMed] [Google Scholar]
- 19.Chen A, Ganor Y, Rahimipour S, Ben-Aroya N, Koch Y, Levite M. The neuropeptides GnRH II and GnRH I are produced by human T cells and trigger laminin receptor gene expression, adhesion, chemotaxis and homing to specific organs. Nat Med. 2002;8:1421–1426. doi: 10.1038/nm1202-801. [DOI] [PubMed] [Google Scholar]
- 20.Cheng MF. Role of gonadotropin releasing hormones in the reproductive behavior of female ring doves (Streptopelia risoria) J Endo Metab. 1977;73:37–45. doi: 10.1677/joe.0.0740037. [DOI] [PubMed] [Google Scholar]
- 21.Cheng CK, Leung PC. Molecular biology of gonadotropinreleasing hormone (GnRH)-I, GnRH-II, and their receptors in humans. Endocr Rev. 2005;26:283–306. doi: 10.1210/er.2003-0039. [DOI] [PubMed] [Google Scholar]
- 22.Chou CS, Beristain AG, MacCalman CD, Leung PC. Cellular localization of gonadotropin-releasing hormone(GnRH) I and GnRH II in first-trimester human placenta and decidua. J Clin Endocrinol Metab. 2004;89:1459–1466. doi: 10.1210/jc.2003-031636. [DOI] [PubMed] [Google Scholar]
- 23.Clayton RN, Eccleston L, Gossard F, Thalbard JC, Morel G. Rat granulosa cells express the gonadotrophin-releasing hormone gene: evidence from in-situ hybridization histochemistry. J Mol Endocrinol. 1992;9:189–195. doi: 10.1677/jme.0.0090189. [DOI] [PubMed] [Google Scholar]
- 24.Conn PM, Crowley WF., Jr Gonadotropin-releasing hormone and its analogues. N Engl J Med. 1991;324:93–103. doi: 10.1056/NEJM199101103240205. [DOI] [PubMed] [Google Scholar]
- 25.Conn PM, Crowley WF. Gonadotropin-releasing hormone and its analogues. Annu Rev Med. 1994;45:391–405. doi: 10.1146/annurev.med.45.1.391. [DOI] [PubMed] [Google Scholar]
- 26.Davidson JS, Wakefield IK, Millar RP. Absence of rapid desensitization of the mouse gonadotropin-releasing hormone receptor. Biochem J. 1994;300:299–302. doi: 10.1042/bj3000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidson JS, Assefa D, Pawson A, Davies P, Hapgood J, Becker I, Flanagan C, Roeske R, Millar R. Irreversible activation of the gonadotropin-releasing hormone receptor by photo-affinity cross-linking: localization of attachment site to Cys residue in the N-terminal segment. Biochemistry. 1997;36:12881–12889. doi: 10.1021/bi971377t. [DOI] [PubMed] [Google Scholar]
- 28.Dudas B, Merchenthaler I. Bi-directional associations between galanin and luteinizing hormone-releasing hormone neuronal systems in the human diencephalon. Neuroscience. 2004;127:695–707. doi: 10.1016/j.neuroscience.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Dudas B, Merchenthaler I. Three-dimensional representation of the neurotransmitter systems of the human hypothalamus: inputs of the gonadotrophin hormone-releasing hormone neuronal system. J Neuroendocrinol. 2006;18:79–95. doi: 10.1111/j.1365-2826.2005.01398.x. [DOI] [PubMed] [Google Scholar]
- 30.Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropinreleasing hormone secretion. Endocrinology. 2006;147:1154–1158. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- 31.Eidne KA, Flanagan CA, Millar RP. Gonadotropin-releasing hormone binding sites in human breast carcinoma. Science. 1985;229:989–991. doi: 10.1126/science.2992093. [DOI] [PubMed] [Google Scholar]
- 32.Eidne KA, Flanagan CA, Harris NS, Millar RP. Gonadotropin- releasing hormone (GnRH)-binding sites in human breast cancer cell lines and inhibitory effects of GnRH antagonists. J Clin Endocrinol Metab. 1987;64:425–432. doi: 10.1210/jcem-64-3-425. [DOI] [PubMed] [Google Scholar]
- 33.Emons G, Schally AV. The use of luteinizing hormone releasing hormone agonists and antagonists in gynaecological cancers. Hum Reprod. 1994;9:1364–1379. doi: 10.1093/oxfordjournals.humrep.a138714. [DOI] [PubMed] [Google Scholar]
- 34.Emons G, Grundker C, Gunthert AR, Westphalen S, Kavanagh J, Verschraegen C. GnRH antagonists in the treatment of gynecological and breast cancers. Endocr Relat Cancer. 2003;10:291–299. doi: 10.1677/erc.0.0100291. [DOI] [PubMed] [Google Scholar]
- 35.Favre N, Fanelli F, Missotten M, Nichols A, Wilson J, di Tiani M, Rommel C, Scheer A. The DRY motif as a molecular switch of the human oxytocin receptor. Biochemistry. 2005;44:9990–10008. doi: 10.1021/bi0509853. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson SS. Evolving concepts in G-protein coupled receptor endocytosis: the role in receptor desensitisation and signalling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 37.Filicori M. Gonadotrophin-releasing hormone agonists, A guide to use and selection. Drugs. 1994;48:41–58. doi: 10.2165/00003495-199448010-00005. [DOI] [PubMed] [Google Scholar]
- 38.Fraser HM. GnRH analogues for contraception. Br Med Bull. 1993;49:62–72. doi: 10.1093/oxfordjournals.bmb.a072606. [DOI] [PubMed] [Google Scholar]
- 39.Flanagan CA, Millar RP, Illing N. Advances in understanding gonadotrophin-releasing hormone receptor structure and ligand interactions. Rev Reprod. 1997;2:113–120. doi: 10.1530/ror.0.0020113. [DOI] [PubMed] [Google Scholar]
- 40.Foster DL, Jackson LM, Padmanabhan V. Programming of GnRH feedback controls timing of puberty and adult reproductive activity. Mol Cell Endocrinol. 2006;255:109–119. doi: 10.1016/j.mce.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Granger A, Ngo-Muller V, Bleux C, Guigon C, Pincas H, Magre S, Daegelen D, Tixier-Vidal A, Counis R, Laverriere JN. The promoter of the rat gonadotropin-releasing hormone receptor gene directs the expression of the human placental alkaline phosphatase reporter gene in gonadotrope cells in the anterior pituitary gland as well as in multiple extrapituitary tissues. Endocrinology. 2004;145:983–993. doi: 10.1210/en.2003-0881. [DOI] [PubMed] [Google Scholar]
- 42.Grundker C, Volker P, Emons G. Antiproliferative signaling of luteinizing hormone-releasing hormone in human endometrial and ovarian cancer cells through G protein alpha(I)-mediated activation of phosphotyrosine phosphatase. Endocrinology. 2001;142:2369–2380. doi: 10.1210/endo.142.6.8190. [DOI] [PubMed] [Google Scholar]
- 43.Grundker C, Gunthert AR, Westphalen S, Emons G. Biology of the gonadotropin-releasing hormone system in gynecological cancers. Eur J Endocrinol. 2002;146:1–14. doi: 10.1530/eje.0.1460001. [DOI] [PubMed] [Google Scholar]
- 44.Grundker C, Schlotawa L, Viereck V, Eicke N, Horst A, Kairies B, Emons G. Antiproliferative effects of the GnRH antagonist cetrorelix and of gnRH-II on human endometrial and ovarian cancer cells are not mediated through the GnRH type I receptor. Eur J Endocrinol. 2004;151:141–149. doi: 10.1530/eje.0.1510141. [DOI] [PubMed] [Google Scholar]
- 45.Guarnieri F, Weinstein H. Conformational memories and the exploration of biologically relevant peptide conformations: an illustration for the gonadotropin-releasing hormone. J Am Chem Soc. 1996;118:5580–5589. [Google Scholar]
- 46.Harris GW. Neural control of the pituitary gland, physiological reviews. Amer Physiol Soc. 1948;28:139–179. doi: 10.1152/physrev.1948.28.2.139. [DOI] [PubMed] [Google Scholar]
- 47.Harris N, Dutlow C, Eidne K, Dong KW, Roberts JL, Millar R. Gonadotropin-releasing hormone gene expressin in MDSMB- 231 and ZR-75-1 breast carcinoma cell lines. Cancer Res. 1991;51:2577–2581. [PubMed] [Google Scholar]
- 48.Harrison GS, Wierman ME, Nett TM, Glode LM. Gonadotropin- releasing hormone and its receptor in normal and malignant cells. Endocr Relat Cancer. 2004;11:725–748. doi: 10.1677/erc.1.00777. [DOI] [PubMed] [Google Scholar]
- 49.Harvey HA, Lipton A, Max DT. LHRH analogs for human mammary carcinomas. MTP Press Ltd; Lancaster: 1984. [Google Scholar]
- 50.Hauser F, Sondergaard L, Grimmelikhuijzen CJ. Molecular cloning, genomic organization and developmental regulation of a novel receptor from Drosophila melanogaster structurally related to gonadotropin-releasing hormone receptors for vertebrates. Biochem Biophys Res Commun. 1998;249:822–828. doi: 10.1006/bbrc.1998.9230. [DOI] [PubMed] [Google Scholar]
- 51.Hislop JN, Madziva MT, Everest HM, Harding T, Uney JB, Willars GB, Millar RP, Troskie BE, Davidson JS, McArdle CA. Desensitization and internalization of human and xenopus gonadotropin-releasing hormone receptors expressed in alphaT4 pituitary cells using recombinant adenovirus. Endocrinology. 2000;141:4564–4575. doi: 10.1210/endo.141.12.7813. [DOI] [PubMed] [Google Scholar]
- 52.Horvat RD, Roess DA, Nelson SE, Barisas BG, Clay CM. Binding of agonist but not antagonist leads to fluorescence resonance energy transfer between intrinsically fluorescent gonadotropin- releasing hormone receptors. Mol Endocrinol. 2001;15:695–703. doi: 10.1210/mend.15.5.0633. [DOI] [PubMed] [Google Scholar]
- 53.Hovelmann S, Hoffmann SH, Kuhne R, ter Laak T, Reilander H, Beckers T. Impact of aromatic residues within transmembrane helix 6 of the human gonadotropin-releasing hormone receptor upon agonist and antagonist binding. Biochemistry. 2002;41:1129–1136. doi: 10.1021/bi0113162. [DOI] [PubMed] [Google Scholar]
- 54.Imai A, Takagi H, Horibe S, Fuseya T, Tamaya T. Coupling of gonadotropin-releasing hormone receptor to Gi protein in human reproductive tract tumors. J Clin Endocrinol Metab. 1996;81:3249–3253. doi: 10.1210/jcem.81.9.8784077. [DOI] [PubMed] [Google Scholar]
- 55.Jan LY, Jan YN, Brownfield MS. Peptidergic transmitters in synaptic boutons of sympathetic ganglia. Nature. 1980;288:380– 382. doi: 10.1038/288380a0. [DOI] [PubMed] [Google Scholar]
- 56.Johnston JD, Messager S, Ebling FJ, Williams LM, Barrett P, Hazlerigg DG. Gonadotrophin-releasing hormone drives melatonin receptor down-regulation in the developing pituitary gland. Proc Natl Acad Sci USA. 2003;100:2831–2835. doi: 10.1073/pnas.0436184100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones SW. Chicken II luteinizing hormone-releasing hormone inhibits the M-current of bullfrog sympathetic neurons. Neurosci Lett. 1987;80:180–184. doi: 10.1016/0304-3940(87)90650-1. [DOI] [PubMed] [Google Scholar]
- 58.Kaiser UB, Conn PM, Chin WW. Studies of gonadotropinreleasing hormone (GnRH) action using GnRH receptor-expressing pituitary cell lines. Endocr Rev. 1997;18:46–70. doi: 10.1210/edrv.18.1.0289. [DOI] [PubMed] [Google Scholar]
- 59.Kauffman AS. Emerging functions of gonadotropin-releasing hormone II in mammalian physiology and behaviour. J Neuroendocrinol. 2004;16:794–806. doi: 10.1111/j.1365-2826.2004.01229.x. [DOI] [PubMed] [Google Scholar]
- 60.Kauffman AS, Wills A, Millar RP, Rissman EF. Evidence that the type-2 gonadotrophin-releasing hormone (GnRH) receptor mediates the behavioural effects of GnRH-II on feeding and reproduction in musk shrews. J Neuroendocrinol. 2005;17:489– 497. doi: 10.1111/j.1365-2826.2005.01334.x. [DOI] [PubMed] [Google Scholar]
- 61.Kawamura K, Fukuda J, Kumagai J, Shimizu Y, Kodama H, Nakamura A, Tanaka T. Gonadotropin-releasing hormone I analog acts as an antiapoptotic factor in mouse blastocysts. Endocrinol Metab Clin North Am. 2005;146:4105–4116. doi: 10.1210/en.2004-1646. [DOI] [PubMed] [Google Scholar]
- 62.Kim KY, Choi KC, Park SH, Auersperg N, Leung PC. Extracellular signal-regulated protein kinase, but not c-Jun Nterminal kinase, is activated by type II gonadotropin-releasing hormone involved in the inhibition of ovarian cancer cell proliferation. J Clin Endocrinol Metab. 2005;90:1670–1677. doi: 10.1210/jc.2004-1636. [DOI] [PubMed] [Google Scholar]
- 63.Kimura A, Ohmichi M, Kurachi H, Ikegami H, Hayakawa J, Tasaka K, Kanda Y, Nishio Y, Jikihara H, Matsuura N, Murata Y. Role of mitogen-activated protein kinase/extracellular signal-regulated kinase cascade in gonadotropin-releasing hormone-induced growth inhibition of a human ovarian cancer cell line. Cancer Res. 1999;59:5133–5142. [PubMed] [Google Scholar]
- 64.King JA, Millar RP. Heterogeneity of vertebrate luteinizing hormone-releasing hormone. Science. 1979;206:67–69. doi: 10.1126/science.384514. [DOI] [PubMed] [Google Scholar]
- 65.King JA, Millar RP. Comparative aspects of luteinizing hormonereleasing hormone structure and function in vertebrate phylogeny. Endocrinology. 1980;106:707–717. doi: 10.1210/endo-106-3-707. [DOI] [PubMed] [Google Scholar]
- 66.King JA, Millar RP. Structure of chicken hypothalamic luteinizing hormone-releasing hormone. I. Structural determination of partially purified material. J Biol Chem. 1982;257:10722–10728. [PubMed] [Google Scholar]
- 67.King JA, Millar RP. Structure of chicken hypothalamic luteinizing hormone-releasing hormone. II. Isolation and characterization. J Biol Chem. 1982;257:10729–10732. [PubMed] [Google Scholar]
- 68.Kogo H, Fujimoto T, Park MK, Mori T. Gonadotropinreleasing hormone receptor mRNA expression in the ovaries of neonatal and adult rats. Cells Tissues Organs. 1999;164:14–22. doi: 10.1159/000016638. [DOI] [PubMed] [Google Scholar]
- 69.Kraus S, Levy G, Hanoch T, Naor Z, Seger R. Gonadotropinreleasing hormone induces apoptosis of prostate cancer cells: role of c-Jun NH2-terminal kinase, protein kinase B, and extracellular signal-regulated kinase pathways. Cancer Res. 2004;64:5736–5744. doi: 10.1158/0008-5472.CAN-04-1156. [DOI] [PubMed] [Google Scholar]
- 70.Kraus S, Naor Z, Seger R. Gonadotropin-releasing hormone in apoptosis of prostate cancer cells. Cancer Lett. 2006;234:109–123. doi: 10.1016/j.canlet.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 71.Latimer VS, Rodrigues SM, Garyfallou VT, Kohama SG, White RB, Fernald RD, Urbanski HF. Two molecular forms of gonadotropin-releasing hormone (GnRH-I and GnRH-II) are expressed by two separate populations of cells in the rhesus macaque hypothalamus. Brain Res Mol Brain Res. 2000;75:287–292. doi: 10.1016/s0169-328x(99)00316-2. [DOI] [PubMed] [Google Scholar]
- 72.Latimer VS, Kohama SG, Garyfallou VT, Urbanski HF. A developmental increase in the expression of messenger ribonucleic acid encoding a second form of gonadotropin-releasing hormone in the rhesus macaque hypothalamus. J Clin Endocrinol Metab. 2001;86:324–329. doi: 10.1210/jcem.86.1.7106. [DOI] [PubMed] [Google Scholar]
- 73.Limonta P, Moretti RM, Marelli MM, Dondi D, Parenti M, Motta M. The luteinizing hormone-releasing hormone receptor in human prostate cancer cells: messenger ribonucleic acid expression, molecular size, and signal transduction pathway. Endocrinology. 1999;140:5250–5256. doi: 10.1210/endo.140.11.7087. [DOI] [PubMed] [Google Scholar]
- 74.Limonta P, Moretti RMMM, Jarelli M, Motta M. The biology of gonadotropin hormone-releasing hormone: role in the control of tumor growth and progression in humans. Front Neuroendocrinol. 2003;24:279–295. doi: 10.1016/j.yfrne.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 75.Lu ZL, Gallagher R, Sellar R, Coetsee M, Millar RP. Mutations remote from the human gonadotropin-releasing hormone (GnRH) receptor binding sites specifically increase binding affinity for GnRH II, but not GnRH I: evidence for ligand-selective receptor active conformations. J Bio Chem. 2005;280:29796–29803. doi: 10.1074/jbc.M413520200. [DOI] [PubMed] [Google Scholar]
- 76.Lu ZL, Coetsee M, White CD, Millar RP. Structural determinants for ligand–receptor conformational selection in a peptide G protein-coupled receptor. J Biol Chem. 2007;282:17921–17929. doi: 10.1074/jbc.M610413200. [DOI] [PubMed] [Google Scholar]
- 77.Maliekal JC, Jackson GE, Flanagan CA, Millar RP. Solution conformations of gonadotropin releasing hormone (GnRH) and [Gln(8)]GnRH. S Afr Med J. 1997;50:217–219. [Google Scholar]
- 78.Maney D, Richardson R, Wingfield JC. Central administration of chicken gonadotropin-releasing hormone-II enhances courtship behavior in a female sparrow. Horm Behav. 1997;32:11–18. doi: 10.1006/hbeh.1997.1399. [DOI] [PubMed] [Google Scholar]
- 79.Marelli MM, Moretti RM, Januszkiewicz-Caulier J, Motta M, Limonta P. Gonadotropin-releasing hormone (GnRH) receptors in tumors: a new rationale for the therapeutical application of GnRH analogs in cancer patients? Cancer Drug Targets. 2006;6:257–269. doi: 10.2174/156800906776842966. [DOI] [PubMed] [Google Scholar]
- 80.Matsuo H, Baba Y, Nair RM, Arimura A, Schally AV. Structure of the porcine LH- and FSH-releasing hormone I. The proposed amino acid sequence. Biochem Biophys Res Commun. 1971;43:1334–1339. doi: 10.1016/s0006-291x(71)80019-0. [DOI] [PubMed] [Google Scholar]
- 81.Maudsley S, Davidson L, Pawson AJ, Chan R. Gonadotropinreleasing hormone (GnRH) antagonists promote proapoptotic signaling in peripheral reproductive tumor cells by activating a Galphai-coupling state of the type I GnRH receptor. Cancer Res. 2004;64:7533–7544. doi: 10.1158/0008-5472.CAN-04-1360. [DOI] [PubMed] [Google Scholar]
- 82.McArdle CA, Davidson JS, Willars GB. The tail of the gonadotrophin-releasing hormone receptor: desensitization at, and distal to, G protein-coupled receptors. Mol Cell Endocrinol. 1999;151:129–136. doi: 10.1016/s0303-7207(99)00024-6. [DOI] [PubMed] [Google Scholar]
- 83.McArdle CA, Franklin J, Green L, Hislop JN. Signalling, cycling and desensitisation of gonadotrophin-releasing hormone receptors. J Endocrinol. 2002;173:1–11. doi: 10.1677/joe.0.1730001. [DOI] [PubMed] [Google Scholar]
- 84.Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron. 1990;5:1– 10. doi: 10.1016/0896-6273(90)90028-e. [DOI] [PubMed] [Google Scholar]
- 85.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G proteincoupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Millar RP. GnRHs and GnRH receptors. In: De Groot LJ, Jameson JL, editors. Endocrinology. 4. W.B. Saunders; Philadelphia, PA: 2001. [Google Scholar]
- 87.Millar RP. Gonadotropin-releasing hormones and their receptors. In: Fauser BCJM, editor. Reproductive medicine: molecular cellular and genetic fundamentals. Parthenon Publishing; Lancaster: 2002. pp. 199–224. [Google Scholar]
- 88.Millar RP. GnRH II and Type II GnRH receptors. Trends Endocrinol Metab. 2003;14:35–43. doi: 10.1016/s1043-2760(02)00016-4. [DOI] [PubMed] [Google Scholar]
- 89.Millar RP, Pawson AJ. Outside-in and inside-out signaling: the new concept that selectivity of ligand binding at the gonadotropinreleasing hormone receptor is modulated by the intracellular environment. Endocrinology. 2004;145:3590–3593. doi: 10.1210/en.2004-0461. [DOI] [PubMed] [Google Scholar]
- 90.Millar RP, Aehnelt C, Rossier G. Higher molecular weight immunoreactive species of luteinizing hormone releasing hormone: possible precursors of the hormone. Biochem Biophys Res Commun. 1977;79:720–731. doi: 10.1016/0006-291x(77)90362-x. [DOI] [PubMed] [Google Scholar]
- 91.Millar RP, Tobler CJ, King JA, Arimura A. Region-specific antisera in molecular biology of neuropeptides: application in quantitation, structural characterisation and metabolism of luteinizing hormone-releasing hormone. In: Soreq H, editor. Molecular Biology Approach to the Neurosciences. Wiley; New York: 1984. pp. 221–230. [Google Scholar]
- 92.Millar RP, King JA, Davidson JS, Milton RC. Gonadotropinreleasing hormone—diversity of functions and clinical applications. S Afr Med J. 1987;72:748–755. [PubMed] [Google Scholar]
- 93.Millar RP, Zhu YF, Chen C, Struthers RS. Progress towards the development of non-peptide orally-active gonadotropin-releasing hormone (GnRH) antagonists: therapeutic implications. Br Med Bull. 2000;56:761–772. doi: 10.1258/0007142001903346. [DOI] [PubMed] [Google Scholar]
- 94.Millar R, Lowe S, Conklin D, Pawson A, Maudsley S, Troskie B, Ott T, Millar M, Lincoln G, Sellar R, Faurholm B, Scobie G, Kuestner R, Terasawa E, Katz A. A novel mammalian receptor for the evolutionarily conserved type II GnRH. Proc Natl Acad Sci USA. 2001;98:9636–9641. doi: 10.1073/pnas.141048498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev. 2004;25:235–275. doi: 10.1210/er.2003-0002. [DOI] [PubMed] [Google Scholar]
- 96.Miller WR, Scott WN, Morris R, Fraser HM, Sharpe R. Growth of human breast cancer cells inhibited by a luteinizing hormone-releasing hormone agonist. Nature. 1985;313:231– 233. doi: 10.1038/313231a0. [DOI] [PubMed] [Google Scholar]
- 97.Milton RCdL, King JA, Badminton MN, Tobler CJ, Lindsey GG, Fridkin M, Millar RP. Comparative structure-activity studies on mammalian [Arg8] LH-RH and chicken [Gln8] LH-RH by fluorimetric titration. Biochem Biophys Res Commun. 1983;111:1082–1088. doi: 10.1016/0006-291x(83)91410-9. [DOI] [PubMed] [Google Scholar]
- 98.Mitchell R, McCulloch D, Lutz E, Johnson M, MacKenzie C, Fennell M, Fink G, Zhou W, Sealfon SC. Rhodopsin-family receptors associate with small G proteins to activate phospholipase D. Nature. 1998;392:411–414. doi: 10.1038/32937. [DOI] [PubMed] [Google Scholar]
- 99.Moghissi KS. Clinical applications of gonadotropin-releasing hormones in reproductive disorders. Endocrinol Metab Clin North Am. 1992;21:125–140. [PubMed] [Google Scholar]
- 100.Morgan K, Millar RP. Evolution of GnRH ligand precursors and GnRH receptors in protochordate and vertebrate species. Gen Comp Endocrinol. 2004;139:191–197. doi: 10.1016/j.ygcen.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 101.Morgan K, Conklin D, Pawson AJ, Sellar R, Ott TR, Millar RP. A transcriptionally active human Type II gonadotropin releasing hormone receptor gene homologue overlaps two genes in the antisense orientation on chromosome 1q.12. Endocrinology. 2003;144:423–436. doi: 10.1210/en.2002-220622. [DOI] [PubMed] [Google Scholar]
- 102.Morgan K, Sellar R, Pawson AJ, Lu ZL, Millar RP. Bovine and ovine gonadotropin-releasing hormone (GnRH)-II ligand precursors and type II GnRH receptor genes are functionally inactivated. Endocrinology. 2006;147:5041–5051. doi: 10.1210/en.2006-0222. [DOI] [PubMed] [Google Scholar]
- 103.Moss RL. Actions of hypothalamic-hypophysiotropic hormones on the brain. Annu Rev Physiol. 1979;41:617–631. doi: 10.1146/annurev.ph.41.030179.003153. [DOI] [PubMed] [Google Scholar]
- 104.Naor Z, Harris D, Shacham S. Mechanism of GnRH receptor signaling: combinatorial cross-talk of Ca2+ and protein kinase C. Front Neuroendocrinol. 1998;19:1–19. doi: 10.1006/frne.1997.0162. [DOI] [PubMed] [Google Scholar]
- 105.Naor Z, Benard O, Seger R. Activation of MAPK cascades by Gprotein- coupled receptors: the case of gonadotropin-releasing hormone receptor. Trends Endocrinol Metab. 2000;11:91–99. doi: 10.1016/s1043-2760(99)00232-5. [DOI] [PubMed] [Google Scholar]
- 106.Navratil AM, Bliss SP, Berghorn KA, Haughian JM, Farmerie TA, Graham JK, Clay CM, Roberson MS. Constitutive localization of the gonadotropin-releasing hormone (GnRH) receptor to low density membrane microdomains is necessary for GnRH signaling to ERK. J Biol Chem. 2003;278:31593–31602. doi: 10.1074/jbc.M304273200. [DOI] [PubMed] [Google Scholar]
- 107.Nelson S, Horvat RD, Malvey J, Roess DA, Barisas BG, Clay CM. Characterization of an intrinsically fluorescent gonadotropinreleasing hormone receptor and effects of ligand binding on receptor lateral diffusion. Endocrinology. 1999;140:950–957. doi: 10.1210/endo.140.2.6518. [DOI] [PubMed] [Google Scholar]
- 108.Nieschlag E, Behre HM, Weinbauer GF. Hormonal male contraception: a real chance? In: Nieschlag E, Habenicht UF, editors. Spermatogenesis–Fertilization–Contraception. Molecular, Cellular and Endocrine Events in Male Reproduction. Springer- Verlag; Berlin: 1992. pp. 477–501. [Google Scholar]
- 109.Nikolaev VO, Hoffmann C, Bunemann M, Lohse MJ, Vilardaga JP. Molecular basis of partial agonism at the neurotransmitter alpha2A-adrenergic receptor and Gi-protein heterotrimer. J Biol Chem. 2006;281:24506–24511. doi: 10.1074/jbc.M603266200. [DOI] [PubMed] [Google Scholar]
- 110.Okada T, Fujiyoshi Y, Silow M, Navarro J, Laundau EM, Shichida Y. Functional role of internal water molecules in rhodopsin revealed by X-ray crystallography. Proc Natl Acad Sci USA. 2002;99:5982–5987. doi: 10.1073/pnas.082666399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Parhar IS, Soga T, Sakuma Y, Millar RP. Spatio-temporal expression of gonadotropin-releasing hormone receptor subtypes in gonadotropes, somatotropes and lactotropes in the cichlid fish. J Neuroendocrinol. 2001;14:657–665. doi: 10.1046/j.1365-2826.2002.00817.x. [DOI] [PubMed] [Google Scholar]
- 112.Pawson AJ, Katz A, Sun YM, Lopes J, Illing N, Millar RP, Davidson JS. Contrasting internalization kinetics of human and chicken gonadotropin-releasing hormone receptors mediated by Cterminal tail. J Endocrinol. 1998;156:R9–R12. doi: 10.1677/joe.0.156r009. [DOI] [PubMed] [Google Scholar]
- 113.Pawson AJ, Morgan K, Maudsley SR, Millar RP. Type II gonadotropin-releasing hormone (GnRH-II) in reproductive biology. Reproduction. 2003;126:271–278. doi: 10.1530/rep.0.1260271. [DOI] [PubMed] [Google Scholar]
- 114.Pawson AJ, Maudsley SR, Lopes J, Katz AA, Sun YM, Davidson JS, Millar RP. Multiple determinants for rapid agonistinduced internalization of a nonmammalian gonadotropin-releasing hormone receptor: a putative palmitoylation site and threonine doublet within the carboxyl-terminal tail are critical. Endocrinology. 2003;144:3860–3871. doi: 10.1210/en.2003-0028. [DOI] [PubMed] [Google Scholar]
- 115.Pawson AJ, Faccenda E, Maudsley SR, Lu ZL, Naor Z, Millar RP. Agonist-independent trafficking of mammalian type I GnRH receptors. The Endocrine Society’s 88th Annual Meeting; 2006. pp. P1–374. [Google Scholar]
- 116.Phillips JA, Alexander N, Karesh WB, Millar RP, Lasley BL. Stimulating male sexual behaviour with repetitive pulses of GnRH in female green iguanas, Iguana iguana. J Exp Zool. 1985;234:481– 484. [Google Scholar]
- 117.Plant TM, Ramaswamy S, Dipietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147:1007–1013. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- 118.Quanbeck C, Sherwood NM, Millar RP, Terasawa E. Two populations of luteinizing hormone-releasing hormone neurons in the forebrain of the rhesus macaque during embryonic development. J Comp Neurol. 1997;380:293–309. [PubMed] [Google Scholar]
- 119.Radovick S, Wray S, Lee E, Nicols DK, Nakayama Y, Weintraub BD, Westphal H, Cutler GB, Jr, Wondisford FE. Migratory arrest of gonadotropin-releasing hormone neurons in transgenic mice. Proc Natl Acad Sci USA. 1991;88:3402–3406. doi: 10.1073/pnas.88.8.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ramakrishnappa N, Rajamahendran R, Lin YM, Leung PC. GnRH in non-hypothalamic reproductive tissues. Anim Reprod Sci. 2005;88:95–113. doi: 10.1016/j.anireprosci.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 121.Rissman EF, Li X. Sex differences in mammalian and chicken-II gonadotropin-releasing hormone immunoreactivity in musk shrew brain. Gen Comp Endocrinol. 1998;112:346–355. doi: 10.1006/gcen.1998.7135. [DOI] [PubMed] [Google Scholar]
- 122.Rodet F, Lelong C, Dubos MP, Costil K, Favrel P. Molecular cloning of a molluscan gonadotropin-releasing hormone receptor orthologue specifically expressed in the gonad. Biochem Biophys Acta. 2005;1730:187–195. doi: 10.1016/j.bbaexp.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 123.Ronacher K, Matsiliza N, Nkwanyana N, Pawson AJ, Adam T, Flanagan CA, Millar RP, Katz AA. Serine residues 338 and 339 in the carboxyl-terminal tail of the type II gonadotropin-releasing hormone receptor are critical for beta-arrestin-independent internalization. Endocrinology. 2004;145:4480–4488. doi: 10.1210/en.2004-0075. [DOI] [PubMed] [Google Scholar]
- 124.Salom D, Lodowski DT, Stenkamp RE, Le Trong M, Golczak M, Jastrzebska B, Harris T, Ballesteros JA, Palczewski K. Crystal structure of a photoactivated deprotonated intermediate of rhodopsin. Proc Natl Acad Sci USA. 2006;103:16123–16128. doi: 10.1073/pnas.0608022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schally AV, Arimura A, Baba Y, Nair RM, Matsuo H, Redding TW, Debeljuk L. Isolation and properties of the FSH and LHreleasing hormone. Biochem Biophys Res Commun. 1971;43:393–399. doi: 10.1016/0006-291x(71)90766-2. [DOI] [PubMed] [Google Scholar]
- 126.Sealfon SC, Weinstein H, Millar RP. Molecular mechanisms of ligand interaction with the gonadotropin–releasing hormone receptor. Endocr Rev. 1997;18:180–205. doi: 10.1210/edrv.18.2.0295. [DOI] [PubMed] [Google Scholar]
- 127.Seeburg PH, Mason AJ, Stewart TA, Nikolics K. The mammalian GnRH gene and its pivotal role in reproduction. Recent Prog Horm Res. 1987;43:69–98. doi: 10.1016/b978-0-12-571143-2.50008-3. [DOI] [PubMed] [Google Scholar]
- 128.Segal-Abramson T, Kitroser H, Levy J, Schally AV, Sharoni Y. Direct effects of luteinizing hormone-releasing hormone agonists and antagonists on MCF-7 mammary cancer cells. Proc Natl Acad Sci USA. 1992;89:2336–2339. doi: 10.1073/pnas.89.6.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sherwood NM, Lovejoy DA, Coe IR. Origin of mammalian gonadotropin-releasing hormones. Endocr Rev. 1993;14:241–254. doi: 10.1210/edrv-14-2-241. [DOI] [PubMed] [Google Scholar]
- 130.Shinitzky M, Fridkin M. Structural features of luliberin (luteinising hormone-releasing hormone) inferred from fluorescence measurements. Biochim Biophys Acta. 1976;434:137–143. doi: 10.1016/0005-2795(76)90043-x. [DOI] [PubMed] [Google Scholar]
- 131.Skynner MJ, Slater R, Sim JA, Allen ND, Herbison AE. Promoter transgenics reveal multiple gonadotropin-releasing hormone- I-expressing cell populations of different embryological origin in mouse brain. J Neurosci. 1999;19:5955–5966. doi: 10.1523/JNEUROSCI.19-14-05955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Soderhall JA, Polymeropoulos EE, Paulini K, Gunther E, Kuhne R. Antagonist and agonist binding models of the human gonadotropin-releasing hormone receptor. Biochem Biophys Res Commun. 2005;333:568–582. doi: 10.1016/j.bbrc.2005.05.142. [DOI] [PubMed] [Google Scholar]
- 133.Somoza GM, Miranda LA, Strobl-Mazzulla P, Guilgur LG. Gonadotropin-releasing hormone (GnRH): from fish to mammalian brains. Cell Mol Neurobiol. 2002;22:589–609. doi: 10.1023/A:1021888420271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Staubli F, Jorgensen TJD, Cazzamali G, Williamson M, Lenz C, Sondergaard L, Roepstor P, Grimmelikhuijzen CJ. Molecular identification of the insect adipokinetic hormone receptors. Proc Natl Acad Sci USA. 2002;99:3446–3451. doi: 10.1073/pnas.052556499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stojilkovic SS, Catt KJ. Expression and signal transduction pathways of gonadotropin-releasing hormone receptors. Recent Prog Horm Res. 1995;50:161–205. doi: 10.1016/b978-0-12-571150-0.50012-3. [DOI] [PubMed] [Google Scholar]
- 136.Tanaka Y, Gavrielides MV, Mitsuuchi Y, Fuki T, Kazanietz MG. Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through activation of p38 MAPK and inhibition of the Akt survival pathway. J Biol Chem. 2003;278:33753–33762. doi: 10.1074/jbc.M303313200. [DOI] [PubMed] [Google Scholar]
- 137.Temple JL, Millar RP, Rissman EF. An evolutionarily conserved form of gonadotropin-releasing hormone coordinates energy and reproductive behavior. Endocrinology. 2002;144:13–19. doi: 10.1210/en.2002-220883. [DOI] [PubMed] [Google Scholar]
- 138.Tena-Sempere M. Kiss-1 and reproduction: focus on its role in the metabolic regulation of fertility. Neuroendocrinology. 2006;83:275– 281. doi: 10.1159/000095549. [DOI] [PubMed] [Google Scholar]
- 139.Tieva A, Stattin P, Wikstrom P, Bergh A, Damber JE. Gonadotropin-releasing hormone receptor expression in the human prostate. Prostate. 2001;47:276–284. doi: 10.1002/pros.1072. [DOI] [PubMed] [Google Scholar]
- 140.Troskie B, King JA, Millar RP, Peng YY, Kim J, Figueras H, Illing N. Chicken GnRH II-like peptides and a GnRH receptor selective for chicken GnRH II in amphibian sympathetic ganglia. Neuroendocrinology. 1997;65:396–402. doi: 10.1159/000127202. [DOI] [PubMed] [Google Scholar]
- 141.Tsutsumi M, Zhou W, Millar RP, Mellon PL, Roberts JL, Flanagan CA, Dong K, Gillo B, Sealfon SC. Cloning and functional expression of a mouse gonadotropin-releasing hormone receptor. Mol Endocrinol. 1992;6:1163–1169. doi: 10.1210/mend.6.7.1324422. [DOI] [PubMed] [Google Scholar]
- 142.Volko H, Peter RE. Actions of two forms of gonadotropin releasing hormone and a GnRH antagonist on spawning behavior of the goldfish Carassius auratus. Gen Comp Endocrinol. 1999;116:347–355. doi: 10.1006/gcen.1999.7377. [DOI] [PubMed] [Google Scholar]
- 143.Vrecl M, Anderson L, Hanyaloglu A, McGregor AM, Groarke AD, Milligan G, Taylor PL, Eidne KA. Agonist-induced endocytosis and recycling of the gonadotropin-releasing hormone receptor: effect of beta-arrestin on internalization kinetics. Mol Endocrinol. 1998;12:1818–1829. doi: 10.1210/mend.12.12.0207. [DOI] [PubMed] [Google Scholar]
- 144.Wang L, Bogerd J, Choi HS, Seong JY, Soh JM, Chun SY, Blomenrör M, Troskie BE, Millar RP, Kwon HB. Three distinct types of gonadotropin-releasing hormone receptor characterized in the bullfrog. Proc Natl Acad Sci USA. 2001;98:361–366. doi: 10.1073/pnas.011508498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wolfe A, Kim HH, Tobet S, Stafford DE, Radovick S. Identification of a discrete promoter region of the human GnRH gene that is sufficient for directing neuron-specific expression: a role for POU homeodomain transcription factors. Mol Endocrinol. 2002;16:435–449. doi: 10.1210/mend.16.3.0780. [DOI] [PubMed] [Google Scholar]
- 146.Zhou W, Flanagan C, Ballesteros JA, Konvicka K, Davidson JS, Weinstein H, Millar RP, Sealon SC. A reciprocal mutation supports helix 2 and helix 7 proximity in the gonadotropin- releasing hormone receptor. Mol Pharmacol. 1994;45:165–170. [PubMed] [Google Scholar]