Abstract
Dehydroepiandrosterone (DHEA) is commonly used in the USA as a nutritional supplement for antiaging, metabolic support or other uses. Investigations into understanding the effects of DHEA on human prostate cancer progression have posed more questions than answers and highlight the importance of communications between stromal and epithelial elements within the prostate that contribute to the regulation of DHEA metabolism. Intracrine metabolism of DHEA to androgens (A) and/or estrogens (E) may occur in one cell compartment (stromal) which may release paracrine hormones or growth/inhibitory factors to the epithelial cells. Alternatively no metabolism of DHEA may occur, resulting in no harmful consequences of high levels of DHEA in prostate tissues. We herein review the tissue components involved and interactions with the prohormone, DHEA and/or resulting metabolites, including dihydrotestosterone (DHT) or 17β-Estradiol (E2) in an in-vitro model of endocrine-immune-paracrine interactions within the prostate. This work raises questions and hypotheses concerning the role of DHEA in prostate in normal tissues, vs. preneoplastic tissues.
Keywords: DHEA, TGF β1, Androgen Receptor, Estrogen Receptor, stromal, epithelial prostate, PSA, testosterone, coculture, red clover isoflavones
Introduction
DHEA and its sulphated form DHEA-S are the most abundant steroids in humans, produced by the adrenal cortex. Humans and other primates are unique among animal species in that their adrenal glands secrete large amounts [1]. DHEA and DHEAS have also been characterized as an important neuroactive neurosteroids [2.]. Serum levels of adrenal DHEA and DHEAS peak in both men and women in their 20’s and 30’s and decrease progressively and profoundly with increasing age. The circulating, sulphated form of DHEA, DHEAS, provides a large reservoir of substrate, up to 100–500 times the levels of testosterone and 1000 to 10,000 times higher than those of estradiol [3] for conversion into androgens and/or estrogens in peripheral intracrine tissues. In the U.S., DHEA is widely available as an over-the-counter dietary supplement, and is increasingly self-prescribed for its alleged anabolic and anti-aging effects, with unsubstantiated claims of beneficial effects as well as uncertain long-term safety [4]. In aged adults, the use of DHEA as a dietary supplement is of potential concern in that its androgenic or estrogenic actions may stimulate proliferation, and other adverse effects in, cancer cells within the prostate or breast. DHEA has been shown to exert many of its effects via the androgen receptor (AR) and/or estrogen receptor (ERβ or ERα) following its enzymatic conversion to androgenic or estrogenic ligands, although direct effects of DHEA on the AR and ER have also been demonstrated. The following chapters unfold the potential complexity concerning DHEA metabolism in the prostate and review the in vitro methods developed in this laboratory to address these questions.
1. Controversy remains as to whether DHEA enhances or reduces the risk of prostate cancer
An important concept to understand in the context of DHEA effects in prostate is that of “intracrinology” as coined by Fernand Labrie, where steroid synthesis and metabolism takes place in peripheral target tissues [5,6]. Prostate cells control the level of intracellular active sex steroids using catalyzing enzymes 17β-hydroxysteroid dehydrogenase (17β-HSD), 3β-HSD, and 5α-reductase [7]. It is understood that the adrenal steroid, DHEA, is an important source of androgens, which, when metabolized by the prostate cells, contribute up to 1/6 of DHT present in the prostate [8] This very large pool of DHEAS/DHEA is at its peak in when men are young, yet we do not see high rates of cancer in young men. Could DHEA or DHEAS may play a protective role in normal prostate, but contribute to prostate cancer progression in the context of reactive or senescent stromal microenvironment or tumor environment represented in prostatic tissues at advanced ages? When one considers the latency period for cancers, the early high exposure to DHEA in young men, hypothetically could be confounded by risk factors such as smoking, inflammation or diet, providing a tissue microenvironment that alters DHEA metabolism, and thus, an altered androgen/estrogen balance [9]. (Also see reactive stromal effects in Chapters 4 and 5 below) Similar hypotheses of early hormonal exposure are proposed for increasing breast cancer risk due resulting form early menarche or late full term pregnancy [10,11]
Alternatively, DHEA has been shown to be cancer preventive in carcinogen-induced rodent prostate cancers in vivo and in vitro studies,[12] [13–16], whether by inhibition of glucose-6-phosphate dehydrogenase [17] or other carcinogen-metabolizing enzymes (15). The relevance of these studies to human biology is uncertain, however, as the amounts of DHEA and DHEAS are much lower in rodents than in humans, and the physiological importance of these adrenal steroids are unknown in rodents.
2. Experimental methodologies to determine DHEA effects in human prostate
We have developed an in vitro model of stromal-epithelial cell coculture to dissect pathways and mechanisms of DHEA effects in the human prostate. The evolution of this model came from surveying the existing available human cell lines and primary cells from prostate epithelium. The difficulties of studying hormonal responsiveness with primary prostate cells cell lines and many cancer cell lines stem from the lack of functional androgen receptors in these cells (including normal primary epithelial cells or preneoplastic high grade prostate intraepithelial neoplastic ‘HGPIN’ cells.) The androgen non-responsive cancer cells such as Du145 or PC-3 cells contain ERβ and have been useful for studying estrogenic metabolites of DHEA. The few androgen responsive epithelial cancer cells include LNCaP and 22RV1, which contain a mutated AR and LAPC-4 with a wild type AR. Prostatic stromal cells can be grown as primary cell lots from radical prostatectomy patients and may include phenotypes of normal, reactive myofibroblasts and ‘cancer-associated fibroblasts’
Prostate cancer epithelial cells exhibit differential responses to DHEA depending in part upon their androgen receptor (AR) status. We have demonstrated that LNCaP cells with a mutated AR are responsive to DHEA treatment as measured by cell proliferation, and expression of prostate specific antigen (PSA), and IGF axis proteins [18]. In contrast, LAPC-4 cells, which harbor a normal AR, are minimally responsive [19]. Prostatic primary stromal cells were responsive to androgens, such as DHT by increasing secretory IGF-1, whereas DHEA did not produce the same effect [20].
3. Development of stromal-epithelial coculture model
Extrapolation of data concerning DHEA effects in prostate can only be as good as the experimental model. Cultures of only epithelial or only stromal cells may miss crucial mechanisms of DHEA effects. In fact, both stromal and epithelial cells are involved in prostate response to DHEA; thus, we have investigated effects of DHEA on epithelial and stromal cells, grown separately and in co-culture allowing improved representation of the prostate tissue microenvironment. The importance of stromal cells in regulating epithelial function and especially in cancer has been extensively reviewed [21]. Morphogenic and regulatory interactions between mesenchyme and epithelium that are present during development remain in adult tissue functioning [22]. Early lesions of cancer may contain epithelial cell genetic and signal transduction abnormalities which may be suppressed or promoted by surrounding stromal tissue. Thus loss or alteration of stromal regulation may lead to epithelial dysfunction. Thus, cancer is a disease of the whole tissue and “It’s not just the cancer cells’ fault” [23]
When DHEA is added to monocultures of prostate epithelial cancer cells (LAPC-4) or primary stromal cells (“6S”), the effects of DHEA on the cells grown separately were minimal. But, when LAPC-4 cells were grown in the presence of 6S stromal cells in coculture, DHEA stimulated LAPC4 PSA protein and gene expression with levels approaching induction by DHT. Also 6S stromal cells responded to increasing doses of DHEA with increased testosterone (T) secretion, suggesting that stromal cells can metabolize DHEA to T, and possibly DHT, and thus induce increased PSA expression in the cocultured epithelial cells. [19]. These studies also indicate involvement of stromal cell paracrine mediators beyond production of DHEA metabolites that may contribute to DHEA-modulated PSA production in LAPC-4 prostate cancer epithelial cells. Interestingly, multiple primary stromal cell lots with phenotypes in the range of normal to reactive or cancer-associated stroma were cocultured with LAPC4 cells and showed levels of induction of epithelial PSA inverse to the stromal expression of smooth muscle markers. This gave a hint that that normal, non-cancerous stromal cells may not have the same capability induce androgenic effects in epithelial cells.
4. Role of stromal environment and inflammation in DHEA metabolism
Alterations in stromal phenotype have been reported in many types of human cancer [24]. Reactive or activated prostate stromal cells have myofibroblastic characteristics, including increased levels of smooth muscle α actin [25]. TGFβ1 and other pro-inflammatory cytokines mediate the reactive stromal response and promote a wound-repair-type reactive myofibroblast phenotype in prostate cancer [26,27] [28] [29] [30].
It has been suggested that about 20% of human cancers are associated with chronic infection or inflammation [31]. Such lesions have been characterized in the prostate as proliferative inflammatory atrophy (PIA) and illustrate the association between inflammation and unusually high proliferation [32]. Increased 3β-HSD type 1 gene expression has been found in the presence of cytokines IL-4 and IL-13 [33], In the inflammatory prostate tissue microenvironment, such as in PIA, is it possible that one of the mechanisms of cancer promotion includes increased metabolism of endogenous DHEA, either to androgens or estrogens, and also increased induction of paracrine factors (including cytokines, chemokines and growth factors), that induce proliferation or inhibit apoptosis. Interestingly, in our previous studies, there was a differential induction of stromal production of IGF axis proteins in the reactive stroma cell lots compared to normal stroma [20] suggesting differentially expressed stromal paracrine factors in reactive stroma may further contribute to the cascade towards cancer promotion. The microenvironment of the prostate may dictate the ultimate fate of DHEA metabolism towards androgenic or estrogenic ligands. This is important, not only in consideration of DHEA used as a dietary supplement, but in also in determining physiological role of DHEA in prostate.
5. DHEA effects in normal vs. reactive stromal microenvironment
We aimed to mimic the increased levels of cytokines and characteristics of reactive stroma, such as are present in PIA, PIN and prostate cancer by adding pro-inflammatory cytokines, TGFβ1 or IL-6, to the coculture model of 6S stromal plus LAPC4 prostate cancer epithelial cells. In our studies, LAPC-4 cells were grown in coculture with prostate stromal cells (6S), and treated with DHEA +/− TGFβ1 or IL-6 [34]. PSA expression and testosterone (T) secretion in LAPC4/6S cocultures were compared with those in monocultured epithelial and stromal cells using real time PCR and/or ELISA. Combined administration of TGFβ1+DHEA increased coculture production of testosterone over DHEA treatment alone, and cocultures increased PSA protein secretion 2–4 times, and PSA gene expression up to 50-fold. TGFβ1 greatly increased stromal-mediated DHEA effects on T production and epithelial cell PSA production.
TGFβ-1 is known to influence steroid metabolizing enzymes such as decreasing 3β HSD in adrenocortical cells [35] and 17β HSD in breast cancer cells [36]. TGFβ1 can decrease activity of CYP7B, which metabolizes DHEA to 7-alpha OH-DHEA, a ligand for ERβ [37] as measured in inflammatory tissues. In our studies, TGFβ1 modulation of the DHEA metabolic pathway may alter the balance of androgenic and estrogenic ligands affecting growth and function of the prostate. To summarize the work of this laboratory on DHEA effects in human prostate cells, we have found that DHEA has minimal effects on prostate stromal OR epithelial cells when cultured alone, but the principal mechanism of DHEA effects is found by coculture of these cells. Stromal cell paracrine and intracrine activities play an important role in DHEA effects on epithelial prostate cells. TGF-β1 induces a reactive stromal phenotype and results in increased androgenicity of DHEA. Further studies aim to elucidate the role of TGF-β1 on expression and activity of the HSD enzymes by altering associations of TGF-β1 receptors with the enzymes.
6. Is DHEA androgenic or estrogenic in the prostate? Direct vs. indirect effects
DHEA can directly activate AR or ERβ in the prostatic epithelium or the AR or ERα in the prostatic stroma. DHEA can be a direct ligand for the AR in mutant prostate epithelial cells such as LNCaP and induce weak androgenic effects, potentially promoting prostate cancer growth, as shown by its stimulation of prostate cancer LNCaP cell proliferation, and modulation of cellular PSA, AR, ERβ, and IGF axis gene and protein expression, in a pattern similar to DHT and T, although on a lesser scale and delayed in time [18].
ERβ is an important target in prostate[38] for endogenous and exogenous estrogens and phytoestrogens and may play a role in modulating androgen activity. DHEA has been shown to exert direct agonist effects on ERβ as observed in competitive receptor binding assays, in which DHEA displayed a higher affinity for ERβ than for AR or ERα, with ERβ being the preferred target for the transcriptional effects of DHEA [39].
Indirect effects refer to DHEA metabolism to androgenic ligands (including androstenedione, testosterone and DHT) or estrogenic ligands (including 7-OH-DHEA, 3β Adiol, or 17β estradiol). Receptors for DHEA or DHEAS have not been definitively isolated [40]. DHEA sulfate (DHEAS) is present in high levels in the prostate, as is the sulfatase that converts DHEAS to DHEA [41]. Prostate stromal and epithelial cells possess the enzymatic machinery to metabolize DHEA (intracrine) to more active androgenic and/or estrogenic steroids [1,42,43] and express secondary mediators (paracrine) for epithelial growth and differentiation. Alternatively, DHEA metabolites may act on ERβ in the prostate, potentially antagonizing androgenic effects on prostate cancer growth. such as metabolism to 7α-hydroxy-DHEA (7HD), a known ligand for ERβ [44].
The complexity of the balance between androgenic and estrogenic effects whether as direct ligands or metabolites of DHEA on the prostate is matched by the complexity of estrogen action through the ERα vs. ERβ. The intratissular balance of androgen and estrogen levels is most important for prostatic development and differentiation. Likewise, the balance between ERα and ERβ including the temporal and spatial expression determines response of prostate to estrogen and is crucial for prostate health [45]. Estrogens have been long used in prostate cancer therapy and the role of estrogens in the prostate have been elegantly studied and reviewed [46] [47]. Estrogens have beneficial effects that support normal growth of the prostate but can also be detrimental to prostate growth and differentiation. [48]. Estrogens acting through the prostate stromal ERα may be growth promoting while estrogens acting through the epithelial ERβ may be antagonistic to ERα-or AR-activated pathways [49,50]. Excessive estrogen induces squamous metaplasia and can act synergistically with androgens to induce glandular hyperplasia. [51]. To the contrary, estrogens can inhibit prostate cancer xenograft growth in female intact and ovariectomized mice, in the absence of androgens [52]. These inhibitory effects were postulated to occur by direct actions via the ER or by E2 effects on other cells secreting secondary factors, which influence cancer cell growth. Additionally, ERβ knock-out mice exhibit increased epithelial proliferation compared with that observed in wild-type mice [53] suggesting that ERβ may inhibit prostate growth.
What regulates the direction of estrogen action? What are downstream signal transduction pathways or gene effects of ER ligand/receptor complexes in either stromal cells or in epithelial cells? How do non-genomic effects of estrogen influence prostate functioning [54,55]? Also beyond the scope of this discussion, what are effects of estrogens as converted to catechol estrogens which could react with DNA and lead to mutations initiating cancer [56]? Paracrine functions become important when considering that stromal cells possess aromatase allowing conversion of testosterone to estrogens [57,58]. Ellem and Risbridger propose a positive-feedback cycle where increased stromal aromatase production may increase local estrogens which then promote inflammation. The inflammation may further stimulate aromatase expression leading to progression of prostate cancer [59]. In the context of reactive stroma, the relationship of reactive stroma to aromatase expression has not been validated.
A final possibility is that DHEA remains as a prohormone, and is not metabolized, at the increased levels over other steroids, provides a ‘hormonal buffer’ [60] against endogenous androgen or estrogen levels.
In our studies aiming to determine downstream androgenic vs. estrogenic effects of DHEA, we evaluated the extent to which DHEA-modulated effects in LNCaP and LAPC-4 cells were mediated via the AR and/or ERβ. We found both receptors were involved. In both LNCaP and LAPC-4 prostate cancer cells, inhibitors of AR(Casodex) and ER (ICI 182,780) suppressed hormone-induced PSA mRNA and protein expression. These studies, in addition to others employing siRNAs to AR or ERβ, western blotting and confocal microscopic analyses, suggest that both AR and ERβ contribute to PSA expression induced by DHEA, DHT and E2 in LNCaP cells, and by DHT in LAPC-4 cells [61].
7. What regulates DHEA metabolism in the prostate?
Based on our studies showing that cancer-derived reactive stromal cells have increased capacity to mediate DHEA metabolism and DHEA-induced PSA production than do normal stromal cells, we hypothesize that DHEA exerts minimal negative effects in the normal prostate, whereas in cancer-associated tissues or inflammatory microenvironment, the stromal component may promote prostate cancer progression in the presence of DHEA via increased stromal metabolism to androgenic or estrogenic metabolites and induction of secondary growth factors.
Many questions remain to be clarified in regulation of DHEA metabolism in the prostate. What endogenous or exogenous factors promote androgenic vs. estrogenic metabolism of DHEA? What homeostatic mechanisms regulate levels and activity of intraprostatic steroid metabolic enzymes, 3β HSD1 or 17β HSD’s? Does the tissue microenvironment affect the levels or activity of metabolic enzymes? What is the role of inflammatory cytokines and chemokines in upregulating the metabolic enzymes? How do changing levels of the metabolic enzyme cofactors, NADPH and NADH, govern metabolic capacity [62]? What is the affect of reactive oxygen species (ROS) on the activity of the metabolic enzymes or cofactors? These are all active areas of research, many addressed by other authors in this issue. The complexities of intratissular androgen and estrogen balance along with the contribution of DHEA to prostate function are becoming increasingly appreciated with less clarity of the role of serum androgen levels in risk of prostate cancer[63].
There is a need to clarify the questions above for normal prostate physiology, and understand how these factors are shifted in a disease state. And finally, curiously, why are endogenous DHEA levels so high in humans and primates, and not in other species?
8. Endo-Immuno-Paracrine model of prostate hormonal microenvironment can be applied to investigating mechanisms of phytomedicines
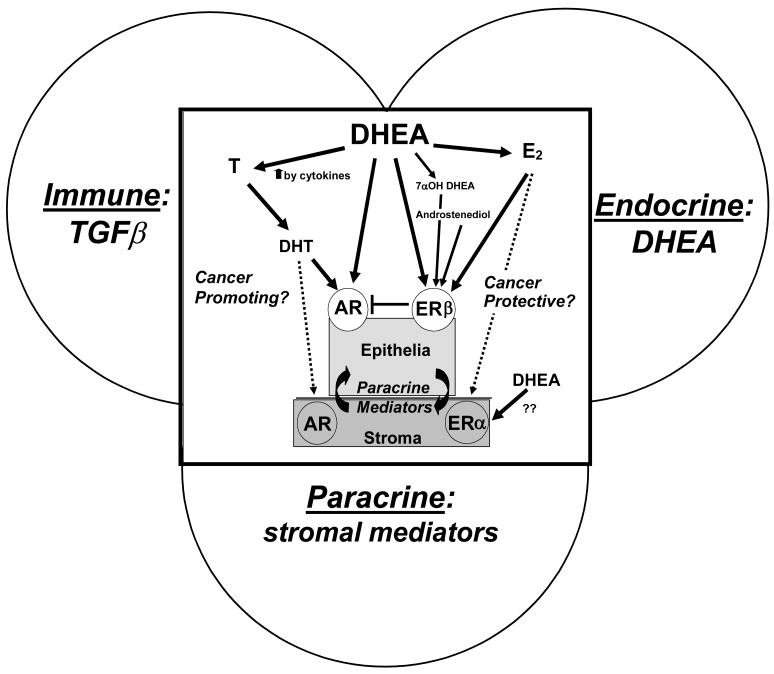
The coculture model developed by this laboratory addresses endocrine (DHEA) - immune (TGFβ1) - paracrine (stromal-epithelial) interactions in the prostate. The addition of TGFβ1 to DHEA–treated prostate stromal plus epithelial cells reproduced a reactive stromal microenvironment and significantly increased the androgenicity of both cell types, as measured by increased PSA (epithelial) and T (stromal) production. Figure 1 depicts the interrelatedness of the endocrine, immune and paracrine functions as they each contribute to overall potential effect of DHEA metabolism in the prostate. (see Figure 1). The question of whether DHEA can be cancer promoting or cancer preventive continues to be debated. [64].
Figure 1. Endo-Immuno-Paracrine influences of DHEA effects on prostate epithelium depends on prostate hormonal microenvironment metabolism and local microenvironment.
The complexity of the prospective effects of DHEA in prostate tissues includes such contributing factors as 1- the possible metabolism of DHEA to androgenic or estrogenic ligands. Androgen Receptor ligands include androstenedione, T and DHT. DHEA is a ligand for the mutant AR as exemplified in the LNCaP cell line. Estrogenic metabolites such as 17β Estradiol, 7-OH-DHEA and serve as ligands for the ERβ. 2- the potential contribution of inflammatory cytokines may increased steroid metabolic enzyme levels or activity; 3- the stromal and epithelial cell components including their cross-talk, 4- the AR and ERβ (or ERα in stromal cells) and downstream effectors; 5- the hormone-induced paracrine signaling; and 6- the importance of the stromal component (normal vs. reactive) as a basis for the differences in the metabolism of DHEA.
This in vitro model cannot provide data on all the complex physiological regulatory mechanisms inherent in in vivo models. It is also difficult to extrapolate from cell cultures, to humans. Nevertheless, DHEA-treated LAPC-4 and 6S cocultures provide a useful preclinical model to elaborate immune/hormone-mediated stromal cell signaling and to identify the mechanisms involved in metabolism of DHEA in prostate growth and gene regulation as well as intracrine and paracrine pathways and mediators of hormone and immunological action in human prostate cells. The stromal-epithelial interface is an important target for various botanical agents or traditional medicines acting as therapeutic agents to normalize hormone metabolism or stromal regulation of epithelial cells. To this end, we treated cells with red clover isoflavones which were combined in the same proportions as in commercially available preparations [65,66]. Red clover isoflavone administration significantly inhibited TGFβ-1+DHEA induction of expression of PSA and T in a dose-dependant manner at final concentrations similar to those achieved clinically (30–300nM) [67].
The development of this model assists in addressing the challenge of research in botanical agents or complementary and alternative medicines which is integrative in nature, not reductive. “Reduction gains precision about parts but at each step loses information about the larger organization it leaves behind.” (Sir David Smithers) [68]). The model can explore multiple pathways, endocrine, paracrine, immune as they all contribute to final effect of DHEA metabolism. Also the botanical or complementary and alternative medicine (CAM) agents may have many different chemical constituents that are symbiotic and multi-mechanistic. This represents a real challenge in its antireductionist nature, yet addresses a vital need of the frontier of CAM research.
These studies highlight the need for further rigorous well-designed laboratory, translational and clinical investigations of the mechanisms of action, efficacy and safety of DHEA, so that questions regarding its potential for improving or compromising human health can finally be answered.
Acknowledgments
This work was supported by the Intramural Research Program, National Center for Complementary and Alternative Medicine, National Institutes of Health, Bethesda, MD.
Abbreviations
- 6S
primary prostate stromal cell
- AR
androgen receptor
- CC
coculture
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone sulphate
- DHT
dihydrotestosterone
- E2
17β estradiol
- ELISA
enzyme-linked immunosorbent assay
- ER
estrogen receptor
- HSD
hydroxysteroid dehydrogenase
- IGF
insulin-like growth factor
- PrSC
primary normal prostate stromal cell
- PSA
prostate specific antigen
- RT-PCR
reverse transcriptase polymerase chain reaction
- T
testosterone
- TGFβ1
Transforming Growth Factor Beta 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Labrie F, Belanger A, Luu-The V, Labrie C, Simard J, Cusan L, Gomez JL, Candas B. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids. 1998;63:322–8. doi: 10.1016/s0039-128x(98)00007-5. [DOI] [PubMed] [Google Scholar]
- 2.Akwa Y, Baulieu EE. Dehydroepiandrosterone sulfate and dehydroepiandrosterone: Neuroactive neurosteroids. Current Opinion in Endocrinology and Diabetes. 2000;7:160–167. [Google Scholar]
- 3.Belanger A, Candas B, Dupont A, Cusan L, Diamond P, Gomez JL, Labrie F. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J Clin Endocrinol Metab. 1994;79:1086–90. doi: 10.1210/jcem.79.4.7962278. [DOI] [PubMed] [Google Scholar]
- 4.Alesci S, Manoli I, Blackman MR. Dehydrodepiandrosterone (DHEA) In: P Coates, et al., editors. Encyclopedia of Dietary Supplements. 1. Marcel Dekker, Inc; New York, NY: 2005. pp. 167–176. [Google Scholar]
- 5.Labrie F. Intracrinology. Mol Cell Endocrinol. 1991;78:C113–8. doi: 10.1016/0303-7207(91)90116-a. [DOI] [PubMed] [Google Scholar]
- 6.Labrie F, Dupont A, Simard J, Luu-The V, Belanger A. Intracrinology: the basis for the rational design of endocrine therapy at all stages of prostate cancer. Eur Urol. 1993;24(Suppl 2):94–105. doi: 10.1159/000474399. [DOI] [PubMed] [Google Scholar]
- 7.Gingras S, Simard J. Induction of 3beta-hydroxysteroid dehydrogenase/isomerase type 1 expression by interleukin-4 in human normal prostate epithelial cells, immortalized keratinocytes, colon, and cervix cancer cell lines. Endocrinology. 1999;140:4573–84. doi: 10.1210/endo.140.10.7038. [DOI] [PubMed] [Google Scholar]
- 8.Geller J. Rationale for blockade of adrenal as well as testicular androgens in the treatment of advanced prostate cancer. Semin Oncol. 1985;12:28–35. [PubMed] [Google Scholar]
- 9.Carruba G. Estrogens and mechanisms of prostate cancer progression. Ann N Y Acad Sci. 2006;1089:201–17. doi: 10.1196/annals.1386.027. [DOI] [PubMed] [Google Scholar]
- 10.Pike MC, Krailo MD, Henderson BE, Casagrande JT, Hoel DG. ‘Hormonal’ risk factors, ‘breast tissue age’ and the age-incidence of breast cancer. Nature. 1983;303:767–70. doi: 10.1038/303767a0. [DOI] [PubMed] [Google Scholar]
- 11.Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res. 2008;10:201. doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green JE, Shibata MA, Shibata E, Moon RC, Anver MR, Kelloff G, Lubet R. 2-difluoromethylornithine and dehydroepiandrosterone inhibit mammary tumor progression but not mammary or prostate tumor initiation in C3(1)/SV40 T/t-antigen transgenic mice. Cancer Res. 2001;61:7449–55. [PubMed] [Google Scholar]
- 13.Rao KV, Johnson WD, Bosland MC, Lubet RA, Steele VE, Kelloff GJ, McCormick DL. Chemoprevention of rat prostate carcinogenesis by early and delayed administration of dehydroepiandrosterone. Cancer Res. 1999;59:3084–9. [PubMed] [Google Scholar]
- 14.Lubet RA, Gordon GB, Prough RA, Lei XD, You M, Wang Y, Grubbs CJ, Steele VE, Kelloff GJ, Thomas CF, Moon RD. Modulation of methylnitrosourea-induced breast cancer in Sprague Dawley rats by dehydroepiandrosterone: dose-dependent inhibition, effects of limited exposure, effects on peroxisomal enzymes, and lack of effects on levels of Ha-Ras mutations. Cancer Res. 1998;58:921–6. [PubMed] [Google Scholar]
- 15.Perkins SN, Hursting SD, Haines DC, James SJ, Miller BJ, Phang JM. Chemoprevention of spontaneous tumorigenesis in nullizygous p53-deficient mice by dehydroepiandrosterone and its analog 16alpha-fluoro-5-androsten-17-one. Carcinogenesis. 1997;18:989–94. doi: 10.1093/carcin/18.5.989. [DOI] [PubMed] [Google Scholar]
- 16.Ciolino H, MacDonald C, Memon O, Dankwah M, Yeh GC. Dehydroepiandrosterone inhibits the expression of carcinogen-activating enzymes in vivo. Int J Cancer. 2003;105:321–5. doi: 10.1002/ijc.11075. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz AG, Pashko LL. Cancer prevention with dehydroepiandrosterone and non-androgenic structural analogs. J Cell Biochem Suppl. 1995;22:210–7. doi: 10.1002/jcb.240590826. [DOI] [PubMed] [Google Scholar]
- 18.Arnold JT, Le H, McFann KK, Blackman MR. Comparative effects of DHEA vs. testosterone, dihydrotestosterone, and estradiol on proliferation and gene expression in human LNCaP prostate cancer cells. Am J Physiol Endocrinol Metab. 2005;288:E573–84. doi: 10.1152/ajpendo.00454.2004. [DOI] [PubMed] [Google Scholar]
- 19.Arnold JT, Gray NE, Jacobowitz K, Viswanathan L, Cheung PW, McFann KK, Le HD, Blackman MR. Human Prostate Stromal Cells Stimulate Increased PSA Production in DHEA-treated Prostate Cancer Epithelial Cells. J Steroid Biochem Mol Biol. 2008 doi: 10.1016/j.jsbmb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le H, Arnold JT, McFann KK, Blackman MR. Dihydrotestosterone and Testosterone, but not DHEA or Estradiol, Differentially Modulate IGF-I, IGFBP - 2 and IGFBP-3 Gene and Protein Expression in Primary Cultures of Human Prostatic Stromal Cells. Am J Physiol Endocrinol Metab. 2005 doi: 10.1152/ajpendo.00451.2005. [DOI] [PubMed] [Google Scholar]
- 21.Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 2004;67:417–34. doi: 10.1679/aohc.67.417. [DOI] [PubMed] [Google Scholar]
- 22.Donjacour AA, Cunha GR. Stromal regulation of epithelial function. Cancer Treat Res. 1991;53:335–64. doi: 10.1007/978-1-4615-3940-7_16. [DOI] [PubMed] [Google Scholar]
- 23.Arnold JT, Isaacs JT. Mechanisms involved in the progression of androgen-independent prostate cancers: it is not only the cancer cell’s fault. Endocr Relat Cancer. 2002;9:61–73. doi: 10.1677/erc.0.0090061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schedin P, Elias A. Multistep tumorigenesis and the mircroenvironment. Breast Cancer Research. 2004;6:93–101. doi: 10.1186/bcr772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166:2472–83. [PubMed] [Google Scholar]
- 26.Peehl DM, Sellers RG. Induction of smooth muscle cell phenotype in cultured human prostatic stromal cells. Exp Cell Res. 1997;232:208–15. doi: 10.1006/excr.1997.3525. [DOI] [PubMed] [Google Scholar]
- 27.Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8:2912–23. [PubMed] [Google Scholar]
- 28.Rowley DR. What might a stromal response mean to prostate cancer progression? Cancer Metastasis Rev. 1998;17:411–9. doi: 10.1023/a:1006129420005. [DOI] [PubMed] [Google Scholar]
- 29.Deutsch E, Maggiorella L, Eschwege P, Bourhis J, Soria JC, Abdulkarim B. Environmental, genetic, and molecular features of prostate cancer. The Lancet Oncology. 2004;5:303–313. doi: 10.1016/S1470-2045(04)01468-8. [DOI] [PubMed] [Google Scholar]
- 30.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 31.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–69. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–92. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simard J, Gingras S. Crucial role of cytokines in sex steroid formation in normal and tumoral tissues. Mol Cell Endocrinol. 2001;171:25–40. doi: 10.1016/s0303-7207(00)00387-7. [DOI] [PubMed] [Google Scholar]
- 34.Gray NE, Liu X, Choi R, Blackman MR, JTA Endocrine-Immune-Paracrine Interactions In Prostate Cells: A Model For Mechanistic Studies Of Phytomedicines. Cancer Prevention Research. doi: 10.1158/1940-6207.CAPR-08-0062. (in revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rainey WE, Naville D, Mason JI. Regulation of 3 beta-hydroxysteroid dehydrogenase in adrenocortical cells: effects of angiotensin-II and transforming growth factor beta. Endocr Res. 1991;17:281–96. doi: 10.1080/07435809109027202. [DOI] [PubMed] [Google Scholar]
- 36.Ee YS, Lai LC, Reimann K, Lim PK. Effect of transforming growth factor-beta1 on oestrogen metabolism in MCF-7 and MDA-MB-231 breast cancer cell lines. Oncol Rep. 1999;6:843–6. doi: 10.3892/or.6.4.843. [DOI] [PubMed] [Google Scholar]
- 37.Dulos J, Boots AH. DHEA metabolism in arthritis: a role for the p450 enzyme Cyp7b at the immune-endocrine crossroad. Ann N Y Acad Sci. 2006;1069:401–13. doi: 10.1196/annals.1351.038. [DOI] [PubMed] [Google Scholar]
- 38.Weihua Z, Warner M, Gustafsson JA. Estrogen receptor beta in the prostate. Mol Cell Endocrinol. 2002;193:1–5. doi: 10.1016/s0303-7207(02)00089-8. [DOI] [PubMed] [Google Scholar]
- 39.Chen F, Knecht K, Birzin E, Fisher J, Wilkinson H, Mojena M, Moreno CT, Schmidt A, Harada S, Freedman LP, Reszka AA. Direct agonist/antagonist functions of dehydroepiandrosterone. Endocrinology. 2005;146:4568–76. doi: 10.1210/en.2005-0368. [DOI] [PubMed] [Google Scholar]
- 40.Widstrom RL, Dillon JS. Is there a receptor for dehydroepiandrosterone or dehydroepiandrosterone sulfate? Semin Reprod Med. 2004;22:289–98. doi: 10.1055/s-2004-861546. [DOI] [PubMed] [Google Scholar]
- 41.Klein H, Molwitz T, Bartsch W. Steroid sulfate sulfatase in human benign prostatic hyperplasia: characterization and quantification of the enzyme in epithelium and stroma. J Steroid Biochem. 1989;33:195–200. doi: 10.1016/0022-4731(89)90294-x. [DOI] [PubMed] [Google Scholar]
- 42.Klein H, Bressel M, Kastendieck H, Voigt KD. Quantitative assessment of endogenous testicular and adrenal sex steroids and of steroid metabolizing enzymes in untreated human prostatic cancerous tissue. J Steroid Biochem. 1988;30:119–30. doi: 10.1016/0022-4731(88)90084-2. [DOI] [PubMed] [Google Scholar]
- 43.Voigt KD, Bartsch W. Intratissular androgens in benign prostatic hyperplasia and prostatic cancer. J Steroid Biochem. 1986;25:749–57. doi: 10.1016/0022-4731(86)90304-3. [DOI] [PubMed] [Google Scholar]
- 44.Martin C, Ross M, Chapman KE, Andrew R, Bollina P, Seckl JR, Habib FK. CYP7B generates a selective estrogen receptor beta agonist in human prostate. J Clin Endocrinol Metab. 2004;89:2928–35. doi: 10.1210/jc.2003-031847. [DOI] [PubMed] [Google Scholar]
- 45.McPherson SJ, Ellem SJ, Risbridger GP. Estrogen-regulated development and differentiation of the prostate. Differentiation. 2008;76:660–70. doi: 10.1111/j.1432-0436.2008.00291.x. [DOI] [PubMed] [Google Scholar]
- 46.Carruba G. Estrogen and prostate cancer: an eclipsed truth in an androgen-dominated scenario. J Cell Biochem. 2007;102:899–911. doi: 10.1002/jcb.21529. [DOI] [PubMed] [Google Scholar]
- 47.Risbridger GP, Ellem SJ, McPherson SJ. Estrogen action on the prostate gland: a critical mix of endocrine and paracrine signaling. J Mol Endocrinol. 2007;39:183–8. doi: 10.1677/JME-07-0053. [DOI] [PubMed] [Google Scholar]
- 48.McPherson SJ, Ellem SJ, Simpson ER, Patchev V, Fritzemeier KH, Risbridger GP. Essential role for estrogen receptor beta in stromal-epithelial regulation of prostatic hyperplasia. Endocrinology. 2007;148:566–74. doi: 10.1210/en.2006-0906. [DOI] [PubMed] [Google Scholar]
- 49.Chang WY, Prins GS. Estrogen receptor-beta: implications for the prostate gland. Prostate. 1999;40:115–24. doi: 10.1002/(sici)1097-0045(19990701)40:2<115::aid-pros7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 50.Signoretti S, Loda M. Estrogen receptor beta in prostate cancer: brake pedal or accelerator? Am J Pathol. 2001;159:13–6. doi: 10.1016/s0002-9440(10)61666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isaacs JT. The aging ACI/Seg versus Copenhagen male rat as a model system for the study of prostatic carcinogenesis. Cancer Res. 1984;44:5785–96. [PubMed] [Google Scholar]
- 52.Corey E, Quinn JE, Emond MJ, Buhler KR, Brown LG, Vessella RL. Inhibition of androgen-independent growth of prostate cancer xenografts by 17beta-estradiol. Clin Cancer Res. 2002;8:1003–7. [PubMed] [Google Scholar]
- 53.Weihua Z, Makela S, Andersson LC, Salmi S, Saji S, Webster JI, Jensen EV, Nilsson S, Warner M, Gustafsson JA. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proc Natl Acad Sci U S A. 2001;98:6330–5. doi: 10.1073/pnas.111150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petricoin EF, 3rd, Ornstein DK, Paweletz CP, Ardekani A, Hackett PS, Hitt BA, Velassco A, Trucco C, Wiegand L, Wood K, Simone CB, Levine PJ, Linehan WM, Emmert-Buck MR, Steinberg SM, Kohn EC, Liotta LA. Serum proteomic patterns for detection of prostate cancer. J Natl Cancer Inst. 2002;94:1576–8. doi: 10.1093/jnci/94.20.1576. [DOI] [PubMed] [Google Scholar]
- 55.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–42. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 56.Cavalieri EL, Rogan EG. A unified mechanism in the initiation of cancer. Ann N Y Acad Sci. 2002;959:341–54. doi: 10.1111/j.1749-6632.2002.tb02105.x. [DOI] [PubMed] [Google Scholar]
- 57.Ellem SJ, Risbridger GP. Aromatase and prostate cancer. Minerva Endocrinol. 2006;31:1–12. [PubMed] [Google Scholar]
- 58.Ho CK, Nanda J, Chapman KE, Habib FK. Oestrogen and benign prostatic hyperplasia: effects on stromal cell proliferation and local formation from androgen. J Endocrinol. 2008;197:483–91. doi: 10.1677/JOE-07-0470. [DOI] [PubMed] [Google Scholar]
- 59.Ellem SJ, Risbridger GP. Treating prostate cancer: a rationale for targeting local oestrogens. Nat Rev Cancer. 2007;7:621–7. doi: 10.1038/nrc2174. [DOI] [PubMed] [Google Scholar]
- 60.Regelson W, Loria R, Kalimi M. Hormonal intervention: “buffer hormones” or “state dependency”. The role of dehydroepiandrosterone (DHEA), thyroid hormone, estrogen and hypophysectomy in aging. Ann N Y Acad Sci. 1988;521:260–73. doi: 10.1111/j.1749-6632.1988.tb35284.x. [DOI] [PubMed] [Google Scholar]
- 61.Arnold JT, Liu X, Allen JD, Le H, McFann KK, Blackman MR. Androgen receptor or estrogen receptor-beta blockade alters DHEA-, DHT-, and E2-induced proliferation and PSA production in human prostate cancer cells. Prostate. 2007;67:1152–1162. doi: 10.1002/pros.20585. [DOI] [PubMed] [Google Scholar]
- 62.Agarwal AK, Auchus RJ. Minireview: cellular redox state regulates hydroxysteroid dehydrogenase activity and intracellular hormone potency. Endocrinology. 2005;146:2531–8. doi: 10.1210/en.2005-0061. [DOI] [PubMed] [Google Scholar]
- 63.Hsing AW, Chu LW, Stanczyk FZ. Androgen and prostate cancer: is the hypothesis dead? Cancer Epidemiol Biomarkers Prev. 2008;17:2525–30. doi: 10.1158/1055-9965.EPI-08-0448. [DOI] [PubMed] [Google Scholar]
- 64.Arnold JT, Blackman MR. Does DHEA Exert Direct Effects on Androgen and Estrogen Receptors, and Does It Promote or Prevent Prostate Cancer? Endocrinology. 2005;146:4565–7. doi: 10.1210/en.2005-0901. [DOI] [PubMed] [Google Scholar]
- 65.Howes J, Waring M, Huang L, Howes LG. Long-term pharmacokinetics of an extract of isoflavones from red clover (Trifolium pratense) J Altern Complement Med. 2002;8:135–42. doi: 10.1089/107555302317371424. [DOI] [PubMed] [Google Scholar]
- 66.Rannikko A, Petas A, Rannikko S, Adlercreutz H. Plasma and prostate phytoestrogen concentrations in prostate cancer patients after oral phytoestogen supplementation. Prostate. 2006;66:82–7. doi: 10.1002/pros.20315. [DOI] [PubMed] [Google Scholar]
- 67.Gray NE, Liu X, Choi R, Blackman MR, JTA Endocrine-Immune-Paracrine Interactions In Prostate Cells: A Model For Mechanistic Studies Of Phytomedicines. Cancer Prevention Research. doi: 10.1158/1940-6207.CAPR-08-0062. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smithers D. On some general concepts in oncology with special reference to Hodgkins’s disease. Int J Radiat Oncol Biol Phys. 1983;9:731–738. doi: 10.1016/0360-3016(83)90242-0. [DOI] [PubMed] [Google Scholar]