Abstract
Recent studies have indicated a role for the endocannabinoid system in ethanol-related behaviors. This study examined the effect of pharmacological activation, blockade, and genetic deletion of the CB1 receptors on ethanol-drinking behavior in ethanol preferring C57BL/6J (B6) and ethanol nonpreferring DBA/2J (D2) mice. The deletion of CB1 receptor significantly reduced the ethanol preference. Although the stimulation of the CB1 receptor by CP-55,940 markedly increased the ethanol preference, this effect was found to be greater in B6 than in D2 mice. The antagonism of CB1 receptor function by SR141716A led to a significant reduction in voluntary ethanol preference in B6 than D2 mice. A significant lower hypothermic and greater sedative response to acute ethanol administration was observed in both the strains of CB1 -/- mice than wild-type mice. Interestingly, genetic deletion and pharmacological blockade of the CB1 receptor produced a marked reduction in severity of handling-induced convulsion in both the strains. The radioligand binding studies revealed significantly higher levels of CB1 receptor-stimulated G-protein activation in the striatum of B6 compared to D2 mice. Innate differences in the CB1 receptor function might be one of the contributing factors for higher ethanol drinking behavior. The antagonists of the CB1 receptor may have therapeutic potential in the treatment of ethanol dependence.
Keywords: alcohol, striatum, CB1 receptor, G-protein, endocannabinoid, anandamide
INTRODUCTION
Chronic alcoholism is a serious mental disorder characterized by impaired control over drinking, leading to tolerance and physical dependence. Although, genetic and environmental factors have been shown to play important role in the development of ethanol dependence, the underlying mechanism is still unclear. Understanding the molecular mechanism of ethanol dependence is critical to the treatment of ethanol-related disorders, as withdrawal severity is a major risk factor for relapse.
In the recent years, a number of studies have suggested a close interaction between the endocannabinoid system and ethanol-related behaviors (Colombo et al., 2007; Lallemand et al., 2001; Vinod and Hungund, 2006). The endocannabinoid system, which consists of cannabinoid (CB) receptors, endocannabinoids, and proteins that are involved in the regulation and metabolism of endocannabinoids, has received much attention in understanding of its functional significance in drug abuse and other neuropsychiatric disorders. Two types of CB receptors, CB1 and CB2, have been identified till to date, which belong to the G-protein coupled receptor (GPCRs) family. The endocannabinoids, N-arachidonyl ethanolamide anandamide (AEA) is a partial agonist whereas 2-arachidonylglycerol is a full agonist for the CB receptors. Recent studies have also demonstrated that AEA act as a full agonist (endovanilloid) at the vanilloid (VR1) receptors (Sugiura et al., 2006).
The CB1 receptor is one of the most abundant neuromodulatory GPCRs that is expressed in the cortex, striatum, hippocampus, and cerebellum (Howlett, 2005). Recent studies have also revealed the existence of CB2 receptors in the brain (Onaivi et al., 2006; Van Sickle et al., 2005). In the CNS, the endocannabinoids are synthesized in the postsynaptic neurons and act as retrograde messengers to activate presynaptically located Gi/o-coupled CB1 receptors that modulate adenylyl cyclase, ion channels, and extracellular signal-regulated kinases (Howlett, 2005). Studies from our laboratory and others have suggested a role for both CB1 and CB2 receptors in alcohol addiction (Ishiguro et al., 2007; Vinod and Hungund, 2006).
Epidemiological studies have established that alcoholism is a familial disorder with significant genetic contribution (Heath et al., 2001). Several animal models have been developed to understand a genetic basis of alcoholism. In this regard, C57BL/6J (B6; ethanol preferring) and DBA/2J (D2; ethanol nonpreferring) mice have been shown to exhibit striking differences in ethanol related behavior. This study was designed to investigate a role of the CB1 receptor in ethanol-related behaviors using pharmacological and genetic manipulation of the CB1 receptor function. This study further examined whether the genetically predetermined differences in the brain CB1 receptor function could explain the changes in behavioral consequences of ethanol drinking behavior.
MATERIALS AND METHODS
Animals
The CB1 heterozygous (±) mice with mixed B6.129Sv(B6)-Cnr1tm|Zim background were generated as described previously (Zimmer et al., 1999). These mice were further backcrossed for 10 generations with B6 background to reach genetic homogeneity. In addition, the heterozygous mice (CB1 ±) with B6 background were interbred with D2 strain to generate CB1 ± mice (F1) and were further backcrossed with D2 mice for 10 generations. A marker-assisted selection protocol was used to evaluate the amount of B6 and D2 background genes in each generation using microsatellite markers (Invitrogen, Carlsbad, CA) as previously described (Hospital et al., 1992). This technique is advantageous in that it significantly accelerates the pace of congenic strain development. Twelve to thirteen-week old male homozygous wild (+/+) and knockout (-/-) mice used in this study were derived from the intercrosses between the 10th generation heterozygous mice. Animal care and handling procedures were in accordance with Institutional and National Institutes of Health guidelines.
Chemicals
CP-55,940, HU-210 (Tocris Cookson, Ellisville, MO), and SR141716A (NIDA, Bethesda, MD) were dissolved in physiological saline with 1% Tween 80 and 1% DMSO. Ethanol (Pharmaco Products, Brook-field, CT) solution was prepared in physiological saline for ethanol tolerance and dependence studies. Ethanol (95%, David Sherman Corporation, St Louis, MO) solutions were prepared in tap water and used for drinking studies. Pyrazole (Sigma-Aldrich, St. Louis, MO) was dissolved in saline and injected at a volume of 10 ml/kg (68 mg/kg).
Ethanol drinking behavior
Mice (-/- and +/+) were individually housed for at least 1 week prior to testing for ethanol preference using a “two-bottle” choice paradigm as previously described (Hungund et al., 2003; Vadasz et al., 2000). Briefly, the test consisted of six 3-day trials, in which mice were allowed to choose between ethanol and water. Animals were acclimatized to escalating concentrations of ethanol; a 3% solution for trial 1 (days 1-3) was increased to 6% in trial 2 (days 3-6), and further increased to 12% for trials 3 (days 6-9), 4 (days 9-12), 5 (days 12-15), and 6 (days 15-18). This arrangement provided multiple measures of ethanol preference at 12% concentration (v/v). The positions of water and ethanol tubes were alternated in each 3-day trial to avoid place preference. Data obtained from the trials 4, 5, and 6 were used to calculate the ethanol preference.
Acute effects of CP-55,940 (10, 30, and 50 μg/kg body wt, i.p.) and SR141716A (1, 3, and 5 mg/kg body wt, i.p.) on ethanol preference was examined using limited access protocol in B6 and D2 mice that were acclimatized to escalating concentrations of ethanol as previously described for unlimited access paradigm. One hour after the treatment with either drug or vehicle, mice were offered 12% ethanol and water for 6 h in the beginning of the dark cycle (reverse light/dark cycle). The consumption of ethanol and water was measured at the end of sixth hour. Data were calculated as grams of ethanol or water consumed/kg body wt/day and then the ratio of ethanol preference (ethanol intake/ethanol + water intake) was determined.
Ethanol-induced hypothermia and sedation
Sensitivity to ethanol-induced hypothermia and sedation was measured in ethanol-naïve CB1 -/- and +/+ mice. Ethanol-induced hypothermia was assessed by monitoring rectal temperatures (Physiotemp Instruments, Clifton, NJ) after administration of a challenge dose of ethanol (2 g/kg body wt, i.p.). The rectal temperatures were recorded immediately prior to the ethanol challenge and 30 min thereafter. The difference in body temperature between pre- and postinjection was used to determine the degree of hypothermia. Ethanol-induced sedation was measured by determining the time between loss of the righting reflex (LORR) and time to regain the righting reflex after the administration of a challenge dose of ethanol (3 g/kg body wt, i.p.) as previously described (Nowak et al., 2006).
Handling-induced convulsions
The ethanol inhalation paradigm was used to evaluate handling-induced convulsions (HIC). This procedure has been proven to be an effective and reliable method for producing functional dependence to ethanol (De Witte et al., 2003; Kliethermes et al., 2004; Nowak et al., 2006; Ripley et al., 2002). Briefly, mice were given a priming dose of ethanol (2 g/kg body wt, i.p.) in addition to pyrazole before being placed in the chamber. An alcohol dehydrogenase inhibitor, pyrazole (68 mg/kg body wt, i.p.), was given to stabilize the blood ethanol level (BEL). Animals were exposed to ethanol vapors for 72 h, with daily pyrazole injections. The BEL was determined every 24 h by an enzymatic method as described previously (Vinod et al., 2006). Briefly, the tail blood (10 μL) was mixed with perchloric acid (90 μL) and then immediately vortexed and centrifuged. An aliquot of supernatant (50 μL) was incubated with reaction buffer [semicarbazide HCl buffer (67 mM); sodium pyrophosphate (67 mM), and glycine (20 mM)] containing NAD and alcohol dehydrogenase for 1 h at room temperature. Absorption was measured spectrophotometrically at 340 nm. The control mice were subjected to identical housing and treatment conditions, with the exception of pre-exposure and infusion of ethanol into the chamber. The HIC was assessed in CB1 -/- and +/+ mice and also following four experimental conditions in regular B6 and D2 mice; (a) coadministration of SR141716A (3 mg/kg body wt, i.p.) with ethanol, (b) ethanol exposure, (c) SR141716A treatment, and (d) vehicle administration. Mice were tested for severity of HIC 8 h following withdrawal from ethanol as previously described (Ripley et al., 2002). Briefly, animals were first lifted by the tail and body tremor and convulsions were assessed. Mice were then twisted clockwise 180° and rescored. The scoring system included; 0-no response; 1-mild tremor; 2-severe tremor; 3-intermittent convulsion, and 4-continuous convulsion. A final score was given following another 180° counter-clockwise twist. Three numerical scores were summed to obtain the total measure. Animals were scored while blind to the experimental conditions and videotaped for later scoring. The dose of SR141716A used in the present experiments was chosen based on the literature (Gessa et al., 2005; Lallemand et al., 2001) and our preliminary findings on the effects of SR141716A on ethanol tolerance (Nowak et al., 2006) and voluntary ethanol consumption.
Agonist-stimulated [35S]GTPγS binding Assay
The CB1 receptor-stimulated [35S]GTPγS activation was measured in the crude synaptic membrane isolated from the cerebral cortex, hippocampus, striatum, and cerebellum of B6 and D2 mice (ethanol naïve) as previously described with minor modifications (Vinod et al., 2006). Briefly, an aliquot of membrane (20-50 μg protein) was incubated in Tris-magnesium-ethylene diamine tetra acetic acid buffer containing 0.1% fatty acid-free BSA, 100 mM NaCl, 30 μM GDP, and 0.05 nM [35S]GTPγS in silicone-treated test tubes for 1 h at 37°C. The CB1 receptor agonist, CP-55,940 (1.5 μM) was used to examine the CB1 receptor-stimulated [35S]GTPγS binding to Gi-protein. Basal activity was measured in absence of CP-55940. Nonspecific binding of the radioligand was determined in the presence of 10 μM GTPγS. Reaction mixture was rapidly filtered through nonpresoaked GF/B glass filters (Brandel, Gaithersburg, MD). Radioactivity was measured by liquid scintillation spectroscopy at an efficiency of 95% for 35S. The CP-55940 stimulated [35S]GTPγS binding was presented as percentage over the basal value: ([stimulation - basal]/basal) × 100.
Data analysis
Statistical analysis was performed by independent-sample t test or two-way analysis of variance (ANOVA) followed by Bonferroni post hoc test as appropriate (GraphPad software, San Diego, CA). Data are presented as mean ± SEM. Results were considered to be statistically significant at P < 0.05.
RESULTS
Effects of CB1 receptor deletion, SR141716A, and CP-55,940 on ethanol preference
The results of two-bottle choice between ethanol and water revealed that mice lacking CB1 receptor gene displayed a significantly lower preference for ethanol compared to their +/+ counterparts in both the strains (Fig. 1). There were main effects of drug (F1,26 = 38.32, P < 0.0001) and strain (F1,26 = 786.8, P < 0.0001) on ethanol preference with a significant drug × strain interaction (F1,26 = 4.54, P < 0.05; Fig. 1).
Fig. 1.
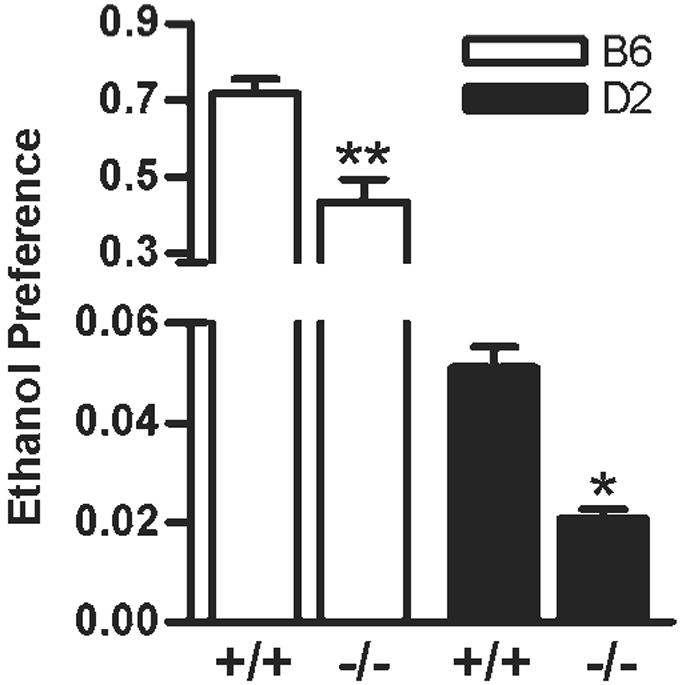
The preference for ethanol was found to be significantly lower in B6.CB1 -/- (P < 0.001) and D2.CB1 -/- (P < 0.05) compared to their corresponding +/+ mice (n = 8-12 each per group) in an unlimited access paradigm.
Two-way ANOVA analysis also revealed an effect of drug (F3,66 = 58.4, P < 0.0001) and strain (F3,26 = 301.0, P < 0.0001) on ethanol preference with significant drug x strain interaction (F3,66 = 30.59, P < 0.0001; Fig. 2A) when mice were administered with CB1 receptor antagonist, SR141716A. Interestingly, a lower dose of SR141716A (1 mg/kg) was able to significantly decrease the ethanol preference in B6 (P < 0.01) but not in D2 mice compared to vehicle-treated control groups (Fig. 2A). Pharmacological manipulation with the CB1 receptor agonist, CP-55940, resulted in drug (F3,63 = 8.76, P < 0.0001) and strain (F1,63 = 119.8, P < 0.0001) dependent increase in ethanol preference with a significant drug × strain interaction (F3,63 = 2.85, P < 0.05; Fig. 2B). Interestingly, a trend toward increase in ethanol preference with a higher dose (50 μg/kg) was observed only in B6 (P < 0.001) but not in D2 mice compared to their respective control groups.
Fig. 2.
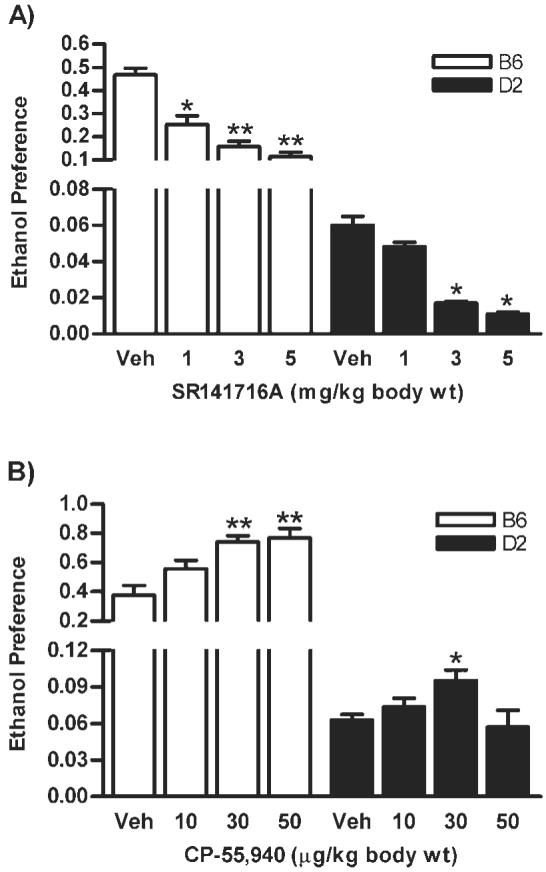
In a limited access paradigm, the CB1 receptor antagonist, SR141716A, reduced ethanol preference in B6 and D2 strains compared to vehicle treated groups (A). Conversely, the CB1 receptor agonist, CP-55,940 increased ethanol preference in B6; however, a small but statistically significant increase was seen in D2 mice (B). *P < 0.05 and **P < 0.001 compared to control groups (n = 8-10).
Ethanol-induced hypothermic and sedative effects in CB1 -/- mice
There was a main effect of ethanol-induced hypothermia on genotype (F1,35 = 35.0, P < 0.0001) and strain (F1,35 = 15.5, P < 0.001) without a significant genotype x strain interaction (F1,35 = 0.54, P = 0.466; Fig. 3A). Similarly, acute ethanol-induced a genotype (F1,37 = 19.11, P < 0.0001) and strain (F1,37 = 33.08, P < 0.0001) dependent increase in the duration of LORR without a significant interaction between genotype × strain (F1,37 = 0.15, P = 0.69; Fig. 3B).
Fig. 3.
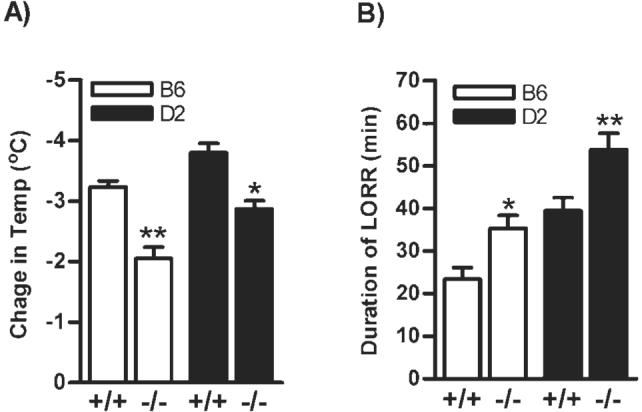
A significant reduction in hypothermic effect of ethanol (2.0 g/kg) was observed in B6.CB1 -/- (P < 0.001; n = 7-10) and D2.CB1 -/- (P < 0.01; n = 7-10; (A). However, duration of LORR was markedly increased in B6.CB1 -/- (P < 0.05; n = 7-10) and D2.CB1 -/- (P < 0.01; n = 7-10) than +/+ mice (B).
Effect of genetic deletion and pharmacological blockade of the CB1 receptor on HIC
A marked effect of HIC on genotype (F1,33 = 48.9, P < 0.0001) and strain (F1,33 = 6.1, P < 0.01) with significant genotype x strain interaction (F1,33 = 4.5, P < 0.05; Fig. 4A) was observed following 8 h after withdrawal from chronic ethanol exposure. The basal scores of HIC were not different between +/+ and -/- mice, which were exposed to circulating air without ethanol (data not shown). This finding was further supported by the pharmacological study in which cotreatment with SR141716A (3 mg/kg body wt) during chronic exposure led to drug (F1,47 = 35.3, P < 0.0001) and strain (F1,47 = 70.9, P < 0.0001) dependent reduction in severity of HIC with significant drug × strain interaction (F1,47 = 4.2, P < 0.05; Fig. 4B). However, SR141716A alone did not show any effect on the HIC in control mice (data not shown). The SR141716A treatment, however, did not alter the BELs in B6 (1.93 ± 0.07 mg/ml) and D2 (3.56 ± 0.29 mg/ml) mice compared to their respective vehicle treated control groups (1.90 ± 0.06 and 3.51 ± 0.38 mg/ml) for B6 and D2 mice, respectively.
Fig. 4.
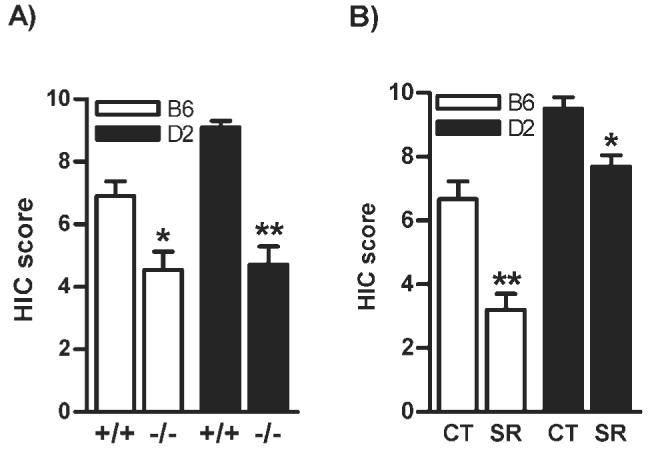
The B6.CB1 -/- (P < 0.01) and D2.CB1 -/- mice (P < 0.001) showed a significant reduction in severity of HIC following 8 h after withdrawal from chronic ethanol exposure compared to +/+ mice (A, n = 8-10). Similarly, the CB1 receptor blockade with SR141716A during alcoholization led to a significant reduction in severity of the HIC in regular B6 (P < 0.001) and D2 mice (P < 0.05) compared to vehicle treated mice, respectively (B, n = 8-12). CT-Control; SR-SR141716A.
CB1 receptor function in the brain of B6 and D2 mice
The CP-55,940-stimulated [35S]GTPγS activation was found to be significantly higher in the striatum of ethanol naïve B6 than D2 mice (30%, P < 0.05; Fig. 5). However, no differences in the G-protein activation were found in the cerebral cortex, hippocampus, and cerebellum between the groups.
Fig. 5.
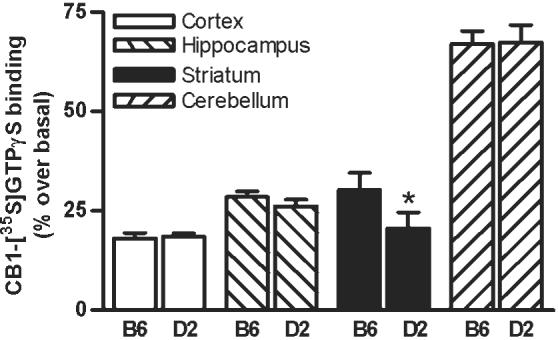
The CB1 receptor-mediated G-protein activation was measured in the crude synaptic membrane isolated from various brain regions of ethanol naïve B6 and D2 mice. The CB1 receptor-mediated G-protein activation was found to be significantly higher (P < 0.05) in the striatum of B6 compared to D2 mice (B). Data presented as percentage over the basal value (n = 5-8 in each group).
DISCUSSION
This study examined whether stimulation and blockade of the CB1 receptor regulate ethanol preference in mice with genetically predetermined differences in ethanol-related behaviors. Activation of the CB1 receptor led to a marked increase in ethanol preference in both the strains with greater stimulation in B6 than D2 strains. The ethanol-reinforcing behavior via CB1 receptor stimulation has been reported in ethanol-preferring rats (Colombo et al., 2002). Conversely, pharmacological blockade with SR141716A or genetic deletion of the CB1 receptor significantly reduced ethanol preference in these mice. These results confirm earlier findings of decreased ethanol intake and conditioned place preference in the CB1 -/- mice (Hungund et al., 2003; Lallemand et al., 2001; Naassila et al., 2004; Thanos et al., 2005; Wang et al., 2003). Interestingly, effects of CP-55,940 and SR141716A on ethanol preference were greater in B6 than D2 mice. Although a reason for this differential effect is currently unknown, a higher efficiency of the striatal CB1 receptor coupling detected in B6 than D2 mice might be one of the contributing factors. This would certainly amplify responses to an agonist and may also unmask an endogenous tone, which could explain the greater opposite effect of SR141716A.
The reduction in ethanol preference particularly in D2 mice at higher dose of CP-55,940 (50 μg/kg) appears to be associated with catalepsy and decreased spontaneous activity by the CB1 receptor stimulation (Chaperon and Thiebot, 1999; Wang et al., 2003). Similarly, no further increase in ethanol preference with this higher dose was also evident in B6 strain. The strain-dependent differences in WIN 55,212-2 (CB1 receptor agonist) and SR141617A induced ethanol preference in B6 and D2 mice has been recently reported (Kelai et al., 2006). In addition, it is also of importance to note a critical role of the CB2 receptor in the neurobiology of alcohol addiction. For instance, enhancement of ethanol preference in mice treated with CB2 receptor agonist under chronic stress model was prevented by CB2 receptor antagonist (Ishiguro et al., 2007) suggesting a potential utility of the CB2 receptor antagonist in the treatment of alcohol addiction.
Effects of acute ethanol challenge on hypothermia and sedation revealed genotype dependent changes. The CB1 -/- mice of both the strains showed a reduction in hypothermic effect. This finding is in agreement with the fact that the CBs elicit hypothermic effect via CB1 receptor (Rawls et al., 2002; Zimmer et al., 1999) and therefore, absence of this receptor could have attributed to a lower hypothermic response. Although, this is not consistent with the previous study (Naassila et al., 2004), the possible explanation for the contrasting results could be due to differences in ethanol dosage employed. Conversely, ethanol-induced sedative effect was greater in CB1 -/- mice, which is similar to the observation made by Naassila et al. (2004).
To test whether genetically predetermined brain regional changes in the endocannabinoid system are associated with differences in ethanol preference in B6 and D2 mice, the status of CB1 receptor function (G-protein activation) was examined. We observed a higher CB1 receptor-mediated [35S]GTPγS binding in the striatum of B6 mice. This is consistent with our previous finding that demonstrated a higher G-protein activation in the whole brain of B6 than D2 mice (Basavarajappa and Hungund, 2001). However, other brain regions that are associated with reward mechanism need to be also further examined. Although, the underlying mechanism(s) of varied ethanol-reinforcing behavior in these strains is yet to be determined, the higher CB1 receptor function (CB1 receptor-mediated G-protein signaling) in the striatum of B6 mice seems to play a critical role in the ethanol consummatory behavior. This hypothesis was partly supported by the existence of correlation between age-dependent decrease in ethanol preference and decline in the limbic CB1 receptor signaling (Wang et al., 2003).
Ethanol intake has been shown to increase the limbic dopamine (DA) and AEA contents (Cheer et al., 2007; Gonzalez et al., 2002a; Hungund et al., 2003; Weiss et al., 1993). AEA also activates the mesolimbic dopaminergic system by increasing the DA content (Gessa et al., 1998; Solinas et al., 2006). A lack of ethanol-induced DA release has been shown in the NAc of CB1 -/- mice or wild-type mice treated with a CB1 receptor antagonist (Cheer et al., 2007; Hungund et al., 2003). These functional interactions between endocannabinoid and DA systems might be partly associated with positive reinforcing effect of ethanol. The observed effect could also be due to a lower DA content in the mesostriatal region in B6 than in D2 mice (George et al., 1995). The hypodopaminergic activity and hyperactivity of the CB1 receptor function in limbic system might play a role in determining the susceptibility for a greater ethanol drinking behavior. Besides this difference, protein kinase A and CREB, which are downstream to the CB1 receptor, have been found to be lower in the NAc of B6 than D2 mice (Misra and Pandey, 2003, 2006; Pandey et al., 2006).
A clinical report correlated the severity of ethanol withdrawal symptoms with the CB1 gene polymorphism (Schmidt et al., 2002), lending indirect support for a role of the CB1 receptor in withdrawal. To further assess the role of the endocannabinoid system in ethanol withdrawal symptoms, the effect of CB1 receptor deletion was investigated. Severity of HIC was markedly decreased in the CB1 -/- mice. This observation was further ascertained by the CB1 receptor antagonist, SR141716A. The concurrent administration of SR141716A (3 mg/kg) with ethanol exposure reduced severity of HIC. This anticonvulsant effect is not due to altered ethanol metabolism since SR141716A did not change the BEL. To our knowledge, this is the first pharmacological evidence linking the CB1 receptor in ethanol withdrawal. In agreement with these findings, reduction of morphine withdrawal signs were also reported in CB1 -/- mice (Ledent et al., 1999) and mice cotreated with SR141716A (Mas-Nieto et al., 2001). We have previously demonstrated that mice treated with SR141716A during chronic exposure are more tolerant to challenge dose of ethanol (Nowak et al., 2006), suggesting a role for the CB1 receptor in neuronal substrate mediating ethanol tolerance and dependence. However, the question of whether genetic differences in the endocannabinoid system associated with symptoms following ethanol withdrawal remains to be elucidated. Our findings are not consistent with a previous report that indicated increased ethanol-induced withdrawal signs in CB1 -/- mice in a liquid-diet paradigm (Naassila et al., 2004). On the contrary, lack of ethanol-induced withdrawal signs in these mice has been shown after voluntary ethanol consumption (Racz et al., 2003). This could be associated with the fact that CB1 -/- mice consume significantly less ethanol than +/+ mice. Besides this, genetic makeup of the mice, dosage, mode, and period of ethanol exposure and time at which the HIC were analyzed might also be important confounding factors for these contrasting results.
Chronic ethanol exposure increases the inhibitory and decreases the excitatory neurotransmissions. Subsequently, the physical signs of withdrawal have primarily been thought to result from reduced GABAergic and increased glutamatergic function (De Witte, 2003). Chronic ethanol exposure has also been shown to increase the endocannabinoid content and concomitant downregulation of CB1 receptors (Basavarajappa et al., 2000; Gonzalez et al., 2002b; Ortiz S et al., 2004; Vinod et al., 2006). Thus, SR141716A could block this effect and the endocannabinoid induced-desensitization of the CB1 receptors. Activation of the CB1 receptors that are located on the GABAergic and glutamatergic interneurons might also play a critical role in regulating the inhibitory and excitatory neurotransmission (Schlicker and Kathmann, 2001). An elevation in the AEA level following NMDA-induced excitotoxicity could be prevented by SR141716A (Hansen et al., 2002; Sommer et al., 2006). It is possible that the administration of SR14176A during ethanol exposure might counteract with functions of GABAergic and glutamatergic system. A recent study further revealed that deletion of CB1 receptors impairs neuroadaptations of both the NMDA and GABA receptors following chronic ethanol exposure suggesting a role for the endocannabinoid system in alcohol dependence (Warnault et al., 2007). Although, the classical tetrad effects of AEA are CB1 receptor-mediated (Wise et al., 2007), SR141716A has been shown to only partially attenuate the AEA-mediated antinociception, catalepsy, hypothermia, and suppression of spontaneous activity (Wiley et al., 2006). This raises the question whether AEA exerts its effect through non-CB1 receptors, for example, VR-1 receptor, DA, and Ca2+/K+ channels (Di Marzo et al., 2000; van der Stelt and Di Marzo, 2005). Because each of these systems may potentially influence the physiology and behavior, further systematic studies are essential in determining the underlying mechanism of ethanol withdrawal signs.
In conclusion, the present findings provide evidence for a critical role for the CB1 receptor in ethanol preference, sensitivity, and dependence. Genetically determined differences in the activity of the endocannabinoid system under basal conditions and in response to ethanol exposure may exist between ethanol-preferring and nonpreferring strains. The CB1 receptor antagonist might be a potential therapeutic agent in the treatment for ethanol dependence.
ACKNOWLEDGMENTS
We are thankful to Dr. A. Zimmer (National Institutes of Health, Bethesda, MD) for providing the CB1 receptor heterozygous mice.
Contract grant sponsor: NIAAA; Contract grant numbers: N01AA22008, AA13003
Footnotes
This article is a US government work and, as such, is in the public domain in the United States of America.
REFERENCES
- Basavarajappa BS, Hungund BL. Cannabinoid receptor agonist-stimulated [35S]guanosine triphosphate gammas binding in the brain of C57BL/6 and DBA/2 mice. J Neurosci Res. 2001;64:429–436. doi: 10.1002/jnr.1094. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Stimulation of cannabinoid receptor agonist 2-arachidonylglycerol by chronic ethanol and its modulation by specific neuromodulators in cerebellar granule neurons. Biochem Biophys Acta. 2000;1535:78–86. doi: 10.1016/s0925-4439(00)00085-5. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Thiebot MH. Behavioral effects of cannabinoid agents in animals. Crit Rev Neurobiol. 1999;13:243–281. doi: 10.1615/critrevneurobiol.v13.i3.20. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Serra S, Brunetti G, Gomez R, Melis S, Vacca G, Carai MM, Gessa L. Stimulation of voluntary ethanol intake by cannabinoid receptor agonists in ethanol-preferring sP rats. Psychopharmacology. 2002;159:181–187. doi: 10.1007/s002130100887. [DOI] [PubMed] [Google Scholar]
- Colombo G, Orru A, Lai P, Cabras C, Maccioni P, Rubio M, Gessa GL, Carai MA. The cannabinoid CB1 receptor antagonist, rimonabant, as a promising pharmacotherapy for alcohol dependence: Preclinical evidence. Mol Neurobiol. 2007;36:102–112. doi: 10.1007/s12035-007-0017-y. [DOI] [PubMed] [Google Scholar]
- De Witte P, Pinto E, Ansseau M, Verbanck P. Alcohol and withdrawal: From animal research to clinical issues. Neurosci Biobehav Rev. 2003;27:189–197. doi: 10.1016/s0149-7634(03)00030-7. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Breivogel CS, Tao Q, Bridgen DT, Razdan RK, Zimmer AM, Zimmer A, Martin BR. Levels, metabolism, and pharmacological activity of anandamide in CB(1) cannabinoid receptor knockout mice: Evidence for non-CB(1), non-CB(2) receptor-mediated actions of anandamide in mouse brain. J Neurochem. 2000;76:2434–1444. doi: 10.1046/j.1471-4159.2000.0752434.x. [DOI] [PubMed] [Google Scholar]
- George SR, Fan T, Ng GY, Jung SY, O'Dowd BF, Naranjo CA. Low endogenous dopamine function in brain predisposes to high alcohol preference and consumption: Reversal by increasing synaptic dopamine. J Pharmacol Exp Ther. 1995;273:373–379. [PubMed] [Google Scholar]
- Gessa GL, Melis M, Muntoni AL, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol. 1998;341:39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Serra S, Vacca G, Carai MA, Colombo G. Suppressing effect of the cannabinoid CB1 receptor antagonist. SR147778, on alcohol intake and motivational properties of alcohol in alcohol-preferring sP rats. Alcohol Alcohol. 2005;40:46–53. doi: 10.1093/alcalc/agh114. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Cascio MG, Fernandez-Ruiz J, Fezza F, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2000a;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Fernandez-Ruiz J, Sparpaglione V, Parolaro D, Ramos JA. Chronic exposure to morphine, cocaine or ethanol in rats produced different effects in brain cannabinoid CB1 receptor binding and mRNA levels. Drug Alcohol Depend. 2002b;66:77–84. doi: 10.1016/s0376-8716(01)00186-7. [DOI] [PubMed] [Google Scholar]
- Hansen HHI, Azcoitia S, Pons J, Romero LM, Garcia-Segura JA, Ramos HS, Hansen J, Fernandez-Ruiz JJ. Blockade of cannabinoid CB1 receptor function protects against in vivo disseminating brain damage following NMDA-induced excitotoxicity. J Neurochem. 2002;82:154–158. doi: 10.1046/j.1471-4159.2002.00961.x. [DOI] [PubMed] [Google Scholar]
- Heath AC, Whitfield JB, Madden PA, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DB, Martin NG. Towards a molecular epidemiology of alcohol dependence: Analysing the interplay of genetic and environmental risk factors. Br J Psychiatry. 2001;40:33–40. doi: 10.1192/bjp.178.40.s33. [DOI] [PubMed] [Google Scholar]
- Hospital F, Chevalet C, Mulsant P. Using markers in gene introgression breeding programs. Genetics. 1992;132:1199–1210. doi: 10.1093/genetics/132.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC. Cannabinoid receptor signaling. Handbook Exp Pharmacol. 2005;168:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Szakall I, Adam A, Basavarajappa B, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary ethanol consumption and lack ethanol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Iwasaki S, Teasenfitz L, Higuchi S, Horiuchi Y, Saito T, Arinami T, Onaivi ES. Involvement of cannabinoid CB2 receptor in alcohol preference in mice and alcoholism in humans. Pharmacogenom J. 2007;7:380–385. doi: 10.1038/sj.tpj.6500431. [DOI] [PubMed] [Google Scholar]
- Kelai S, Hanoun N, Aufrere G, Beauge F, Hamon M, Lanfumey L. Cannabinoid-serotonin interactions in alcohol-preferring vs. alcohol-avoiding mice. J Neurochem. 2006;99:308–20. doi: 10.1111/j.1471-4159.2006.04054.x. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL, Cronise K, Crabbe JC. Anxiety-like behavior in mice in two apparatuses during withdrawal from chronic ethanol vapor inhalation. Alcoholism Clin Exp Res. 2004;28:1012–1019. doi: 10.1097/01.alc.0000131976.40428.8f. [DOI] [PubMed] [Google Scholar]
- Lallemand F, Soubrie PH, De Witte PH. Effects of CB1 cannabinoid receptor blockade on ethanol preference after chronic ethanol administration. Alcoholism Clin Exp Res. 2001;25:1317–1323. [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Mas-Nieto M, Pommier B, Tzavara ET, Caneparo A, Da Nascimento S, Le Fur G, Roques BP, Noble F. Reduction of opioid dependence by the CB1 antagonist SR141716A in mice: Evaluation of the interest in pharmacotherapy of opioid addiction. Br J Pharmacol. 2001;132:1809–1816. doi: 10.1038/sj.bjp.0703990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra K, Pandey SC. Differences in basal levels of CREB and NPY in nucleus accumbens regions between C57BL/6 and DBA/2 mice differing in inborn alcohol drinking behavior. J Neurosci Res. 2003;74:967–975. doi: 10.1002/jnr.10831. [DOI] [PubMed] [Google Scholar]
- Misra K, Pandey SC. The decreased cyclic-AMP dependent-protein kinase a function in the nucleus accumbens: A role in alcohol drinking but not in anxiety-like behaviors in rats. Neuropsychopharmacology. 2006;31:1406–1419. doi: 10.1038/sj.npp.1300900. [DOI] [PubMed] [Google Scholar]
- Naassila M, Pierrefiche O, Ledent C, Daoust M. Decreased alcohol self-administration and increased alcohol sensitivity and withdrawal in CB1 receptor knockout mice. Neuropharmacol. 2004;46:243–253. doi: 10.1016/j.neuropharm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Nowak KL, Vinod KY, Hungund BL. Pharmacological manipulation of CB1 receptor function alters development of tolerance to alcohol. Alcohol Alcohol. 2006;41:24–32. doi: 10.1093/alcalc/agh217. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola BE, Liu QR, Hope B, Iwasaki S, Arinami T, Teasenfitz L, Uhl GR. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- Ortiz S, Oliva JM, Perez-Rial S, Palomo T, Manzanares J. Chronic ethanol consumption regulates cannabinoid CB1 receptor gene expression in selected regions of rat brain. Alcohol Alcohol. 2004;39:88–92. doi: 10.1093/alcalc/agh036. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Misra K. Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci. 2006;26:8320–8331. doi: 10.1523/JNEUROSCI.4988-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz I, Bilkei-Gorzo A, Toth ZE, Michel K, Palkovits M, Zimmer A. A critical role for the cannabinoid CB1 receptors in ethanol dependence and stress-stimulated ethanol drinking. J Neurosci. 2003;23:2453–2458. doi: 10.1523/JNEUROSCI.23-06-02453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Cabassa J, Geller EB, Adler MW. CB1 receptors in the preoptic anterior hypothalamus regulate WIN 55212-2 [(4,5-dihydro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenylcarboyl)-6H-pyrrolo[3,2,1ij]quinolin-6-one]-induced hypothermia. J Pharmacol Exp Ther. 2002;301:963–968. doi: 10.1124/jpet.301.3.963. [DOI] [PubMed] [Google Scholar]
- Ripley TL, Dunworth SJ, Stephens DN. Effect of CGP39551 administration on the kindling of ethanol-withdrawal seizures. Psychopharmacology. 2002;163:157–165. doi: 10.1007/s00213-002-1138-7. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Kathmann M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol Sci. 2001;22:565–572. doi: 10.1016/s0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- Schmidt LG, Samochowiec J, Finckh U, Fiszer-Piosik E, Horodnicki J, Wendel B, Rommelspacher H, Hoehe MR. Association of a CB1 cannabinoid receptor gene (CNR1) polymorphism with severe alcohol dependence. Drug Alcohol Depend. 2002;65:221–224. doi: 10.1016/s0376-8716(01)00164-8. [DOI] [PubMed] [Google Scholar]
- Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;98:408–419. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- Sommer C, Schomacher M, Berger C, Kuhnert K, Muller HD, Schwab SSchabitz WR. Neuroprotective cannabinoid receptor antagonist SR141716A prevents downregulation of excitotoxic NMDA receptors in the ischemic penumbra. Acta Neuropathol. 2006;112:277–286. doi: 10.1007/s00401-006-0110-8. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kishimoto S, Oka S, Gokoh M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog Lipid Res. 2006;45:405–446. doi: 10.1016/j.plipres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Dimitrakakis ES, Rice O, Gifford A, Volkow ND. Ethanol self-administration and ethanol conditioned place preference are reduced in mice lacking cannabinoid CB1 receptors. Behav Brain Res. 2005;164:206–213. doi: 10.1016/j.bbr.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Vadasz C, Saito M, Gyetvai B, Mikics E, Vadasz C., II Scanning of five chromosomes for alcohol consumption loci. Alcohol. 2000;22:25–34. doi: 10.1016/s0741-8329(00)00098-7. [DOI] [PubMed] [Google Scholar]
- van der Stelt M, Di Marzo V. Anandamide as an intracellular messenger regulating ion channel activity. Prostagland Other Lipid Mediat. 2005;77:111–122. doi: 10.1016/j.prostaglandins.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Hungund BL. Cannabinoid-1 receptor: A novel target for the treatment of neuropsychiatric disorders. Expert Opin Ther Targets. 2006;10:203–210. doi: 10.1517/14728222.10.2.203. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Xie S, Cooper TB, Hungund BL. Effect of chronic ethanol exposure and its withdrawal on the endocannabinoid system. Neurochem Int. 2006;49:619–625. doi: 10.1016/j.neuint.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci USA. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnault V, Houchi H, Barbier E, Pierrefiche O, Vilpoux C, Ledent C, Daoust M, Naassila M. The lack of CB1 receptors prevents neuroadapatations of both NMDA and GABA(A) receptors after chronic ethanol exposure. J Neurochem. 2007;102:741–752. doi: 10.1111/j.1471-4159.2007.04577.x. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol selfadministration stimulates dopamine release in the rat nucleus accumbens: Genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Wiley JL, Razdan RK, Martin BR. Evaluation of the role of the arachidonic acid cascade in anandamide's in vivo effects in mice. Life Sci. 2006;80:24–35. doi: 10.1016/j.lfs.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Wise LE, Shelton CC, Cravatt BF, Martin BR, Lichtman AH. Assessment of anandamide's pharmacological effects in mice deficient of both fatty acid amide hydrolase and cannabinoid CB1 receptors. Eur J Pharmacol. 2007;557:44–48. doi: 10.1016/j.ejphar.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]


