Abstract
Steroid hormones act on developing neural circuits that regulate the hypothalamic-pituitary-gonadal axis and are involved in hormone-sensitive behaviours. To test the hypothesis that developmental exposure to oestradiol (E2) organises the quantity of adult oestrogen receptors (ERα and ERβ), we used male mice with a targeted mutation of the aromatase enzyme gene (ArKO) and their wild-type (WT) littermates. These mice are unable to aromatise testosterone to E2, but still express both ERα and β. To evaluate adult responsiveness to E2, gonadectomised males were implanted with Silastic capsules containing E2, or an empty implant, 5 days prior to sacrifice. Immunoreactivity for ERα and ERβ was quantified in the caudal ventromedial nucleus (VMN) and the medial preoptic area (POA). Regardless of genotype, adult treatment with E2 reduced ERα-immunoreactive (ir) and ERβ-ir cell numbers in the POA, as well as ERβ-ir, but not ERα-ir, cell numbers in the VMN. Genotype, and thus endogenous exposure to E2, produced opposite effects on ER expression in the two brain areas. In the VMN, ArKO males had more ERα-ir and ERβ-ir cells than did WT males. In the POA, ArKO males had fewer ERα-ir and ERβ-ir cells than did WT males. Thus, numbers of immunoreactive neurones containing both ERs in the adult ArKO male were enhanced in the POA, but decreased in the VMN, and most likely these patterns were established during the developmental critical period. Furthermore, although both ERα and β-ir cell numbers are altered by the disruption of the aromatase gene, ERβ is altered in a more robust and region-specific manner.
Oestradiol (E2) is a steroid hormone produced by the gonads and also in the brain where it affects neural structure and function both in perinatal and adult individuals (1–3). Like other steroid hormones, E2 serves as ligand for specialised receptors that affect gene transcription. To date, two oestrogen receptors (ERα and ERβ) have been cloned (4, 5) and characterised in the murine brain (6). The regulation of ERs by ligand has been examined using a number of techniques including binding assays, autoradiography, in situ hybridisation and immunocytochemistry (7–12). In accordance with the principles of negative feedback, when the primary source of E2 (i.e. the gonads) is removed, ER expression levels increase, and E2 replacement causes a subsequent decline in ER (11). However, during the rat oestrous cycle, different neural regions exhibit differences in the concentrations of ER mRNA (11). Another dimension of the relationship between E2, ERα and ERβ is that exposure to E2 during development (during gestation, shortly after birth and/or puberty) may influence the feedback dynamics of each ER to its ligand in the adult brain. For example, when male rat pups are deprived of E2 just after birth and analysed as adults, levels of ER are elevated compared to untreated littermates (7, 8). Thus, the quantity of neural ER is closely related to and influenced by endogenous levels of oestradiol during development.
In the present study, we utilised a new approach to re-examine a question that has been asked previously: how do perinatal and adult E2 exposure differentially affect the concentrations of adult neural ERs? First, to remove both pre- and postnatal endogenous production of E2, without using pharmacology or surgery, we conducted these studies in the aromatase knockout (ArKO) mouse. These mutants carry targeted disruptions in both the transcriptional and translational sites of the CYP19 gene, do not produce aromatase enzyme, and are unable to convert testosterone to E2 (13). Both ERα and ERβ are present in ArKO brains and they are capable of responding to exogenous oestradiol (13, 14). Second, although many of the studies examining developmental actions of E2 on adult receptor function compared males and females, we chose to examine males only. By using males, we not only can examine effects resulting from the lack of oestradiol during the hormone critical period, but also circumvent potential confounds attributable to sex and sex chromosome genes that manifest at different time-points (15, 16). Finally, we used immunocytochemistry to examine the impact of E2 on both ERα and ERβ in the same individuals in consecutive sections. Few studies have asked about the relationship between E2 and ERβ (17, 18) or assessed both receptors in the same individuals (6, 19).
We predicted that if E2 acts during neural development to establish adult ER levels, then castrated adult male ArKO mice should have different numbers of ER immunoreactive cells compared to wild-type (WT) littermates. After the administration of E2 to adults we predicted that ArKO males would be less responsive to E2 and have a blunted decrease in ERs compared to WT males. We focused our analysis on two areas that have been examined in previosu studies: the caudal aspect of the ventromedial nucleus of the hypothalamus (VMN) and the medial preoptic area (POA). These two regions are highly involved in regulating adult displays of reproductive behaviour, are oestrogen-sensitive, and exhibit sexually dimorphic characteristics known to be organised by developmental hormone exposure. In addition, several studies suggest suboptimal sexual behaviour in ArKO mice (13, 14, 20, 21) and ER functioning is certainly essential for normal expression of these behaviours.
Materials and methods
Animals
Mice used in this study were produced by heterozygous breeding pairs; each mouse had one normal and one disrupted copy of the aromatase enzyme gene (13). At the time of this experiment, animals had been back-crossed at least three times into the C57BL/6J strain. Animals were genotyped by polymerase chain reaction amplification of tail DNA (13). All mice were born and housed in the University of Virginia Animal Care Facility, located in Gilmer Hall, under a 12: 12 h light/dark cycle (lights on at 00.00 h).
Mice were given food and water ad libitum. All animals were fed a standard laboratory mouse chow that contains phytoestrogens (Harlan Teklad Global Diet 8604) (Harlan, Bicester, UK)). We selected this diet because it is a standard rodent chow and we did not want to complicate the interpretation of the data by introducing dietary phytoestrogens as another variable. Moreover, standard phytoestrogen-containing diets have been used for most studies of sexual differentiation. The groups formed included WT (n = 13) and ArKO (n = 19) mice. Adult mice (age 10–16 weeks) were gonadectomised and, 1 week later, received a Silastic implant (1.98 mm inner diameter ×3.17 mm outer diameter) containing 50 μg 17-β-oestradiol dissolved in 25 μl sesame oil or an empty implant. Implants were placed subcutaneously in the midscapular region. All animals were sacrificed 5 days later. In total, the four groups of castrates had the following compositions; WT + blank (n = 8), WT + E2 (n = 5), ArKO + blank (n = 9) and ArKO + E2 (n = 10).
At the time of brain collection, animals were deeply anaesthetised with sodium pentobarbital and brains were removed and fixed via a 5% acrolein immersion (22). Following overnight immersion in 0.1 M of phosphate buffer containing 30% sucrose, serial coronal sections (30 μm) were made through the forebrain and stored in cryoprotectant (containing sucrose, PVP-40, and ethylene glycol) at −20 °C. One in four sections were processed for immunocytochemical analysis of ERα and another quarter for ERβ.
Immunocytochemistry
Brain sections were removed from cryoprotectant and rinsed in 0.2 M Tris buffered saline (5 × 10 min), pH 7.8. These rinses were followed by a 30-min incubation in a 1% NaBH4 solution and a 10-min incubation in 0.3% H2O2, with three 10-min rinses in between. Sections were incubated at 4 °C for 48 h in a primary antibody directed against either ERα or β. For ERα, we used a polyclonal antibody directed against the last 14 amino acids of the rat ERα C-terminus (C1355, 1: 5000; generously provided by Dr Margaret Shupnik, University of Virginia) (23). One reason for selecting this antibody is that immunoreactivity is not influenced by ligand availability (24). For ERβ detection, we used a polyclonal antibody directed against the C-terminus of the mouse ERβ protein (1: 1000; Z8P Zymed Laboratories, San Franciso, CA, USA). Control studies on the effects of ligand on numbers of immunopositive ERβ cells have not been conducted with this antiserum; thus, it is possible that some of the effects of oestrogen might be caused by altered efficiency of antibody binding rather than a decrease in receptor expression or its degradation. Both antibodies have been used in mouse brain previously (25, 26). Next, tissue was incubated for 1 h in a biotinylated goat antirabbit secondary antibody (1: 500; Vector Laboratories, Burlingame, CA, USA). Following rinses, sections were incubated for 90 min in avidin–biotin complex (1: 1000; Vectastain Elite; Vector Laboratories). After 30 min of rinsing, tissues were incubated for 5 min with a nickel intensified diaminobenzidine (DAB) solution (0.25% nickel ammonium sulphate and 0.05% DAB) activated by 0.001% hydrogen peroxide. All sections were processed for ERα and β in the same staining run.
Image analysis and statistics
Oestrogen receptor immunoreactive cell bodies were quantified in one section from each brain using Metamorph Image Analysis (Molecular Devices, Chester, PA, USA). Sections were best matched based on landmarks defined in the Franklin and Paxinos mouse brain atlas (27). As described previously (26), areas quantified were the POA (0.14 mm anterior to bregma) and the caudal aspect of the VMN (−1.82 mm posterior to bregma) (27). In the POA, the two major fibre tracts served as landmarks when choosing sections where the optic tract is barely present at the base of the brain, and the anterior commisure is present at its greatest lateral extent. Within the VMN, matching sections were chosen based on the shape of the third ventricle and arcuate nucleus in addition to the lateral and caudal appearance of the ERα staining within the VMN. We intentionally excluded the region of the POA representing the anteroventral periventricular nucleus by avoiding counting immunoreactive cells located within a distance of ten cell bodies from the ventricle. Relationships among groups were assessed using a two-way ANOVA for each ER with genotype and hormone condition as the two factors. Differences among groups were analysed using Bonferroni’s planned comparison tests. P < 0.05 was considered statistically significant.
Results
Developmental exposure to, and adult treatment with, E2 decreases numbers of both ERα-immunoreactive (ir) and ERβ-ir neurones in the VMN
In the VMN, numbers of ERα-ir cells were affected by genotype [F(1,31) = 5.92, P < 0.02], with ArKO males possessing more ERα-ir cells than WT males in this area. Hormone treatment had no independent influence [F(1,31) = 0.10], however, an interaction between genotype and hormone treatment was observed [F(1,31) = 6.61, P < 0.02]. Planned comparisons revealed that the effect was caused by ArKO castrates that did not receive E2 treatment in adulthood (P < 0.05; Fig. 1). For ERβ, genotype [F(1,31) = 5.82, P < 0.022] and hormone treatment [F(1,31) = 23.7, P < 0.0001] both had independent effects on cell numbers, and an interaction was also found [F(1,31) = 7.01.92, P < 0.01]. Again, ArKO males had more ERβ-ir cells than did WT males and E2 treatment decreased ERβ-ir cell numbers. Planned comparisons revealed that the castrated ArKO mice that did not receive E2 in adulthood had more ERβ-ir cells in the VMN than males in any of the other groups (P < 0.05; Figs 1 and 2).
Fig. 1.
Mean numbers of oestrogen receptor-immunoreactive (ER-ir) neurones in the ventromedial nucleus (VMN) in adult male aromatase knockout (ArKO) mice and wild-type (WT) littermates. Animals were castrated and given an oestradiol implant or a blank implant for 5 days prior to sacrifice. (A) Numbers of ERα-ir neurones. (B) ERβ-ir cell counts. *Significantly more ER-ir cells than males in any of the other groups (P < 0.05). Numbers of animals per group: WT + blank (n = 8), WT + E2 (n = 5), ArKO + blank (n = 9), ArKO + E2 (n = 10).
Fig. 2.
Photomicrograph of the ventromedial nucleus. Both oestrogen receptor (ER)α-immunoreactive (ir) ir cells (A,B) and ERβ-ir neurones (C,D) are shown. Brains were collected from castrated adult males of two genotypes; aromatase knockouts (B,D) and their wild-type littermates (A,C). Scale bar = 100 μm.
Developmental exposure to E2 increases, while adult E2 decreases, numbers of both ER-ir neurones in the POA
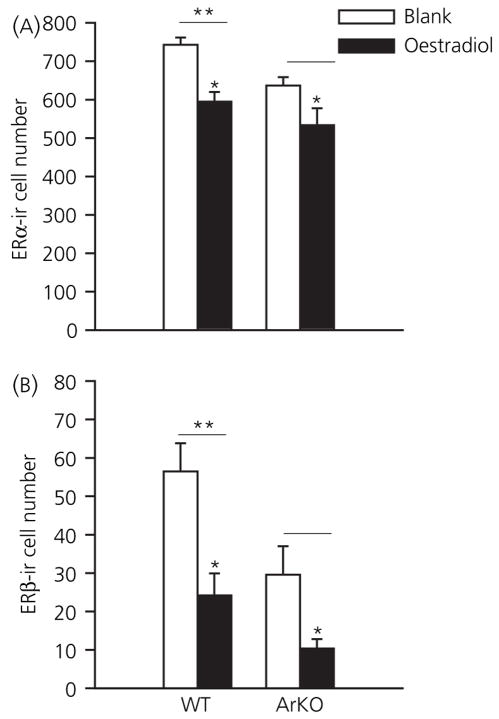
Oestrogen receptor α-ir cell numbers were affected by genotype [F(1,31) = 7.13, P < 0.01] and adult hormone condition [F(1,31) = 16.01, P < 0.001], but no interaction between these factors was revealed [F(1,31) = 0.51]. The same pattern was found for ERβ-ir cells; independent effects of genotype [F(1,29) = 10.90, P < 0.005] and hormone [F(1,29) = 17.53, P < 0.001], but no interaction between the two [F(1,29) = 1.13], was observed. Post-hoc analysis showed that ArKO mice had fewer ERα-ir and ERβ-ir cells in the POA compared to WT males (Fig. 3). Furthermore, WT and ArKO males that received E2 treatment had lower numbers of ER-ir cells than untreated castrates. Both effects were more striking for ERβ-ir than ERα-ir in part because there are ten-fold more ERα then ERβ containing neurones in this region.
Fig. 3.
Mean numbers of oestrogen receptor-immunoreactive (ER-ir) neurones in the medial preoptic area (POA) in adult male aromatase knockout (ArKO) mice and wild-type (WT) littermates. Animals were castrated and given an oestradiol implant or a blank implant for 5 days prior to sacrifice. (A) Numbers of ERα-ir neurones. (B) ERβ-ir cell counts. *Significantly reduced ER-ir cell numbers in animals with oestradiol treatment (P < 0.05). **Significantly more ER-ir cells in WT than ArKO males (P < 0.05). Numbers of animals per group: WT + blank (n = 8), WT + E2 (n = 5), ArKO + blank (n = 9), ArKO + E2 (n = 10).
Discussion
Our results show that endogenous E2 production in males during perinatal development and/or puberty affects adult neural ER-ir cell number in a receptor and region-specific manner. In the VMN, males that lacked both developmental E2 and received no adult hormone treatment after castration (ArKO males) had the largest quantities of both ERα-ir and ERβ-ir cell numbers. Adult treatment with E2 down-regulated both oestrogen receptors in ArKO brains. Interestingly, in the WT male VMN, ERα-ir cells were unaffected by administration of E2 to castrated adults. The implication here is that developmental exposure to E2 decreases adult responses to the same hormone in the adult VMN. Also, although adult E2 treatment did reduce ERβ-ir cell numbers in both genotypes, there were fewer ERβ-ir neurones in the brains of WT males; the castrated untreated ArKO males had three- to four-fold more ERβ-ir than did the WT untreated castrates. Thus, the lack of E2 during development leads to an augmented expression of this receptor in adult VMN. By contrast, in the POA, the lack or presence of endogenous E2 prior to adult castration did not change the response to E2; after castration, we noted a decrease in both ERα-ir and ERβ-ir cell numbers. Furthermore, in castrates that did not receive E2, ArKO males had fewer ER-ir cells than did WT males, thus showing region-specific differences in constitutive ER content. This relationship was more striking for ERβ than ERα.
The only immunocytochemical data similar to these were collected from gonad-intact male ArKO and WT mice, and ERα was the only ER examined (9). In this previous study, ArKO mice had more ERα-ir cells in the POA and arcuate/VMN region of the hypothalamus compared to WT littermates (9). None of our data are directly comparable with these in terms of the ArKO mouse strain used, hormone milieu, ERα antibody or brain regions examined. For example, the arcuate/VMH region previously examined contained both the rostral and caudal VMN as well as the part of the arcuate nucleus, whereas our analysis was localised to the caudal VMN. The only meaningful comparison that we can make is between the POA data. We can consider data from gonad-intact ArKO males in the previous study as being comparable with our ArKO castrate males given an empty implant (both lack E2), and the gonad-intact WT in the previous study as being comparable with our E2-treated WT males. When we made this comparison, we noted a strong trend for more ERα in the ArKO POA compared to the E2-treated WT male. Thus, similar patterns were observed in both studies.
In male rats, postnatal administration of an aromatase inhibitor (on the day of birth through 21 days of age) produces a significant increase in ERα-ir concentration in the POA and VMN of adults compared to control castrates (7). Our data in the VMN are in agreement with this report but, in the POA, our findings are in the opposite direction. It is possible that two critical periods exist for the POA (i.e. before and after birth), and only one of these is affected by the postnatal drug treatment. It is also possible that species differences between rats and mice can account for these discrepancies.
In addition to the ArKO mouse, studies using ERKO mice have been conducted in which the effects of E2 on the WT ER were examined (17, 18). The assumption behind these experiments is that the lack of one ER provides information about its role in the actions of E2 on the remaining ER. When ERβ is knocked out in male mouse brain, down-regulation of ERα-ir by long-term E2 treatment is blocked in both the POA and the VMN (18). These results do not match the present data in the ArKO male showing that ERα-ir cell numbers can be reduced by E2 in both these regions. Thus, selective blockade of the actions of ERβ is not equivalent to complete E2 deprivation. Male reproductive behaviour in ERβKO males is in agreement with this conclusion (28, 29).
Although the removal of one ER may not be equivalent to complete E2 deprivation, it does enable us to parse out combined actions of the receptors. For example, ERβ-ir has been quantified in adult male WT and ERαKO mice (17). Adult males were either gonad-intact or castrated and given short- or long-term E2 treatment. In the WT males, castrates had higher ERβ-ir numbers in the POA and VMN than did the WT given long-term E2, although the effect was only significant in the POA. This is in agreement with the present findings. The lack of functional ERα reduced ERβ-ir in the POA regardless of hormone treatment. In the VMN, ERαKO males in all the groups without gonads had very high ERβ-ir counts. Considered along with the present data, these studies suggest a role for ERα in regulating ERβ in the POA and VMN. Although ArKO males display a decrease in ERβ-ir in response to adult E2, ERαKO males fail to do so (17). The ERβKO male has a reduced response to E2 in terms of ERα-ir in the POA, and the ArKO male as a similar response but not as blunt; thus, E2 can decrease ERα-ir significantly when both ERs are activated.
Brain region-specific discrepancies in ER regulation following ligand administration are well documented and are dependent upon several factors. Previous studies using rodents provide evidence for both up and down regulation of neural ERs during the cycle or following adult E2 treatment (11, 30, 31). Within the rat brain, ERα distribution in the postnatal POA and VMN is comparable to that seen in adults. Moreover, female rats display more robust ERα-immunoreactivity than do males, suggesting that neonatal testosterone aromatised to E2 decreases ERα-ir cell numbers (32). Pharmacological disruption of the aromatisation of testosterone to E2, however, alters neural ER-ir cell numbers. For example, in male rat brains, there is a down regulation of ERα following testosterone treatment that can be reversed upon coadministration of the non-steroidal aromatase inhibitor, fadrozole (33). These data indicate that perinatal disruption of ER activation causes permanent alterations in adult hormone receptor content.
The ArKO model has proven useful in parsing out organisational and activational effects of oestradiol in the rodent brain. Although these animals are incapable of synthesising E2 from testosterone, the classic ERα and β are still present. Several behaviours in the male ArKO mouse are disrupted, but these can be rescued by adult treatment with E2. This suggests a degree of plasticity which makes it important to evaluate how and whether ER dynamics in the adult brain are altered by the absence of endogenous ligand and/or restoration of ligand in adults. The VMN is essential for the expression of adult female sexual receptivity, a set of behaviours that are highly sexually dimorphic; male mice appropriately primed to do not show lordosis (34). Our data suggest that VMN-related behaviours may differ between adult WT and ArKO males. Recently, we have reported that male ARKO mice, raised on a low phytoestrogen containing chow, tend to show more lordosis behaviour than do WT males on the same diet (21). In the case of behaviours involving the POA, our data predict that WT and ArKO males treated with E2 may be equivalent or have only small differences. In the case of male aggression and copulatory behaviours, this appears to hold true (14, 35, 36). In summary, some of the specificity of the actions of oestradiol are produced by regional differences in the ER-containing cell populations and by the two different ER subtypes within these populations.
Acknowledgments
The authors would like to thank Ms Aileen Wills and Ms Savera Shetty for technical assistance. This work was supported by NIH grants R01 MH57759 and K02 MH01349 (E.F.R.). A.E.K. was supported by an NIH training grant T32 HD072323 and an individual NRSA F31 MH70092.
References
- 1.McCarthy MM, Schlenker EH, Pfaff DW. Enduring consequences of neonatal treatment with antisense oligodeoxynucleotides to estrogen receptor messenger ribonucleic acid on sexual differentiation of rat brain. Endocrinology. 1993;133:433–439. doi: 10.1210/endo.133.2.8344188. [DOI] [PubMed] [Google Scholar]
- 2.Toran-Allerand CD, Hashimoto K, Greenough WT, Saltarelli M. Sex steroids and the development of the newborn mouse hypothalamus and preoptic area in vitro. III Effects of estrogen on dendritic differentiation. Brain Res. 1983;283:97–101. doi: 10.1016/0165-3806(83)90085-8. [DOI] [PubMed] [Google Scholar]
- 3.Toran-Allerand CD. Estrogen and the brain. Beyond ER-alpha, ER-beta, and 17beta-estradiol. Ann NY Acad Sci. 2005;1052:136–144. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]
- 4.White R, Lees JA, Needham M, Ham J, Parker M. Structural organization and expression of the mouse estrogen receptor. Mol Endocrinol. 1987;1:735–744. doi: 10.1210/mend-1-10-735. [DOI] [PubMed] [Google Scholar]
- 5.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shughrue PJ, Scrimo PJ, Merchenthaler I. Evidence for the colocalization of estrogen receptor-beta mRNA and estrogen receptor-alpha immunoreactivity in neurons of the rat forebrain. Endocrinology. 1998;139:5267–5270. doi: 10.1210/endo.139.12.6525. [DOI] [PubMed] [Google Scholar]
- 7.Bakker J, Pool CW, Sonnemans M, van Leeuwen FW, Slob AK. Quantitative estimation of estrogen and androgen receptor-immunoreactive cells in the forebrain of neonatally estrogen-deprived male rats. Neuroscience. 1997;77:911–919. doi: 10.1016/s0306-4522(96)00423-x. [DOI] [PubMed] [Google Scholar]
- 8.Kuhnemann S, Brown TJ, Hochberg RB, MacLusky NJ. Sexual differentiation of estrogen receptor concentrations in the rat brain: effects of neonatal testosterone exposure. Brain Res. 1995;691:229–234. doi: 10.1016/0006-8993(95)00640-c. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal VR, Sinton CM, Liang C, Fisher C, German DC, Simpson ER. Upregulation of estrogen receptors in the forebrain of aromatase knockout (ArKO) mice. Mol Cell Endocrinol. 2000;162:9–16. doi: 10.1016/s0303-7207(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 10.McGinnis MY, Krey LC, MacLusky NJ, McEwen BS. Steroid receptor levels in intact and ovariectomized estrogen-treated rats: an examination of quantitative, temporal and endocrine factors influencing the efficacy of an estradiol stimulus. Neuroendocrinology. 1981;33:158–165. doi: 10.1159/000123222. [DOI] [PubMed] [Google Scholar]
- 11.Shughrue PJ, Bushnell CD, Dorsa DM. Estrogen receptor messenger ribonucleic acid in female rat brain during the estrous cycle: a comparison with ovariectomized females and intact males. Endocrinology. 1992;131:381–388. doi: 10.1210/endo.131.1.1612018. [DOI] [PubMed] [Google Scholar]
- 12.Yuan H, Bowlby DA, Brown TJ, Hochberg RB, MacLusky NJ. Distribution of occupied and unoccupied estrogen receptors in the rat brain: effects of physiological gonadal steroid exposure. Endocrinology. 1995;136:96–105. doi: 10.1210/endo.136.1.7828562. [DOI] [PubMed] [Google Scholar]
- 13.Honda S, Harada N, Ito S, Takagi Y, Maeda S. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem Biophys Res Commun. 1998;252:445–449. doi: 10.1006/bbrc.1998.9672. [DOI] [PubMed] [Google Scholar]
- 14.Bakker J, Honda S, Harada N, Balthazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm Behav. 2004;46:1–10. doi: 10.1016/j.yhbeh.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26:2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold AP, Burgoyne PS. Are XX and XY brain cells intrinsically different? Trends Endocrinol Metab. 2004;15:6–11. doi: 10.1016/j.tem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Nomura M, Korach KS, Pfaff DW, Ogawa S. Estrogen receptor beta (ERbeta) protein levels in neurons depend on estrogen receptor alpha (ERalpha) gene expression and on its ligand in a brain region-specific manner. Brain Res Mol Brain Res. 2003;110:7–14. doi: 10.1016/s0169-328x(02)00544-2. [DOI] [PubMed] [Google Scholar]
- 18.Temple JL, Fugger HN, Li X, Shetty SJ, Gustafsson J, Rissman EF. Estrogen receptor beta regulates sexually dimorphic neural responses to estradiol. Endocrinology. 2001;142:510–513. doi: 10.1210/endo.142.1.8054. [DOI] [PubMed] [Google Scholar]
- 19.Greco B, Allegretto EA, Tetel MJ, Blaustein JD. Coexpression of ER beta with ER alpha and progestin receptor proteins in the female rat forebrain: effects of estradiol treatment. Endocrinology. 2001;142:5172–5181. doi: 10.1210/endo.142.12.8560. [DOI] [PubMed] [Google Scholar]
- 20.Bakker J, Honda S, Harada N, Balthazart J. Sexual partner preference requires a functional aromatase (cyp19) gene in male mice. Horm Behav. 2002;42:158–171. doi: 10.1006/hbeh.2002.1805. [DOI] [PubMed] [Google Scholar]
- 21.Kudwa AE, Boon WC, Simpson ER, Handa RJ, Rissman EF. Dietary phytoestrogens dampen female sexual behavior in mice with a disrupted aromatase enzyme gene. Behav Neurosci. 2007;121:356–361. doi: 10.1037/0735-7044.121.2.356. [DOI] [PubMed] [Google Scholar]
- 22.Scordalakes EM, Shetty SJ, Rissman EF. Roles of estrogen receptor alpha and androgen receptor in the regulation of neuronal nitric oxide synthase. J Comp Neurol. 2002;453:336–344. doi: 10.1002/cne.10413. [DOI] [PubMed] [Google Scholar]
- 23.Friend KE, Chiou YK, Lopes MB, Laws ER, Jr, Hughes KM, Shupnik MA. Estrogen receptor expression in human pituitary: correlation with immunohistochemistry in normal tissue, and immunohistochemistry and morphology in macroadenomas. J Clin Endocrinol Metab. 1994;78:1497–1504. doi: 10.1210/jcem.78.6.7515390. [DOI] [PubMed] [Google Scholar]
- 24.Greco B, Blasberg ME, Kosinski EC, Blaustein JD. Response of ERalpha-IR and ERbeta-IR cells in the forebrain of female rats to mating stimuli. Horm Behav. 2003;43:444–453. doi: 10.1016/s0018-506x(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 25.Kudwa AE, Gustafsson JA, Rissman EF. Estrogen receptor beta modulates estradiol induction of progestin receptor immunoreactivity in male, but not in female, mouse medial preoptic area. Endocrinology. 2004;145:4500–4506. doi: 10.1210/en.2003-1708. [DOI] [PubMed] [Google Scholar]
- 26.Moffatt CA, Rissman EF, Shupnik MA, Blaustein JD. Induction of progestin receptors by estradiol in the forebrain of estrogen receptor-alpha gene-disrupted mice. J Neurosci. 1998;18:9556–9563. doi: 10.1523/JNEUROSCI.18-22-09556.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. New York, NY: Academic Press; 1997. [Google Scholar]
- 28.Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor beta gene-deficient (betaERKO) male and female mice. Proc Natl Acad Sci USA. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Temple JL, Scordalakes EM, Bodo C, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor beta gene disrupts pubertal male sexual behavior. Horm Behav. 2003;44:427–434. doi: 10.1016/j.yhbeh.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Liposits Z, Kallo I, Coen CW, Paull WK, Flerko B. Ultrastructural analysis of estrogen receptor immunoreactive neurons in the medial preoptic area of the female rat brain. Histochemistry. 1990;93:233–239. doi: 10.1007/BF00266383. [DOI] [PubMed] [Google Scholar]
- 31.Simerly RB, Carr AM, Zee MC, Lorang D. Ovarian steroid regulation of estrogen and progesterone receptor messenger ribonucleic acid in the anteroventral periventricular nucleus of the rat. J Neuroendocrinol. 1996;8:45–56. doi: 10.1111/j.1365-2826.1996.tb00685.x. [DOI] [PubMed] [Google Scholar]
- 32.Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. J Comp Neurol. 1997;389:81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 33.Clancy AN, Michael RP. Effects of testosterone and aromatase inhibition on estrogen receptor-like immunoreactivity in male rat brain. Neuroendocrinology. 1994;59:552–560. doi: 10.1159/000126705. [DOI] [PubMed] [Google Scholar]
- 34.Kudwa AE, Bodo C, Gustafsson JA, Rissman EFA. previously uncharacterized role for estrogen receptor beta: defeminization of male brain and behavior. Proc Natl Acad Sci USA. 2005;102:4608–4612. doi: 10.1073/pnas.0500752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toda K, Saibara T, Okada T, Onishi S, Shizuta Y. A loss of aggressive behaviour and its reinstatement by oestrogen in mice lacking the aromatase gene (Cyp19) J Endocrinol. 2001;168:217–220. doi: 10.1677/joe.0.1680217. 2005. [DOI] [PubMed] [Google Scholar]
- 36.Toda K, Okada T, Takeda K, Akira S, Saibara T, Shiraishi M, Onishi S, Shizuta Y. Oestrogen at the neonatal stage is critical for the reproductive ability of male mice as revealed by supplementation with 17beta-oestradiol to aromatase gene (Cyp19) knockout mice. J Endocrinol. 2001;168:455–463. doi: 10.1677/joe.0.1680455. [DOI] [PubMed] [Google Scholar]