Abstract
BACKGROUND
Breast cancer is the most common cancer in women in developed countries, and 12% of breast cancer occurs in women 20–34 years. Survival from breast cancer has significantly improved, and the potential late effects of treatment and the impact on quality of life have become increasingly important. Young women constitute a minority of breast cancer patients, but commonly have distinct concerns and issues compared with older women, including queries regarding fertility, contraception and pregnancy. Further, they are more likely than older women to have questions regarding potential side effects of therapy and risk of relapse or a new primary. In addition, many will have symptoms associated with treatment and they present a management challenge. Reproductive medicine specialists and gynaecologists commonly see these women either shortly after initial diagnosis or following adjuvant therapy and should be aware of current management of breast cancer, the options for women at increased genetic risk, the prognosis of patients with early stage breast cancer and how adjuvant systemic treatments may impact reproductive function.
METHODS
No systematic literature search was done. The review focuses on the current management of breast cancer in young women and the impact of treatment on reproductive function and subsequent management. With reference to key studies and meta-analyses, we highlight controversies and current unanswered questions regarding patient management.
RESULTS
Chemotherapy for breast cancer is likely to negatively impact on reproductive function. A number of interventions are available which may increase the likelihood of future successful pregnancy, but the relative safety of these interventions is not well established. For those who do conceive following breast cancer, there is no good evidence that pregnancy is detrimental to survival. We review current treatment; effects on reproductive function; preservation of fertility; contraception; pregnancy; breastfeeding and management of menopausal symptoms following breast cancer.
CONCLUSION
This paper provides an update on the management of breast cancer in young women and is targeted at reproductive medicine specialists and gynaecologists.
Keywords: breast cancer, fertility, contraception, pregnancy, menopause
Background
Breast cancer affects up to one in eight women in developed countries with a median age of 61 years at diagnosis (www.cancer.org). The incidence of breast cancer by age is shown in Table I. Approximately 2% of breast cancers occur in young women between 20 and 34 years of age and 11% between 35 and 44 years of age, which translates into over 1500 women under 45 each year in Australia. These women present unique and specific management issues and they are the focus of this review (Ries et al., 2008).
Table I.
Incidence of breast cancer by age
| Age | Annual incidence/100 000 women |
|---|---|
| <20 | 0.1 |
| 20–24 | 1.4 |
| 25–29 | 8.1 |
| 30–34 | 24.8 |
| 35–39 | 58.4 |
| 40–44 | 116.1 |
| 45–49 | 198.5 |
Reproduced from Future Oncol 2007;3(5):569–574 with permission of Future Medicine Ltd. and the authors Pagani and Goldhirsch (2006).
Survival following breast cancer is improving, with over 88% of patients alive at 5 years (Ries et al., 2008) although the prognosis appears to be worse in young women under 35 years at diagnosis (Aebi et al., 2000). In the USA, around 3 million women are survivors of breast cancer, and this number is likely to increase with earlier diagnosis and improvements in adjuvant therapy (Ganz and Hahn, 2008). Breast cancer survivors may present to the reproductive medicine specialist and gynaecologist with a range of queries and symptoms, and younger women have additional concerns particularly regarding fertility and premature menopause that need to be addressed and managed (Partridge et al., 2007). Clinicians who do not specialize in breast cancer, but who nevertheless see these younger women for advice regarding fertility and the gynaecological consequences of adjuvant treatment should have a clear understanding of the current management of breast cancer, the common gynaecological complications of treatment and the evidence regarding their optimal management. In particular, the potential impact of fertility treatments on hormone-receptor positive breast cancer needs to be considered and discussed. In addition, the reproductive medicine specialist and gynaecologist should be aware of current protocols for counselling, surveillance and management of women who carry genetic mutations which may increase their risk for breast, endometrial and ovarian cancer. The majority of breast cancers are diagnosed at an early stage, which is defined as cancers that are potentially curable and have not spread beyond the breast or the axillary lymph nodes (ductal carcinoma in situ and stages I, IIA, IIB and IIIA).
Methods
No systematic literature search was done. This review focuses on early breast cancer in young women by providing a brief overview and summary of breast cancer management with reference to some key studies and meta-analyses. We highlight the controversies and current unanswered questions regarding patient management.
Current treatment of breast cancer
Surgical management
Level 1 evidence from large randomized controlled trials (RCT) and meta-analyses demonstrate equivalent survival rates with breast conserving therapy (BCT) and radiotherapy (RT) compared with mastectomy in women with early breast cancer (Early Breast Cancer Trialists’ Collaborative Group, 1995; Jatoi and Proschan, 2005; Yang et al., 2008). Mastectomy is usually advised in patients at increased risk of loco-regional recurrence (in the breast or draining lymph nodes) with BCT and these risk factors include larger tumour size, multi-centricity, an extensive intra-ductal component or extensive lymphovascular invasion (Newman and Kuerer, 2005; Schwartz et al., 2006; White et al., 2008). Young age is also a risk factor for loco-regional recurrence, and the place of BCT in young women, particularly those <35 years old is controversial as they appear to be at higher risk of local recurrence than older women (Kim et al., 1998; Voogd et al., 2001; Arriagada et al., 2003; Zhou and Recht, 2004; Schwartz et al., 2006). BCT is the standard of care for early breast cancer, and is unlikely to compromise survival in young women (Kroman et al., 2004). However, the relative risks and benefits of BCT versus mastectomy in very young women remain uncertain since this population has not been well represented in large RCT. In addition, as up to 10% of very young women (Malone et al., 2000) may have an inherited predisposition to breast cancer (even in the absence of a family history), unilateral or bilateral mastectomy may be a good option.
Reconstructive surgery should be offered to most women after mastectomy. This is either via an implant placed under the skin and pectoral muscles or using myocutaneous tissue transfer such as a latissimus dorsi or transverse rectus myocutaneous flaps. These will not impede the ability to detect recurrent disease, and although a woman does not require imaging follow-up of the reconstructed breasts, clinical examination is important to exclude local recurrence.
Axillary node dissection and sentinel node biopsy
The clinical management of the axilla in women with early breast cancer has changed radically over the last decade. Axillary node dissection is associated with morbidity including lymphoedema in 10–20% of patients as well as sensory loss and restricted shoulder movement (Mansel et al., 2006; Schulze et al., 2006; Del Bianco et al., 2008). Over two-thirds of women have negative axillary nodes and therefore have a potentially unnecessary surgical procedure. Sentinel node biopsy (SNB) is a minimally invasive surgical technique to assess nodal involvement using blue dye and/or radioisotope mapping to identify and map the location of sentinel nodes that drain the breast. It is appropriate for women with smaller (<3 cm) tumours that are unifocal. Axillary dissection is only required in those patients with an involved or positive SNB. The false-negative rate of SNB is low (∼5–8%), and the risk of isolated axillary recurrences is very low—<1% in women who have been staged with SNB (Naik et al., 2004). The procedure has significantly less morbidity than axillary node dissection and is associated with an improved quality of life with no adverse impact on survival (Veronesi et al., 2006; Krag et al., 2007).
Radiotherapy
RT significantly reduces loco-regional recurrence which is associated with increased morbidity and potentially increased mortality. RT is recommended to all women after BCT and significantly reduces local recurrence from about 26% without radiation to 7% with radiation (Clarke et al., 2005). There is evidence that a radiation boost to the tumour bed is of benefit in young women and further reduces the risk of local recurrence after BCT (Vrieling et al., 2003). Radiation is also advised following mastectomy to all patients who have a high risk of local relapse, including those with tumours >5 cm and/or those with at least four positive nodes or extensive lymphovascular permeation (Overgaard et al., 1997; Ragaz et al., 2005; Gebski et al., 2006). The role of RT in women with 1–3 involved axillary nodes is currently being addressed in clinical trials, but there is evidence to support its role particularly in higher risk patients. Age <45 years, >25% of nodes positive, medial tumour location and estrogen receptor (ER) negative (ER–ve) status have been found to be statistically significant independent factors associated with greater local recurrence, meriting consideration and discussion of post-mastectomy RT (Truong et al., 2005).
Adjuvant systemic therapy
Systemic adjuvant therapy includes all forms of endocrine therapy (sometimes called hormone therapy (HT)) and/or cytotoxic therapy used in conjunction with local surgical treatment for early breast cancer. The aim of adjuvant systemic therapy is to eradicate micrometastases. There is Level 1 evidence to demonstrate a significant benefit from adjuvant chemotherapy and endocrine therapy both in terms of relapse-free survival and overall survival. In younger women (<50 years), six cycles of an anthracycline-based combination chemotherapy (e.g. with FEC [5-fluorouracil/epirubicin/cyclophosphamide] or FAC [5-fluorouracil/doxorubicin/cyclophosphamide]) are associated with a proportional reduction in mortality of ∼38% which approximates to a 5–15% absolute improvement in survival at 15 years of follow-up, depending on the underlying risk of recurrence (Early Breast Cancer Trialists’ Collaborative Group, 2005; Early Breast Cancer Trialists’ Collaborative Group et al., 2008). This may now be even higher with more current chemotherapy regimens (such as ‘dose dense chemotherapy’ where chemotherapy is administered every 2 weeks rather than 3 weeks with white cell growth factor support) and the addition of taxanes (De Laurentiis et al., 2008). The absolute benefit of chemotherapy is also related to patient age and receptor status as well as a number of other pathological factors. Human epidermal growth factor receptor-2 (HER2) testing is now routine, and there is very good evidence from RCT to demonstrate a significant improvement in relapse-free survival and overall survival with adjuvant trastuzumab in women with HER2 positive breast cancer (as reviewed by Baselga et al. (2006)). The details of all the chemotherapy regimens and current trials are beyond the scope of this review, but the drugs used and the doses as well as the number of cycles can increase the likelihood of amenorrhoea and infertility.
For pre-menopausal women with hormone receptor positive (HR+ve) tumours, 5 years of tamoxifen will reduce the annual breast cancer death rate by 31%, independent of the use of chemotherapy or age. HR+ve indicates ER and/or progesterone receptor (PR) positive. Fifteen years after initial therapy, these benefits translate into average absolute reductions in recurrence of ∼11.8% (SE 1.3) and in breast cancer mortality of 9.2% (SE 1.2) which approximates a 4–12% absolute gain depending on the underlying risk. Meta-analysis of multiple randomized trials confirms that combining anthracycline-based chemotherapy with 5 years of tamoxifen, in women with HR+ve disease, reduces the risk of dying from breast cancer by ∼57% (Early Breast Cancer Trialists’ Collaborative Group, 2005). For example, if the risk of dying over the next 15 years is 25% without adjuvant therapy, this may be reduced to ∼12%. The greater the risk of relapse, the greater the potential of benefit from adjuvant treatment.
Current areas of controversy include the existence of any additional benefit of ovarian suppression by gonadotrophin-releasing hormone (GnRH) analogues as endocrine therapy for young women with ER positive early breast cancer, particularly in those who retain ovarian function after chemotherapy and who are on tamoxifen (Bao and Davidson, 2007). The frequency of use of GnRH analogues in community practice is unknown, and their role is being addressed in the multi-national intergroup Suppression of Ovarian Function Trial (SOFT). Similarly, ongoing RCTs are addressing the role of hormonal therapy alone with either ovarian suppression and tamoxifen, or tamoxifen alone instead of chemotherapy in selected young women with ER positive early breast cancer. In a recently reported study in pre-menopausal women with HR+ve disease, adjuvant oophorectomy plus tamoxifen compared with observation alone lead to a significantly improved disease-free survival rate at 5 years (83 versus 61%) and 10 years (66 versus 47%) (Love et al., 2008).
Extensive evidence derived from RCT’s of systemic adjuvant therapy in early breast cancer has lead to the development of decision-making tools, such as adjuvant online (http://www.adjuvantonline.com), which help health professionals make individualized estimates of the risk of cancer-related mortality or relapse without systemic adjuvant therapy and estimates of the reduction of these risks with therapy based on patient age, tumour size, nodal involvement, histological grade, etc. (Ravdin et al., 2001). These estimates are then provided on printed sheets in simple graphical and text formats that can be used in consultations and allow individual women to be informed and involved in their treatment decisions. The accuracy of these estimates of treatment benefit in the very young is uncertain as relatively few would have participated in the large randomized trials that contributed to the evidence.
Prognosis in younger women
The majority of breast cancers presenting in young women are invasive cancers and most are infiltrating ductal cancers. Younger women are more likely to present with larger tumours and appear to have worse outcomes stage for stage in some but not in all studies (Aebi et al., 2000; Maggard et al., 2003; El Saghir et al., 2006). This apparent difference in prognosis may relate to differences in the biology of breast cancer in younger women (Anders et al., 2008). The most important prognostic factors include tumour size and nodal status as well as histological grade and HER2 and HR status. Younger women are more likely to have higher grade, HR–ve tumours with a high proliferative fraction and lymph vascular invasion. Colleoni et al. (2002) reported that young women <35 years were more likely to have ER−ve (38.8 versus 21.6%, P < 0.001), PR−ve (49.1 versus 35.3%, P = 0.001) tumours compared with older pre-menopausal women. The impact of HR status on prognosis in younger women is controversial, but a large population study from Denmark has recently found that a positive HR status does not confer a negative impact on survival in young women as has been previously reported (Bentzon et al., 2008). It is now well accepted that breast cancer is a heterogeneous disease encompassing several distinct entities with different morphological and immunohistochemical features and varying biological behaviour and prognosis. For example, it is now recognized that ‘triple negative’ (ER–ve, PR–ve and HER2–ve) and basal-like breast cancers are more common in younger women and have an aggressive clinical behaviour and are more likely to relapse within the first 5 years (Rakha et al., 2008).
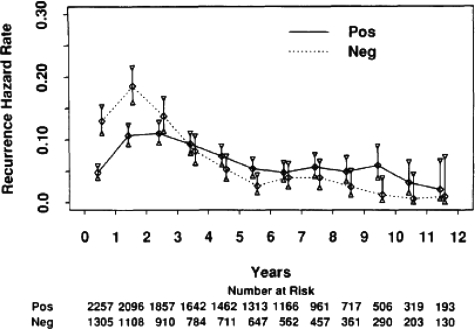
An understanding of all these prognostic factors and an appreciation of the difficulty of accurately predicting prognosis in an individual are of particular importance when counselling the patient about fertility interventions (see below). Close communication with the treating oncologists should provide the fertility team with a better appreciation of the likely prognosis, and this should be factored into decision-making. This ideally should have been discussed in detail with the patient prior to referral for fertility intervention. Novel tools for predicting recurrence using gene arrays such as oncotype DX™ and MammaPrint® are being used and their role under investigation (reviewed by Ross et al. (2008)). An important question apart from overall prognosis that often is raised is when the patient is at most risk of relapse and how long this risk persists? There are data to suggest that the hazard of recurrence is greatest (i.e. 13%) between years 1 and 2 after surgery and then it falls consistently over time. After 5 years, the recurrence risk averages around 4% per year in the study reported by Saphner et al. (1996). Patients with HR–ve tumours had a higher recurrence risk in years 0–5, whereas the hazard of recurrence for women with HR+ve cancers was relatively constant in the first 5 years after diagnosis and from years 5 to12, highlighting the very different natural history between HR+ve and HR–ve tumours and raising the question as to what the optimal duration of hormonal therapy should be in women with HR+ve breast cancers, as shown in Fig. 1 (Saphner et al., 1996). It should also be pointed out that these data on the annual risks of recurrence were derived from seven adjuvant studies carried out over 20 years ago and included both pre-menopausal and post-menopausal women with node negative and node positive breast cancers. Furthermore, the adjuvant treatments used would not be considered standard of care today and the study also included controls who did not receive adjuvant systemic therapy, suggesting that the annual hazard of recurrence is likely to lower than presented in Fig. 1. Pre-menopausal patients had a lower annual risk of recurrence than post-menopausal patients in this study. More recently, Demicheli et al. (2004) reported the results of a study of the hazard rate for recurrence in the first 4 years after mastectomy alone in more than 1000 patients. They found a sharp two-peaked hazard function for node positive pre-menopausal patients with a steep peak at 8–10 months after mastectomy and a broader second peak that reached its maximum at 28–30 months. These data may help women decide on the timing of pregnancy after diagnosis.
Figure 1.
Annual hazard of recurrence of 3563 patients separated by ER status. ER, estrogen receptor. Reproduced from Saphner et al. (1996) with permission. The mean follow-up times for ER+ve and ER–ve patients were 8.1 and 8.0 years, respectively.
Breast cancer susceptibility gene carriers
Hereditary breast cancer accounts for 5–10% of all breast carcinomas and most are attributed to autosomal dominant germline mutations in breast cancer susceptibility gene 1 (BRCA1) and breast cancer susceptibility gene 2 (BRCA2) (Malone et al., 2000). The likelihood of a BRCA mutation is higher in women with breast cancer under the age of 45 with a strong family history of breast and/or ovarian cancer. Although there is some variability between different series, there are population-based studies that have demonstrated BRCA1 and BRCA2 mutations in 9% of women under 40 (Loman et al., 2001). In the study of Loman et al., the subset of women with a strong family history of breast or breast/ovarian cancer had almost a 40% risk of carrying a BRCA1 or BRCA2 mutation. There are also a number of other less common breast cancer susceptibility genes and syndromes that are beyond the scope of this review. Women with BRCA gene mutations tend to develop breast cancer at a younger age and are at greater risk of bilateral breast cancer at presentation as well as increased risk of subsequently developing a contralateral breast cancer (Ford et al., 1994). BRCA1 mutation carriers have a 50–80% lifetime risk of breast cancer and a 40–60% risk of ovarian cancer, with the median age at diagnosis in the mid-40s. The breast cancer risk is similar in BRCA2 mutation carriers, but the lifetime risk of ovarian cancer is ∼15% and tends to occur in post-menopausal women in their 60s. For BRCA mutation carriers diagnosed with breast cancer, the risk of a recurrence or a new primary in the ipsilateral breast in women who have breast conserving surgery is estimated to be as high as 20–50% at 10 years, and the lifetime risk for a contralateral second primary cancer is ∼40–60% in women diagnosed with breast cancer under 40 years (Chen et al., 1999; Weitzel et al., 2003).
Breast cancers in BRCA1 cancers are more commonly associated with worse prognostic features, and outcome may be improved by adjuvant chemotherapy. Hence, even small node negative tumours in BRCA1 carriers are offered adjuvant chemotherapy. Breast cancers in BRCA2 carriers are more similar to sporadic tumours. The optimal chemotherapy for BRCA-related breast cancers is being actively investigated and is beyond the scope of this review (Quinn et al., 2007; Rottenberg et al., 2007). Newer targeted therapies such as poly (ADP-ribose) polymerase inhibitors which selectively target BRCA-related breast and ovarian cancers and spare normal heterozygous cells are being investigated in clinical trials (www.clinicaltrials.gov).
The management of women with BRCA mutations is complex, requires a multi-disciplinary team approach and includes counselling about cancer risk, surveillance options and discussion of risk-reducing surgery including prophylactic mastectomy and/or oophorectomy. Prophylactic mastectomy is very effective and reduces the risk of breast cancer by over 90% (Hartmann et al., 2001; Rebbeck et al., 2004).
Prophylactic bilateral salpingo-ophorectomy (BSO) significantly reduces the risk of both ovarian and breast cancer in BRCA1/2 carriers (Bermejo-Perez et al., 2007) BSO reduces the risk of ovarian cancers by 85–90% and reduces the risk of breast cancer by 50% (Kauff and Barakat, 2007). The reduction in the risk of breast cancer even in women with BRCA1-associated breast cancer which is commonly ER−ve may seem counterintuitive, but there is growing evidence to suggest that many ER−ve breast cancers evolve from ER+ve precursors (Allred, 2004). Risk reduction following BSO may differ for BRCA1/2 carriers. Case–control studies suggest that prophylactic oophorectomy may result in a greater reduction in breast cancer risk in BRCA1 carriers who undergo surgery before 40 years of age, compared with BRCA2 carriers (Eisen et al., 2005). Both ovaries and fallopian tubes should be removed since both are at increased risk for malignant transformation (Levine et al., 2003). Indeed, there is growing evidence to suggest that most BRCA-related ‘ovarian cancers’ actually arise in the fimbrial end of the fallopian tube which probably explains why transvaginal ultrasound screening for ovarian cancer does not appear to be an effective screening modality (Callahan et al., 2007). Hysterectomy at the time of BSO is controversial, and the additional morbidity needs to be taken into account and weighed against the potential risks of combined HT for the management of menopausal symptoms and bone protection.
Effects of breast cancer treatment on reproductive function
Around 2.7% of breast cancers occur in women of peak reproductive age (25–35 years) (Axelrod et al., 2008). The growing tendency in developed countries for delayed childbearing may increase breast cancer risk and also increases the number of women who have not yet started or completed their families when breast cancer is diagnosed. In addition to possibly having an inferior outcome compared with older women as discussed above (Chen et al., 2003; Maggard et al., 2003; Rapiti et al., 2005; El Saghir et al., 2006), young breast cancer patients may face dilemmas regarding fertility, pregnancy and contraception (Partridge et al., 2004) and report having difficulty obtaining information in these areas (Thewes et al., 2005).
Breast cancer is likely to have a negative impact on reproductive function for a number of reasons. First, from the toxic effect of chemotherapy on ovarian follicles, secondly from the advice commonly given to patients to delay pregnancy for at least 2 years following a diagnosis of breast cancer and thirdly because endocrine therapy commonly continues for at least 5 years, after which fertility is likely to be reduced due to age-related decline. In addition, ovarian ablation or bilateral oophorectomy may be advised for some younger women with HR+ve cancers (Early Breast Cancer Trialists’ Collaborative Group, 2005; Bao and Davidson, 2007) or as risk-reducing surgery in BRCA1/2 gene mutation carriers as discussed earlier.
Ovarian function following chemotherapy for breast cancer
Ovarian dysfunction following chemotherapy for breast cancer is related to patient age, to ovarian function at the time of treatment and to the specific agents used, particularly the dose of alkylating agents such as cyclophosphamide (Lee et al., 2006). Common effects of chemotherapy on ovarian function include temporary amenorrhoea due to loss of the developing cohort of ovarian follicles or permanent amenorrhoea due to loss of remaining follicles (Oktay et al., 2006a, b). Chemotherapy causes depletion of the primordial follicle pool in a drug- and dose-dependent manner (Sonmezer and Oktay, 2004). Prevalence rates of temporary and permanent amenorrhoea vary due to differences in treatment regimens, patient characteristics and outcome measures used. For those who do resume normal menstrual cycles, ovarian damage due to chemotherapy can still be identified. There is marked follicular depletion (Oktem and Oktay, 2007), fertility is impaired and the mean age at menopause is reduced (Partridge et al., 2007).
Amenorrhoea rates following combination chemotherapy consisting of cyclophosphamide + methotrexate + 5-flurouracil (CMF regimen) range from 21 to 71% in women aged ≤40 years, and from 40–100% in older women (Gadducci et al., 2007), although this combination is now rarely used. In most series, anthracycline-based adjuvant chemotherapy regimens appear to have a lower incidence of amenorrhoea, which is probably due to the lower cumulative cyclophosphamide dose administered compared with that given in the CMF regimen.
The impact of taxanes on the incidence of amenorrhoea is uncertain with conflicting results. Some studies suggest no additional effect (Tham et al., 2007), whereas others report that the rates of amenorrhoea may be increased (Martin et al., 2005; Swain et al., 2005; Petrek et al., 2006). There may also be a difference between docetaxel and paclitaxel (Alton et al., 2004), but there is insufficient data to comment on the impact of different schedules of paclitaxel (e.g. weekly, dose dense or three-weekly dosing) on the rate of amenorrhoea.
As might be expected, younger breast cancer patients are less likely to experience amenorrhoea or menstrual cycle changes following chemotherapy. A recent retrospective review of 160 patients aged from 18 to 34 years (median age 32 years) treated with alkylating agent-based chemotherapy regimens (CMF) and 80 with anthracycline-based regimens (AD) and a median follow-up period of 54 months reported that treatment-induced menstrual change (amenorrhoea) occurred in 59 (36.9%) patients, 25 (31.3%) of those treated with CMF and 34 (42.5%) with AD. Amenorrhoea occurred after a median two cycles of chemotherapy (range: 1–6 cycles). Menstruation resumed in 49 (83.1%) patients, 20 (80%) of those treated with CMF and 29 (85.3%) with AD. The median time to resumption of menstruation was 3.5 months (Kil et al., 2006).
As a rule of thumb, chemotherapy for breast cancer appears to add about 10 years to ovarian age in terms of reproductive function. Unfortunately, many young women are not fully aware or well informed of the potential adverse reproductive effects of chemotherapy on fertility or fail to understand the possible consequences of treatment while making treatment decisions shortly after the diagnosis of breast cancer (Duffy et al., 2005).
Endocrine therapy and reproductive function
Around 60% of pre-menopausal breast cancer patients will have HR+ve cancers and will be given endocrine therapy either alone (in selected patients) or more commonly chemotherapy followed by endocrine therapy. Endocrine therapy regimens vary according to patient and tumour characteristics, but may include tamoxifen or ovarian suppression or ablation or both. Aromatase inhibitors (AIs) are currently only used in post-menopausal women, and their role in conjunction with GnRH agonists in younger women is under investigation.
Tamoxifen
Tamoxifen is a mixed estrogen agonist and antagonist which commonly induces hot flushes, night sweats, vaginal discharge, itching or dryness (Mourits et al., 2001). Tamoxifen is often reported to cause menstrual disorders but evidence for this is lacking. Tamoxifen may also stimulate ovulation (Oktay et al., 2005) and is licensed in the UK for the treatment of anovulatory infertility. It is important that younger women realize that tamoxifen is not a contraceptive and carries a risk of stimulating multiple ovulations and hence multiple pregnancy. In post-menopausal women, tamoxifen increases the risk of endometrial polyps, hyperplasia and a 2-fold increased risk of endometrial cancer (van Leeuwen et al., 1994), but this is far less likely in pre-menopausal women.
Tamoxifen has a similar chemical structure to diethylstilbestrol. Concern that tamoxifen may be teratogenic arose from animal studies showing increased genital tract malformations (Barthelmes and Gately, 2004). However, there relatively little human evidence that tamoxifen is teratogenic. There have been four reports of craniofacial abnormalities associated with tamoxifen use in the first trimester (Cullins et al., 1994). A further case report linked tamoxifen with genital tract abnormalities in humans (Tewari et al., 1997). No fetal abnormalities were seen in the offspring of 85 women who became pregnant while taking prophylactic tamoxifen for the prevention of breast cancer (Clark, 1993). However, there are no long-term data from children exposed to tamoxifen during development. This is important because the effects of diethylstilbestrol only became evident in later life.
Assessment of fertility following breast cancer treatment
Lack of prospective studies with current chemotherapeutic regimens makes it difficult to predict their impact on future fertility. Most studies have used amenorrhoea or menstrual irregularity as an endpoint, but these do not reliably relate to fertility. Recent studies have assessed the impact of chemotherapy on ovarian function using composite measures derived from assisted reproductive technology (ART) studies. Ovarian reserve testing (ORT) includes timed (Day 2–5 of the menstrual cycle) follicle-stimulating hormone (FSH) and lutenizing hormone, estradiol, inhibin B and anti-Mullerian hormone (AMH) in combination with ovarian antral follicle count determined at transvaginal ultrasound. Declining ovarian reserve is reflected in lower circulating levels of estradiol, inhibin B and AMH produced by the granulosa cells of the ovarian follicle and reduced numbers of antral follicles. These tests appear to predict the outcome in ART (Bukulmez and Arici, 2004), and control levels have been published in a healthy and subfertile populations (Broekmans et al., 2006). ORT has not been shown to reliably predict pregnancy, but appears to predict earlier age at menopause (Lutchman Singh et al., 2005). The validity of ORT following chemotherapy for breast cancer is not yet established, and this battery of tests is not commonly available in clinical practice. ORT following chemotherapy suggests that it does not reliably predict the response to ovarian stimulation (Lutchman Singh et al., 2005). Prospective studies in breast cancer patients indicate that ovarian reserve declines during chemotherapy, with regimens containing taxanes in addition to cyclophosphamide showing increased gonadotoxicity. Gonadotrophin suppression with endocrine therapy resulted in expected falls in estradiol (P < 0.05) and inhibin B (P < 0.001) levels, but also resulted in a delayed fall in AMH level after 6 months (P < 0.0001). A fall in AMH may precede other changes in ORT following breast cancer chemotherapy (Anderson et al., 2006).
Preservation of fertility in breast cancer patients
Breast cancer patients who have not started or completed their families may wish to consider available options to try and increase the chances of successful pregnancy following chemotherapy. Currently, there are no treatments which are guaranteed to preserve fertility. For women with breast cancer, the issue of fertility preservation is more complex than in other cancers with concerns that fertility preservation strategies and/or subsequent pregnancy may increase the risk of cancer recurrence, particularly in women with hormone-receptor positive disease (Partridge et al., 2007). The potential risks and benefits of treatment should be considered on an individual basis (Table II). In evaluating patient options, a critical factor is whether the patient has a fertile male partner with whom she is planning a family. For single women, the options are currently very limited.
Table II.
Advantages and disadvantages of fertility-preserving strategies
| Option | Advantage | Disadvantage |
|---|---|---|
| Potential fertility preserving strategies | ||
| 1. IVF and embryo cryopreservation | Relatively effective in achieving pregnancy | Requires a male partner and embryos legally owned by both partners |
| Clinically available | Likely to increase circulating estrogen levels which may impact on prognosis of ER positive breast cancer | |
| May delay chemotherapy | ||
| In gene mutation, carriers may transmit increased cancer risk to offspring | ||
| 2. Ovarian stimulation and oocyte cryopreservation | Does not require a male partner | Very few successful pregnancies |
| Likely to increase circulating estrogen levels which may impact on prognosis of ER positive breast cancer | ||
| May delay chemotherapy | ||
| In gene mutation, carriers may transmit increased cancer risk to offspring | ||
| 3. Ovarian tissue cryopreservation and xenotransplantation |
Does not require a male partner | Very few successful pregnancies |
| Does not require ovarian stimulation and increased estradiol levels | May reimplant ovarian tissue affected by micrometastases | |
| Unlikely to delay chemotherapy | In gene mutation, carriers may transmit increased cancer risk to offspring | |
| Surgical procedure | ||
| 4. Ovarian suppression with GnRH agonists | Does not require a male partner | Efficacy in fertility preservation not confirmed |
| Simple to administer | Side effects unknown | |
| Unlikely to delay chemotherapy | ||
| Relatively less invasive | ||
ER, estrogen receptor; GnRH, luteinizing hormone releasing hormone; IVF, in vitro fertilization.
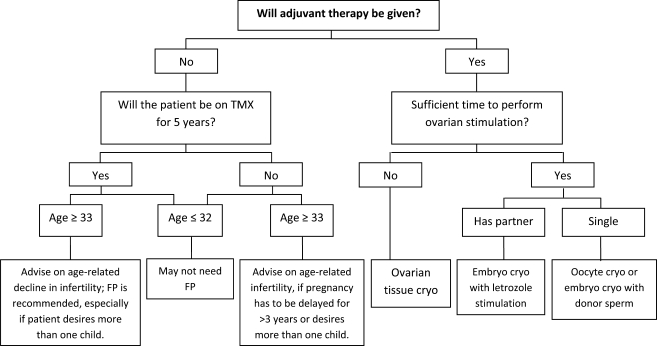
Fertility preservation options can be divided into those which aim to reduce the impact of chemotherapy on ovarian function, those which aim to remove and preserve ovarian tissue before starting chemotherapy and those which aim to produce mature oocytes or fertilized embryos for future use. Sonmezer and Oktay (2006) have proposed an algorithm to aid decisions regarding fertility preservation based on cancer treatment choices and age that may be a useful tool in clinical practice (Fig. 2).
Figure 2.
A proposed algorhythmic approach to decision-making for fertility preservation in breast cancer patients. Reproduced from Sonmezer and Oktay (2006) with permission. Embryo cryopreservation using letrozole is a novel stimulation protocol in breast cancer patients, and long-term follow-up data are awaited. Ovarian tissue and oocyte cryopreservation are experimental technologies. cryo, cryopreservation; FP, fertility preservation; TMX, tamoxifen
Multiple small observational studies have demonstrated a protective effect on ovarian function when GnRH agonists are given concurrently with chemotherapy (Blumenfeld, 2007). A recent small RCT demonstrated that significantly more breast cancer patients given an GnRH agonist before chemotherapy resumed spontaneous menses and ovulation (Badawy et al., 2008) and this requires confirmation.
The mechanisms of action of GnRH agonists in preserving ovarian function are not fully understood, but may include interruption of FSH secretion, decrease in utero-ovarian perfusion, activation of GnRH receptors, up-regulation of intra-gonadal antiapoptotic molecules such as sphingosine-1-phosphate or by protection of undifferentiated germ line stem cells (Blumenfeld, 2007).
Embryo cryopreservation is a standard, widely available treatment for infertility that may be used in breast cancer patients who have a male partner or who have access to donor sperm. Although success rates with frozen embryos are somewhat reduced, these women can undergo ovarian stimulation, oocyte harvesting followed by IVF and embryo freezing for attempted pregnancy when breast cancer treatment is completed. Advantages of this strategy are the relatively high success rates. Disadvantages include the need for ovarian stimulation (and subsequent high estradiol levels), cost (variable), possible delays in commencing adjuvant treatment (Lee et al., 2006) and the risk of cancelled cycles. Strategies to avoid high estrogen levels include ‘natural cycle’ IVF, albeit with very low pregnancy rates (Oktay, 2005). Ovarian stimulation protocols using the AI letrozole, together with gonadotrophin treatment (FSH), resulted in significantly lower peak estradiol levels than tamoxifen alone or plus FSH, and 44% reduction in the amount of gonadotrophin needed but similar length of stimulation and number of embryos obtained. Fertilization rates were similar to conventional ovarian stimulation protocols and 81% completed their IVF cycles within 8 weeks of surgery (Oktay et al., 2006a, b). Short-term follow-up (around 1 year) is reassuring that this intervention does not appear to affect breast cancer prognosis (Azim et al., 2008), but long-term data are not yet available. In patients with potentially fatal disease, the issue of what to do with the embryos if the patient dies prior to their use should be addressed.
For those who do not have a male partner, oocyte cryopreservation may be considered. However, success rates with this method are three to four times lower than that seen with embryo cryopreservation, at ∼20% at best (Partridge et al., 2007). Like embryo cryopreservation, this approach may incur expense, treatment delay and requires ovarian stimulation. Ovarian tissue cryopreservation for future orthotopic or heterotopic reimplantation and stimulation has limited international experience and relatively few successful pregnancies (Oktay and Tilly, 2004). Survival of transplanted ovarian follicles appears to be reduced in older women (Partridge et al., 2007). It remains unclear whether removing ovarian tissue for this highly experimental technique augments or reduces fertility in younger women with breast cancer. Further, it is possible that reimplanted ovarian tissue may harbour breast cancer micrometastases that may increase recurrence risk, although this is more a theoretical risk and there are no supportive clinical data. Women who carry a pathogenic BRCA1 or 2 gene mutation have a 50% risk of transmitting this mutation to their offspring. Recent advances in pre-implantation genetic diagnosis have allowed the selection of unaffected embryos during IVF protocols in these women (Jasper et al., 2008).
Ovum donation is another option and has the benefit of using fresh ova from a donor (with higher success rates than frozen ova). This may be particularly appealing to women who do not, at the time of diagnosis, have a partner with whom they are considering having a child. For BRCA1 or 2 mutation carriers, there is a potential benefit that the child will not inherit their gene mutation. There is no required delay in cancer treatment as this process would occur after cancer treatment is completed, however, similar to IVF, the recipient of the ova may require hormonal stimulation (to prepare the endometrium for embryo transfer) and subsequent increased estradiol levels. The recipient will incur not only the cost for her own stimulation and transfer but also the costs for the ovarian stimulation and collection of the ova from the donor and other medical costs including counselling. Also, the patient will usually need to find the oocyte donor herself.
Contraception following breast cancer
Pregnancy should be avoided during active treatment of breast cancer, so adequate, acceptable and effective contraception is a priority. Hormonal contraceptives remain contraindicated, although the evidence for harm is difficult to establish. This is particularly relevant given the current availability of low-dose long-acting progestogen only contraceptive such as the levonorgestrel-releasing intra-uterine system (Mirena) which delivers high local but low systemic doses of progestogen and offer highly effective contraception. Mirena has been used following breast cancer, and may reduce the risk of endometrial pathology in tamoxifen users (Chan et al., 2007), but studies are generally small and have not included recurrence or new cancers as an endpoint. Subgroup analysis of a recent retrospective cohort study reported a trend towards increased breast cancer recurrence rate in women using Mirena at the time of diagnosis who continued with the device in situ. This was not seen in those who had Mirena inserted after a breast cancer diagnosis (Trinh et al., 2008). This highlights the need for prospective studies to address the safety of Mirena after breast cancer. Meanwhile, patients should be advised that the safety of Mirena on breast cancer recurrence is unknown and alternative non-hormonal contraception is recommended.
Observational studies in BRCA1 and 2 unaffected mutation carriers have demonstrated that the oral contraceptive pill reduces the risk of ovarian cancer (McLaughlin et al., 2007), but may be associated with an increased risk of breast cancer, although the data regarding this are conflicting (Brohet et al., 2007). Reassuringly, Lee et al. recently reported the results of a population-based study that found no association between oral contraceptive use and breast cancer risk in BRCA1/2 mutation carriers. Further confirmation that currently available low-dose oral contraceptives do not increase breast cancer risk in carriers is important from a public health perspective, given the high prevalence of oral contraceptive use. There is still a paucity of evidence-based information on oral contraception and risk of breast cancer in BRCA1 and 2 carriers to make definitive recommendations.
Pregnancy following breast cancer
Less than 10% of women previously diagnosed with breast cancer subsequently become pregnant (Danforth, 1991; Kroman et al., 1997). This is around half the pregnancy rate seen in an age-matched group who have not had breast cancer (Ives et al., 2007). Of these pregnancies, between 14 and 44% are terminated (Barthelmes and Gately, 2004). Relatively few studies have addressed the impact of pregnancy following breast cancer (Ives et al., 2007). Further, those who become pregnant following breast cancer may be a self-selected group when may not be representative of the larger population. Women carrying hereditary gene mutations that increase the risk of cancer in their offspring may be particularly reluctant to have children (Smith et al., 2004).
The use of adjuvant chemotherapy does not appear to affect the outcome of pregnancy in women who become pregnant at least 6 months after diagnosis, with more women having a successful live birth than having an abortion or miscarrying (Clark and Chua, 1989; Reichman et al., 1994; Ives et al., 2007). Moreover, pregnancy does not appear to adversely affect prognosis following a prior history of breast cancer. The overall survival rates at 5 and 10 years were found to be better for women who subsequently conceived in a Western Australian population study (Ives et al., 2007) than has been reported in similar cohorts (Velentgas et al., 1999; Blakely et al., 2004). This apparent survival benefit is probably due to a ‘healthy mother effect’ (Sankila et al., 1994), suggesting that breast cancer survivors who subsequently conceive are a self-selecting group of women with better prognosis. Retrospective case–controlled studies report that subsequent live birth does not adversely effect prognosis (Kroman et al., 1997; Barthelmes et al., 2005). The little available information appears to show no increase in the incidence of prematurity, stillbirth or congenital malformations in their babies. Small series following these children are also reassuring (Mintzer et al., 2002), but little is known about the long-term impact of chemotherapy or endocrine therapy on offspring (Cardonick and Iacobucci, 2004). The largest reported series included 84 children who were exposed to a variety of chemotherapy agents in utero and followed for a median of 18 years. Reassuringly, no adverse sequelae were documented although the numbers are relatively small (Aviles and Neri, 2001).
Women are commonly recommended to delay pregnancy for at least 2 years following a diagnosis of breast cancer as most recurrences will develop in that time (Averette et al., 1999). The optimal time to delay pregnancy following the diagnosis and treatment is unknown and is an important issue for all patients considering pregnancy. The risk of relapse and the time to recurrence is related to many factors including the stage, grade and nodal status as well as the HR status. Saphner et al. (1996) have reported annual hazard rates of recurrence after primary therapy. These results should be interpreted with caution since chemotherapy regimens have now changed and the number of younger women included is not stated. For the entire group, the risk of recurrence was greatest (13.3%) for the interval between years 1 and 2 after surgery. This risk then decreased consistently, and beyond 5 years averaged about 4.3% per annum in women with HR+ve breast cancer in the Saphner study (Fig. 1), but there are some caveats with these figures, as discussed earlier. Patients with HR–ve tumours had a higher hazard of recurrence in years 0–5 which then decreased over time, whereas the hazard of recurrence for women with HR+ve cancers was relatively constant in the first 5 years after diagnosis and from years 5 to 12. This highlights the distinct natural history of HR+ve and HR–ve tumours. More recently, we have recognized that there are a number of different sub-types of breast cancer, some of which are more common in younger women and they appear to have a higher risk of relapse in the first 2–3 years after diagnosis. These estimates of recurrence should be considered in decisions regarding subsequent pregnancy. However, there is little evidence to show benefit to patients in waiting more than 2 years after diagnosis of breast cancer to attempt pregnancy, as long as adjuvant therapy has been completed. In addition, patients need to understand that there is a risk that breast cancer may return and this may affect their ability to care for future offspring. Over 50% (of 126) of breast cancer patients in a population-based series in Western Australia conceived within 2 years of diagnosis and this did not seem to adversely affect their survival (Ives et al., 2007).
In women with BRCA1 or 2 gene mutations, the risks of pregnancy are not well established. Observational studies suggest that BRCA1 mutation carriers who have their first child at 30 years or older may have a reduced personal risk of breast cancer (Andrieu et al., 2006). However, the opposite effect has been observed in BRCA2 mutation carriers where late first pregnancies (over 30 years) are linked with increased risk of breast cancer (Andrieu et al., 2006).
Breastfeeding after breast cancer
It is well established that breast milk is the best source of nutrients for babies (Helewa et al., 2002) Following breast cancer treatment, women may need advice about their ability and the safety of breastfeeding. Observational data suggest that breastfeeding does not impact on breast cancer prognosis and that infants breastfed by mothers with a history of breast cancer or current cancer do not have an elevated risk of cancer (American Academy of Pediatrics. Work Group on Breastfeeding, 1997; Michels et al., 2001; Helewa et al., 2002).
All younger women having breast conserving surgery will be advised to also have RT and will then be unlikely to be able to feed from the treated breast (Tralins, 1995; Moran et al., 2005). Women can successfully breastfeed from the other breast after breast cancer if they do not undergo pharmacological suppression of lactation (Higgins and Haffty, 1994; Moran et al., 2005), and it is possible to breastfeed exclusively from one breast (Sacchini et al., 2006).
Menopausal symptoms following breast cancer treatment
Menopausal symptoms are a frequent and troublesome side effect of breast cancer therapy in women of all ages. Hot flashes, night sweats, sexual dysfunction, poor sleep and tiredness are common (Saunders et al., 2008). Vasomotor symptoms, particularly hot flushes, appear to be more severe than in women who have not had breast cancer treatment (Couzi et al., 1995; Carpenter et al., 1998; Santoro, 2004). Vasomotor symptoms such as hot flashes are the most common side effect (Cella and Fallowfield, 2008). Up to 20% of breast cancer patients consider stopping or actually cease endocrine therapy because of menopausal symptoms, primarily hot flashes (Fellowes et al., 2001; Barron et al., 2007), despite its established role in reducing recurrence. Atrophic vaginitis affects many women using endocrine therapy for breast cancer, particularly those using AIs (Howell et al., 2005). Sexual dysfunction may be related to atrophic vaginitis but also to changes in body image, libido and self-esteem and may be more common in younger women (Canney and Hatton, 1994; Schover, 1994; Andersen et al., 2007).
The recommended duration of initial adjuvant endocrine therapy is 5 years and some patients may benefit from a further 5 years of treatment. With such long-term treatment duration, it is critical to address morbidity associated with treatment side effects in an effort to optimize adherence to therapy and quality of life.
Use of estrogen and progestin in breast cancer patients
Estrogen-containing HT is the most effective and well-studied treatment for menopausal vasomotor symptoms and atrophic vaginitis in healthy post-menopausal women (MacLennan et al., 2004), but the efficacy and safety of HT following breast cancer is contentious. There are two large RCTs investigating the use of HT after diagnosis of early stage breast cancer having contradictory results. The Stockholm and HABITS trials were similar in design, though a goal of the Stockholm protocol was to minimize the use of progestogen combined with estrogen. The HABITS trial was stopped prematurely because it identified a significantly higher risk of recurrence in women taking menopausal HT (relative hazard [RH] = 3.3, 95% confidence interval [CI] = 1.5–7.4 at a median follow-up of 2.1 years), whereas at a median follow-up of 4.1 years the risk of recurrence was not associated with HT in the Stockholm trial (RH = 0.82, 95% CI = 0.35–1.9) (Holmberg et al., 2004; von Schoultz and Rutqvist, 2005). HT was not effective in controlling hot flashes in tamoxifen users in one retrospective study (Sestak et al., 2006). Long-term use of combined HT has been associated with an increased risk of new breast cancers in some studies (Rossouw et al., 2002). In breast cancer survivors, one RCT reports a 2- to 3-fold increased risk of new primary or recurrent breast cancers in HT users (Holmberg et al., 2008). Further, HT may compromise the effects of endocrine therapy aimed at blocking the effects of estrogen, or reduce its production in ER+ve disease. In addition, combined HT increases breast density, which may compromise the ability of mammography to detect early cancers (Chiarelli et al., 2006). Consequently, many women wish to avoid HT following breast cancer (Biglia et al., 2003). Progestins are also effective for menopausal hot flashes following breast cancer (Loprinzi et al., 1994; Barton et al., 2002; Bordeleau et al., 2007), but their safety is not established and their effects may depend on circulating levels of estradiol. Of concern is that the addition of progestin to estrogen for HT appears to increase the risk of a primary breast cancer (Anderson et al., 2004).
The recent trial of tibolone after breast cancer (LIBERATE) has concluded that tibolone increases the risk of new breast cancers or recurrence (Peter Kenemans, personal communication).
Use of estrogen and progestin in BRCA1/2 mutation carriers
Risk-reducing BSO in pre-menopausal BRCA1/2 mutation carriers will induce surgical menopause. There are surprisingly few published studies on the consequences of surgical menopause, but the observational literature suggests that symptoms may be more severe and prolonged following BSO compared with spontaneous menopause (Bachmann, 1999). Estrogen-containing HT may be of limited efficacy in relieving menopausal symptoms in these younger women (Madalinska et al., 2006). Further, the impact of HT on subsequent breast or ovarian cancer risk in these women is poorly understood. One retrospective study (Rebbeck et al., 2005) was reassuring that the reduction in breast cancer risk associated with BSO is not modified by short-term use of HT. In the absence of other data, a decision model has been developed which allows individualized assessment of the impact of prophylactic BSO, bilateral prophylactic mastectomy and HT use on life expectancy in BRCA1/2 carriers at age 30, 35 and 40. The overall impact of HT use ranged from a gain of 0.79 years to a loss of 1.09 years dependent on the age of BSO and the duration of HT use. This suggests that the use of HT in BRCA1/2 users should be guided by patient symptoms and issues around quality of life, in the absence of clearer information about relative risks (Gallagher, 2007; Metcalfe et al., 2007).
Management of menopausal symptoms following breast cancer
Non-hormonal treatments for hot flushes following breast cancer have been extensively reviewed (Hickey et al., 2008). Currently, there is no evidence that complementary or natural therapies are consistently effective or safe following breast cancer. Further, although behavioural or lifestyle changes have shown promise, efficacy data are lacking. A number of pharmaceutical therapies have been tested and appear moderately effective in reducing the number and severity of hot flushes in breast cancer patients. These non-hormonal therapies do not appear to impact on other common menopausal symptoms, although some may improve sleep when sleep disturbance is due to night sweats.
For breast cancer patients with moderate-to-severe hot flashes, it is reasonable to consider either serotonin-norepinephrine reuptake inhibitor (SNRI) or selective serotonin reuptake inhibitor (SSRI) either venlafaxine or paroxetine as first-line approach. There are few head-to-head studies between preparations, but venlafaxine (37.5 mg daily increasing to 75 mg daily after 1 week) or paroxetine (10 mg daily increasing to 20 mg daily after 1 week if symptoms persist) has been extensively studied and appears effective, at least in the short-term and adequately tolerated. If venlafaxine is not effective, it is reasonable to consider trying paroxetine and vice versa. In those who are taking tamoxifen, preparations which induce CYP2D6 (e.g. paroxetine and fluoxetine) should be avoided since these may interfere with the breakdown of tamoxifen to its active metabolite (Stearns et al., 2003). SSRI and SNRI are contraindicated in women taking monoamine oxidase inhibitors and should be used cautiously or potentially avoided in women with bipolar disorder/manic depression, because of the risk of inducing mania. If there is no response in 4 weeks, then the treatment is unlikely to be effective.
Gabapentin (300 mg tds) appears be an effective alternative and may be used as an alternative first-line treatment, instead of SSRI/SNRIs or where these are unsuitable (Pandya et al., 2005). There does not appear to be a benefit of adding gabapentin to SSRI/SNRI (Loprinzi et al., 2007). Gabapentin may also be considered if sexual dysfunction is a problem prior to SSRI/SNRIs or develops on this therapy. For those with milder symptoms requesting treatment, clonidine (25 mg bd) or vitamin E (800 IU/day) shows moderate efficacy (Nelson et al., 2006). Endocrine therapy is a common cause of menopausal symptoms in both pre and post-menopausal women. Changing or stopping endocrine therapy may be considered, but should be managed in consultation with the oncologists, preferably within a multi-disciplinary treatment approach (Hickey et al., 2008).
Atrophic vaginitis following breast cancer
Symptomatic atrophic vaginitis affects around one-third of post-menopausal women after breast cancer (Leining et al., 2006) and is particularly troublesome in those using AIs (Fallowfield et al., 2004). Increasing use of AIs in post-menopausal breast cancer patients means that the number of women complaining of symptomatic atrophic vaginitis following breast cancer treatment is likely to increase (Cella et al., 2006).
Vaginal estrogens are an effective treatment for vaginal dryness (Suckling et al., 2007) and are more effective than non-hormonal vaginal lubricants such as Replens® in women who have not had breast cancer (Nachtigall, 1994). Although systemic estrogens are avoided following estrogen-receptor positive breast cancer, vaginal estrogens are commonly used via an estradiol-releasing vaginal ring, estrogen-based vaginal creams, pessaries containing estriol and a slow-release 17β estradiol tablet. However, there are few data on either safety or efficacy (Ponzone et al., 2005). Small retrospective studies in breast cancer patients suggest that vaginal estrogens do not adversely affect the outcome (Dew et al., 2003). Similarly, vaginal estrogens were permitted in the placebo-controlled MA.17 trial of letrozole as extended adjuvant therapy following 5 years of tamoxifen without seeming to interfere with the observed efficacy (Goss et al., 2003). Although systemic absorption of vaginal estrogens is not sufficient to cause endometrial hyperplasia, it may be sufficient to increase circulating estradiol in breast cancer patients taking AIs (Kendall et al., 2006). More information is needed about the safety of vaginal estrogens following breast cancer. An alternative option is to use the less potent estrogen, estriol which cannot be converted to estradiol. Vaginal preparations containing estriol are as effective as those containing estradiol in treating symptomatic women (Barentsen et al., 1997).
Sexual dysfunction following breast cancer
Both the diagnosis and treatment of breast cancer may impact negatively on sexual function in women (Burwell et al., 2006). These are likely to vary according to age, menopausal status and relationship factors as well as the nature of surgical and endocrine treatments (Cella and Fallowfield, 2008). Sexual dysfunction is common in women with atrophic vaginitis (Levine et al.) and may also relate to changes in body image, libido and self-esteem after breast cancer (Canney and Hatton, 1994). There are relatively few safe and effective treatment options for hypoactive sexual desire disorder (HSDD). Sensitive but direct questioning about sexual function may be needed since patients may be reluctant to raise these issues themselves. Vaginal dryness should be excluded as a contributory factor (Loprinzi et al., 1997). Concurrent treatment with SSRI/SNRIs may cause/complicate sexual dysfunction by reducing libido and causing anorgasmia. Testosterone therapy is offered by some clinicians for HSDD, but its safety and efficacy have not been well established in healthy women (Wierman et al., 2006) or after breast cancer (Barton et al., 2007). There is some evidence that sexual problems after breast cancer tend to decrease over time (Burwell et al., 2006). Type 5 phosphodiesterase inhibitors such as sildenafil have been extensively studies for female sexual dysfunction, but RCT have not shown significant benefit (Basson et al., 2002). Small studies suggest that the antidepressant bupropion may improve sexual function in breast cancer survivors (Mathias et al., 2006), but larger trials are needed to confirm this. Sex therapy may be helpful.
Treatment of bone loss
Abrupt withdrawal of circulating estrogen, such as occurs with chemotherapy-induced ovarian failure, oophorectomy, GnRH agonist therapy or with AIs, is accompanied by rapid bone loss (Sverrisdottir et al., 2004). Chemotherapy per se has not been associated with bone loss if it is not followed by ovarian failure (Shapiro et al., 2001). Women who develop breast cancer may have a higher bone density than those who do not, presumably secondary to effects of lifetime exposure to circulating estrogen which has been linked to increased risk of breast cancer (Nguyen et al., 2000; Heshmati et al., 2002). Nevertheless, bone loss and increasing risk of fracture is a risk for breast cancer survivors (Chen et al., 2003). Tamoxifen preserves bone density and reduces fractures in post-menopausal women by its estrogenic action on bone (Love et al., 1992). Conversely, in pre-menopausal women with endogenous circulating estrogen, tamoxifen is associated with bone loss, presumably because of competitive binding to the ER (Powles et al., 1996). AIs are now commonly used as first-line endocrine therapy in HR+ve post-menopausal women. However, bone loss with the AIs is substantial and greatest for women within 4 years of menopause and for women <65 years of age. Fractures are significantly increased following AI use compared with tamoxifen (Coleman et al., 2007). In pre-menopausal women, GnRH analogues are associated with rapid bone loss when used in the setting of breast cancer or endometriosis. It results in greater bone loss than is seen with chemotherapy-induced ovarian failure, although some recovery occurs after its cessation (Fogelman et al., 2003).
The reproductive medicine specialist and gynaecologist may be involved in diagnosing and managing reduced bone density or osteoporosis following breast cancer. Lifestyle guidance on calcium intake, exercise and avoidance of bone toxins, such as smoking and excessive alcohol, is important (Sambrook and Eisman, 2000). Bisphosphonates may minimize the bone loss associated with AI use. Zoledronic acid 4 mg every 6 months effectively inhibited bone loss in breast cancer patients using AIs and GnRH agonists (Gnant et al., 2007). The effect on fracture reduction in breast cancer patients is not yet known. The available data do not support fracture prevention with bisphosponates in women with a femoral neck T score above –2.5 and no prevalent fracture. Indeed, among women with T score > –2.0, more wrist fractures were reported in the ‘FIT’ study in the Alendronate group versus placebo (RH = 1.9; 95% CI = 1.0–4.0) (Cummings et al., 1998). The use of bisphosphonates for fracture prevention should depend on absolute risk of fracture (Prince, 2001).
Conclusions
Increasing numbers of breast cancer survivors are presenting to reproductive medicine specialists and their gynaecologists with symptoms secondary to breast cancer treatments and queries regarding ongoing issues around fertility and menopause. Many of these will be younger women who prefer greater involvement in treatment decision-making and their needs for information may differ from older women (Degner et al., 1997). In general, patients who are better informed experience greater emotional, social and physical well-being (Fallowfield et al., 1994), better clinical outcomes, quality of life (Fallowfield et al., 1994) and satisfaction with care (Weiss et al., 1996). Timing of information is important. Fertility discussions need to be prioritized and considered soon after diagnosis and before chemotherapy. Interventions such as IVF or ovarian tissue freezing should be discussed in detail in relation to the patient’s personal and medical circumstances. Predicting fertility following chemotherapy for breast cancer remains problematic, and more sensitive indices of ovarian function as well as longitudinal data regarding subsequent infertility, pregnancy and pregnancy outcome are needed in order to better inform women about the consequences of chemotherapy and the likelihood of a subsequent pregnancy. Current evidence suggests that pregnancy does not appear to be detrimental following breast cancer, but individualized counselling regarding prognosis and risk relapse based on their age and pathological features of the cancer is required before patients can make informed decisions regarding future childbearing.
The management of menopausal symptoms following breast cancer is a particular challenge. There is a growing literature on safe and effective non-hormonal treatments for hot flushes. However, other common menopausal symptoms such as vaginal dryness and sexual dysfunction should not be overlooked and are likely to require additional management. There is a growing recognition of the importance of developing a ‘survivorship care plan’ to coordinate care of women with a prior history of breast cancer and to address and prevent the long-term side effects and consequences of adjuvant therapies (Ganz and Hahn, 2008). This is best done by a multi-disciplinary team, of which the reproductive medicine specialist and gynaecologist are integral members.
Funding
Martha Hickey is a receipient of an NHMRC Clinical Career Development Award and a Concept Award from the National Breast Cancer Foundation. Funding to pay the Open Access publication charges for this article was provided by The School of Women's and Infants' Health, University of Western Australia.
Acknowledgements
The authors are grateful to Associate Professor Roger Hart for his comments on this manuscript.
References
- Aebi S, Gelber S, Castiglione-Gertsch M, Gelber RD, Collins J, Thurlimann B, Rudenstam CM, Lindtner J, Crivellari D, Cortes-Funes H, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet. 2000;355:1869–1874. doi: 10.1016/s0140-6736(00)02292-3. [DOI] [PubMed] [Google Scholar]
- Allred DC, Brown P, Medina D. The origins of estrogen receptor alpha-positive and estrogen receptor alpha-negative breast cancer. Breast Cancer Res. 2004;6:240–245. doi: 10.1186/bcr938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alton J, Jacobs L, Fox K, Schuchter L, Domchek S, Glick J, Meadows A, DeMichele A. Chemotherapy-related amenorrhea (CRA) in breast cancer survivors: impact of taxanes on ovarian function. Breast Cancer Res Treat. 2004;88:S61–S62. [Google Scholar]
- American Academy of Pediatrics. Work Group on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 1997;100:1035–1039. doi: 10.1542/peds.100.6.1035. [DOI] [PubMed] [Google Scholar]
- Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, Febbo PG, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- Anderson G, Limacher M, Assaf A, Bassford T, Beresford S, Black H, Bonds D, Brunner R, Brzyski R, Caan B, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Themmen AP, Al-Qahtani A, Groome NP, Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod. 2006;21:2583–2592. doi: 10.1093/humrep/del201. [DOI] [PubMed] [Google Scholar]
- Andersen BL, Carpenter KM, Yang HC, Shapiro CL. Sexual well-being among partnered women with breast cancer recurrence. J Clin Oncol. 2007;25:3151–3157. doi: 10.1200/JCO.2006.09.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrieu N, Goldgar DE, Easton DF, Rookus M, Brohet R, Antoniou AC, Peock S, Evans G, Eccles D, Douglas F, et al. Pregnancies, breast-feeding, and breast cancer risk in the International BRCA1/2 Carrier Cohort Study (IBCCS) J Natl Cancer Inst. 2006;98:535–544. doi: 10.1093/jnci/djj132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriagada R, Le MG, Guinebretiere JM, Dunant A, Rochard F, Tursz T. Late local recurrences in a randomised trial comparing conservative treatment with total mastectomy in early breast cancer patients. Ann Oncol. 2003;14:1617–1622. doi: 10.1093/annonc/mdg452. [DOI] [PubMed] [Google Scholar]
- Averette HE, Mirhashemi R, Moffat FL. Pregnancy after breast carcinoma: the ultimate medical challenge. Cancer. 1999;85:2301–2304. doi: 10.1002/(sici)1097-0142(19990601)85:11<2301::aid-cncr1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Aviles A, Neri N. Hematological malignancies and pregnancy: a final report of 84 children who received chemotherapy in utero. Clin Lymphoma. 2001;2:173–177. doi: 10.3816/clm.2001.n.023. [DOI] [PubMed] [Google Scholar]
- Axelrod D, Smith J, Kornreich D, Grinstead E, Singh B, Cangiarella J, Guth AA. Breast cancer in young women. J Am Coll Surg. 2008;206:1193–1203. doi: 10.1016/j.jamcollsurg.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26:2630–2635. doi: 10.1200/JCO.2007.14.8700. [DOI] [PubMed] [Google Scholar]
- Bachmann GA. Vasomotor flushes in menopausal women. Am J Obstet Gynecol. 1999;180:S312–S316. doi: 10.1016/s0002-9378(99)70725-8. [DOI] [PubMed] [Google Scholar]
- Badawy A, Elnashar A, El-Ashry M, Shahat M. Gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage: prospective randomized study. Fertil Steril. 2008 doi: 10.1016/j.fertnstert.2007.12.044. doi:10.1016/j.fertnstert.2007.12.044 (3 August 2008, date last accessed) [DOI] [PubMed] [Google Scholar]
- Bao T, Davidson NE. Adjuvant endocrine therapy for premenopausal women with early breast cancer. Breast Cancer Res. 2007;9:115. doi: 10.1186/bcr1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barentsen R, van de Weijer PH, Schram JH. Continuous low dose estradiol released from a vaginal ring versus estriol vaginal cream for urogenital atrophy. Eur J Obstet Gynecol Reprod Biol. 1997;71:73–80. doi: 10.1016/s0301-2115(96)02612-7. [DOI] [PubMed] [Google Scholar]
- Barron TI, Connolly R, Bennett K, Feely J, Kennedy MJ. Early discontinuation of tamoxifen: a lesson for oncologists. Cancer. 2007;109:832–839. doi: 10.1002/cncr.22485. [DOI] [PubMed] [Google Scholar]
- Barthelmes L, Gately CA. Tamoxifen and pregnancy. Breast. 2004;13:446–451. doi: 10.1016/j.breast.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Barthelmes L, Davidson LA, Gaffney C, Gateley CA. Pregnancy and breast cancer. BMJ. 2005;330:1375–1378. doi: 10.1136/bmj.330.7504.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton D, Loprinzi C, Quella S, Sloan J, Pruthi S, Novotny P. Depomedroxyprogesterone acetate for hot flashes. J Pain Symptom Manage. 2002;24:603–607. doi: 10.1016/s0885-3924(02)00525-0. [DOI] [PubMed] [Google Scholar]
- Barton DL, Wender DB, Sloan JA, Dalton RJ, Balcueva EP, Atherton PJ, Bernath AMJ, DeKrey WL, Larson T, Bearden JD, et al. Randomized controlled trial to evaluate transdermal testosterone in female cancer survivors with decreased libido; North Central Cancer Treatment Group protocol N02C3. J Natl Cancer Inst. 2007;99:672–679. doi: 10.1093/jnci/djk149. [DOI] [PubMed] [Google Scholar]
- Baselga J, Perez EA, Pienkowski T, Bell R. Adjuvant trastuzumab: a milestone in the treatment of HER-2-positive early breast cancer. Oncologist. 2006;11(Suppl. 1):4–12. doi: 10.1634/theoncologist.11-90001-4. [DOI] [PubMed] [Google Scholar]
- Basson R, McInnes R, Smith MD, Hodgson G, Koppiker N. Efficacy and safety of sildenafil citrate in women with sexual dysfunction associated with female sexual arousal disorder. J Womens Health Gend Based Med. 2002;11:367–377. doi: 10.1089/152460902317586001. [DOI] [PubMed] [Google Scholar]
- Bentzon N, During M, Rasmussen BB, Mouridsen H, Kroman N. Prognostic effect of estrogen receptor status across age in primary breast cancer. Int J Cancer. 2008;122:1089–1094. doi: 10.1002/ijc.22892. [DOI] [PubMed] [Google Scholar]
- Bermejo-Perez MJ, Marquez-Calderon S, Llanos-Mendez A. Effectiveness of preventive interventions in BRCA1/2 gene mutation carriers: a systematic review. Int J Cancer. 2007;121:225–231. doi: 10.1002/ijc.22817. [DOI] [PubMed] [Google Scholar]
- Biglia N, Cozzarella M, Cacciari F, Ponzone R, Roagna R, Maggiorotto F, Sismondi P. Menopause after breast cancer: a survey on breast cancer survivors. Maturitas. 2003;45:29–38. doi: 10.1016/s0378-5122(03)00087-2. [DOI] [PubMed] [Google Scholar]
- Blakely LJ, Buzdar AU, Lozada JA, Shullaih SA, Hoy E, Smith TL, Hortobagyi GN. Effects of pregnancy after treatment for breast carcinoma on survival and risk of recurrence. Cancer. 2004;100:465–469. doi: 10.1002/cncr.11929. [DOI] [PubMed] [Google Scholar]
- Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist. 2007;12:1044–1054. doi: 10.1634/theoncologist.12-9-1044. [DOI] [PubMed] [Google Scholar]
- Bordeleau L, Pritchard K, Goodwin P, Loprinzi C. Therapeutic options for the management of hot flashes in breast cancer survivors: an evidence-based review. Clin Ther. 2007;29:230–241. doi: 10.1016/j.clinthera.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12:685–718. doi: 10.1093/humupd/dml034. [DOI] [PubMed] [Google Scholar]
- Brohet RM, Goldgar DE, Easton DF, Antoniou AC, Andrieu N, Chang-Claude J, Peock S, Eeles RA, Cook M, Chu C, et al. Oral contraceptives and breast cancer risk in the international BRCA1/2 carrier cohort study: a report from EMBRACE, GENEPSO, GEO-HEBON, and the IBCCS Collaborating Group. J Clin Oncol. 2007;25:3831–3836. doi: 10.1200/JCO.2007.11.1179. [DOI] [PubMed] [Google Scholar]
- Bukulmez O, Arici A. Assessment of ovarian reserve. Curr Opin Obstet Gynecol. 2004;16:231–237. doi: 10.1097/00001703-200406000-00005. [DOI] [PubMed] [Google Scholar]
- Burwell SR, Case LD, Kaelin C, Avis NE. Sexual problems in younger women after breast cancer surgery. J Clin Oncol. 2006;24:2815–2821. doi: 10.1200/JCO.2005.04.2499. [DOI] [PubMed] [Google Scholar]
- Callahan MJ, Crum CP, Medeiros F, Kindelberger DW, Elvin JA, Garber JE, Feltmate CM, Berkowitz RS, Muto MG. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol. 2007;25:3985–3990. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- Canney PA, Hatton MQ. The prevalence of menopausal symptoms in patients treated for breast cancer. Clin Oncol (R Coll Radiol) 1994;6:297–299. doi: 10.1016/s0936-6555(05)80270-5. [DOI] [PubMed] [Google Scholar]
- Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncol. 2004;5:283–291. doi: 10.1016/S1470-2045(04)01466-4. [DOI] [PubMed] [Google Scholar]
- Carpenter S, Andrykowski AM, Cordova M. Hot flashes in postmenopausal women treated for breast carcinoma: prevalence, severity, correlates, management and relation to quality of life. Cancer. 1998;82:1682–1691. [PubMed] [Google Scholar]
- Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat. 2008;107:167–180. doi: 10.1007/s10549-007-9548-1. [DOI] [PubMed] [Google Scholar]
- Cella D, Fallowfield L, Barker P, Cuzick J, Locker G, Howell A and ATAC Trialistsa9 Group. Quality of life of postmenopausal women in the ATAC (‘Arimidex’, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for early breast cancer. Breast Cancer Res Treat. 2006;100:273–284. doi: 10.1007/s10549-006-9260-6. [DOI] [PubMed] [Google Scholar]
- Chan SSC, Tam WH, Yeo W, Yu MMY, Ng DPS, Wong AWY, Kwan WH, Yuen PM. A randomised controlled trial of prophylactic levonorgestrel intrauterine system in tamoxifen-treated women. BJOG. 2007;114:1510–1515. doi: 10.1111/j.1471-0528.2007.01545.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Thompson W, Semeciw R, Mao Y. Epidemiology of contralateral breast cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:855–861. [PubMed] [Google Scholar]
- Chen Z, Maricic M, Bassford TL, Ritenbaugh C, Lopez AM, Leboff MS, Gass M, Barad D. Increased fracture risk among breast cancer survivors results from the women’s health initiative. J Bone Miner Res. 2003;18:S22–S22. doi: 10.1001/archinte.165.5.552. [DOI] [PubMed] [Google Scholar]
- Chiarelli AM, Kirsh VA, Klar NS, Shumak R, Jong R, Fishell E, Yaffe MJ, Boyd NF. Influence of patterns of hormone replacement therapy use and mammographic density on breast cancer detection. Cancer Epidemiol Biomarkers Prev. 2006;15:1856–1862. doi: 10.1158/1055-9965.EPI-06-0290. [DOI] [PubMed] [Google Scholar]
- Clark S. Prophylactic tamoxifen. Lancet. 1993;342:168. [Google Scholar]
- Clark RM, Chua T. Breast cancer and pregnancy: the ultimate challenge. Clin Oncol (R Coll Radiol) 1989;1:11–18. doi: 10.1016/s0936-6555(89)80004-4. [DOI] [PubMed] [Google Scholar]
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- Coleman RE, Banks LM, Girgis SI, Kilburn LS, Vrdoljak E, Fox J, Cawthorn SJ, Patel A, Snowdon CF, Hall E, et al. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol. 2007;8:119–127. doi: 10.1016/S1470-2045(07)70003-7. [DOI] [PubMed] [Google Scholar]
- Colleoni M, Rotmensz N, Robertson C, Orlando L, Viale G, Renne G, Luini A, Veronesi P, Intra M, Orecchia R, et al. Very young women (<35 years) with operable breast cancer: features of disease at presentation. Ann Oncol. 2002;13:273–279. doi: 10.1093/annonc/mdf039. [DOI] [PubMed] [Google Scholar]
- Couzi RJ, Helzlsouer KJ, Fetting JH. Prevalence of menopausal symptoms among women with a history of breast cancer and attitudes towards estrogen replacement therapy. J Clin Oncol. 1995;13:12737–12744. doi: 10.1200/JCO.1995.13.11.2737. [DOI] [PubMed] [Google Scholar]
- Cullins SL, Pridjian G, Sutherland CM. Goldenhar’s syndrome associated with tamoxifen given to the mother during gestation. JAMA. 1994;271:1905–1906. [PubMed] [Google Scholar]
- Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077–2082. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- Danforth DN., Jr How subsequent pregnancy affects outcome in women with a prior breast cancer. Oncology (Williston) 1991;5:23–30. discussion 30–21. [PubMed] [Google Scholar]
- De Laurentiis M, Cancello G, D’Agostino D, Giuliano M, Giordano A, Montagna E, Lauria R, Forestieri V, Esposito A, Silvestro L, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol. 2008;26:44–53. doi: 10.1200/JCO.2007.11.3787. [DOI] [PubMed] [Google Scholar]
- Degner LF, Kristjanson LJ, Bowman D, Sloan JA, Carriere KC, O’Neil J, Bilodeau B, Watson P, Mueller B. Information needs and decisional preferences in women with breast cancer. JAMA. 1997;277:1485–1492. [PubMed] [Google Scholar]
- Del Bianco P, Zavagno G, Burelli P, Scalco G, Barutta L, Carraro P, Pietrarota P, Meneghini G, Morbin T, Tacchetti G, et al. Morbidity comparison of sentinel lymph node biopsy versus conventional axillary lymph node dissection for breast cancer patients: results of the sentinella-GIVOM Italian randomised clinical trial. Eur J Surg Oncol. 2008;34:508–513. doi: 10.1016/j.ejso.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Demicheli R, Bonadonna G, Hrushesky WJM, Retsky MW, Valagussa P. Menopausal status dependence of the timing of breast cancer recurrence after surgical removal of the primary tumour. Breast Cancer Res. 2004;6:R689–R696. doi: 10.1186/bcr937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dew JE, Wren BG, Eden JA. A cohort study of topical vaginal estrogen therapy in women previously treated for breast cancer. Climacteric. 2003;6:45–52. [PubMed] [Google Scholar]
- Duffy CM, Allen SM, Clark MA. Discussions regarding reproductive health for young women with breast cancer undergoing chemotherapy. J Clin Oncol. 2005;23:766–773. doi: 10.1200/JCO.2005.01.134. [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group. Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. N Engl J Med. 1995;333:1444–1455. doi: 10.1056/NEJM199511303332202. [erratum appears in N Engl J Med 1996 Apr 11;334(15):1003] [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- Clarke M, Coates AS, Darby SC, Davies C, Gelber RD, Godwin J, Goldhirsch A, Gray R, Peto R, et al. Early Breast Cancer Trialists’ Collaborative Group. Adjuvant chemotherapy in oestrogen-receptor-poor breast cancer: patient-level meta-analysis of randomised trials. Lancet. 2008;371:29–40. doi: 10.1016/S0140-6736(08)60069-0. [DOI] [PubMed] [Google Scholar]
- Eisen A, Lubinski J, Klijn J, Moller P, Lynch HT, Offit K, Weber B, Rebbeck T, Neuhausen SL, Ghadirian P, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: an international case–control study. J Clin Oncol. 2005;23:7491–7496. doi: 10.1200/JCO.2004.00.7138. [DOI] [PubMed] [Google Scholar]
- El Saghir NS, Seoud M, Khalil MK, Charafeddine M, Salem ZK, Geara FB, Shamseddine AI. Effects of young age at presentation on survival in breast cancer. BMC Cancer. 2006;6:194. doi: 10.1186/1471-2407-6-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallowfield LJ, Hall A, Maguire P, Baum M, A’Hern RP. Psychological effects of being offered choice of surgery for breast cancer. BMJ. 1994;309:448. doi: 10.1136/bmj.309.6952.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trial. J Clin Oncol. 2004;22:4261–4271. doi: 10.1200/JCO.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Fellowes D, Fallowfield LJ, Saunders CM, Houghton J. Tolerability of hormone therapies for breast cancer: how informative are documented symptom profiles in medical notes for ‘well-tolerated’ treatments? Breast Cancer Res Treat. 2001;66:73–81. doi: 10.1023/a:1010684903199. [DOI] [PubMed] [Google Scholar]
- Fogelman I, Blake GM, Blamey R, Palmer M, Sauerbrei W, Schumacher M, Serin D, Stewart A, Wilpshaar W. Bone mineral density in premenopausal women treated for node-positive early breast cancer with 2 years of goserelin or 6 months of cyclophosphamide, methotrexate and 5-fluorouracil (CMF) Osteoporos Int. 2003;14:1001–1006. doi: 10.1007/s00198-003-1508-y. [DOI] [PubMed] [Google Scholar]
- Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994;343:692–695. doi: 10.1016/s0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- Gadducci A, Cosio S, Genazzani AR. Ovarian function and childbearing issues in breast cancer survivors. Gynecol Endocrinol. 2007;23:625–631. doi: 10.1080/09513590701582406. [DOI] [PubMed] [Google Scholar]
- Gallagher K. What should I do If I’m at High Risk for Breast Cancer? USA: Healthwise; 2007. [Google Scholar]
- Ganz P, Hahn E. Implementing a survivorship care plan for patients with breast cancer. J Clin Oncol. 2008;26:759–767. doi: 10.1200/JCO.2007.14.2851. [DOI] [PubMed] [Google Scholar]
- Gebski V, Lagleva M, Keech A, Simes J, Langlands AO. Survival effects of postmastectomy adjuvant radiation therapy using biologically equivalent doses: a clinical perspective [erratum appears in J Natl Cancer Inst 2006;98(12):876] J Natl Cancer Inst. 2006;98:26–38. doi: 10.1093/jnci/djj002. [DOI] [PubMed] [Google Scholar]
- Gnant MF, Mlineritsch B, Luschin-Ebengreuth G, Grampp S, Kaessmann H, Schmid M, Menzel C, Piswanger-Soelkner JC, Galid A, Mittlboeck M, et al. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol. 2007;25:3194–3197. doi: 10.1200/JCO.2005.02.7102. [DOI] [PubMed] [Google Scholar]
- Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- Hartmann LC, Sellers TA, Schaid DJ, Frank TS, Soderberg CL, Sitta DL, Frost MH, Grant CS, Donohue JH, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J Natl Cancer Inst. 2001;93:1633–1637. doi: 10.1093/jnci/93.21.1633. [DOI] [PubMed] [Google Scholar]
- Helewa M, Lévesque P, Provencher D, Lea RH, Rosolowich V, Shapiro HM Breast Disease Committee Executive Committee, Council and Society of Obstetricians and Gynaecologists of Canada. Breast cancer, pregnancy, and breastfeeding. J Obstet Gynaecol Can. 2002;24:164–180. [PubMed] [Google Scholar]
- Heshmati HM, Khosla S, Robins SP, O’Fallon WM, Melton LJ, 3rd, Riggs BL. Role of low levels of endogenous estrogen in regulation of bone resorption in late postmenopausal women. J Bone Miner Res. 2002;17:172–178. doi: 10.1359/jbmr.2002.17.1.172. [DOI] [PubMed] [Google Scholar]
- Hickey M, Saunders CM, Partridge A, Santoro N, Joffe H, Stearns V. Practical clinical guidelines for assessing and managing menopausal symptoms after breast cancer. Ann Oncol. 2008;19:1669–1680. doi: 10.1093/annonc/mdn353. [DOI] [PubMed] [Google Scholar]
- Higgins S, Haffty BG. Pregnancy and lactation after breast-conserving therapy for early stage breast cancer. Cancer. 1994;73:2175–2180. doi: 10.1002/1097-0142(19940415)73:8<2175::aid-cncr2820730823>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Holmberg L, Anderson H and committees Hsadm. HABITS (hormonal replacement therapy after breast cancer—is it safe?), a randomised comparison: trial stopped. Lancet. 2004;363:453–455. doi: 10.1016/S0140-6736(04)15493-7. [DOI] [PubMed] [Google Scholar]
- Holmberg L, Iversen OE, Rudenstam CM, Hammar M, Kumpulainen E, Jaskiewicz J, Jassem J, Dobaczewska D, Fjosne HE, Peralta O, et al. Increased risk of recurrence after hormone replacement therapy in breast cancer survivors. J Natl Cancer Inst. 2008;100:475–482. doi: 10.1093/jnci/djn058. [DOI] [PubMed] [Google Scholar]
- Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- Ives A, Saunders C, Bulsara M, Semmens J. Pregnancy after breast cancer: population based study. BMJ. 2007;334:194. doi: 10.1136/bmj.39035.667176.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper MJ, Liebelt J, Hussey ND. Preimplantation genetic diagnosis for BRCA1 exon 13 duplication mutation using linked polymorphic markers resulting in a live birth. Prenat Diagn. 2008;28:292–298. doi: 10.1002/pd.1925. [DOI] [PubMed] [Google Scholar]
- Jatoi I, Proschan MA. Randomized trials of breast-conserving therapy versus mastectomy for primary breast cancer: a pooled analysis of updated results. Am J Clin Oncol. 2005;28:289–294. doi: 10.1097/01.coc.0000156922.58631.d7. [DOI] [PubMed] [Google Scholar]
- Kauff ND, Barakat RR. Risk-reducing salpingo-oophorectomy in patients with germline mutations in BRCA1 or BRCA2. J Clin Oncol. 2007;25:2921–2927. doi: 10.1200/JCO.2007.11.3449. [DOI] [PubMed] [Google Scholar]
- Kendall A, Dowsett M, Folkerd E, Smith I. Caution: vaginal estradiol appears to be contraindicated in postmenopausal women on adjuvant aromatase inhibitors. Ann Oncol. 2006;17:584–587. doi: 10.1093/annonc/mdj127. [DOI] [PubMed] [Google Scholar]
- Kil WJ, Ahn SD, Shin SS, Lee SW, Choi EK, Kim JH, Son BH, Ahn SH, Kim WK, Kim SB. Treatment-induced menstrual changes in very young (<35 years old) breast cancer patients. Breast Cancer Res Treat. 2006;96:245–250. doi: 10.1007/s10549-005-9059-x. [DOI] [PubMed] [Google Scholar]
- Kim SH, Simkovich-Heerdt A, Tran KN, Maclean B, Borgen PI. Women 35 years of age or younger have higher locoregional relapse rates after undergoing breast conservation therapy. J Am Coll Surg. 1998;187:1–8. doi: 10.1016/s1072-7515(98)00114-8. [DOI] [PubMed] [Google Scholar]
- Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, Weaver DL, Miller BJ, Jalovec LM, Frazier TG, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- Kroman N, Jensen MB, Melbye M, Wohlfahrt J, Mouridsen HT. Should women be advised against pregnancy after breast-cancer treatment? Lancet. 1997;350:319–322. doi: 10.1016/S0140-6736(97)03052-3. [DOI] [PubMed] [Google Scholar]
- Kroman N, Holtveg H, Wohlfahrt J, Jensen M-B, Mouridsen HT, Blichert-Toft M, Melbye M. Effect of breast-conserving therapy versus radical mastectomy on prognosis for young women with breast carcinoma. Cancer. 2004;100:688–693. doi: 10.1002/cncr.20022. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K American Society of Clinical O. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- Leining MG, Gelber S, Rosenberg R, Przypyszny M, Winer EP, Partridge AH. Menopausal-type symptoms in young breast cancer survivors. Ann Oncol. 2006;17:1777–1782. doi: 10.1093/annonc/mdl299. [DOI] [PubMed] [Google Scholar]
- Levine DA, Argenta PA, Yee CJ, Marshall DS, Olvera N, Bogomolniy F, Rahaman JA, Robson ME, Offit K, Barakat RR, et al. Fallopian tube and primary peritoneal carcinomas associated with BRCA mutations. J Clin Oncol. 2003;21:4222–4227. doi: 10.1200/JCO.2003.04.131. [DOI] [PubMed] [Google Scholar]
- Loman N, Johannsson O, Kristoffersson U, Olsson H, Borg A. Family history of breast and ovarian cancers and BRCA1 and BRCA2 mutations in a population-based series of early-onset breast cancer. J Natl Cancer Inst. 2001;93:1215–1223. doi: 10.1093/jnci/93.16.1215. [DOI] [PubMed] [Google Scholar]
- Loprinzi CL, Michalak JC, Quella SK, O’Fallon JR, Hatfield AK, Nelimark RA, Dose AM, Fischer T, Johnson C, Klatt NE, et al. Megestrol acetate for the prevention of hot flashes. N Engl J Med. 1994;331:347–352. doi: 10.1056/NEJM199408113310602. [DOI] [PubMed] [Google Scholar]
- Loprinzi CL, Abu-Ghazaleh S, Sloan JA, vanHaelst-Pisani C, Hammer AM, Rowland KM, Jr, Law M, Windschitl HE, Kaur JS, Ellison N. Phase III randomized double-blind study to evaluate the efficacy of a polycarbophil-based vaginal moisturizer in women with breast cancer. J Clin Oncol. 1997;15:969–973. doi: 10.1200/JCO.1997.15.3.969. [DOI] [PubMed] [Google Scholar]
- Loprinzi CL, Kugler JW, Barton DL, Dueck AC, Tschetter LK, Nelimark RA, Balcueva EP, Burger KN, Novotny PJ, Carlson MD, et al. Phase III trial of gabapentin alone or in conjunction with an antidepressant in the management of hot flashes in women who have inadequate control with an antidepressant alone: NCCTG N03C5. J Clin Oncol. 2007;25:308–312. doi: 10.1200/JCO.2006.07.5390. [DOI] [PubMed] [Google Scholar]
- Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, Jordan VC, Carbone PP, DeMets DL. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992;326:852–856. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- Love RR, Van Dinh N, Quy TT, Linh ND, Tung ND, Shen T-Z, Hade EM, Young GS, Jarjoura D. Survival after adjuvant oophorectomy and tamoxifen in operable breast cancer in premenopausal women. J Clin Oncol. 2008;26:253–257. doi: 10.1200/JCO.2007.11.6061. [DOI] [PubMed] [Google Scholar]
- Lutchman Singh K, Davies M, Chatterjee R. Fertility in female cancer survivors: pathophysiology, preservation and the role of ovarian reserve testing. Hum Reprod Update. 2005;11:69–89. doi: 10.1093/humupd/dmh052. [DOI] [PubMed] [Google Scholar]
- MacLennan A, Lester S, Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev. 2004;18 doi: 10.1002/14651858.CD002978.pub2. CD002978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madalinska JB, van Beurden M, Bleiker EM, Valdimarsdottir HB, Hollenstein J, Massuger LF, Gaarenstroom KN, Mourits MJ, Verheijen RH, van Dorst EB, et al. The impact of hormone replacement therapy on menopausal symptoms in younger high-risk women after prophylactic salpingo-oophorectomy. J Clin Oncol. 2006;24:3576–3582. doi: 10.1200/JCO.2005.05.1896. [DOI] [PubMed] [Google Scholar]
- Maggard MA, O’Connell JB, Lane KE, Liu JH, Etzioni DA, Ko CY. Do young breast cancer patients have worse outcomes? J Surg Res. 2003;113:109–113. doi: 10.1016/s0022-4804(03)00179-3. [DOI] [PubMed] [Google Scholar]
- Malone K, Daling J, Neal C, Suter N, O’Brien C, Cushing-Haugen K, Jonasdottir T, Thompson J, Ostra E. Frequency of BRCA1/BRCA2 mutations in a population-based sample of young breast carcinoma cases. Cancer Epidemiol Biomarkers Prev. 2000;88:1393–1402. doi: 10.1002/(sici)1097-0142(20000315)88:6<1393::aid-cncr17>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Mansel R, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, Yiangou C, Horgan K, Bundred N, Monypenny I, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC trial. J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla J-P, Weaver C, Tomiak E, Al-Tweigeri T, Chap L, Juhos E, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- Mathias C, Cardeal Mendes CM, Pondé de Sena E, Dias de Moraes E, Bastos C, Braghiroli MI, Nuñez G, Athanazio R, Alban L, Moore HC, et al. An open-label, fixed-dose study of bupropion effect on sexual function scores in women treated for breast cancer. Ann Oncol. 2006;17:1792–1796. doi: 10.1093/annonc/mdl304. [DOI] [PubMed] [Google Scholar]
- McLaughlin JR, Risch HA, Lubinski J, Moller P, Ghadirian P, Lynch H, Karlan B, Fishman D, Rosen B, Neuhausen SL, et al. Reproductive risk factors for ovarian cancer in carriers of BRCA1 or BRCA2 mutations: a case–control study. Lancet Oncol. 2007;8:26–34. doi: 10.1016/S1470-2045(06)70983-4. [DOI] [PubMed] [Google Scholar]
- Metcalfe KA, Poll A, O’Connor A, Gershman S, Armel S, Finch A, Demsky R, Rosen B, Narod SA. Development and testing of a decision aid for breast cancer prevention for women with a BRCA1 or BRCA2 mutation. Clin Genet. 2007;72:208–217. doi: 10.1111/j.1399-0004.2007.00859.x. [DOI] [PubMed] [Google Scholar]
- Michels KB, Trichopoulos D, Rosner BA, Hunter DJ, Colditz GA, Hankinson SE, Speizer FE, Willett WC. Being breastfed in infancy and breast cancer incidence in adult life: results from the two nurses’ health studies. Am J Epidemiol. 2001;153:275–283. doi: 10.1093/aje/153.3.275. [DOI] [PubMed] [Google Scholar]
- Mintzer D, Glassburn J, Mason BA, Sataloff D. Breast cancer in the very young patient: a multidisciplinary case presentation. Oncologist. 2002;7:547–554. doi: 10.1634/theoncologist.7-6-547. [DOI] [PubMed] [Google Scholar]
- Moran MS, Colasanto JM, Haffty BG, Wilson LD, Lund MW, Higgins SA. Effects of breast-conserving therapy on lactation after pregnancy. Cancer J. 2005;11:399–403. doi: 10.1097/00130404-200509000-00007. [DOI] [PubMed] [Google Scholar]
- Mourits MJ, De Vries EG, Willemse PH, Ten Hoor KA, Hollema H, Van der Zee AG. Tamoxifen treatment and gynecologic side effects: a review. Obstet Gynecol. 2001;97:855–866. doi: 10.1016/s0029-7844(00)01196-0. [DOI] [PubMed] [Google Scholar]
- Nachtigall LE. Comparative study: replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178–180. doi: 10.1016/s0015-0282(16)56474-7. [DOI] [PubMed] [Google Scholar]
- Naik AM, Fey J, Gemignani M, Heerdt A, Montgomery L, Petrek J, Port E, Sacchini V, Sclafani L, VanZee K, et al. The risk of axillary relapse after sentinel lymph node biopsy for breast cancer is comparable with that of axillary lymph node dissection: a follow-up study of 4008 procedures. Ann Surg. 2004;240:462–468. doi: 10.1097/01.sla.0000137130.23530.19. discussion 468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HD, Vesco KK, Haney E, Fu R, Nedrow A, Miller J, Nicolaidis C, Walker M, Humphrey L. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295:2057–2071. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- Newman LA, Kuerer HM. Advances in breast conservation therapy. J Clin Oncol. 2005;23:1685–1697. doi: 10.1200/JCO.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Center JR, Eisman JA. Association between breast cancer and bone mineral density: the Dubbo Osteoporosis Epidemiology Study. Maturitas. 2000;36:27–34. doi: 10.1016/s0378-5122(00)00133-x. [DOI] [PubMed] [Google Scholar]
- Oktay K. Further evidence on the safety and success of ovarian stimulation with letrozole and tamoxifen in breast cancer patients undergoing in vitro fertilization to cryopreserve their embryos for fertility preservation. J Clin Oncol. 2005;23:3858–3859. doi: 10.1200/JCO.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Oktay K, Tilly J. Livebirth after cryopreserved ovarian tissue autotransplantation. Lancet. 2004;364:2091–2092. doi: 10.1016/S0140-6736(04)17541-7. [DOI] [PubMed] [Google Scholar]
- Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23:4347–4353. doi: 10.1200/JCO.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Oktay K, Hourvitz A, Sahin G, Oktem O, Safro B, Cil A, Bang H. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;a 91:3885–3890. doi: 10.1210/jc.2006-0962. [DOI] [PubMed] [Google Scholar]
- Oktay K, Oktem O, Reh A, Vahdat L. Measuring the impact of chemotherapy on fertility in women with breast cancer. J Clin Oncol. 2006;b 24:4044–4046. doi: 10.1200/JCO.2006.06.9823. [DOI] [PubMed] [Google Scholar]
- Oktem O, Oktay K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer. 2007;110:2222–2229. doi: 10.1002/cncr.23071. [DOI] [PubMed] [Google Scholar]
- Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, Kjaer M, Gadeberg CC, Mouridsen HT, Jensen MB, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- Pagani O, Goldhirsch A. Breast cancer in young women: climbing for progress in care and knowledge. Women’s Health. 2006;2:717–732. doi: 10.2217/17455057.2.5.717. [DOI] [PubMed] [Google Scholar]
- Pandya KJ, Morrow GR, Roscoe JA, Zhao H, Hickok JT, Pajon E, Sweeney T, Banerjee TK, Flynn PJ. Gabapentin for hot flashes in 420 women with breast cancer: a randomised double-blind placebo-controlled trial. Lancet. 2005;366:818–824. doi: 10.1016/S0140-6736(05)67215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, Rosenberg R, Przypyszny M, Rein A, Winer EP. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22:4174–4183. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- Partridge A, Gelber S, Gelber RD, Castiglione-Gertsch M, Goldhirsch A, Winer E. Age of menopause among women who remain premenopausal following treatment for early breast cancer: long-term results from International Breast Cancer Study Group Trials V and VI. Eur J Cancer. 2007;43:1646–1653. doi: 10.1016/j.ejca.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Petrek JA, Naughton MJ, Case LD, Paskett ED, Naftalis EZ, Singletary SE, Sukumvanich P. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. J Clin Oncol. 2006;24:1045–1051. doi: 10.1200/JCO.2005.03.3969. [DOI] [PubMed] [Google Scholar]
- Ponzone R, Biglia N, Jacomuzzi ME, Maggiorotto F, Mariani L, Sismondi P. Vaginal oestrogen therapy after breast cancer: is it safe? Eur J Cancer. 2005;41:2673–2681. doi: 10.1016/j.ejca.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol. 1996;14:78–84. doi: 10.1200/JCO.1996.14.1.78. [DOI] [PubMed] [Google Scholar]
- Prince RL. How to diagnose the presence of osteoporosis and assess the risk of fracture. Best Pract Res Clin Rheumatol. 2001;15:345–358. doi: 10.1053/berh.2001.0154. [DOI] [PubMed] [Google Scholar]
- Quinn JE, James CR, Stewart GE, Mulligan JM, White P, Chang GKF, Mullan PB, Johnston PG, Wilson RH, Harkin DP. BRCA1 mRNA expression levels predict for overall survival in ovarian cancer after chemotherapy. Clin Cancer Res. 2007;13:7413–7420. doi: 10.1158/1078-0432.CCR-07-1083. [DOI] [PubMed] [Google Scholar]
- Ragaz J, Olivotto IA, Spinelli JJ, Phillips N, Jackson SM, Wilson KS, Knowling MA, Coppin CML, Weir L, Gelmon K, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: a critical review. J Clin Oncol. 2008;26:2568–2581. doi: 10.1200/JCO.2007.13.1748. [DOI] [PubMed] [Google Scholar]
- Rapiti E, Fioretta G, Verkooijen HM, Vlastos G, Schafer P, Sappino A-P, Kurtz J, Neyroud-Caspar I, Bouchardy C. Survival of young and older breast cancer patients in Geneva from 1990 to 2001. Eur J Cancer. 2005;41:1446–1452. doi: 10.1016/j.ejca.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Ravdin PM, Siminoff LA, Davis GJ, Mercer MB, Hewlett J, Gerson N, Parker HL. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, van’t Veer L, Garber JE, Evans GR, Narod SA, Isaacs C, Matloff E, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2004;22:1055–1062. doi: 10.1200/JCO.2004.04.188. [DOI] [PubMed] [Google Scholar]
- Rebbeck TR, Friebel T, Wagner T, Lynch HT, Garber JE, Daly MB, Isaacs C, Olopade OI, Neuhausen SL, van’t Veer L, et al. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2005;23:7804–7810. doi: 10.1200/JCO.2004.00.8151. [DOI] [PubMed] [Google Scholar]
- Reichman BS, Green KB. Breast cancer in young women: effect of chemotherapy on ovarian function, fertility, and birth defects. J Natl Cancer Inst Monogr. 1994;16:125–129. [PubMed] [Google Scholar]
- Ries L, Melbert D, Krapcho M, Stinchcomb D, Howlader N, Horner M, Mariotto A, Miller B, Feuer E, Altekruse S, et al. Natl Cancer Inst. Bethesda, MD: 2008. SEER Cancer Statistics Review, 1975–2005. http://seer.cancer.gov/csr/1975_2005/ (based on November 2007 SEER data submission, posted to the SEER web site). [Google Scholar]
- Ross JS, Hatzis C, Symmans WF, Pusztai L, Hortobagyi GN. Commercialized multigene predictors of clinical outcome for breast cancer. Oncologist. 2008;13:477–493. doi: 10.1634/theoncologist.2007-0248. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Rottenberg S, Nygren AOH, Pajic M, van Leeuwen FWB, van der Heijden I, van de Wetering K, Liu X, de Visser KE, Gilhuijs KG, van Tellingen O, et al. Selective induction of chemotherapy resistance of mammary tumors in a conditional mouse model for hereditary breast cancer. Proc Natl Acad Sci USA. 2007;104:12117–12122. doi: 10.1073/pnas.0702955104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchini V, Pinotti JA, Barros ACSD, Luini A, Pluchinotta A, Pinotti M, Boratto MG, Ricci MD, Ruiz CA, Nisida AC, et al. Nipple-sparing mastectomy for breast cancer and risk reduction: oncologic or technical problem? J Am Coll Surg. 2006;203:704–714. doi: 10.1016/j.jamcollsurg.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Sambrook PN, Eisman JA. Osteoporosis prevention and treatment. Med J Aust. 2000;172:226–229. [PubMed] [Google Scholar]
- Sankila R, Heinavaara S, Hakulinen T. Survival of breast cancer patients after subsequent term pregnancy: ‘healthy mother effect. Am J Obstet Gynecol. 1994;170:818–823. doi: 10.1016/s0002-9378(94)70290-x. [DOI] [PubMed] [Google Scholar]
- Santoro N. What a SWAN can teach us about menopause. Contemp Obstet/Gynecol. 2004;49:69–79. [Google Scholar]
- Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–2746. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- Schover LR. Sexuality and body image in younger women with breast cancer. J Natl Cancer Inst Monogr. 1994;16:177–182. [PubMed] [Google Scholar]
- Schulze T, Mucke J, Markwardt J, Schlag PM, Bembenek A. Long-term morbidity of patients with early breast cancer after sentinel lymph node biopsy compared to axillary lymph node dissection. J Surg Oncol. 2006;93:109–119. doi: 10.1002/jso.20406. [DOI] [PubMed] [Google Scholar]
- Schwartz GF, Veronesi U, Clough KB, Dixon JM, Fentiman IS, Heywang-Kobrunner SH, Holland R, Hughes KS, Mansel RE, Margolese R, et al. Breast J; Consensus conference on breast conservation; April 28–May 1, 2005; Milan, Italy. 2006. pp. 398–407. [DOI] [PubMed] [Google Scholar]
- Sestak I, Kealy R, Edwards R, Forbes J, Cuzick J. Influence of hormone replacement therapy on tamoxifen-induced vasomotor symptoms. J Clin Oncol. 2006;24:3991–3996. doi: 10.1200/JCO.2005.04.3745. [DOI] [PubMed] [Google Scholar]
- Shapiro CL, Manola J, Leboff M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J Clin Oncol. 2001;19:3306–3311. doi: 10.1200/JCO.2001.19.14.3306. [DOI] [PubMed] [Google Scholar]
- Smith KR, Ellington L, Chan AY, Croyle RT, Botkin JR. Fertility intentions following testing for a BRCA1 gene mutation. Cancer Epidemiol Biomarkers Prev. 2004;13:733–740. [PubMed] [Google Scholar]
- Sonmezer M, Oktay K. Fertility preservation in female patients. Hum Reprod Update. 2004;10:251–266. doi: 10.1093/humupd/dmh021. [DOI] [PubMed] [Google Scholar]
- Sonmezer M, Oktay K. Fertility preservation in young women undergoing breast cancer therapy. Oncologist. 2006;11:422–434. doi: 10.1634/theoncologist.11-5-422. [DOI] [PubMed] [Google Scholar]
- Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, Hayes DF, Desta Z, Flockhart DA. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;4 doi: 10.1002/14651858.CD001500.pub2. CD001500. [DOI] [PubMed] [Google Scholar]
- Sverrisdottir A, Fornander T, Jacobsson H, von Schoultz E, Rutqvist LE. Bone mineral density among premenopausal women with early breast cancer in a randomized trial of adjuvant endocrine therapy. J Clin Oncol. 2004;22:3694–3699. doi: 10.1200/JCO.2004.08.148. [DOI] [PubMed] [Google Scholar]
- Swain SM, Land SR, Sundry R, Ritter M, Costantino J, Wolmark N, Ganz PA. Amenorrhea in premenopausal women on the doxorubicin (A) and cyclophosphamide (C)-> docetaxel (T) arm of NSABP B-30: Preliminary results. J Clin Oncol. 2005;23:13S–13S. [Google Scholar]
- Tewari K, Bonebrake RG, Asrat T, Shanberg AM. Ambiguous genitalia in infant exposed to tamoxifen in utero. Lancet. 1997;350:183. doi: 10.1016/S0140-6736(97)24029-8. [DOI] [PubMed] [Google Scholar]
- Tham YL, Sexton K, Weiss H, Elledge R, Friedman LC, Kramer R. The rates of chemotherapy-induced amenorrhea in patients treated with adjuvant doxorubicin and cyclophosphamide followed by a taxane. Am J Clin Oncol. 2007;30:126–132. doi: 10.1097/01.coc.0000251398.57630.4f. [DOI] [PubMed] [Google Scholar]
- Thewes B, Meiser B, Taylor A, Phillips KA, Pendlebury S, Capp A, Dalley D, Goldstein D, Baber R. Fertility- and menopause-related information needs of younger women with a diagnosis of early breast cancer. J Clin Oncol. 2005;23:5155–5165. doi: 10.1200/JCO.2005.07.773. [DOI] [PubMed] [Google Scholar]
- Tralins AH. Lactation after conservative breast surgery combined with radiation therapy. Am J Clin Oncol. 1995;18:40–43. doi: 10.1097/00000421-199502000-00008. [DOI] [PubMed] [Google Scholar]
- Trinh XB, Tjalma WAA, Makar AP, Buytaert G, Weyler J, van Dam PA. Use of the levonorgestrel-releasing intrauterine system in breast cancer patients. Fertil Steril. 2008;90:17–22. doi: 10.1016/j.fertnstert.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Truong PT, Olivotto IA, Kader HA, Panades M, Speers CH, Berthelet E. Selecting breast cancer patients with T1-T2 tumors and one to three positive axillary nodes at high postmastectomy locoregional recurrence risk for adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1337–1347. doi: 10.1016/j.ijrobp.2004.08.009. [DOI] [PubMed] [Google Scholar]
- van Leeuwen FE, Benraadt J, Coebergh JW, Kiemeney LA, Gimbrere CH, Otter R, Schouten LJ, Damhuis RA, Bontenbal M, Diepenhorst FW, et al. Risk of endometrial cancer after tamoxifen treatment of breast cancer. Lancet. 1994;343:448–452. doi: 10.1016/s0140-6736(94)92692-1. [DOI] [PubMed] [Google Scholar]
- Velentgas P, Daling JR, Malone KE, Weiss NS, Williams MA, Self SG, Mueller BA. Pregnancy after breast carcinoma: outcomes and influence on mortality. Cancer. 1999;85:2424–2432. doi: 10.1002/(sici)1097-0142(19990601)85:11<2424::aid-cncr17>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, Intra M, Veronesi P, Maisonneuve P, Gatti G, et al. Sentinel-lymph-node biopsy as a staging procedure in breast cancer: update of a randomised controlled study. Lancet Oncol. 2006;7:983–990. doi: 10.1016/S1470-2045(06)70947-0. [DOI] [PubMed] [Google Scholar]
- von Schoultz E, Rutqvist LE. Menopausal hormone therapy after breast cancer: the Stockholm randomized trial. J Natl Cancer Inst. 2005;97:533–535. doi: 10.1093/jnci/dji071. [DOI] [PubMed] [Google Scholar]
- Voogd AC, Nielsen M, Peterse JL, Blichert-Toft M, Bartelink H, Overgaard M, van Tienhoven G, Andersen KW, Sylvester RJ, van Dongen JA, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: pooled results of two large European randomized trials [erratum appears in J Clin Oncol 2001 May 1; 19(9):2583] J Clin Oncol. 2001;19:1688–1697. doi: 10.1200/JCO.2001.19.6.1688. [DOI] [PubMed] [Google Scholar]
- Vrieling C, Collette L, Fourquet A, Hoogenraad WJ, Horiot JC, Jager JJ, Bing Oei S, Peterse HL, Pierart M, Poortmans PM, et al. Can patient-, treatment- and pathology-related characteristics explain the high local recurrence rate following breast-conserving therapy in young patients? Eur J Cancer. 2003;39:932–944. doi: 10.1016/s0959-8049(03)00123-0. [DOI] [PubMed] [Google Scholar]
- Weiss SM, Wengert PA, Jr, Martinez EM, Sewall W, Kopp E. Patient satisfaction with decision-making for breast cancer therapy. Ann Surg Oncol. 1996;3:285–289. doi: 10.1007/BF02306284. [DOI] [PubMed] [Google Scholar]
- Weitzel J, McCaffrey S, Nedelcu R, MacDonald D, Blazer K, Cullinane C. Effect of genetic cancer risk assessment on surgical decisions at breast cancer diagnosis. Arch Surg. 2003;138:1323–1328. doi: 10.1001/archsurg.138.12.1323. discussion 1329. [DOI] [PubMed] [Google Scholar]
- White JR, Halberg FE, Rabinovitch R, Green S, Haffty BG, Solin LJ, Strom EA, Taylor ME, Edge SB. American College of Radiology appropriateness criteria on conservative surgery and radiation: stages I and II breast carcinoma. J Am Coll Radiol. 2008;5:701–713. doi: 10.1016/j.jacr.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Wierman ME, Basson R, Davis SR, Khosla S, Miller KK, Rosner W, Santoro N. Androgen therapy in women: an Endocrine Society Clinical Practice guideline. J Clin Endocrinol Metab. 2006;91:3697–3710. doi: 10.1210/jc.2006-1121. [DOI] [PubMed] [Google Scholar]
- Yang SH, Yang KH, Li YP, Zhang YC, He XD, Song AL, Tian JH, Jiang L, Bai ZG, He LF, et al. Breast conservation therapy for stage I or stage II breast cancer: a meta-analysis of randomized controlled trials. Ann Oncol. 2008;19:1039–1044. doi: 10.1093/annonc/mdm573. [DOI] [PubMed] [Google Scholar]
- Zhou P, Recht A. Young age and outcome for women with early-stage invasive breast carcinoma. Cancer. 2004;101:1264–1274. doi: 10.1002/cncr.20523. [DOI] [PubMed] [Google Scholar]