Abstract
Background
Herpes simplex virus (HSV) remains latent in nerve root ganglia of infected persons and is thought to reactivate several times yearly. Recent in situ data show the localization of HSV-specific CD8+ T cells at the dermal epidermal junction next to peripheral sensory nerve endings, suggesting that viral reactivation may occur more frequently than previously appreciated.
Methods
Twenty-five HSV-2–seropositive and 18 HSV-1–seropositive healthy adults collected anogenital and oral swabs, respectively, 4 times per day for 60 days. Swabs were assayed for HSV, using a quantitative polymerase chain reaction assay.
Results
Twenty-four percent of anogenital reactivations and 21% of oral reactivations lasted ≤6 h, and 49% of anogenital reactivations and 39% of oral reactivations lasted ≤12 h. Lesions were reported in only 3 (7%) of 44 anogenital reactivations and 1 (8%) of 13 oral reactivations lasting ≤12 h. The median HSV DNA levels at initial and last detection were 103.5 and 103.3 copies/mL, respectively, during anogenital reactivation and 103.7 and 103.0 copies/mL, respectively, during oral reactivation.
Conclusions
This high frequency of short subclinical HSV reactivation in immunocompetent hosts strongly suggests that the peripheral mucosal immune system plays a critical role in clearing HSV reactivations.
Seventeen percent of the US adult population is seropositive for herpes simplex virus type 2 (HSV-2) and 58% are seropositive for HSV-1, indicating chronic infection with the viruses that cause genital and oral herpes [1]. HSV acquired from sexual exposure infects and remains latent in the sacral ganglia [2], and HSV acquired nonsexually usually infects and remains latent in the trigeminal ganglia. Intermittent reactivations can be clinical, causing typical herpetic genital or oral lesions, or subclinical, causing asymptomatic viral shedding [3]. Studies in the past decade have highlighted the importance of subclinical reactivation in the transmission of genital herpes to sexual partners and newborns [4, 5]. With daily genital mucosa sampling, HSV-2 shedding as detected by polymerase chain reaction (PCR) is observed on 12%–25% of days; ~60% of episodes are subclinical [6–8]. With daily oral mucosal sampling, HSV-1 shedding as detected by PCR is observed on 5%–9% of days [9, 10]. Recent studies have raised important questions about the mechanism and frequency of HSV reactivation in humans. Mathematical modeling of daily genital shedding patterns in a cohort of women with genital herpes suggested that shedding episodes of several days duration may be caused by multiple short overlapping HSV reactivations rather than by single ganglionic HSV reactivations [11, 12]. In addition, recent immunohistologic studies have shown the persistence of HSV-2–specific T cells in genital skin contiguous to sensory neuronal nerve endings, suggesting that peripheral mucosal immune responses may help rapidly clear ganglionic HSV reactivations [13]. The studies described above suggest that the frequency of mucosal HSV reactivation may be underestimated and that the typical reactivation duration may be overestimated. We designed a study to determine whether frequent short subclinical bursts of mucosal HSV reactivations occur, and, if so, to determine their frequency, duration, and pattern.
SUBJECTS, MATERIALS, AND METHODS
Study participants and procedures
During 2004–2007, HSV-2–seropositive participants were asked to collect anogenital swab specimens and HSV-1–seropositive participants were asked to collect oral swab specimens for HSV DNA PCR 4 times/ day at home for 60 days. All participants were HIV-negative healthy men and women aged ≥18 years. Participants were recruited by word of mouth and advertising and were enrolled if they met the serologic criteria described above and could comply with the intensive study protocol. Three participants who were both HSV-1 and HSV-2 seropositive collected both anogenital and oral swab specimens. Participants took no antiviral medication for the study duration and collected swab specimens at ~6-h intervals upon awakening, in the midmorning, in the afternoon, and at bedtime and recorded in a diary the exact swabbing time and any symptoms present. Anogenital swabs were obtained by rubbing a polyester fiber–tipped swab across the surface of the penile and perianal areas (in that order) for men or across the posterior cervical/vaginal, vulvar, and perianal areas (in that order) for women. Oral swabs were performed by rubbing a polyester fiber–tipped swab across the buccal mucosa and tongue. A separate swab was collected from any lesions present. These sampling and collection methods were identical to those used in our previous studies of daily sampling, except that sampling frequency was increased [6, 7, 10]. The reliability of self-collected swab specimens as a measure of detecting HSV reactivations is similar to that of clinician-collected swabs [3]. Participants were seen in the clinic every 2 weeks for collection of samples and diary review. These studies were approved by the University of Washington institutional review board, and all participants gave written informed consent.
Laboratory methods
HSV serologic testing was performed by Western blot [14]. Swabs were placed into vials containing 1 mL of PCR transport medium and refrigerated until laboratory processing. HSV DNA was detected using a quantitative PCR assay, and the HSV DNA level was expressed as copies per milliliter of transport medium [15, 16]. The initial PCR assay uses type-common primers to the HSV gene encoding glycoprotein B. Positive samples were subsequently analyzed using type-specific primers to examine whether the DNA detected was that of HSV-1, HSV-2, or both [15, 17]. An internal control was included in the PCR reaction to ensure that HSV-negative findings were not due to inhibition. Samples were considered positive for HSV if we detected ≥3 copies of HSV DNA per 20 µL of specimen (i.e.,≥150 copies of HSV DNA per mL of transport media) [16]. Laboratory personnel were blinded to clinical data.
Statistical analysis
HSV anogenital shedding was considered to have occurred if an anogenital or lesion sample at a given time was positive for HSV, and oral shedding was considered to have occurred if an oral or lesion sample at a given time was positive for HSV. If both samples were positive for HSV, the sample with the higher HSV DNA copy number was used in further analyses. Shedding rates were calculated as the number of swab specimens with HSV DNA detected divided by the total number of swab specimens collected. A shedding episode of known duration was defined as a series of HSV-positive swab specimens that were collected immediately before and after at least 2 HSV-negative swab specimens [18]. Since not all periods had swab specimens available, some subjects shed HSV for an unknown period. These are referred to as shedding episodes of uncertain duration, because the episodes may have been longer than observed. Any shedding episode (of known or uncertain duration) could include 1 missing or 1 HSV-negative swab specimen within the episode.
To compute episode duration, we estimated start and stop times for shedding. At times when sampling was consistent, start times were estimated as the chronological midpoint between the last HSV-negative and first HSV-positive swab specimen, and stop times were estimated as the midpoint between last HSV-positive and first HSV-negative swab specimen. For shedding episodes of uncertain duration, we assumed missing swab specimens at 2 time points before and 2 time points after the positive swab specimen(s) were HSV negative, and we estimated start and stop times as described above. We compared duration and median HSV DNA level detected between episodes of known and uncertain length to assess potential bias in our interpolation method.
To compare the calculated percentage of time shedding that would have been obtained if only the first morning swab specimen had been collected instead of all 4 daily swab specimens, we calculated the total hours of shedding in each group as above, first using results for only the first morning swab specimen and then using results for all 4 daily swab specimens. We then divided this value by the total hours of follow-up, defined as the interval between collection of the first and final swab specimens. To calculate a reactivation rate per 30 days, we counted all episodes (certain and uncertain length) in the numerator and used follow-up time as defined above in the denominator. Generalized estimating equation models were used to test for significant associations between episode duration or mean HSV DNA level at onset of shedding and other factors. Median values are reported because they are more robustly resistant to the effect of outliers. The Wilcoxon rank-sum test was used to compare median shedding rates and numbers of shedding episodes of certain duration between men and women.
RESULTS
Twenty-five participants who performed genital swabbing and 18 who performed oral swabbing collected samples for a median of 61 days (range, 5–73 days), with 38 participants (88%) collecting samples for ≥30 days, 34 (79%) for ≥50 days, and 32 (74%) for ≥60 days (table 1). Twenty-one participants (84%) in the genital swabbing group and 15 (83%) in the oral swabbing group had ≥1 sample in which HSV was detected.
Table 1.
Demographic and clinical characteristics of study subjects who performed genital and/or oral swabbing for detection of herpes simplex virus (HSV).
| Baseline characteristic | Genital swabbing group (n = 25) |
Oral swabbing group (n = 18) |
|---|---|---|
| Age, years | 44 (24–66) | 43 (28–75) |
| Sex | ||
| Male | 10 (40) | 12 (67) |
| Female | 15 (60) | 6 (33) |
| Race/ethnicity | ||
| African American | 3 (12) | 0 |
| Asian | 0 | 3 (17) |
| Native American | 1 (4) | 0 |
| White | 21 (84) | 12 (67) |
| Multiracial | 0 | 3 (17) |
| Genital herpes history | ||
| Any | 19 (76) | 8 (44) |
| Recurrences in past 6 months, no.a | 2.3 (0–6) | 1.4 (1–3) |
| Oral herpes history | ||
| Any | 5 (20) | 14 (78) |
| Recurrences in past 6 months, no.b | 0 | 0.75 (0–1) |
| HSV serostatus | ||
| HSV-1 and HSV-2 | 13 (52) | 7 (39) |
| HSV-1 only | 0 | 11 (61) |
| HSV-2 only | 12 (48) | 0 |
NOTE. Data are no. (%) of study subjects or mean value (range). Three subjects participated in both studies
Data are for subjects with a history of genital herpes.
Data are for subjects with a history of oral herpes.
Genital samples
Genital samples were collected on 1287 days and at 4706 time points. HSV DNA was detected on 246 days (19%) and at 640 time points (14%). HSV typing results were available for samples collected at 572 of these 640 time points. An additional 63 samples were assumed to contain HSV-2 because the participant was HSV-2 seropositive only, and the copy number in the remaining 5 samples (0.8%) was too low for typing. HSV-2 alone was found in 598 samples (93.4%), both HSV-1 and HSV-2 in 25 (3.9%), and HSV-1 alone in 12 (1.9%).
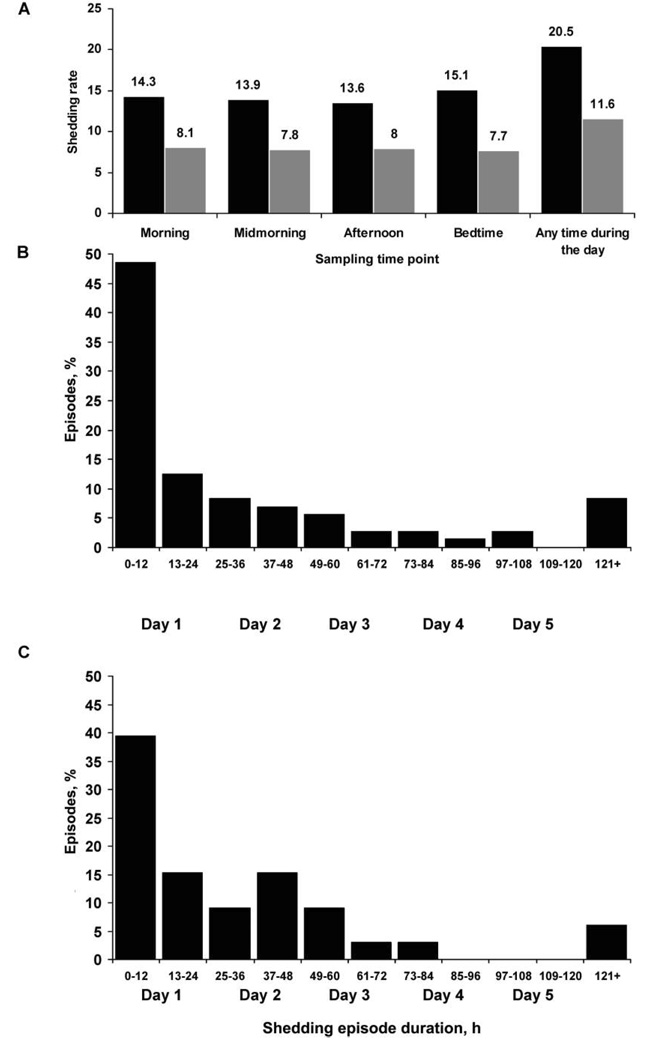
Genital samples were collected at all 4 time points on 962 days (75%) and at 3 of 4 time points on 232 days (18%); thus, ≥3 genital samples were collected on 1194 days (93%). We initially analyzed data from the 962 days on which all 4 samples were collected. Overall, HSV DNA was detected on 197 (20%) of 962 days, including 94 days (9.8%) on which HSV DNA was detected during all 4 time points and 56 days (5.8%), 25 days (2.6%), and 22 days (2.3%) on which HSV DNA was detected at 1, 2, and 3 time points, respectively. The detection of HSV DNA was not influenced by the time of collection (figure 1A).
Figure 1.
Rates of herpes simplex virus shedding for 72 genital and 33 oral episodes of known duration. A, Rates of genital shedding (black) and oral shedding (grey), by sampling time point. B, Duration of genital shedding episodes. C, Duration of oral shedding episodes.
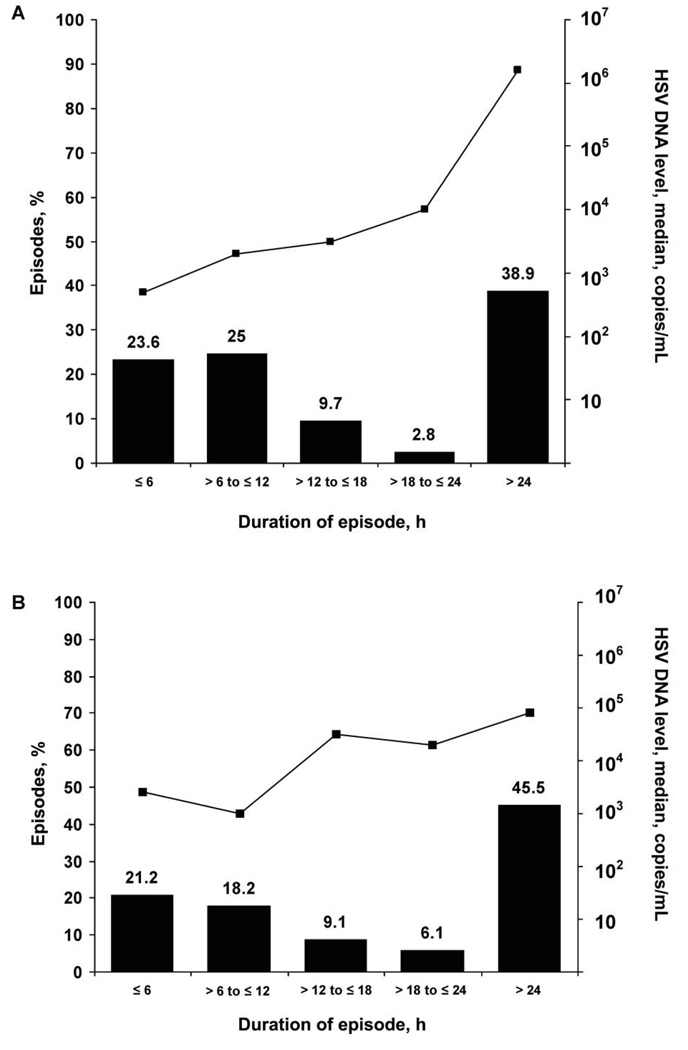
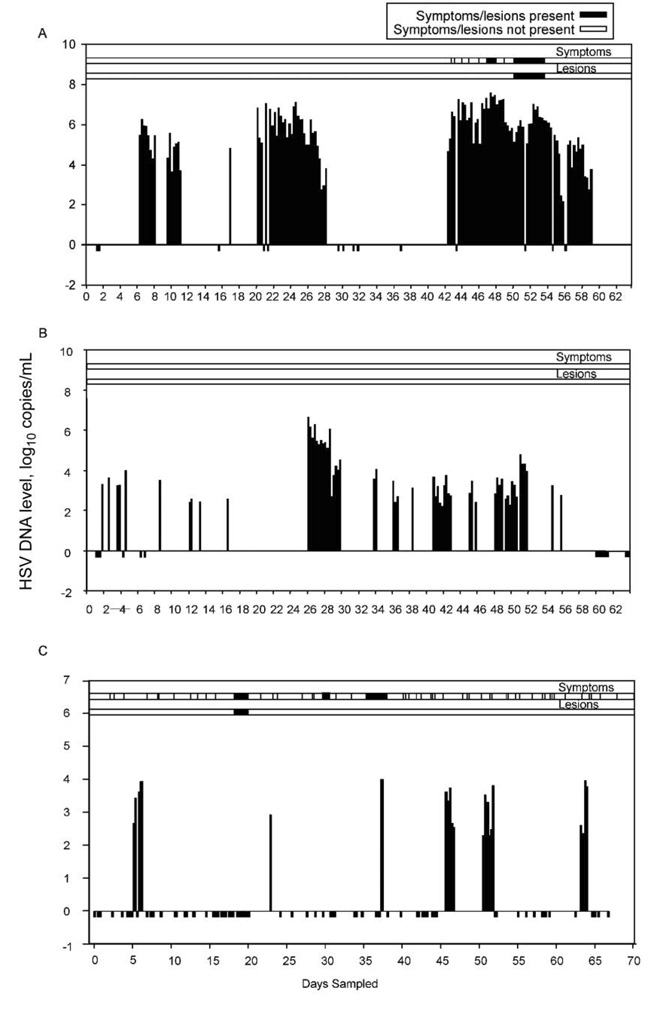
We identified 109 separate episodes of genital HSV shedding in 21 participants. For 72 episodes (66%), the duration of genital shedding was known with certainty because sampling was performed on all 4 time points/day. The median duration of genital HSV reactivation among episodes for which the duration of shedding was known was 13 h (range, 4 h to 17 days) (table 2), and the estimated duration of reactivation among 37 episodes for which the duration of shedding was uncertain was 11 h (range, 2 h to 10 days) (P = .9). Of the 72 genital shedding episodes of known duration, 35 (49%) lasted ≤12 h (figure 1B). The median maximum HSV DNA level detected during an episode increased with episode duration (figure 2A). Seven participants (28%) had ≥1 genital shedding episode that lasted ≤6 h, and 14 (56%) had ≥1 that lasted ≤18 h. Of the 72 genital shedding episodes of known duration, 61 were HSV-2 episodes (median duration, 14 h [range, 4 h to 17 days]), 6 were HSV-1 episodes (median duration, 6 h [range, 5–7 h]), 2 included shedding of both HSV-1 and HSV-2, and 3 were untypeable. The participant with the 17-day HSV-2 shedding episode had recently received a diagnosis of genital herpes (although the participant was seropositive for both HSV-1 and HSV-2) and had the highest shedding rate in the study (48% of genital swab specimens collected had HSV detected). The 17-day shedding episode consisted of 7 days of intermittent pruritic prodrome followed by 5 days of vulvar lesions and 5 days of asymptomatic shedding. In addition to this 17-day episode, she had 3 asymptomatic HSV-2 shedding episodes lasting 42 h, 48 h, and 8 days, and one 7-h asymptomatic genital HSV-1 shedding episode (figure 3).
Table 2.
Characteristics of herpes simplex virus (HSV) shedding episodes.
| Characteristic | Genital shedding episodes (n = 72) |
P | Oral shedding episodes (n = 33) |
P |
|---|---|---|---|---|
| HSV reactivation duration | ||||
| Overall, median (range) | 13 h (4 h to 17 days) | 24 h (4 h to 12 days) | ||
| ≤12 h, no. (%), episodes | 35 (49) | 13 (39) | ||
| ≤6 h, no. (%), episodes | 17 (24) | 7 (21) | ||
| HSV level, median (range), copies/mL | ||||
| At episode onset | 103.5 (102.2–107.5) | 103.7 (102.2–106.1) | ||
| In last positive sample | 103.3 (102.2–105.8) | 103.0 (102.4–105.6) | ||
| HSV level at episode onset, median, copies/mL | ||||
| By episode duration | <.001 | .14 | ||
| >12 h | 104.2 | 103.9 | ||
| ≤12 h | 103.1 | 103.1 | ||
| By sex | <.0001 | .95 | ||
| Women | 104.5 | 103.6 | ||
| Men | 103.2 | 103.7 |
NOTE. Data are for reactivations during which swab samples were obtained at 4 time points daily (shedding episodes of known duration). Twenty-five subjects collected genital swab specimens and 18 subjects collected oral swab specimens, including 3 subjects who collected both genital and oral swab specimens.
Figure 2.
Herpes simplex virus levels for 72 genital and 33 oral episodes of known duration, by durations of genital (A) and oral (B) shedding episodes.
Figure 3.
Representative herpes simplex virus (HSV) shedding patterns for 3 participants. Negative values indicate missing samples. A, Data for a 27-year-old woman, HSV-1 and HSV-2 seropositive, with genital herpes diagnosed just before study entry. All pictured genital reactivations are HSV-2 except the 7-h reactivation on day 16, during which HSV-1 was detected. B, Data for a 50-year-old man, HSV-1 and HSV-2 seropositive, with genital herpes diagnosed 25 years before study entry. All pictured genital reactivation episodes are HSV-2, except 1 swab specimen collected at 1 time point on day 41, had both HSV-1 and HSV-2 detected. C, Data for a 35-year-old man, HSV-1 seropositive, with oral herpes diagnosed 5 years before study entry.
Oral samples
Oral samples were collected on 1045 days and at 3651 time points. HSV DNA was detected on 98 (9%) days and at 254 (7%) time points. HSV-1 alone was found in 253 samples (99.6%) and HSV-2 alone in 1 sample (0.4%).
Oral samples were collected at all 4 time points on 691 days (66%) and at 3 of 4 time points on 218 days (21%); thus, ≥3 daily oral samples collected were collected on 909 days (87%). We initially analyzed data from 691 days on which samples at all 4 time points were collected. Overall, HSV DNA was detected on 80 (12%) of these 691 days. The detection of HSV DNA on oral mucosa was also not influenced by collection time (figure 1A).
We identified 43 separate episodes of oral HSV shedding, of which 33 were of known duration. Thirty-two of these 33 episodes had HSV-1 detected. The median duration of an oral HSV reactivation episode during which samples at all 4 time points were collected daily was 24 h (range, 4 h to 12 days) (table 2). Oral shedding episodes with a duration of ≤12 h occurred in 13 (39%) of 33 episodes (figure 1C). Six participants (43%) had ≥1 oral episode that lasted ≤6 h, and 10 (71%) had ≥1 episode that lasted ≤18 h. As with genital herpes, the median maximum HSV-1 DNA level detected during an oral-labial shedding episode increased with episode duration (figure 2B). The single episode of oral HSV-2 shedding lasted 7 h.
Associations between shedding and symptoms
Representative patterns of reactivation are shown in figure 3. Shorter genital shedding episodes were less likely to be symptomatic than longer ones. Only 3 genital shedding episodes (7%) lasting ≤24 h were associated with reported genital lesions, compared with 8 genital episodes (29%) lasting >24 h (P = .028). Similarly, only 5 genital episodes (11%) lasting ≤24 h were associated with reported genital symptoms, compared with 9 genital episodes (32%) lasting >24 h (P = .027). The median HSV DNA level was higher for swab specimens taken directly from lesions (105.9 copies/mL [range, 100–108.1 copies/mL]) than for swab specimens obtained at the same time from sampling the entire anogenital area (104.2 copies/mL [range, 100–107.3 copies/mL]). The median number of genital shedding episodes lasting <12 h was greater among men (1.5 episodes [range, 0–7 episodes)] than among women (0 episodes [range, 0–2 episodes]; P = .06). Only 2 oral reactivations, both in the same person, were accompanied by lesions; both reactivations involved classic labial lesions. Two additional oral reactivations in another person were associated with oral tingling but had no definable ulcerations.
HSV reactivation rates as measured by 4 times daily sampling
The median number of HSV reactivations of known duration per person among subjects who shed during the 60-day sampling period was 3 genital reactivations (range, 1–14 reactivations) and 3 oral reactivations (range, 1–4 reactivations). The median genital HSV reactivation rate was 1.5 reactivations per 30 days (range, 0–10.7 reactivations per 30 days), or 18 reactivations annually, compared with 0.5 reactivations per 30 days and 6 reactivations annually if calculated from once daily morning sampling. Similarly, the median oral HSV reactivation rate was 1.4 reactivations per 30 days (range, 0–3.0 reactivations per 30 days), or 16.2 reactivations annually, compared with 0.9 reactivations per 30 days and 10.8 reactivations annually, if calculated from once daily morning sampling. These rates are 3 times higher for HSV-2 and 1.5 times higher for HSV-1, compared with studies using once daily sampling.
DISCUSSION
Our study demonstrates several new concepts about HSV infection. HSV reactivation has both a more frequent onset and more rapid clearance than previously appreciated. Collection of samples 4 times daily revealed that approximately half of HSV mucosal reactivations last ≤12 h and that these short reactivation episodes are largely asymptomatic, characterized by rapid emergence of 103–104 copies/mL of HSV DNA in the skin or mucosa, and accompanied by rapid viral clearance by the host. Prolonged genital shedding was associated with a higher initial viral level and a greater likelihood of symptoms and lesions. Median genital HSV reactivation frequency was 18 episodes annually, 81% of genital mucosal HSV reactivations were subclinical, and half lasted <12 h. Only 19% of genital shedding episodes were associated with symptoms and 15% with overt genital lesions, illustrating the importance of subclinical HSV reactivation in the biology of HSV reactivation. Similarly, reported oral ulcerations accompanied only 2 of 33 oral HSV reactivations of known duration.
Our data raise several issues pertinent to the pathogenesis of HSV reactivation. Most samples were collected from a large surface area and placed into 1 mL of viral transport solution. Although this method of collection is consistent and reproducible, HSV reactivations are exquisitely anatomically localized [13]. Hence, the in vivo number of HSV virions released from subclinical ulcerations is undoubtedly markedly higher then the 103–104 copies/mL we detected. This hypothesis is supported by the higher viral level found from swab specimens of identifiable lesions than samples collected at the same time from the entire anogenital area (105.9 vs. 104.2 copies/mL). Rapid HSV clearance within 6–12 h after shedding appearance illustrates prompt and effective immunocompetent host defense mechanisms. Prior work has shown that HSV-2 clearance from genital lesions is associated with HSV-2–specific cytotoxic T cell activity in the CD8+ fraction of T lymphocytes [19–21]. HSV-specific CD8+ T cells have also been found in ganglia, suggesting that some control occurs in ganglia [22–26]. However, when control at the ganglia fails and virus travels via anterograde transport to genital mucosa, the fact that mucosal replication is effectively eliminated within hours after appearance suggests that host T cells must either move extremely rapidly to the mucosal site of replication or remain in the genital mucosa between recurrences to rapidly control and eliminate HSV mucosal replication when virus first appears from peripheral nerves. Recent immunohistologic studies suggest that HSV-2–specific CD8+ T cells can persist in genital skin for extended periods and are associated with localized clearance of subclinical HSV-2 reactivation [13]. The rapid host elimination of HSV-2 suggests that much HSV-2 control rests within the peripheral mucosal immune system.
Our study also helps explain the large body of data implicating HSV infections as an important factor in HIV acquisition. The rapid reactivation, release, and resolution of relatively high copy numbers of HSV DNA in genital mucosa may help explain the high rate of sexual transmission of HSV-2 and the increased risk that HSV-2 confers in HIV acquisition [27–29]. Frequent, short, subclinical genital mucosal reactivations place large numbers of activated CD4+ T lymphocytes at risk for HIV infection at the genital mucosa [20]. If subclinical ulcerations are also present during these reactivations, then they would provide portals of entry for HIV, further enhancing the risk owing to increased numbers of CD4+ T lymphocytes at the mucosa. Our findings also put into perspective the use of episodic therapy for mucosal HSV infection. Although effective in relieving the discomfort of individual episodes [30–32], such an approach treats only a small fraction of reactivations. Daily suppressive antiviral therapy can reduce genital HSV-2 shedding, as detected by once daily sampling, by 70%–85% of days but reduces HSV-2 sexual transmission by only 48% [33]. This disparity between antiviral effects on shedding and transmission may suggest that once daily antiviral therapy does not eliminate short reactivations.
Participants included in these studies were persons well versed in the signs and symptoms of genital and oral herpes. Hence, the ratio of subclinical to clinical ulcerations among HSV-2–infected persons in the general population is probably even higher than the 85% ratio found in our study, as recognition of lesions is likely lower. Frequent sampling is difficult for participants and results in a large number of samples for the laboratory. We collected and performed PCR analysis on >8300 separate samples from 43 participants. The genital shedding rate we found among our 25 HSV-2–infected participants is comparable to historical shedding rates among our previously studied HSV-2–infected patients; among 352 participants (145 men and 207 women) in previous genital HSV-2 shedding studies, once-daily samples were PCR positive on 2740 (23.1%) of 11,838 days, with samples collected on a median of 57 days per person. Thus, data from the present study are likely representative of immunocompetent persons with oral and genital HSV infections in the studied age range. Significant individual variability in HSV shedding frequency is seen, and age, sex, immune status, host genetics, and number and density of recurrences in dorsal root ganglia appear to affect the severity of HSV reactivation [10, 34–37]. Whether rapidly cleared episodes of mucosal HSV are seen in immune-suppressed patients remains to be determined. The high combined rate of HSV-1 and HSV-2 reactivation we observed among immunocompetent persons, if subsequently demonstrated among HIV-infected persons, could provide an explanation for how HSV increases plasma HIV level [38, 39], as most HIV-infected persons worldwide have HSV-1 and HSV-2 infections.
In summary, our data indicate that the frequency of mucosal HSV reactivation and the pace of clearance are much faster than previously appreciated. The most common form of HSV reactivation is an asymptomatic reactivation associated with rapid onset and clearance of virus within 12 h, illustrating a dynamic interaction between virus and host in the peripheral skin and mucosa. These short subclinical reactivations help explain the observations that most HSV-2 transmission events occur during subclinical reactivations and that clinical disease manifestations predict neither mother-to-child nor sexual transmission. The frequency of these reactivations also provide a possible explanation for how incident and prevalent HSV-2 infections increase both the risk of HIV acquisition and the HIV level in coinfected persons.
Acknowledgments
Financial support: National Institutes of Health/National Institute of Allergy and Infectious Diseases (grants P01 AI30731, R37 AI42528, K23 AI071257, K24 AI071113, and T32 AI07044).
Footnotes
Potential conflicts of interest: A.W. has received grant support from GSK, Antigenics, 3M, Roche, and Vical; has been a consultant for Novartis, Powdermed, and Medigene; and has been a speaker for Merck Vaccines. L.C. has done consulting for Antigenics. All other authors: no conflicts.
Presented in part: International Herpesvirus Workshop, Seattle, WA, July 2006 (abstract 2.44); Annual Meeting of the Infectious Diseases Society of America, Toronto, Canada, October 2006 (abstract 699); Biennial Meeting of the International Society of Sexually Transmitted Diseases Research, Seattle, WA, July 2007 (abstract O-030).
References
- 1.Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 2.Croen KD, Ostrove JM, Dragovic L, Straus SE. Characterization of herpes simplex virus type 2 latency-associated transcription in human sacral ganglia and in cell culture. J Infect Dis. 1991;163:23–28. doi: 10.1093/infdis/163.1.23. [DOI] [PubMed] [Google Scholar]
- 3.Wald A, Zeh J, Selke S, Ashley RL, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 1995;333:770–775. doi: 10.1056/NEJM199509213331205. [DOI] [PubMed] [Google Scholar]
- 4.Mertz GJ, Benedetti J, Ashley R, Selke SA, Corey L. Risk factors for the sexual transmission of genital herpes. Ann Intern Med. 1992;116:197–202. doi: 10.7326/0003-4819-116-3-197. [DOI] [PubMed] [Google Scholar]
- 5.Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203–209. doi: 10.1001/jama.289.2.203. [DOI] [PubMed] [Google Scholar]
- 6.Wald A, Corey L, Cone R, Hobson A, Davis G, Zeh J. Frequent genital herpes simplex virus 2 shedding in immunocompetent women: effect of acyclovir treatment. J Clin Invest. 1997;99:1092–1097. doi: 10.1172/JCI119237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wald A, Huang ML, Carrell D, Selke S, Corey L. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis. 2003;188:1345–1351. doi: 10.1086/379043. [DOI] [PubMed] [Google Scholar]
- 8.Krone MR, Tabet SR, Paradise M, Wald A, Corey L, Celum CL. Herpes simplex virus shedding among human immunodeficiency virusnegative men who have sex with men: site and frequency of shedding. J Infect Dis. 1998;178:978–982. doi: 10.1086/515666. [DOI] [PubMed] [Google Scholar]
- 9.Tateishi K, Toh Y, Minagawa H, Tashiro H. Detection of herpes simplex virus (HSV) in the saliva from 1,000 oral surgery outpatients by the polymerase chain reaction (PCR) and virus isolation. J Oral Pathol Med. 1994;23:80–84. doi: 10.1111/j.1600-0714.1994.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim HN, Meier A, Huang ML, et al. Oral herpes simplex virus type 2 reactivation in HIV-positive and -negative men. J Infect Dis. 2006;194:420–427. doi: 10.1086/505879. [DOI] [PubMed] [Google Scholar]
- 11.Crespi CM, Cumberland WG, Blower S. A queueing model for chronic recurrent conditions under panel observation. Biometrics. 2005;61:193–198. doi: 10.1111/j.0006-341X.2005.040332.x. [DOI] [PubMed] [Google Scholar]
- 12.Crespi CM, Cumberland WG, Wald A, Corey L, Blower S. Longitudinal study of herpes simplex virus type 2 infection using viral dynamic modelling. Sex Transm Infect. 2007;83:359–364. doi: 10.1136/sti.2006.022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu J, Koelle DM, Cao J, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988;26:662–667. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jerome KR, Huang ML, Wald A, Selke S, Corey L. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J Clin Microbiol. 2002;40:2609–2611. doi: 10.1128/JCM.40.7.2609-2611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magaret A, Wald A, Huang M, Selke S, Corey L. Optimizing PCR positivity criterion for detection of HSV DNA on skin and mucosa. J Clin Microbiol. 2007;45:1618–1620. doi: 10.1128/JCM.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corey L, Huang ML, Selke S, Wald A. Differentiation of herpes simplex virus types 1 and 2 in clinical samples by a real-time taqman PCR assay. J Med Virol. 2005;76:350–355. doi: 10.1002/jmv.20365. [DOI] [PubMed] [Google Scholar]
- 18.Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann InternMed. 1994;121:847–854. doi: 10.7326/0003-4819-121-11-199412010-00004. [DOI] [PubMed] [Google Scholar]
- 19.Cunningham AL, Turner RR, Miller AC, Para MF, Merigan TC. Evolution of recurrent herpes simplex lesions: an immunohistologic study. J Clin Invest. 1985;75:226–233. doi: 10.1172/JCI111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koelle DM, Posavad CM, Barnum GR, Johnson ML, Frank JM, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest. 1998;101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koelle DM, Liu Z, McClurkan CM, et al. Expression of cutaneous lymphocyte-associated antigen by CD8+ T cells specific for a skin-tropic virus. J Clin Invest. 2002;110:537–548. doi: 10.1172/JCI15537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheridan BS, Khanna KM, Frank GM, Hendricks RL. Latent virus influences the generation and maintenance of CD8+ T cell memory. J Immunol. 2006;177:8356–8364. doi: 10.4049/jimmunol.177.12.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verjans GM, Hintzen RQ, van Dun JM, et al. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci U S A. 2007;104:3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khanna KM, Bonneau RH, kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Lint AL, Kleinert L, Clarke SR, Stock A, Heath WR, Carbone FR. Latent infection with herpes simplex virus is associated with ongoing CD8+ T-cell stimulation by parenchymal cells within sensory ganglia. J Virol. 2005;79:14843–14851. doi: 10.1128/JVI.79.23.14843-14851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185:45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 28.Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35:435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 29.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 30.Leone PA, Trottier S, Miller JM. Valacyclovir for episodic treatment of genital herpes: a shorter 3-day treatment course compared with 5-day treatment. Clin Infect Dis. 2002;34:958–962. doi: 10.1086/339326. [DOI] [PubMed] [Google Scholar]
- 31.Wald A, Carrell D, Remington M, Kexel E, Zeh J, Corey L. Two-day regimen of acyclovir for treatment of recurrent genital herpes simplex virus type 2 infection. Clin Infect Dis. 2002;34:944–948. doi: 10.1086/339325. [DOI] [PubMed] [Google Scholar]
- 32.Aoki FY, Tyring S, Diaz-Mitoma F, Gross G, Gao J, Hamed K. Single-day, patient-initiated famciclovir therapy for recurrent genital herpes: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2006;42:8–13. doi: 10.1086/498521. [DOI] [PubMed] [Google Scholar]
- 33.Corey L, Wald A, Patel R, et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med. 2004;350:11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 34.Seppanen M, Meri S, Notkola IL, et al. Subtly impaired humoral immunity predisposes to frequently recurring genital herpes simplex virus type 2 infection and herpetic neuralgia. J Infect Dis. 2006;194:571–578. doi: 10.1086/506477. [DOI] [PubMed] [Google Scholar]
- 35.Whitley RJ, Miller RL. Immunologic approach to herpes simplex virus. Viral Immunol. 2001;14:111–118. doi: 10.1089/088282401750234484. [DOI] [PubMed] [Google Scholar]
- 36.Bochud P, Magaret A, Koelle DM, Aderem A, Wald A. Polymorphisms in toll-like receptor 2 are associated with increased viral shedding and lesional rate in patients with genital HSV-2 infection. J Infect Dis. 2007;196:505–509. doi: 10.1086/519693. [DOI] [PubMed] [Google Scholar]
- 37.Wang K, Mahalingam G, Hoover SE, et al. Diverse herpes simplex virus type 1 thymidine kinase mutants in individual human neurons and ganglia. J Virol. 2007;81:6817–6826. doi: 10.1128/JVI.00166-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mole L, Ripich S, Margolis D, Holodniy M. The impact of active herpes simplex virus infection on human immunodeficiency virus load. J Infect Dis. 1997;176:766–770. doi: 10.1086/517297. [DOI] [PubMed] [Google Scholar]
- 39.Schacker T, Zeh J, Hu H, Shaughnessy M, Corey L. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J Infect Dis. 2002;186:1718–1725. doi: 10.1086/345771. [DOI] [PubMed] [Google Scholar]