Abstract
Serotonergic modulation of acetylcholine (ACh) release after neuron-specific increase of the expression of 5-HT1B receptors by gene transfer was studied in-vitro and in-vivo. The increased expression of the 5-HT1B receptor in-vitro was induced by treating rat primary fetal septal cell cultures for 3 days with a viral vector inducing the expression of green fluorescent protein alone (GFP vector), or, in addition, of 5-HT1B receptors (HA1B/GFP vector). The transfection resulted in a high number of GFP-positive cells, part of which being immunopositive for choline acetyltransferase. In HA1B/GFP-cultures (vs. GFP-cultures), electrically-evoked ACh release was significantly more sensitive to the inhibitory action of the 5-HT1B agonist CP-93,129. Increased expression of the 5-HT1B receptor in-vivo was induced by stereotaxic injections of the vectors into the rat septal region. Three days later, electrically-evoked release of ACh in hippocampal slices of HA1B/GFP-treated rats was lower than in their GFP-treated counterparts, showing a higher inhibitory efficacy of endogenous 5-HT on cholinergic terminals after transfection. Moreover, CP-93,129 had a higher inhibitory potency. In conclusion, the HA1B/GFP vector reveals a useful tool to induce a targeted increase of 5-HT1B heteroreceptors on cholinergic neurons in selected CNS regions, which provides interesting perspectives for functional approaches at more integrated levels.
Keywords: rat septal neurons; rat hippocampus; CP-93,129; septal cell culture; Herpes Simplex Virus
1. Introduction
5-HT1B receptors are G protein-coupled serotonergic receptors involved in the modulation of transmitter release on serotonergic axon terminals, where they act as autoreceptors, or on axon terminals of other neurons, where they operate as heteroreceptors [(for reviews see: [3; 27; 28; 53]]. For example, it is well established that the release of acetylcholine (ACh) from axon terminals of septohippocampal cholinergic neurons is inhibited by 5-HT1B receptors [15; 38; 55]. Interestingly, it has been suggested that similar serotonergic effects on cholinergic transmission might participate in cognitive processes [7; 8; 14; 47; 54]. For instance, from studies using 5-HT1B receptor knock-out (KO) mice, it was concluded that 5-HT1B receptors might be implicated in certain spatial memory processes [e.g., [9; 37; 48; 58]. In addition to cognition, 5-HT1B receptors also seem to be involved in locomotor activity, reinforcement of drugs of abuse, migraine, stress sensitivity, mood, aggressive behaviour, depression and anxiety states [17; 29; 50].
However, although numerous experiments suggest a role of 5-HT1B receptors in the behavioural processes mentioned above, the fact that they are found on various neuronal populations and in most brain areas [50] makes it extremely difficult to link observed serotonergic effects to 5-HT1B receptors located on a well-defined population of neurons. Moreover, it is not possible to manipulate 5-HT1B heteroreceptors in physiological or behavioural models without also impinging on 5-HT1B autoreceptors, thereby complicating the interpretation of findings.
For these reasons, we now investigated the role of 5-HT1B heteroreceptors on cholinergic neurons in a defined brain region using selective and neuron-specific overexpression of this receptor via targeted gene transfer. As in previous studies on other brain nuclei [18; 19; 31; 42], a replication-deficient Herpes simplex virus type 1 (HSV-1) was used as a dual expression vector, carrying both epitope-tagged 5-HT1B receptors and green fluorescent protein (GFP) on separate transcriptional cassettes.
It was the aim of the present study to apply this viral gene transfer technique in the septal region of the rat brain, in order to assess for the first time changes in the presynaptic function of 5-HT1B heteroreceptors following their overexpression in the septohippocampal cholinergic pathway. The physiological interest in this strategy relies on the idea that only the expression of the 5-HT1B hetero- but not of the autoreceptor is increased in a defined brain region, the hippocampus, in which serotonergic-cholinergic interactions seem to play an important role in cognitive processes (see above). Our gene transfer experiments were performed both in-vitro and in-vivo. The in-vitro study, was performed on primary cell cultures of the fetal septal region which contain cholinergic neurons [22] endowed with 5-HT1B heteroreceptors [21; 23; 55]. The in-vivo part relied upon stereotaxic injections of the HSV vectors into the medial septum of the rat brain, to increase the expression of 5-HT1B heteroreceptors in septohippocampal cholinergic neurons [1]. The transfection of cholinergic neurons (in-vitro and in-vivo), was verified using immunocytochemistry, whereas functional consequences of the increased expression were determined by measuring the 5-HT1B receptor-mediated modulation of electrically evoked ACh release.
2. Materials and methods
2.1. Chemicals and drugs
Chemicals and drugs were purchased from the following sources: [methyl-3H]choline chloride from Amersham Biosciences (Freiburg, Germany); hemicholinium-3 from ChemCon (Freiburg, Germany); 3-[3-(Dimethylamino)propyl]-4-hydroxy-N-[4-(4-pyridinyl)phenyl]benzamide (GR-55,562) from Biotrend (Köln, Germany). The drug 3-(1,2,5,6-tetrahydropyrid-4-yl)pyrrollo[3,2-b]pyrid-5-one (CP-93,129) was kindly donated by Pfizer (Groton, USA). Sources of products used during stereotaxic injections of rats or for cell cultivation and immunocytochemistry are indicated hereafter (see below).
2.2. Herpes Simplex Viral vector
Herpes simplex virus (HSV) gene transfer vectors, abbreviated as GFP and HA1B/GFP hereafter, were produced as described in detail previously [18]. Briefly, a reconstructed amplicon containing two transcriptional units terminated by SV40 polyadenylation sites, the first producing HA-5-HT1B (HA, hemagglutinin, tag of the 5-HT1B receptor) from an HSV promoter/enhancer and the second producing GFP (Green Fluorescent Protein) from a CMV promoter/enhancer was created (“HA1B/GFP”). As a control, a second amplicon producing only GFP was also prepared (“GFP”). Both amplicons were then packaged using replication-deficient HSV as described before [43]. This viral preparation was kept in 5 µl-aliquots in a −80°C-freezer until utilization.
2.3. Animal care
All procedures involving animal care and experimentation were conducted in accordance with institutional guidelines that comply with the German law for animal protection (DtTSchG, 25.5.1998, last modification: 21.6.2005) and the European Communities Council Directive of 24 November 1986 (86/609/EEC; official animal experimentation licence to J-C.C.: # 67–215, 04.08.04). All efforts were made to minimize animal suffering and to reduce the number of animals used to the minimum regarding statistical constraints.
2.4. Preparation of primary septal cell cultures
Cell cultures from the fetal septal region were prepared as previously described [21; 22] with minor modifications. Pregnant Wistar rats on embryonic day 17 (ED17) were deeply anaesthetized with sodium pentobarbital, both uterine horns were removed and each embryo was taken out of its individual amniotic sac and placenta and placed in an ice-cold Petri dish filled with phosphate-buffered saline (PBS, Biochrom KG, Berlin, Germany). Embryos were first killed by decapitation and all steps of brain dissection were done under a stereomicroscope (magnification from ×10 to ×25) according to [20].
Tissue pieces of the embryonic septal area were collected in ice-cold phosphate-buffered saline and incubated for 20 min at 37°C in 1 ml of a trypsin-EDTA solution (0.5 %, Sigma-Aldrich, Taufkirchen, Germany). Trypsinization was stopped by the addition of 500 µl of NU-serum (NU-serum IV culture supplement, Becton Dickinson, Franklin Lakes, NJ, USA). Subsequently the cells were dissociated by gentle trituration using a fire-polished Pasteur pipette. Following centrifugation of the suspension obtained (200 g, 5 min), the pellet was resuspended in 1 ml of growth medium (see below). After a second trituration, the suspension was recentrifuged (200 g, 5 min) and the pellet resuspended by gentle trituration in a final volume of 125 µl growth medium per septum. Finally, cells were plated at a density of 1.4 × 106 cells/ml onto 96-well plates, each well containing a circular glass cover slip (5 mm diameter; “cell culture discs”), previously coated with poly d-lysine (poly-d-lysine hydrobromide, Sigma Aldrich, Taufkirchen, Germany).
Cells were cultured at 37°C in a humidified 95% air/5% CO2 atmosphere for 14 days in-vitro (DIV) in Dulbecco’s modified Eagle’s medium (DMEM medium: Nut. Mix. F-12 with Glutamax-1, GibcoBRL, Life Technologies, Eggenstein, Germany) containing 10% of NU-serum IV culture supplement (Becton Dickinson), a mixture of insulin, transferrin and sodium selenite (5 µg/ml, 5µg/ml and 5 ng/ml, Sigma), a mixture of penicillin and streptomycin (50 U/ml and 5 ng/ml; GibcoBRL), as well as mouse nerve growth factor (mNGF, 10 ng/ml, Alomone/ICS, Munich, Germany) and human neurotrophin-3 (hNT-3, 50 ng/ml, Alomone/ICS). The growth medium was changed every 3–4 days.
2.5. Infection of cell cultures with viral vectors
At DIV 9, twelve cell culture discs were transferred to each of a 3.5 cm-Petri dishes filled with 1.5 ml growth medium and further grown for 3 days. At DIV 11 the viral vectors (either GFP or HA1B/GFP) were added to the growth medium at a concentration of 1 µl viral suspension (~100.000 infective units) per ml of growth medium. ACh release experiments using these cultures or fixation of the cells for immunocytochemical stainings, respectively, were performed three days later (DIV 14) because the virus vectors have been shown to induce a maximal expression of the 5-HT1B receptor after 3 days [18].
2.6. Stereotaxic injection of viral vectors in-vivo
The in-vivo study was performed on young adult Long-Evans male rats (Centre d’Elevage R. Janvier, Le Genest St-Isle, France) aged of 3 months at the time of surgery. They were kept in an animal colony in individual transparent cages (42 × 26 × 15 cm) in rooms that were maintained on a 12:12 h dark-light cycle (light on at 7:00) under controlled temperature (19° ± 1°C). The rats were housed with ad libitum access to food and water throughout the experiment. Before surgery, the rats were randomly assigned to one of the two groups, namely GFP and HA1B/GFP. The GFP group refers to animals injected with the viral construct expressing GFP only, the HA1B/GFP group refers to animals injected with the construct expressing both GFP and the 5-HT1B receptor tagged with HA, whereas the CON group refers to animals that were not subjected to any surgery.
Bilateral implantation of guide cannulae
rats were anaesthetised with isoflurane (1-chloro-2,2,2-trifluoroethyl-difluoromethylether; 2–3%; Forene®, Abbott GmbH, Wiesbaden, Germany) and the scalp fur was shaved before the animal was placed in a Stoelting® stereotaxic frame. Rats were kept under anaesthesia during the surgery with the aid of a gas mask fixed to the incisor bar of the stereotaxic frame. After scalp incision, the coordinates of Bregma were determined and two small holes for bilateral stereotaxic injection of the viral vector were drilled at the sites for the implantation of the two guide cannulas. Two stainless steel guide cannulae (length 12 mm; outer diameter 0.40 mm) targeting the medial septal area were then implanted at the following stereotaxic coordinates: A = + 0.8; L = ± 0.5; V = −5.6 in mm from Bregma (with the incisor bar set at 3.3 mm below the interaural line; according to [44] and with A referring to anterior, L to lateral and V to ventral). Guide cannulae were kept in place by a cap of acrylic dental cement tightly fixed to the skull by three stainless steel screws. Following disinfection of the surrounding skin, the cap of dental cement was rounded to avoid any involuntary injury of the rat. At the end of the surgery, a stainless steel dummy was placed in the opening of each of both guide cannulae in order to avoid any obstruction. Finally, each rat received an intramuscular injection of an antibiotic, i.e. benzylpenicillin-benzathine (Pendysin® 1.2 Mio, Jenapharm GmbH, Jena, Germany). After surgery, animals were allowed to recover from anaesthesia in the surgery room before being replaced in their home cages and brought back to the animal colony.
Injection of the viral vectors
Injections of the viral vectors GFP and HA1B/GFP were performed under isoflurane anaesthesia and aseptic conditions between 3 and 13 days after guide cannula implantations. The microinjection needles were connected to a 10 µl Hamilton syringe using a catheter and the syringe was fixed in a microinjection pump (World Precision Instruments, Sarasota; FL, USA). Because the microinjection needles were longer than the guide cannulae, we were able to inject the virus vectors at two different sites in depth, i.e. at −7.6 and −6.6 mm (from Bregma) in order to infect the medial septal area over a significant distance of its dorso-ventral extent. For each site, the injection of 1 µl of the viral suspension (~100.000 infective units) was performed at a rate of 200 nl/min. In order to enable sufficient diffusion of the virus from the injection site, the needle was left in place for each injection depth and site for 5 additional minutes before being slowly retracted. At the end of the surgery, the stainless steel dummy was replaced in the opening of each of both guide cannulae in order to avoid any obstruction. After the injections, animals were allowed to recover from anaesthesia before being replaced in their home cages and brought back to the animal colony. As a maximum of the increased expression of the transferred genes was shown to occur about 3 to 4 days after the injection [18], preparation of brain slices for immunocytochemical characterizations as well as for ACh release experiments were performed on the third day after the microinjection.
2.7. Electrically-evoked release of tritiated acetylcholine ([3H]ACh)
Three days after injection of the viral vectors into the rat septal region in-vivo, or at DIV 14 for septal cell cultures (i.e. 3 days after the infection of the cell cultures with the viral constructs), electrically-evoked release of [3H]ACh from the hippocampal slices, or the cell cultures, respectively, was measured.
Hippocampal slices
Rats previously injected with viral vectors (groups: GFP, HA1B/GFP) or untreated rats (group: CON) were sacrificed by decapitation during anesthesia following carbon dioxide inhalation. Their brain was quickly removed and transferred into ice-cold modified Krebs-Henseleit (KH) buffer of the following composition (in mM): NaCl, 118; KCl, 4.8; CaCl2, 1.3; MgSO4, 1.2; NaHCO3, 25; KH2PO4, 1.2; glucose, 10; ascorbic acid, 0.6; Na2EDTA, 0.03; saturated with carbogen (95% O2/5% CO2), pH adjusted to 7.4. The anterior part of the brain containing the septal region was used for subsequent sectioning and immunostaining (see below). From the posterior part of the brain, both hippocampi were dissected out and cut in 350-µm thick slices along the septotemporal axis with a McIIwain tissue chopper. The hippocampal slices were washed three times with ice cold KH buffer and incubated in 2 ml KH buffer containing [3H]choline (0.1 µ mol/L; [methyl-3H]-choline (82 Ci/mmol), for 45 min at 37°C under a flow of carbogen. After incubation, brain slices were carefully washed with KH buffer (at 37°C), transferred into superfusion chambers (one slice per chamber) and superfused at a rate of 1.2 ml/min and a temperature of 37°C with oxygenated KH buffer routinely supplemented with 10 µM hemicholinium-3. After 15 min of superfusion, the slices were prestimulated by electrical fields (18 rectangular pulses at 3 Hz, 2 ms, 4 V/chamber, 25–28 mA). Collection of 2-min fractions started after 32 min of superfusion. The overflow of [3H] was induced three times by electrical field stimulations (90 rectangular pulses at 3 Hz, 2 ms, 8 V/chamber, ~ 50 mA) after 36 min (S1), 52 min (S2) and 68 min (S3) of superfusion. The drug to be tested (CP-93,129) was added to the superfusion buffer from 8 min before S2 and S3 onwards, with concentration increasing from S2 to S3. At the end of the experiments (i.e. after 76 min of superfusion) the radioactivity of superfusate samples and slices (dissolved in 300 µl Solvable® 350; Perkin-Elmer, Rodgau, Germany) was determined by liquid scintillation counting.
Septal cell cultures
Cell culture discs containing septal cell cultures at DIV 14 (i.e. 3 days after infection with viral constructs) were carefully washed with KH buffer (at least 3 times at 37°C) and preincubated in the presence of [3H]choline as described above for hippocampal slices. Subsequently they were carefully washed again and then placed (two cell culture discs back to back) in the superfusion chambers, where they were superfused at a rate of 0.6 ml/min in the presence of 10 µM hemicholinium-3 and a temperature of 25°C (a temperature previously shown to be optimal for superfusion experiments using cultured neurons [5; 21; 22]). After 49 min of superfusion, collection of 4-min fractions was started. The overflow of [3H] was induced twice by electrical field stimulations (360 rectangular pulses at 3 Hz, 0.5 ms, 9.4 V/chamber, 90 – 100 mA) after 57 min (S1) and 85 min (S2) of superfusion. The drug to be tested (CP-93,129) was added to the superfusion buffer from 8 min before S2 onwards. At the end of the experiment (i.e. after 129 min of superfusion) the radioactivity of superfusate samples and cell culture discs (cells dissolved in 300 µl Solvable® 350) was determined by liquid scintillation counting.
Calculation of release data
As regards [3H]ACh release from hippocampal slices of stereotaxically transfected animals, GFP fluorescence served as an indicator of viral transfection. Thus, before statistics were performed, 40 µm vibratome slices were observed under a fluorescence microscope in order to check for the site of the cannula tracks as well as for GFP-positive cells in the medial septal area. Consequently, data of rats in which the cannulae were not implanted into the appropriate target region or for which no GFP-positive cells could be detected in the medial septum (about in 40% of rats treated with either viral vector) were discarded from all statistical analyses.
The fractional rate of tritium outflow (in per cent of tissue or cell tritium per 4 min) was calculated as: (pmoles tritium outflow per 4 min) × 100 / (pmoles of tritium in the hippocampal slices (or cell culture discs) at the start of the respective 4-min period). The ‘basal tritium outflow’ (b1-value) represents the ‘fractional rate of tritium outflow per 4 min’ in the fraction preceding S1. The stimulation-evoked overflow of tritium at S1, S2 or S3 was expressed as a percentage of the tritium content of the hippocampal slices (or cell culture discs) just before the onset of the respective stimulation period. It was calculated following subtraction of the basal tritium outflow; the latter was assumed to decline linearly from the 4-min fraction immediately before the onset of the stimulation to the 4-min fraction 12 – 16 min after the onset of the stimulation. Effects of drugs added before Sn were estimated as the ratio of the overflow evoked by the corresponding stimulation periods (Sn/S1) and then compared to the appropriate control ratios (no drug addition before Sn). Effects of drugs added before Sn on the basal outflow of [3H] were determined routinely as the ratio (bn/b1) of the fractional rates of [3H]outflow of the fractions preceding the corresponding stimulation periods and then compared to the appropriate control ratios (no drug addition before Sn). However, since no basal effects of drugs were observed in the present study, they are not mentioned in the subsequent text.
2.8. Immunocytochemistry
Fixation of cell cultures and brain slices for immunostaining
Some of the septal cell cultures were pretreated with the viral vectors for 3 days (starting at DIV 11) as described above. At DIV 14, the cells on the culture discs were fixed (2 × 15 min) by treatment with 4% paraformaldehyde in 0.1 M phosphate buffer (PB, Biochrom KG, Berlin, Germany), then washed in 0.1 M PB (2 times for 15 min) and finally further treated as described below.
Fixation and staining of brain slices was performed using the free floating slice technique. The anterior parts of the brains from rats stereotaxically injected with the viral vectors were dissected and fixed in 4% paraformaldehyde dissolved in 0.1 M PB for 24 h before washing and storage (4°C) in 0.1 M PB until sectioning. Forty µm thick coronal sections of each anterior rat brain piece were prepared using a vibratome (Leica VT1000S, Wetzlar, Germany) and collected in a 24-well plate in 0.1 M PB. Due to the presence of GFP in the cell cultures and slices, all steps of immunostaining were conducted under dark conditions.
Glial fibrillary acidic protein (GFAP, only for the cell cultures)
After fixing and washing, septal cell cultures were directly incubated with a Cy3-conjugated mouse anti-GFAP antibody (1:2000; Sigma, Taufkirchen, Germany) in 0.1 M PB for 1 hour. After 3 washings of 10 min each in 0.1 M PB, cells were mounted on glass slides with fluorescence-specific mounting medium (Vectashield® Mounting medium, Vector Laboratories, Burlingame, CA, USA).
Glutamate decarboxylase 67 (GAD67)
Septal cell cultures were treated, with 5 or 10% Normal Goat Serum (NGS, Vector laboratories) and 0.5% Triton X-100 (in 0.1 M PB) for 30 min before incubation with mouse anti-GAD67 antibody (1:5000, affinity purified monoclonal antibody, Chemicon International, Hampshire, United Kingdom) in 0.1 M PB (supplemented with 1% NGS and 0.5% Triton X-100) for 12 hours at 4°C. After washing of the cells with 0.1 M PB, they were subsequently stained by the addition of Alexa 568-conjugated goat anti-mouse antibody (1:500, InVitrogen GmbH, Karlsruhe, Germany) in 0.1 M PB for 1 hour at room temperature. Following a final washing period (4×30 min in 0.1 M PB), septal cultures were placed on glass slides and mounted with Vectashield® mounting medium.
Hemagglutinin (HA)
Septal cell cultures or brain sections, respectively, were treated, with 5 or 10% normal goat serum (NGS; Vector Laboratories) and 0.5% Triton X-100 (in 0.1 M PB) for 30 min (1 hour for the brain sections) before incubation for 12 hours at 4°C with mouse anti-HA antibody (1:1000, HA.11 monoclonal antibody, purified, Covance, San Diego, CA, USA) in 0.1 M PB (supplemented with 1% NGS and 0.5% Triton X-100). After washing of the brain sections of cell culture discs with 0.1 M PB, they were stained by the addition of Cy3-conjugated goat anti-mouse antibody (1:500; Dianova, Hamburg, Germany) in 0.1 M PB for 2 hours at room temperature. Following a final washing period (4 × 30 min in 0.1 M PB), septal cultures or brain sections were placed on glass slides and mounted with Vectashield® mounting medium.
Choline acetyltransferase (ChAT)
Septal cell cultures or brain sections, respectively, were treated, with 5 or 10% Normal Donkey Serum (NDS, Dutscher, Brumath, France) and 0.5% Triton X-100 (in 0.1 M PB) for 30 min (1 hour for the brain sections) before incubation with goat anti-ChAT antibody (1:500, affinity purified polyclonal antibody, Chemicon International, Hampshire, United Kingdom) in 0.1 M PB (supplemented with 1% NDS and 0.5% Triton X-100) for 12 hours at 4°C. After washing of the tissues with 0.1 M PB, they were subsequently stained by the addition of Cy3-conjugated donkey anti-goat antibody (1:500, InVitrogen GmbH, Karlsruhe, Germany) in 0.1 M PB (supplemented with 1% NDS and 0.5% Triton X-100) for 1 hour for the cell cultures (2 hours for the brain sections) at room temperature. Following a final washing period (4×30 min in 0.1 M PB), septal cultures or brain sections were placed on glass slides and mounted with Vectashield® mounting medium.
Microscopy
Brain slices and cell cultures, once stained, were first screened under a fluorescence microscope for overview pictures (AxioPhot with AxioCam, using AxioVision software, Zeiss, Oberkochen, Germany). Subsequently they were further analysed using a confocal laser microscope [AxioVert 135 (Zeiss, Oberkochen, Germany) equipped with the confocal emission unit MRC 1024 (BioRad, Hercules, CA, USA) and the MetaMorph 7.0 software (Universal Imaging, Downingtown, PA, USA)].
2.9. Statistical analysis
All data were analysed using analyses of variance (ANOVA); when appropriate, ANOVA was followed by multiple comparisons using the Newman-Keuls test [57]. All values shown in the Figures are Means ± S.E.M, “n” representing the total number of hippocampal slices or cell culture discs, respectively, used for each experimental condition.
3. Results
3.1. Increased expression of 5-HT1B receptors in septal cell cultures
3.1.1. Immunostaining of cell cultures
Primary cell cultures from the rat fetal septal region were transfected either with the viral construct expressing only GFP (GFP group) or with the viral construct expressing both GFP and the 5-HT1B receptor (tagged with hemagglutinin; HA1B/GFP group). Three days after transfection, the cell culture discs were washed and fixed for immunocytochemical characterization.
Glial Fibrillary Acidic Protein and glutamate decarboxylase
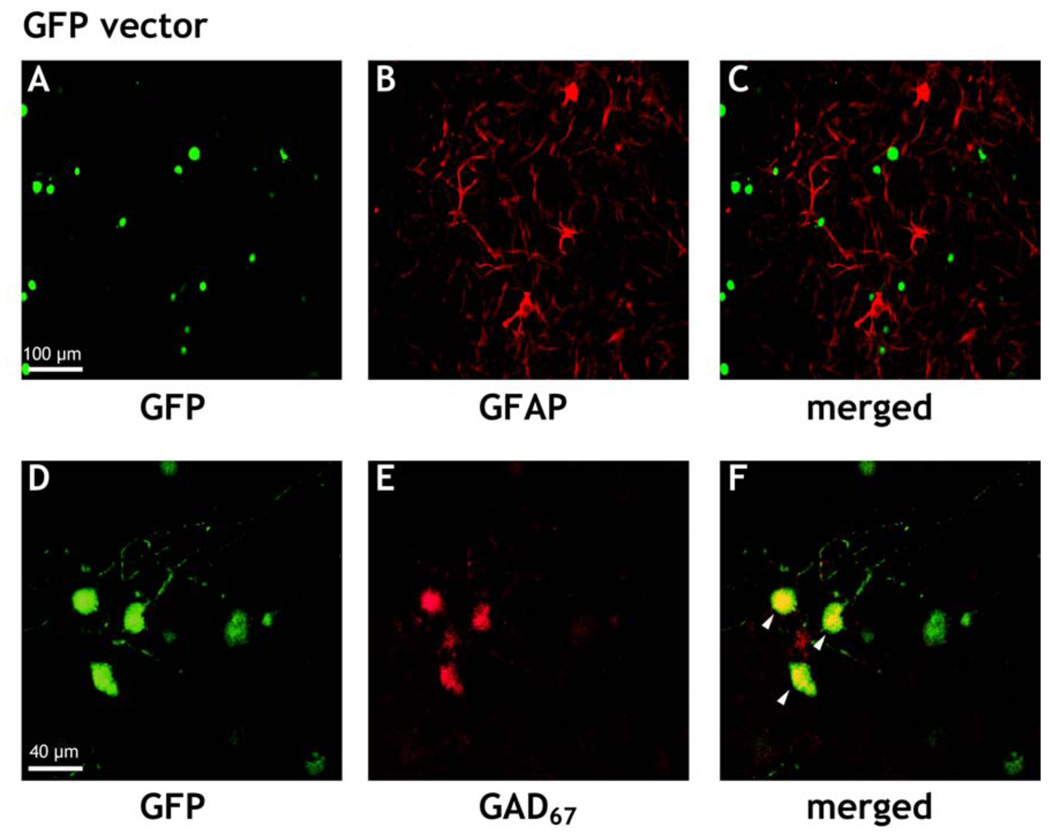
In a previous study on primary cell cultures from the fetal septal region of the rat they were characterized as a mixed culture of non-neuronal and neuronal cells, some of the latter being cholinergic neurons [22]. Using an antibody against the glial marker GFAP, the presence of a large number of glial cells is also visible in the present cultures (Figure 1B). However, in cultures transfected with either the GFP vector (Figure 1) or the HA1B/GFP vector, no colocalization between GFP and GFAP was found. (Figure 1C).
Figure 1.
Typical examples of immunostained septal cell cultures under a confocal microscope. Cells were immunostained with a Cy3-conjugated mouse antibody against glial fibrillary acidic protein (GFAP), or an Alexa 568-conjugated mouse antibody against glutamate decarboxylase (GAD67), respectively, at DIV14, three days after infection of the cultures with the GFP virus, i.e. the viral construct expressing only GFP. In A or D cells expressing GFP, in B or E cells in the corresponding fields expressing GFAP or GAD67, respectively, are shown. C and F represent the corresponding merged pictures (A + B) or (D + E), respectively. The yellow color in F indicates colocalization of the expression of GAD67 and GFP within the same cells (see arrows).
On the other hand Figure 1E also reveals the presence of cells in the cultures, which are stained with an antibody against GAD67. Some of these cells were co-expressing GFP (Figure 1F).
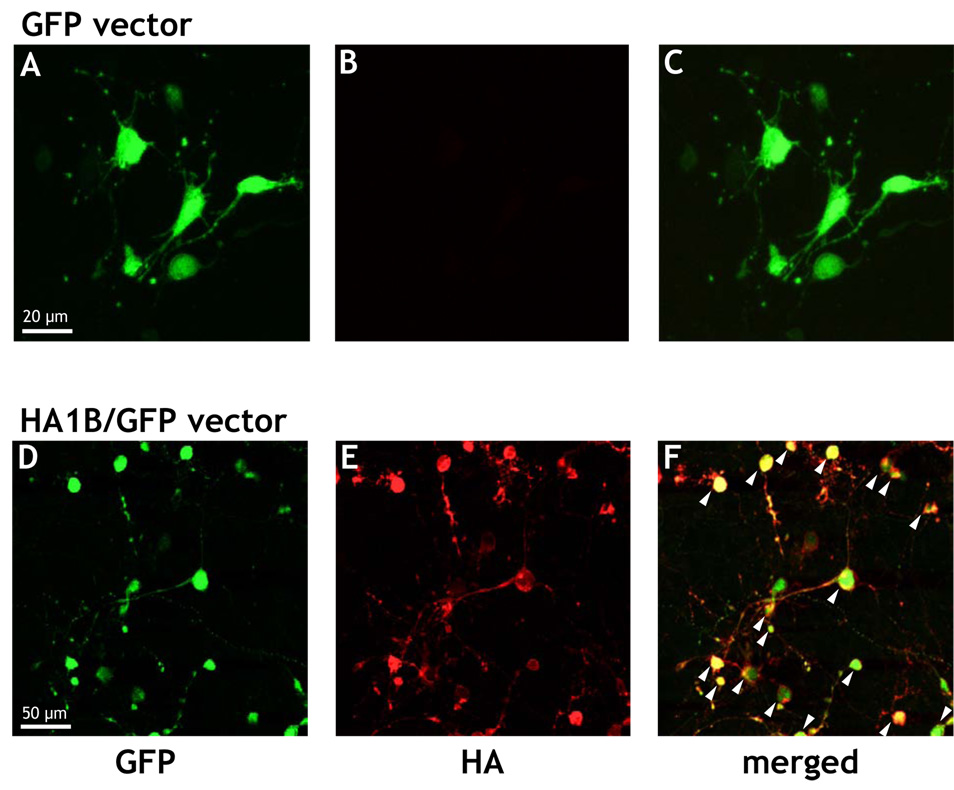
Hemagglutinin immunostaining
Data are shown in Figure 2. As described in Methods, a hemagglutinin (HA) epitope tag was introduced into the N-terminus of the 5-HT1B gene to allow easier detection of 5-HT1B receptor expression with commercially available antibodies. As shown in Figure 2A, neuronal cells transfected with GFP only exhibited the classical green fluorescence of GFP (Figure 2A), whereas a labeling of cells with an antibody against hemagglutinin could not be shown (Figure 2B). In contrast, cell cultures transfected with the HA1B/GFP construct exhibited both GFP (Figure 2D) as well as hemagglutinin labeling (Figure 2E) and these two labels were colocalized in an important proportion of cells (Figure 2F).
Figure 2.
Typical examples of immunostained septal cell cultures under a confocal microscope. Cells were immunostained with a Cy3-conjugated antibody against Hemagglutinin (HA) at DIV14, three days after infection of the cultures with the viral particles. A, B, C: cell cultures infected with the GFP virus, i.e. the viral construct expressing only GFP (scale bar: 20 µm). D, E, and F: cell cultures infected with the HA1B/GFP virus, i.e. the viral construct expressing both HA-tagged 5-HT1B receptor and GFP (scale bar: 50 µm). In A and D cells expressing GFP, in B and E neurons expressing (or not) the HA-tag are shown. C and F represent the corresponding merged pictures (A + B and D + F, respectively). The yellow color in F indicates colocalization of the expression of HA and GFP within the same cells (see arrows).
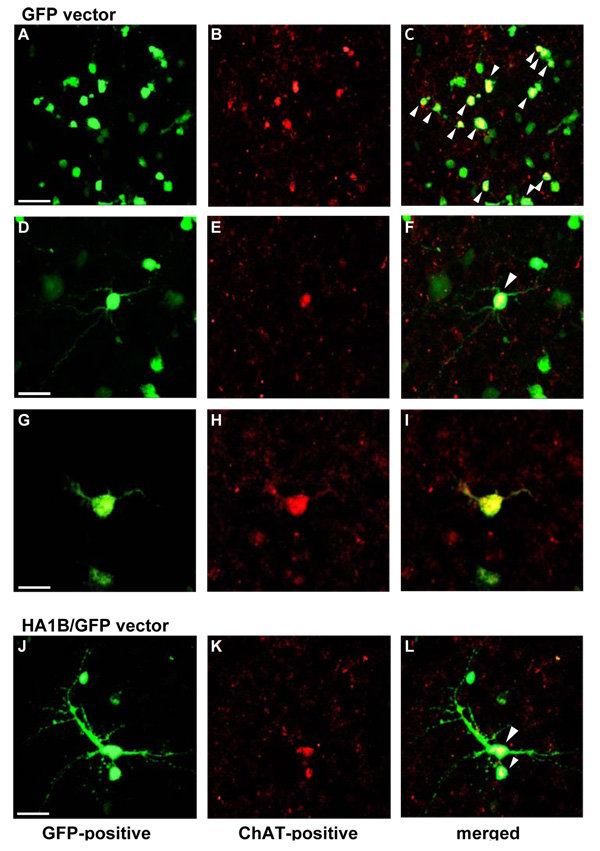
Choline acetyltransferase
As evident from Figure 3, cell cultures of the rat fetal septal area transfected with the GFP (Figure 3 A to I) or the HA1B/GFP vector (Figure 3 J to L) show (at different levels of magnification) cells expressing GFP (Figure 3A, D, G, J). Moreover, some of the cells are also labeled using an antibody against the cholinergic marker enzyme choline acetyltransferase (ChAT; Figure 3B, E, H, K). Finally, as evident from the merged pictures, in some of the neurons, a colocalization of GFP and ChAT-immunostaining can be shown (Figure 3C, F, I, L). In addition to these observations it should also be noticed that some ChAT-positive neurons were negative for GFP (not visible in Figure 3) and that some GFP-positive cells were not stained for ChAT. Because of the high density of the cellular network, it was not possible to quantify the number of cholinergic cells and their proportion with respect to all neuronal cells. Moreover, despite its good quality, the ChAT staining did not always allow us to detect neuritis.
Figure 3.
Microscopic aspect of ChAT immunostained septal cell cultures. At DIV 11, cells were transfected with either the GFP (A to I) or the HA1B/GFP vector (J to L) (1 µl/1 ml). At DIV 14, cells were fixed and further rinsed in PB 0.1 M. Following fixation, cells were immunostained with a Cy3-conjugated antibody against ChAT according to the protocol described in the Methods section. A, D, G and J: GFP-positive cells in the septal cell cultures. B, E, H and K: ChAT labelling at the same position as in pictures A, D, G & J, respectively. C, F, I and L: merged pictures of A & B; D & E; G & H and J & K, respectively. The yellow colour in C, F, I and L indicates colocalization of the expression of ChAT and GFP within the same cells (see white arrow heads). Scale bars in A (50 µ m), D (20 µ m), G (10 µ m) & J (20 µ m) are respectively valid for B & C; E & F; H & I and K & L. All pictures were taken using a confocal microscope.
3.1.2. Electrically-evoked release of [3H]ACh in septal cell cultures
Accumulation of [3H]choline by cell cultures
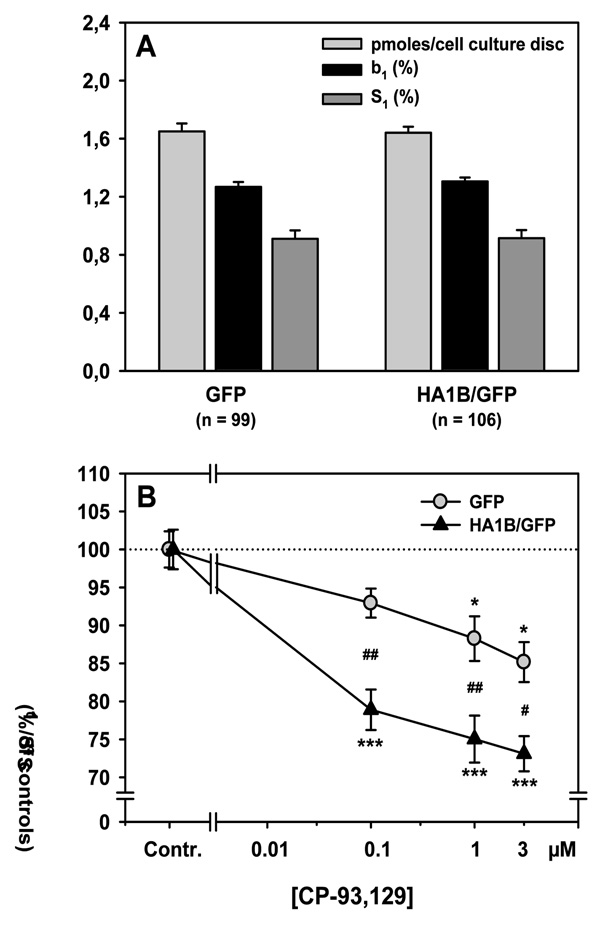
Following two weeks in-vitro the septal cell cultures treated for 3 days with the GFP- or the HA1B/GFP-vector, respectively, were washed carefully and incubated in superfusion medium (KH buffer) in the presence of [3H]choline. Data for the accumulation of [3H]choline (in pmoles/cell culture discs) are shown in Figure 4A (light grey bars). ANOVA of the [3H]choline accumulation showed no significant difference between the two types of transfected cell cultures (F[1,203] = 0.018; n.s.).
Figure 4.
Electrically-evoked release of [3H]ACh and its 5-HT1B receptor mediated modulation in septal cell cultures. Cells were preincubated with [3H]choline at DIV14, i.e. three days after infection of the cultures with either GFP or HA1B/GFP viral vectors. Subsequently they were washed, superfused continuously in the presence of hemicholinium-3 (10 µ M) and stimulated twice by electrical fields (S1, S2: see methods). A: Accumulation of [3H]choline (in pmoles/cell culture disc) into the cultured cells, as well as basal outflow of [3H] (b1 in % of cell culture [3H]) and electrically-evoked overflow of [3H] at S1 (in % of cell culture [3H]) from the cultured cells; means + S.E.M; n = number of slices. B: Effects of various concentrations of the 5-HT1B receptor agonist CP-93,129 added before S2 on the evoked overflow of [3H]. Effects of CP-93,129 are shown as the ratio S2/S1 (in % of drug free controls) of the evoked [3H]overflows elicited at the corresponding stimulation periods. Means ± S.E.M.; n = 9–31 per CP-93,129 concentration; *p<0.05; ***p<0.001 vs. correspondding controls; #p<0.05, ##p<0.01 vs. corresponding value from GFP-virus treated cultures; for further statistics see Results.
Baseline outflow of tritium from cell cultures (b1)
Following preincubation, the cell culture discs were washed carefully and superfused continuously. Data for the baseline [3H]outflow from the cultured cells immediately before the electrical field stimulation (S1) are presented in Figure 4A (black bars). The statistical analysis was done both on the absolute values (in nCi) for the basal [3H]outflow and the relative values (in percent of accumulated [3H] per cell culture disc), but only relative values are shown in Figure 4A. ANOVA of the baseline [3H]outflow from cell cultures showed no significant difference between the GFP and the HA1B/GFP groups, whether on absolute (F[1,203] = 0.062; n.s.) or relative values (F[1,203] = 0.733; n.s.).
Electrically-evoked overflow of tritium in cell cultures
During superfusion, the septal cell cultures were exposed to two electrical field stimulations (S1, S2). Data for the amount of the electrically-evoked overflow of [3H] during the first stimulation period (S1) are shown in Figure 4A (dark grey bars). As for the baseline outflow, statistical analyses was performed on both absolute (in nCi) and relative values of S1 (in percent of accumulated [3H] in each cell culture), but only relative values are presented in Figure 4A. ANOVA of the electrically-evoked overflow of [3H] showed no significant difference between both transfection types, whether on absolute (F[1,203] = 0.071; n.s.) or relative values (F[1,203] = 0.003; n.s.).
Effects of the selective 5-HT1B agonist CP-93,129 on the evoked overflow of [3H]
During the experiment, various concentrations of the 5-HT1B agonist CP-93,129 were added to the superfusion medium before S2. The inhibitory effects of this drug are shown in Figure 4B. Data were analysed using a two-way ANOVA combining the “Vector” as a first factor (GFP or HA1B/GFP virus) and the “Drug condition” as a second factor (slices where either controls or superfused with CP-93,129, at 0.1, 1 or 3 µM). ANOVA showed a significant effect of Vector (F[1,197] = 21.53; p < 0.001) and of Drug condition (F[3,197] = 28.50; p < 0.001), as well as a significant interaction between these factors (F[3,197] = 3.79; p < 0.05). The Drug condition effect was due to a significant effect of CP-93,129 in both groups as compared to control slices. For the GFP group, 1 µM of CP-93,129 decreased the [3H]ACh release by about 12% (p < 0.05) and 3 µM by 15% (p < 0.05), as compared to respective controls. For the HA1B/GFP group, 0.1 µM of CP-93,129 decreased the [3H]ACh release by 22% (p < 0.001), 1 µM by 25% (p < 0.001) and 3 µM by 27% (p < 0.001), as compared to the respective controls. These differences account for the significant interaction, the effects of CP-93,129 being significantly more potent in HA1B/GFP-treated septal cultures as compared to those treated with GFP virus, whatever the concentration (p < 0.01 for 0.1 and 1 µM of CP-93,129 and p < 0.05 for 3 µM of CP-93,129).
3.2. Increased expression of 5-HT1B receptors in septal tissue in-vivo
As described in Methods, the viral vectors GFP or HA1B/GFP were injected stereotaxically into the medial septum of adult Long-Evans rats. Three days later, the effects of the transfection were studied (i) in slices of the septal region using immunocytochemistry, and (ii) in slices of the hippocampus by measuring the electrically-evoked release of ACh.
3.2.1. Immunostaining of brain slices
Hemagglutinin immunostaining
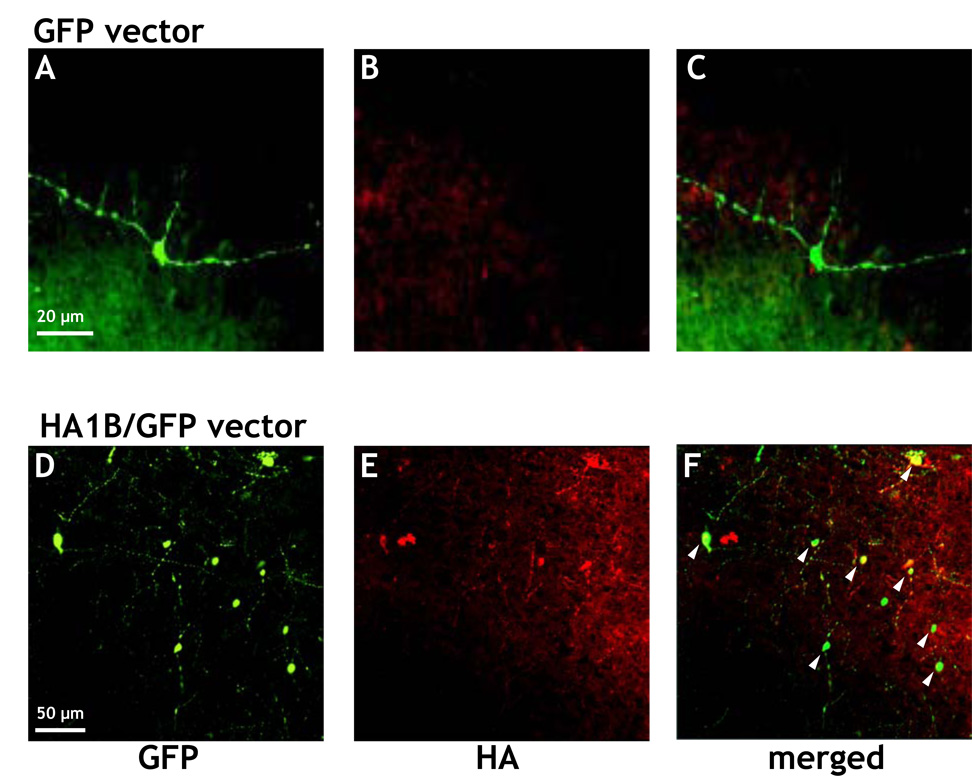
As mentioned above, a hemagglutinin (HA) epitope tag was introduced into the N-terminus of the 5-HT1B gene to allow easier detection of the transfected 5-HT1B receptor with commercially available antibodies. Anterior parts of the transfected brains (containing the septal nuclei) were fixed for subsequent immunostaining. As shown in Figure 5A, many neuronal cells of the septal region of rats treated with the GFP vector showed a green fluorescence, whereas in none of the same cells a “specific” staining for HA could be detected (Figure 5B and C). The red staining of cells in Figures 5B and C was most probably due to unspecific reactions of the HA antibody in the tissue or to autofluorescence phenomena, which are sometimes related to inflammatory processes. Conversely, septal cells of rats injected with the HA1B/GFP vector exhibited both a staining for GFP (Figure 5D), as well as for HA, which indicates the presence of the 5-HT1B receptor (Figure 5E and 5F). It should be noticed that, as mentioned above, unspecific or autofluorescence reactions of the HA antibody are also detectable in Figure 5E and F (for further remarks: see discussion).
Figure 5.
Microscopic aspect of the septal region of the rat brain after immunostaining for hemagglutinin (HA). Three days after intraseptal injection of either the GFP or the HA1B/GFP vector, the brain was fixed and slices of the septal region were prepared for immunocytochemistry using an immunostaining against HA. A, B, C: pictures from a brain infected with the GFP virus, i.e. the viral construct expressing only GFP; D, E, and F: pictures from a brain infected with the HA1B/GFP virus, i.e. the viral construct expressing both HA-5-HT1B (Hemagglutinin tag + 5-HT1B receptor) and GFP. A and D show cells expressing GFP, B and E show neurons expressing the HA-tag. In C and F the corresponding pictures are merged (A + B and D + F, respectively). The yellow color in F (not visible in C) indicates the colocalization of GFP and HA expression within the same cells (see arrows).
Choline acetyltransferase
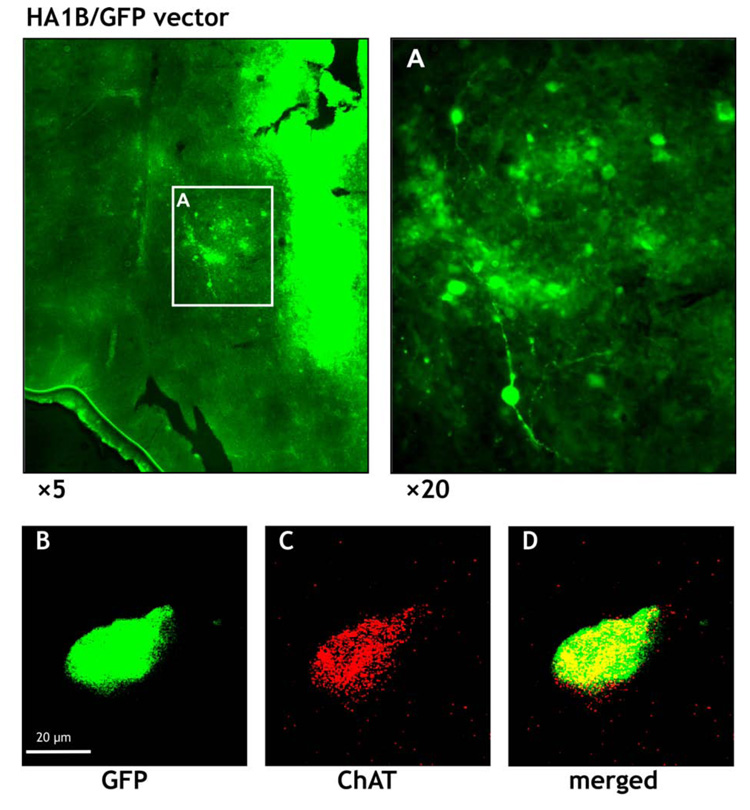
In order to check whether some of the cells transfected with the HA1B/GFP vector were cholinergic, we also performed ChAT immunocytochemistry in slices of the septal region. Figure 6A shows at two different magnifications that many cells in this area were GFP-positive. Moreover, although the staining for ChAT was very weak (Figure 6B) colocalization of ChAT in several GFP-positive cells could be detected: an example is shown in Figure 6B to D. In this context it should be noted, that the colocalization of ChAT and GFP might be underestimated, because ChAT antibodies do not penetrate well into the tissue whereas GFP expression is easily detectable at all levels of the tissue sections. For these reasons, a clearcut demonstration of a colocalization of ChAT and GFP septal neurons of HA1B/GFP treated rats was only possible using confocal laser microscopy on single GFP labelled cells as shown in Figure 6B to D.
Figure 6.
Microscopic aspect of the septal region of the rat brain after immunostaining against choline acetyltransferase (ChAT). Three days after intraseptal injection of the HA1B/GFP vector, the brain was fixed and slices of the septal region were prepared for immunocytochemistry using a Cy3 staining for ChAT positive cells. A: overview of the septal region (at two levels of magnification: ×5 and ×20) showing numerous cells expressing GFP; notice also the light green staining of the injection cannula track in the tissue (right side of the lower magnification picture). B shows a cell expressing GFP at a higher level of magnification (see scale bar) and C the same cell, but stained for ChAT. D represents the corresponding merged picture (B + C). The yellow colour in D indicates a colocalization of GFP and ChAT expression within the same cell.
3.2.2. Electrically-evoked release of [3H]ACh in hippocampal slices
Comparison of untreated rats with virus-treated rats
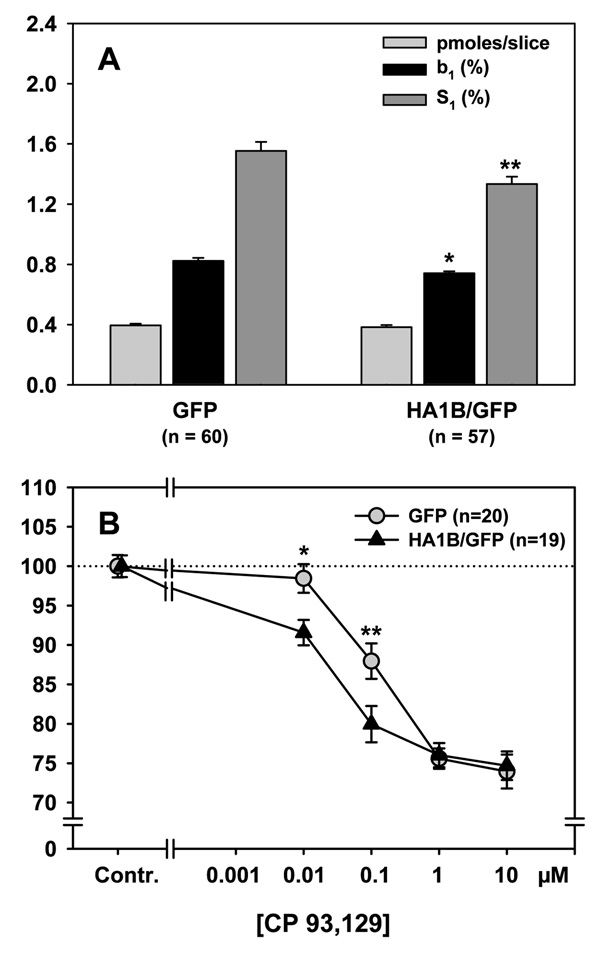
As the goal of this study was to analyse whether the overexpression of the 5-HT1B receptor in the septal area might have functional effects on the release of ACh in the hippocampus, ACh release data of the GFP group were used as controls of the corresponding data of the HA1B/GFP group (see below). Nevertheless, we first checked, whether the transfection surgery alone already affected hippocampal release of ACh. For this purpose we compared data from the GFP group (intraseptal virus injection) with those of the CON group (no surgery at all, see Methods). Data of this comparison are shown in Table 1. ANOVA of the [3H]choline accumulation in hippocampal slices showed no significant difference between GFP and CON rats (F[1,150] = 0.532; n.s.). ANOVA of the values of baseline [3H]outflow showed a significant difference between the two groups (F[1,105] = 52.76; p < 0.001), which was due to a baseline outflow that was significantly lower (by 19%) in the GFP group as compared to the CON group. Concerning the electrically-evoked [3H]overflow, ANOVA showed a weak but significant difference between the two groups (F[1,105] = 4.14; p = 0.04), which was due to a lower evoked overflow (by 17%) in the GFP group as compared to the CON group. Finally, concerning the effect of the 5-HT1B receptor selective agonist CP-93,129 on the evoked overflow of [3H]ACh, there was no difference between GFP and CON groups.
Table 1.
Comparison of hippocampal ACh release models in CON rats (no surgery) with rats (GFP-group) treated with intraseptal virus injection: tissue accumulation of [3H], basal and evoked outflow of [3H], and effects of CP-93,129 on the evoked overflow.
| Parameter | CON | GFP |
|---|---|---|
| pmoles/slice | 0.41 ± 0.01 | 0.40 ± 0.01 |
| b1 (% of tissue-3H) | 1.02 ± 0.02 | 0.82 ± 0.02*** |
| S1 (% of tissue-3H) | 1.87 ± 0.16 | 1.55 ± 0.06* |
| CP-93,129 concentration | Effects of CP-93,129 (in % of controls) | |
| 0.01 µM | 104.83 ± 1.54 | 98.44 ± 1.81 |
| 0.1 µM | 86.13 ± 1.44 | 87.96 ± 2.25 |
| 1 µM | 79.34 ± 2.03 | 75.59 ± 1.29 |
| 10 µM | 71.89 ± 1.51 | 73.95 ± 2.17 |
Hippocampal slices of CON (47 slices) and GFP rats (60 slices) were preincubated with [3H]choline, superfused and electrically stimulated as described in methods. The tissue accumulation of [3H] (in pmoles/slice), the basal [3H]outflow (in % of tissue-3H), as well as the electrically-evoked overflow of [3H] at S1 (in % of tissue-3H) are given. The effects of the 5-HT1B receptor agonist CP-93,129 added before Sn are shown as the ratios (Sn/S1) of the evoked overflows of [3H] elicited at the corresponding stimulations (in % of the respective drug-free control values); means (± S.E.M); statistics
p < 0.05
p < 0.001 vs. group CON (ANOVA, see Results section).
In the subsequent experiments [3H]ACh release data from hippocampal slices of the GFP group were compared with those of the HA1B/GFP group.
Accumulation of [3H]choline by hippocampal slices
Data (in pmoles/slice) are shown in Figure 7A (light grey bars). ANOVA of the [3H]choline accumulation showed no significant difference between the two groups GFP and HA1B/GFP (F[1,115] = 0.491; n.s.).
Figure 7.
Electrically-evoked release of [3H]ACh and its 5-HT1B receptor-mediated modulation in rat hippocampal slices. Three days after stereotaxic injection of either GFP or HA1B/GFP viral vectors into the rat septal region in-vivo, hippocampal slices were prepared from the brains and preincubated with [3H]choline. Subsequently they were washed, superfused continuously in the presence of hemicholinium-3 (10 µM) and stimulated by electrical fields up to three times (S1, S2, S3: see methods). A: Accumulation of [3H]choline (in pmoles/slice) into the hippocampal slices, as well as basal outflow of [3H] (b1 in % of tissue-3H) and electrically-evoked overflow of [3H] at S1 (in % of tissue-3H) from the hippocampal slices; means + S.E.M; n = number of slices. B: Effects of various concentrations of the 5-HT1B receptor agonist CP-93,129 added before Sn on the evoked overflow of [3H]. Effects of CP-93,129 are shown as the ratio Sn/S1 (in % of drug free controls) of the evoked [3H]overflow elicited at the corresponding stimulation periods. Means ± S.E.M.; n = 19–20 per CP-93,129 concentration; *p < 0.05, **p < 0.01 vs. group GFP; for further statistics see Results.
Baseline outflow of tritium in hippocampal slices (b1)
Data are presented in Figure 7A (black bars). Statistical analyses were performed on both absolute (b1-values in nCi) and relative values (b1-values in % of accumulated [3H] in each slice), but only relative values are illustrated in the graph. ANOVA of the baseline [3H]outflow from hippocampal slices showed a significant difference between the GFP and the HA1B/GFP groups on both absolute (F[1,115] = 5.57; p < 0.05) and relative values (F[1,115] = 11.92; p < 0.01). This difference was due to a baseline [3H]outflow that was significantly lower (by about 10%) in rats of the HA1B/GFP group as compared to those of the GFP group.
Electrically-evoked overflow of tritium in hippocampal slices (S1)
Data are shown in Figure 7A (dark grey bars). As for the baseline, statistical analyses were performed on both absolute (S1 in nCi) and relative (S1 in % of accumulated [3H] in each slice) values, but only relative values are illustrated in Figure 7A. ANOVA of the electrically-evoked [3H]overflow from hippocampal slices showed a significant difference between the two groups on both absolute (F[1,115] = 8.98; p < 0.01) and relative values (F[1,115] = 8.43; p < 0.01). This difference was due to an electrically-evoked [3H]overflow that was significantly lower (by 15%) in slices from rats of the HA1B/GFP group as compared to those from rats of the GFP group.
Effect of CP-93,129 on the evoked overflow of [3H] in hippocampal slices
Data are shown in Figure 7B. The selective 5-HT1B receptor agonist CP-93,129 was added to the superfusion buffer from 8 min before S2 and S3 onwards with concentrations increasing from S2 to S3. Indeed, some slices were successively subjected to 0.01 (S2) and 1 µM of CP-93,129 (S3) whereas another subset was subjected to 0.1 (S2) and 10 µM (S3) of the same drug. Therefore, to compare the effect of CP-93,129 in GFP and HA1B/GFP groups on ACh release, a three-way ANOVA, including repeated measures (the same slices were subjected to two concentrations of the same drug) was performed, comprising the following factors: Vector (two levels: GFP or HA1B/GFP vector), Drug condition (three levels: control [no drug] or “CP1” [ 0.01 or 1 µM of CP-93,129] or “CP2” [0.1 or 10 µM of CP-93,129]). The repeated measures factor was considered as Stimulation rank and corresponded to S2/S1 or S3/S1 values in % of the corresponding control. ANOVA showed that there was an overall significant effect of Drug condition (F[2,111] = 66.99; p < 0.001) and of the Stimulation rank (F[1,111] = 153.44; p < 0.001), as well as a significant interaction between Vector and Stimulation rank (F[1,111] = 11.86; p < 0.001) and a significant interaction between Drug condition and Stimulation rank (F[2,111] = 50.99; p < 0.001). The interaction between all factors was close to significance (F[2,111] = 3.03; p = 0.052). The Drug condition effect was due to a significant inhibitory effect of CP-93,129 in both groups as compared to control slices. The interaction between the different factors was due to a significant decrease of the [3H]ACh release when the concentration of CP-93,129 was increased, which was found in both groups. Indeed, for the GFP group, 0.1 µM of CP-93,129 decreased the [3H]ACh release by 12% (p < 0.001), 1 µM by 24% (p < 0.001) and 10 µM by 26% (p < 0.001) as compared to controls. The lowest concentration of CP-93,129 (0.01 µ M) failed to significantly inhibit [3H]ACh release in this group (p = 0.41). For the HA1B/GFP group, 0.01 µM of CP-93,129 decreased the [3H]ACh release by 9% (p < 0.001), 0.1 µM by 20% (p < 0.001), 1 µM by 24% (p < 0.001) and 10 µM by 25% (p < 0.001) as compared to controls, indicating a shift to the left of the concentration-response curve in the HA1B/GFP group as compared to the GFP group. This was confirmed by the post-hoc comparisons, which showed that for the concentrations 0.01 and 0.1 µM, CP-93,129 decreased the release of [3H]ACh with a significantly higher potency in the HA1B/GFP group as compared to the GFP group (p < 0.001 for both concentrations).
Both in the GFP and HA1B/GFP groups the inhibitory effect of 0.1 µM CP-93,129 was completely antagonized in the presence of the selective 5-HT1B antagonist GR-55,562 (1 µM; [56]) throughout superfusion (GFP group: 96.6 ± 5.4% of controls; HA1B/GFP group: 99.3 ± 4.2% of controls, respectively). The presence of GR-55,562 throughout superfusion significantly increased the basal (b1) but not the evoked overflow of [3H] at S1 in the GFP group, whereas no significant changes were observed in the HA1B/GFP group (data not shown).
4. Discussion
The present series of experiments used a viral gene transfer technique to induce an increased expression of the 5-HT1B receptor in cholinergic neurons of the septal region. Herpes simplex viral particles were used as vectors for the expression of GFP or of the 5-HT1B receptor, which were transfected either alone or together (“GFP vector”, expressing only GFP, or “HA1B/GFP vector”, expressing HA-tagged 5-HT1B receptor and GFP, respectively; see [18]). To analyse the ability of these vectors to express the proteins in question, the study was performed in two steps. The first step consisted in an in-vitro approach, in which the growth medium of primary neuronal cultures prepared from the fetal septal region [22] was infected with the viral vectors to allow an analysis of the functional effects of an increased expression of the 5-HT1B receptor at a more cellular level. The second step relied upon stereotaxic injections of the same viral particles into the septal region of the rat basal forebrain in order to enhance the expression of the 5-HT1B receptor in the target region of cholinergic neurons in the medial septum/diagonal band of Broca, namely the hippocampus. In each of these approaches, transfection-induced expression of the 5-HT1B receptor was verified by immunocytochemical techniques, whereas its functional consequences were assessed by measuring the modulation of the electrically-evoked ACh release. For the latter purpose, septal cell cultures or hippocampal slices, respectively, were preincubated with [3H]choline, superfused and stimulated electrically. Since electrically-evoked [3H]overflow both from septal cell cultures [22] and brain slices (e.g. [30; 39; 45]) pre-incubated with [3H]choline has been shown to reflect action-potential-induced, exocytotic release of authentic acetylcholine (ACh), mainly the expression “evoked release of ACh” will be used hereafter.
4.1. Increased expression of 5-HT1B receptors in-vitro: primary cholinergic cell cultures
Since we wanted to study the functional consequences of an increased expression of the 5-HT1B receptor in the axon terminals of the septo-hippocampal cholinergic pathway [34; 46], primary cell cultures were prepared from the embryonic septal region at E17 for the in-vitro study. We have previously shown that such septal cultures consisted of a mixture of non-neuronal (mainly astroglial) and neuronal cells and that some of the latter could be identified immunohistochemically as cholinergic [22]. Also in the present study GFAP-positive astroglial cells (Figure 1) and ChAT-positive cholinergic (Figure 3) were observed in primary cultures of the fetal septal area. Moreover, GAD67-positive GABAergic neurons (Figure 1E) were detected. Besides an increase in ChAT-positive neurons during growth, the previous study [22] also showed that following electrical field stimulation of such cultures, an action potential-induced and exocytotic release of ACh was easily detectable. Moreover, this evoked release of ACh was modulated by presynaptic muscarinic autoreceptors as well as by adenosine A1 and µ-opioid heteroreceptors [22]. As in a recent paper of our group [21], the present study extends this list of presynaptic receptors: the significant inhibitory effects (see Figure 3) of the selective 5-HT1B agonist CP-93,129 (see: [33]), suggests that also ACh release-modulating 5-HT1B receptors are present and functional on cholinergic neurons in cultured septal cells, although their subcellular localization (soma, dendrites or axon terminals) still has to be established.
In the present work, we confirmed the well established neurotropism of the HSV vector: as already described by Clark et al. [18], GFAP-positive astroglial cells visibly did not express GFP (see Figure 1; cf. also Barot et al. [4]). Moreover, as substantiated by the present data, some but not all of the GFP-positive neuronal cells in the septal culture were also positive for the cholinergic marker ChAT (Figure 3); in addition, following infection with the vector HA1B/GFP, GFP-positive neurons were also positive for the hemagglutinin-tag. Since the GFP and HA1B proteins are coded for by the same plasmid but different cassettes, they are necessarily co-expressed. Moreover, because HA is an epitope tag fused to the 5-HT1B receptor coding sequence (with the additional advantage of being a cytoplasmic marker that is heavily expressed in the cell body), the demonstration of colocalization of HA and GFP infers also colocalization of the 5-HT1B receptor and GFP. Taken together, these observations suggest that (at least some of) the cholinergic cells in the culture have been transfected with the gene coding for the 5-HT1B receptor. It should be noted, however, that in addition to cholinergic neurons in the culture, also GABAergic neurons might exhibit an increased expression of the 5-HT1B receptor (Figure 1F; see also below).
The finding (Figure 4A), that both the basal and the evoked release of ACh was unaffected by the increased expression of the 5-HT1B heteroreceptor in HA1B/GFP treated cultures is not surprising: these cultures, due to their origin - i.e., the fetal septal area which is free of serotonergic cell bodies [34; 46] – should also be devoid of serotonergic neurons. Hence, due to the absence of endogenously released 5-HT in the culture, a decrease in the basal and evoked release of ACh should not be expected despite the increased expression of the 5-HT1B heteroreceptor. Nevertheless Figure 4A also shows that the expression of the 5-HT1B receptor in addition to GFP did not change the excitability and basic properties of those cholinergic neurons, although we cannot completely rule out the possibility that, due to an insufficient number of cells overexpressing these proteins, such effects might have been too small to be detected.
The assumption that septal cells treated with the HA1B/GFP virus exhibit an increased expression of functional 5-HT1B receptors is also supported by Figure 4B, which shows that the inhibitory effect of the 5-HT1B agonist CP-93,129 was significantly more pronounced as compared to cells treated with the GFP-virus only. However, the following points should be kept in mind:
As evident from Figure 1F, also GABAergic neurons in the culture might exhibit an increased expression the 5-HT1B receptor following treatment with the viral vectors. Since GABA release has been shown to be inhibited via presynaptic 5-HT1B receptors in several brain regions [2; 6; 16; 59], it may be assumed that in HA1B/GFP-treated cultures, the presence of CP-93,129 more potently inhibits GABA release than in GFP-treated cultures. Since GABA, on the other hand, has been shown to inhibit the release of ACh in various CNS regions via presynaptic GABAB receptors [40; 41; 55], a decrease in the release of GABA should be equivalent to a disinhibition of ACh release, thereby counterbalancing the inhibitory effect of the 5-HT1B receptor agonist CP-93,129.
Nevertheless, even if questions remain to be answered, this in-vitro experiment showed that, using a HSV-1 vector, it was possible to induce functional changes of the 5-HT1B heteroreceptors in septal cell cultures that contained transfected cholinergic neurons.
4.2. Increased expression of 5-HT1B receptors in-vivo: septohippocampal cholinergic neurons
Herpes simplex virus type 1 (HSV-1) is currently the most extensively used herpes virus for purposes of gene transfer in-vivo [11; 12; 35] since it allows a reliable transfer of specific cellular properties to defined brain structures. In addition, targeted gene transfer to adult animals avoids compensations during postnatal development [25; 36], often observed in knock-out (KO) mice ([10; 24; 37; 49; 51; 52; 58] for review see: [26]).
Following injection of the viral particles (GFP vector or HA1B/GFP vector, respectively) into the medial septum/diagonal band of Broca, a sphere of virus-infected, GFP-positive cells around the injection site was observed. In addition, a small but non-negligible gliosis or inflammatory response (showing up as autofluorescence) appeared at the place were the guide cannulae were lowered into the brain. Using immunostaining for HA, the epitope-tag of the transfected 5-HT1B receptor (Figure 5), it was possible to demonstrate that following HA1B/GFP vector injection, some of the GFP-positive cells were also labelled for HA and thus should also overexpress the 5-HT1B receptor. Since not all of the GFP positive cells were stained with the HA antibody it should be realized that (depending on the tissue depth) the HA antibody (like any antibody) usually only labels cells on the tissue surface, whereas every GFP expressing neuron throughout the tissue will exhibit its characteristic green fluorescence. Finally, it was evident (Figure 6) that also (ChAT-positive) cholinergic cell bodies in the medial septum/diagonal band of Broca were infected with the HA1B/GFP vector. Moreover, it should be emphasized that Neumaier’s group recently showed (see Figure 1 in: [4]) that stereotaxical injection of the same viral vectors into the shell of the nucleus accumbens led to an expression of the transgene mostly in medium spiny neurons of this region and subsequently – as confirmed by western blots - to an overexpression of the HA-5-HT1B protein in the projection field of these medium spiny neurons, the ventral tegmental area.
Thus our present results confirm that (1) it is possible to infect neurons of the medial septum/diagonal band of Broca with this type of viral vector, and that (2) some of the infected neurons are cholinergic. However, although a quantitative assessment was not possible, we noticed that not all cholinergic neurons underwent transfection. Therefore, one may wonder whether the number of injected viral particles was high enough. Recently, targeted gene transfer into the ventral tegmental area (VTA) was studied using different combinations of viral concentration and injection volumes [13] (see also [43]). These authors concluded that in the VTA the sphere of transgene-expressing neurons was substantially larger with a 2 µl than with a 1 µl microinjection volume, independent from the vector titer; in addition they underlined that increasing the vector titer would simply cause more infections per cell rather than increasing the number of infected cells [13]. In the present study, the titer of the vector was of 1 – 2 × 108 I.U. /ml and a total of 4 µ l were injected at two depths of the medial septum/diagonal band of Broca.
Although the HSV-1 mediated gene transfer seems to be neuron-specific, one of the most important limitations of this technique is that it is difficult to design vectors that target specific subtypes of neurons, although this problem is less important when the vector is injected in more homogeneous structures. As the medial septum/diagonal band of Broca is the site of origin of both cholinergic and GABAergic fibers projecting to the hippocampus [34; 46], we cannot exclude that some GABAergic projection neurons were also infected. This possibility, which seems to occur following injection of the viral vector into the Nucleus accumbens [31; 42], was not tested in the present study, but the following points may be emphasized: (1) Whereas several studies have shown that presynaptic 5-HT1B receptors inhibit the release of GABA in various other brain regions [2; 6; 16; 59], direct evidence is lacking for their occurrence on GABAergic terminals in the hippocampus [55]. (2) Although the main effect of GABA on septohippocampal cholinergic projection neurons seems to take place in the septal nuclei themselves, there is also some evidence for the presence of inhibitory GABAB receptors on cholinergic axon terminals in the hippocampus [40; 41; 55]. (3) Consequently, an increased expression of 5-HT1B receptors on septohippocampal GABAergic projection neurons might lead to a decrease in hippocampal GABA release and, in turn, to a disinhibition, i.e. a facilitation of hippocampal ACh release. (4) In the present study, however, a decrease rather than an increase of ACh release was observed (Figure 7A), suggesting that the enhanced expression of 5-HT1B receptor in cholinergic projection neurons from the septum overruled a (still possible) increased expression of the 5-HT1B receptor in GABAergic projection neurons. In any event, however, the targeted expression of HA1B/GFP transgene expression in septal neurons makes it very unlikely that 5-HT1B receptors arising from elsewhere (e.g. hippocampal or dorsal raphe neurons) accounted for the increased potency of CP-93,129 on [3H]ACh release from hippocampal slices.
4.3. Functional consequences of an increase in 5-HT1B receptor expression in septohippocampal cholinergic neurons
In order to check whether the intraseptal injection procedure itself affected cholinergic transmission in the hippocampus, ACh release data from hippocampal slices of the GFP group were compared with those of rats that were not subjected to surgery (group CON; see Table 1). Surprisingly it was observed that both the spontaneous (b1-values) as well as the evoked release of ACh (S1-values) were significantly lower in GFP as compared to CON rats. This difference suggests that the lesion resulting from our surgical procedures, and perhaps more particularly from the guide cannula implantation, has damaged some septohippocampal cholinergic afferents. On the other hand, the inhibitory effects of the 5-HT1B agonist CP-93,129 did not differ among GFP and CON groups, suggesting, that the 5-HT1B heteroreceptor mediated modulation of hippocampal ACh release was unaffected by the surgery. Moreover, these obervations clearly attest that the GFP group is the most appropriate control for the rats that received the HA1B/GFP vector.
Three days following stereotaxic intraseptal injection with either GFP or HA1B/GFP viral vectors, hippocampal slices of these two groups accumulated similar amounts of [3H]choline, suggesting that the density of cholinergic afferents to the hippocampus was about similar. On the other hand, it was observed that both the spontaneous (b1-values) as well as the evoked release of ACh (S1-values) were significantly lower in HA1B/GFP treated as compared to GFP treated rats (Figure 7A). This observation suggests, that endogenously released 5-HT arising from raphé projections to the hippocampus inhibits the evoked release of ACh more effectively in rats with increased 5-HT1B receptor expression on its septohippocampal cholinergic axon terminals than in rats of the GFP group. This suggestion is supported by the higher potency of the exogenous 5-HT1B receptor agonist CP-93,129 (Figure 7B) on the evoked release of ACh. Our data thus confirm that intraseptal injection of the HA1B/GFP vector not only resulted in transfection of septal cholinergic neurons, as demonstrated by the immunocytochemical changes described above, but also affected the modulation of ACh release in the target region of the septal projections, the hippocampus. Thus it has to be assumed that the density of presynaptic 5-HT1B receptors on hippocampal cholinergic afferents of the HA1B/GFP group is higher than in the GFP group. Since the concentration response curve of CP-93,129 was shifted to the left but reached the same maximal inhibitory effect, the number of spare receptors (the receptor reserve) might be increased by the transfection, although other explanations (e.g. changes in affinity or improved coupling to signal transduction) are also possible. Thus, lower concentrations of the 5-HT1B receptor agonist resulted in higher levels of inhibition of hippocampal ACh release. Manipulating the level of 5-HT1B receptor expression may also be relevant to pathophysiological phenomena, given the plasticity of 5-HT1B mRNA expression levels that has been detected in various brain regions following behavioural stress exposure [32]. Moreover, septal 5-HT1B mRNA levels may also be altered by various environmental factors; indeed, an about 50% reduction in septal 5-HT1B mRNA in old (24 months) vs. young (3 months) rats has been detected recently (J.F. Neumaier, personal unpublished data).
4.4. Conclusions
The present investigation shows for the first time functional consequences of an increased expression of 5-HT1B heteroreceptors on septohippocampal cholinergic projection neurons. In view of the suggested cholinergic-serotonergic interactions in learning and memory (see Introduction), future experiments will now have to examine if an increased expression of 5-HT1B heteroreceptors on septohippocampal cholinergic neurons is accompanied by behavioural modifications, for instance in cognitive tests. However, especially with regard to behavioural tests of cognition, which are always time consuming and often require experiments extending over days and weeks, the present transfection technique, despite its regional selectivity, may cause technical problems, because the increased expression seems to be only transitory (see above). We want to emphasize, however, that transient expression of the viral transfection vector has also clear-cut advantages: for instance, it may sensitize animals to subsequent treatments with drugs of abuse, and, more generally, enable a powerful within-subject approach of various integrated effects of an increased expression of 5-HT1B receptors, as a control can be used before the transfection, and another one once the overexpression has vanished.
Acknowledgements
The financial support of the Deutsche Forschungsgemeinschaft (Ja 244/5-1/2 to R.J.), of the National Institute of Mental Health (NIMH MH63303 to J.F.N.), as well as the fellowship granted to C.R. by Neurex, the French Ministère de la Recherche and the European Doctoral College of Strasbourg are gratefully acknowledged.
Abbreviations
- ACh
acetylcholine
- ANOVA
analysis of variance
- ChAT
choline acetyltransferase
- CNS
central nervous system
- CP-93,129
3-(1,2,5,6-tetrahydropyrid-4-yl)pyrrollo[3,2-b]pyrid-5-one
- GAD67
glutamate decarboxylase 67
- GFAP
Glial fibrillary acidic protein
- GFP
green fluorescent protein
- GR-55,562
3-[3-(Dimethylamino)propyl]-4-hydroxy-N-[4-(4-pyridinyl)phenyl]benzamide dihydrochloride
- HA
hemagglutinin
- HSV
herpes simplex virus
References
- 1.Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The rat nervous system. New York: Elsevier Academic Press; 1995. pp. 443–493. [Google Scholar]
- 2.Bagdy E, Kiraly I, Harsing LG., Jr Reciprocal innervation between serotonergic and GABAergic neurons in raphe nuclei of the rat. Neurochem Res. 2000;25:1465–1473. doi: 10.1023/a:1007672008297. [DOI] [PubMed] [Google Scholar]
- 3.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 4.Barot SK, Ferguson SM, Neumaier JF. 5-HT(1B) receptors in nucleus accumbens efferents enhance both rewarding and aversive effects of cocaine. Eur. J. Neurosci. 2007;25:3125–3131. doi: 10.1111/j.1460-9568.2007.05568.x. [DOI] [PubMed] [Google Scholar]
- 5.Birthelmer A, Ehret A, Riegert C, Rothmaier AK, Leemhuis J, Jackisch R. Modulation of electrically evoked serotonin release in cultured rat raphe neurons. J. Neurochem. 2007;100:1613–1625. doi: 10.1111/j.1471-4159.2006.04287.x. [DOI] [PubMed] [Google Scholar]
- 6.Bramley JR, Sollars PJ, Pickard GE, Dudek FE. 5-HT1B receptor-mediated presynaptic inhibition of GABA release in the suprachiasmatic nucleus. J Neurophysiol. 2005;93:3157–3164. doi: 10.1152/jn.00770.2004. [DOI] [PubMed] [Google Scholar]
- 7.Buhot MC. Serotonin receptors in cognitive behaviors. Curr. Opin. Neurobiol. 1997;7:243–254. doi: 10.1016/s0959-4388(97)80013-x. [DOI] [PubMed] [Google Scholar]
- 8.Buhot MC, Martin S, Segu L. Role of serotonin in memory impairment. Ann. Med. 2000;32:210–221. doi: 10.3109/07853890008998828. [DOI] [PubMed] [Google Scholar]
- 9.Buhot MC, Wolff M, Benhassine N, Costet P, Hen R, Segu L. Spatial learning in the 5-HT1B receptor knockout mouse: selective facilitation/impairment depending on the cognitive demand. Learn. Memory. 2003;10:466–477. doi: 10.1101/lm.60203. [DOI] [PubMed] [Google Scholar]
- 10.Buhot MC, Wolff M, Savova M, Malleret G, Hen R, Segu L. Protective effect of 5-HT1B receptor gene deletion on the age-related decline in spatial learning abilities in mice. Behav. Brain Res. 2003;142:135–142. doi: 10.1016/s0166-4328(02)00400-x. [DOI] [PubMed] [Google Scholar]
- 11.Burton EA, Fink DJ, Glorioso JC. Gene delivery using herpes simplex virus vectors. DNA Cell Biol. 2002;21:915–936. doi: 10.1089/104454902762053864. [DOI] [PubMed] [Google Scholar]
- 12.Burton EA, Wechuck JB, Wendell SK, Goins WF, Fink DJ, Glorioso JC. Multiple applications for replication-defective herpes simplex virus vectors. Stem Cells. 2001;19:358–377. doi: 10.1634/stemcells.19-5-358. [DOI] [PubMed] [Google Scholar]
- 13.Carlezon WA, Jr, Neve RL. Viral-mediated gene transfer to study the behavioral correlates of CREB function in the nucleus accumbens of rats. Methods Mol Med. 2003;79:331–350. doi: 10.1385/1-59259-358-5:331. [DOI] [PubMed] [Google Scholar]
- 14.Cassel JC, Jeltsch H. Serotonergic modulation of cholinergic function in the central nervous system: cognitive implications. Neurosci. 1995;69:1–41. doi: 10.1016/0306-4522(95)00241-a. [DOI] [PubMed] [Google Scholar]
- 15.Cassel JC, Jeltsch H, Neufang B, Lauth D, Szabo B, Jackisch R. Downregulation of muscarinic- and 5-HT1B-mediated modulation of [3H]acetylcholine release in hippocampal slices of rats with fimbria-fornix lesions and intrahippocampal grafts of septal origin. Brain Res. 1995;704:153–166. doi: 10.1016/0006-8993(95)01092-0. [DOI] [PubMed] [Google Scholar]
- 16.Chadha A, Sur C, Atack J, Duty S. The 5HT(1B) receptor agonist, CP-93129, inhibits [(3)H]-GABA release from rat globus pallidus slices and reverses akinesia following intrapallidal injection in the reserpine-treated rat. Br. J. Pharmacol. 2000;130:1927–1932. doi: 10.1038/sj.bjp.0703526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark MS, Neumaier JF. The 5-HT1B receptor: behavioral implications. Psychopharmacol Bull. 2001;35:170–185. [PubMed] [Google Scholar]
- 18.Clark MS, Sexton TJ, McClain M, Root D, Kohen R, Neumaier JF. Overexpression of 5-HT1B receptor in dorsal raphe nucleus using Herpes Simplex Virus gene transfer increases anxiety behavior after inescapable stress. J. Neurosci. 2002;22:4550–4562. doi: 10.1523/JNEUROSCI.22-11-04550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark MS, Vincow ES, Sexton TJ, Neumaier JF. Increased expression of 5-HT1B receptor in dorsal raphe nucleus decreases fear-potentiated startle in a stress dependent manner. Brain Res. 2004;1007:86–97. doi: 10.1016/j.brainres.2004.01.070. [DOI] [PubMed] [Google Scholar]
- 20.Dunnett SB, Björklund A. Staging and dissection of rat embryos. In: Dunnett SB, Björklund A, editors. Neural transplantation. A practical approach. Oxford: IRL Press; 1992. pp. 1–19. [Google Scholar]
- 21.Ehret A, Birthelmer A, Rutz S, Riegert C, Rothmaier AK, Jackisch R. Agonist-mediated regulation of presynaptic receptor function during development of rat septal neurons in culture. J. Neurochem. 2007;102:1071–1082. doi: 10.1111/j.1471-4159.2007.04598.x. [DOI] [PubMed] [Google Scholar]
- 22.Ehret A, Haaf A, Jeltsch H, Heimrich B, Feuerstein TJ, Jackisch R. Modulation of electrically evoked acetylcholine release in cultured rat septal neurones. J. Neurochem. 2001;76:555–564. doi: 10.1046/j.1471-4159.2001.00030.x. [DOI] [PubMed] [Google Scholar]
- 23.Fink KB, Göthert M. 5-HT receptor-mediated modulation of neurotransmitter release in the CNS: role of presynaptic 5-HT heteroreceptors and GABAergic interneurons. Pharmacol Rev. 2007;59 doi: 10.1124/pr.107.07103. (in press) [DOI] [PubMed] [Google Scholar]
- 24.Gardier AM, Gruwez B, Trillat AC, Jacquot C, Hen R, Bourin M. Interaction between 5-HT(1A) and 5-HT(1B) receptors: effects of 8-OH-DPAT-induced hypothermia in 5-HT(1B) receptor knockout mice. Eur. J. Pharmacol. 2001;421:171–175. doi: 10.1016/s0014-2999(01)01037-8. [DOI] [PubMed] [Google Scholar]
- 25.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 26.Gingrich JA, Hen R. Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology (Berl) 2001;155:1–10. doi: 10.1007/s002130000573. [DOI] [PubMed] [Google Scholar]
- 27.Göthert M. Presynaptic serotonin receptors in the central nervous system. Ann N Y Acad Sci. 1990;604:102–112. doi: 10.1111/j.1749-6632.1990.tb31986.x. [DOI] [PubMed] [Google Scholar]
- 28.Göthert M, Fink K, Frolich D, Likungu J, Molderings G, Schlicker E, Zentner J. Presynaptic 5-HT auto- and heteroreceptors in the human central and peripheral nervous system. Behav. Brain Res. 1996;73:89–92. doi: 10.1016/0166-4328(96)00076-9. [DOI] [PubMed] [Google Scholar]
- 29.Groenink L, van Bogaert MJ, van der Gugten J, Oosting RS, Olivier B. 5-HT1A receptor and 5-HT1B receptor knockout mice in stress and anxiety paradigms. Behav Pharmacol. 2003;14:369–383. doi: 10.1097/01.fbp.0000087737.21047.75. [DOI] [PubMed] [Google Scholar]
- 30.Hertting G, Zumstein A, Jackisch R, Hoffmann I, Starke K. Modulation by endogenous dopamine of the release of acetylcholine in the caudate nucleus of the rabbit. Naunyn-Schmiedeberg's Arch. Pharmacol. 1980;315:111–117. doi: 10.1007/BF00499253. [DOI] [PubMed] [Google Scholar]
- 31.Hoplight BJ, Sandygren NA, Neumaier JF. Increased expression of 5-HT1B receptors in rat nucleus accumbens via virally mediated gene transfer increases voluntary alcohol consumption. Alcohol. 2006;38:73–79. doi: 10.1016/j.alcohol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Hoplight BJ, Vincow ES, Neumaier JF. Cocaine increases 5-HT(1B) mRNA in rat nucleus accumbens shell neurons. Neuropharmacology. 2007;52:444–449. doi: 10.1016/j.neuropharm.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 33.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [Review] [PubMed] [Google Scholar]
- 34.Jakab RL, Leranth C. Septum. In: Paxinos G, editor. The Rat Nervous System. Amsterdam: Elsevier Academic Press; 1995. pp. 405–442. [Google Scholar]
- 35.Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7:33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 36.Luo X, Persico AM, Lauder JM. Serotonergic regulation of somatosensory cortical development: lessons from genetic mouse models. Dev Neurosci. 2003;25:173–183. doi: 10.1159/000072266. [DOI] [PubMed] [Google Scholar]
- 37.Malleret G, Hen R, Guillou JL, Segu L, Buhot MC. 5-HT1B receptor knockout mice exhibit increased exploratory activity and enhanced spatial memory performance in the Morris water maze. J. Neurosci. 1999;19:6157–6168. doi: 10.1523/JNEUROSCI.19-14-06157.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maura G, Raiteri M. Cholinergic terminals in rat hippocampus possess 5-HT1B receptors mediating inhibition of acetylcholine release. Eur J Pharmacol. 1986;129:333–337. doi: 10.1016/0014-2999(86)90443-7. [DOI] [PubMed] [Google Scholar]
- 39.Molenaar PC, Nickolson VJ, Polak RL. Preferential release of newly synthesized 3H-acetylcholine from rat cerebral cortex slices in vitro. British Journal of Pharmacology. 1973;47:97–108. doi: 10.1111/j.1476-5381.1973.tb08162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moor E, DeBoer P, Westerink BH. GABA receptors and benzodiazepine binding sites modulate hippocampal acetylcholine release in vivo. Eur. J. Pharmacol. 1998;359:119–126. doi: 10.1016/s0014-2999(98)00642-6. [DOI] [PubMed] [Google Scholar]
- 41.Nava F, Carta G, Bortolato M, Gessa GL. gamma-Hydroxybutyric acid and baclofen decrease extracellular acetylcholine levels in the hippocampus via GABA(B) receptors. Eur. J. Pharmacol. 2001;430:261–263. doi: 10.1016/s0014-2999(01)01163-3. [DOI] [PubMed] [Google Scholar]
- 42.Neumaier JF, Vincow ES, Arvanitogiannis A, Wise RA, Carlezon WA., Jr Elevated expression of 5-HT1B receptors in nucleus accumbens efferents sensitizes animals to cocaine. J. Neurosci. 2002;22:10856–10863. doi: 10.1523/JNEUROSCI.22-24-10856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neve RL, Neve KA, Nestler EJ, Carlezon WA., Jr Use of herpes virus amplicon vectors to study brain disorders. Biotechniques. 2005;39:381–391. doi: 10.2144/05393PS01. [DOI] [PubMed] [Google Scholar]
- 44.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- 45.Richardson IW, Szerb JC. The release of labelled acetylcholine and choline from cerebral cortical slices stimulated electrically. Br. J. Pharmacol. 1974;52:499–507. doi: 10.1111/j.1476-5381.1974.tb09717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Risold PY. The septal region. In: Paxinos G, editor. The Rat Nervous System. Amsterdam: Elsevier Academic Press; 2004. pp. 605–632. [Google Scholar]
- 47.Ruotsalainen S, Miettinen R, MacDonald E, Riekkinen M, Sirvio J. The role of the dorsal raphe-serotonergic system and cholinergic receptors in the modulation of working memory. Neuroscience and Biobehavioral Reviews. 1998;22:21–31. doi: 10.1016/s0149-7634(96)00065-6. [DOI] [PubMed] [Google Scholar]
- 48.Rutz S, Riegert C, Rothmaier AK, Buhot MC, Cassel JC, Jackisch R. Presynaptic serotonergic modulation of 5-HT and acetylcholine release in the hippocampus and the cortex of 5-HT1B-receptor knockout mice. Brain Res Bull. 2006;70:81–93. doi: 10.1016/j.brainresbull.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Rutz S, Riegert C, Rothmaier AK, Buhot MC, Cassel JC, Jackisch R. Presynaptic serotonergic modulation of 5-HT and acetylcholine release in the hippocampus and the cortex of 5-HT(1B)-receptor knockout mice. Brain Res. Bull. 2006;70:81–93. doi: 10.1016/j.brainresbull.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28:565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot MC, Hen R. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- 52.Shippenberg TS, Hen R, He M. Region-specific enhancement of basal extracellular and cocaine-evoked dopamine levels following constitutive deletion of the Serotonin(1B) receptor. J. Neurochem. 2000;75:258–265. doi: 10.1046/j.1471-4159.2000.0750258.x. [DOI] [PubMed] [Google Scholar]
- 53.Starke K, Göthert M, Kilbinger H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol. Rev. 1989;69:864–989. doi: 10.1152/physrev.1989.69.3.864. [DOI] [PubMed] [Google Scholar]
- 54.Steckler T, Sahgal A. The role of serotonergic-cholinergic interactions in the mediation of cognitive behaviour. Behav. Brain Res. 1995;67:165–199. doi: 10.1016/0166-4328(94)00157-b. [DOI] [PubMed] [Google Scholar]
- 55.Vizi ES, Kiss JP. Neurochemistry and pharmacology of the major hippocampal transmitter systems: synaptic and nonsynaptic interactions. Hippocampus. 1998;8:566–607. doi: 10.1002/(SICI)1098-1063(1998)8:6<566::AID-HIPO2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 56.Walsh DM, Beattie DT, Connor HE. The activity of 5-HT1D receptor ligands at cloned human 5-HT1D alpha and 5-HT1D beta receptors. Eur. J. Pharmacol. 1995;287:79–84. doi: 10.1016/0014-2999(95)00612-1. [DOI] [PubMed] [Google Scholar]
- 57.Winer R. Statistical principles in experimental design. New York, London: McGraw - Hill; 1971. [Google Scholar]
- 58.Wolff M, Savova M, Malleret G, Hen R, Segu L, Buhot MC. Serotonin 1B knockout mice exhibit a task-dependent selective learning facilitation. Neurosci. Lett. 2003;338:1–4. doi: 10.1016/s0304-3940(02)01339-3. [DOI] [PubMed] [Google Scholar]
- 59.Yan QS, Yan SE. Serotonin-1B receptor-mediated inhibition of [(3)H]GABA release from rat ventral tegmental area slices. J. Neurochem. 2001;79:914–922. doi: 10.1046/j.1471-4159.2001.00643.x. [DOI] [PubMed] [Google Scholar]