Abstract
Although studies of Ames and Snell dwarf mice have suggested possible important roles of the growth hormone (GH)/insulin-like growth factor-1 (IGF-1) axis in aging and age-related diseases, the results cannot rule out the possibility of other hormonal changes playing an important role in the life extension exhibited by these dwarf mice. Therefore, growth hormone receptor/binding protein (GHR/BP) knockout (KO) mice would be valuable animals to directly assess the roles of somatotropic axis in aging and age-related diseases because the primary hormonal change is due to GH/IGF-1 deficiency. Our pathological findings showed GHR/BP KO mice to have a lower incidence and delayed occurrence of fatal neoplastic lesions compared with their wild-type littermates. These changes of fatal neoplasms are similar to the effects observed with calorie restriction and therefore could possibly be a major contributing factor to the extended life span observed in the GHR/BP KO mice.
Keywords: Growth hormone receptor/binding protein, Knockout mouse, Neoplastic disease, Aging
RECENT studies of long-lived mutant mice, such as Ames and Snell dwarf mice, strongly suggest that reduced levels of growth hormone (GH) and insulin-like growth factor-1 (IGF-1) may have important antiaging effects that contribute to extended life span of these mice (1–4). Although the results of studies from Ames and Snell dwarf mice indicate important roles of the GH/IGF-1 axis in aging, these mice also show other hormonal changes in addition to reduced GH and IGF-1. Ames and Snell dwarf mice are deficient in prolactin (PRL) and thyroid-stimulating hormone due to the developmental arrest of the anterior pituitary (5–7). Because of these hormonal changes, results from experiments with Ames and Snell dwarf mice cannot rule out other potential factors contributing to their extended life span. To examine the exact role of GH/IGF-1 axis in aging, it is of interest to conduct an aging study using an animal model in which the primary endocrine change is limited only to the GH/IGF-1 axis.
Animals with a targeted disruption of the growth hormone receptor/binding protein (GHR/BP) gene were successfully generated (8). These GHR/BP knockout (KO) mice produce GH but cannot respond to it because of the absence of GHR. Consequently, the GHR/BP KO mice are GH insensitive, exhibit a deficiency of IGF-1 in peripheral circulation, and have retarded postnatal growth (8). Because of the lack of GH action, the body weight of adult GHR/BP KO mice is less than half the weight of their wild-type (WT) littermates (9). Although the GHR/BP KO mice show the dwarf phenotype, the hormonal profile and fertility of these mice are different from those of Ames and Snell dwarf mice. Plasma PRL levels are not suppressed and, in fact, are significantly elevated (10), which may represent a compensatory response to the absence of GH signaling. Plasma levels of thyroid hormones, thyroxine (T4), and triiodo-thyronine (T3) are slightly but significantly suppressed in these animals (11), presumably as a secondary consequence of reduced IGF-1 levels (12). Both females and males are fertile, although puberty is delayed and fertility is reduced (8,10,13). In males, the effects of exogenous luteinizing hormone–releasing hormone and luteinizing hormone (LH) and the release of LH and testosterone are reduced (10). Therefore, the GHR/BP KO mouse is valuable as a model to directly examine the roles of GH/IGF-1 axis in aging.
Coschigano and colleagues (14) examined the longevity of GHR/BP KO mice and their normal siblings and found that GHR/BP KO mice live approximately 40%–55% longer than their normal siblings. The differences in the average life span between GHR/BP KO and normal mice ranged from 38% to 55%, depending on gender and using only WT (+/+) or both WT (+/+) and heterozygous KO (+/−) animals as normal controls. These results indicate that a primary defect in the GH/IGF-1 axis is sufficient to prolong life and that PRL deficiency, infertility, and/or a primary defect in the function of the pituitary–thyroid axis are not necessary for the life-extending effect (14,15).
We previously demonstrated that Ames dwarf mice show a delayed occurrence of fatal neoplastic diseases compared with WT littermates, which could be one of the major contributing factors of extended life span (16). Therefore, it was of great interest to examine the pathological profile of another long-lived mutant, GHR/BP KO mice; document the age-related disease patterns; and examine the relation between age-related disease and extended life span in the mice.
METHODS
Animal Maintenance
The mice used in this study were produced from a founder created by deletion and gene substitution of most of the fourth exon and part of the fourth intron of the GHR/BP gene (8). Their genetic background was a mix of 129Ola and BalbC. The GHR/BP KO mice used in this study were derived from mating female heterozygous (+/−) KO mice with male heterozygous KO (+/−). The basic operations of the animal colony are those previously described (14). The animals were maintained in a fully accredited vivarium by the Association for Assessment and Accreditation of Laboratory Animal Care at Southern Illinois University (Carbondale, IL). Food (LabDiet; PMI Feeds, Inc., St Louis, MO) and tap water were provided ad libitum. A 12:12 hours light/dark cycle was used. Sentinel mice housed in the same room were sacrificed for the monitoring of viral antibodies (Mouse Level II Complete Antibody Profile; CARB, Ectro, EDIM, GDVII, LCM, M. Ad-FL, M. Ad-K87, MCMV, MHV, M. pul., MPV, MVM, Polyoma, PVM, Reo, Sendai; BioReliance, Rockville, MD). Monitoring was repeated every 6 months. All tests were negative for viral antibodies tested. The animals were inspected twice daily. Date of death was recorded as the outcome measure when the mice died spontaneously. From these data, the median, mean, 10th percentile, and maximum survival for each group were determined. Mice found dead were removed from the cage and immediately necropsied.
Procedures for Examination of Pathology in Mice Dying Spontaneously
All mice were inspected at least twice daily (between 07:00 and 08:00 hours and between 15:00 and 16:00 hours). Mice that died spontaneously or sacrificed (approximately 5% of the mice) because of visible tumors were removed from the cage, immediately necropsied under gross pathological examination, and preserved in Bouin's solution. A total of 55 mice were examined: 21 GHR/BP KO mice (16 males and 5 females) and 34 normal siblings (WT: 18 males and 16 females). Approximately 10% of the mice had severe autolysis, for which we were unable to perform histopathological examination and subsequently unable to grade the lesions. Data were obtained from a total of 49 mice (19 GHR/BP KO mice [15 males and 4 females] and 30 WT [16 males and 14 females]). As there were no clear differences in major pathology observed between sexes and the sample size in each sex is too low for an independent effect of gender, the data obtained from males and females were combined.
After the mice were examined for gross pathological lesions, the following organs and tissues were excised and fixed with Bouin's solution: brain, pituitary gland, heart, lung, trachea, thymus, aorta, esophagus, stomach, small intestine, colon, liver, pancreas, spleen, kidneys, urinary bladder, reproductive system (male: prostate, testes, epididymis, seminal vesicles; female: ovaries, uterus), thyroid gland, adrenal glands, parathyroid glands, psoas muscle, knee joint, sternum, and vertebrae. Any other organ or tissue in which lesions were observed by gross inspection was excised. The fixed tissues, which were processed conventionally, were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin–eosin. Diagnosis of each histopathological change was made using histological classifications for aging mice as described by Bronson and Lipman (17).
Pathological Assessment
A list of lesions was compiled for each mouse that included both neoplastic and nonneoplastic diseases. Based on these histopathological data, tumor burden, disease burden, and severity of each lesion in each mouse were assessed. The tumor burden was calculated as the sum of the different types of tumors in each mouse. For example, a mouse that had lymphoma and adenocarcinoma had a tumor burden of 2. The disease burden was similarly calculated as the sum of the histopathological changes in a mouse. The severity of neoplastic and renal lesions was assessed using the grading system described below.
The percentage of tumor-bearing mice and overall incidence of disease were calculated for each experimental group. The percentage of tumor-bearing mice was calculated as the percentage of mice that had one or more neoplastic lesions. For this assessment, all neoplastic lesions were counted without regard to severity and thus included both incidental (not severe enough to be the cause or death) and those that were fatal (severe enough to be the cause of death).
Two pathologists separately examined all the samples without knowledge of their genotype or age.
Grading of Lesions
The severity of neoplastic lesions and glomerulonephritis was determined using grading systems previously described (16). The severity of these lesions was determined because the high prevalence of lesions allowed for a finer dissection of the effect of the genetic manipulation.
Glomerulonephritis was graded in the order of increasing severity: Grade 0, no lesions; Grade 1, minimal change in glomeruli (minimal glomerulosclerosis); Grade 2, minimal glomerulosclerosis with a few (less than 10) casts in renal tubules; Grade 3, minimal glomerulosclerosis with more than 10 casts in renal tubules; and Grade 4, glomerulosclerosis with interstitial fibrosis.
The determination for the severity of neoplastic lesions was based on previously reported criteria (16), that is, the histopathological findings of tumor cell involvement as follows: Grade 1, primary site only; Grade 2, primary site and intraorgan or one other organ metastasis; Grade 3, metastasis to two to three organs; and Grade 4, metastasis to more than four organs or Grade 3 + additional pathology, for example, pleural effusion, ascites, and subcutaneous edema. Hydrothorax, ascites, and subcutaneous edema are common complications associated with advanced neoplastic disease.
Probable Cause of Death
The probable cause of death was determined independently by the two pathologists based on the severity of the pathology found at necropsy. In cases with neoplastic lesions, mice with Grade 3 or 4 lesions were categorized as death by neoplastic disease.
In more than 90% of the cases, there was agreement by the two pathologists. In cases where the two pathologists did not agree or where disease did not appear severe enough, the cause of death was categorized as unknown.
Statistical Analysis
The total frequency and grade of lesions were compared between genotypes using a chi-square test (18). When the expected frequencies were too small for the chi-square test, the data were analyzed using the Fisher's exact test (18). The Kaplan–Meier survival curves for fatal neoplastic lesions and adenocarcinoma were analyzed using a log-rank test (19).
RESULTS
Average Life Span, Tumor-Bearing Mice, and Tumor Burden
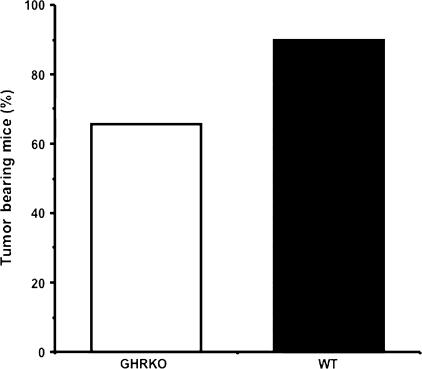
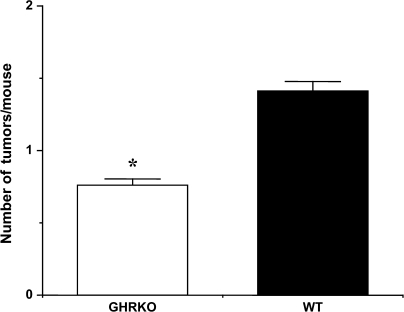
The average life span of GHR/BP KO mice and their WT littermates in this study was 1,046 and 747 days, respectively. The average life span of GHR/BP KO mice was approximately 40% longer than their WT littermates. Because our previous study demonstrated that the Ames dwarf mice and their WT littermates have a similar overall incidence of neoplastic disease (16), we first wanted to compare the percentage of tumor-bearing mice in the GHR/BP KO group and the WT littermates. Interestingly, the GHR/BP KO group had a lower percentage of tumor-bearing mice than the WT littermates. Although more than 90% of the mice in WT group had neoplastic lesions, approximately 68% of GHR/BP KO group had neoplastic lesions (Figure 1). Second, because aging rodents tend to have several different types of tumors at death, we compared the number of different types of tumors (tumor burden) for each mouse in both groups. As the data in Figure 2 show, the tumor burden for the GHR/BP KO mice is significantly less (47.5% less) than that of their WT littermates (p = .005), suggesting that the overall tumor incidence is less for GHR/BP KO mice than for their WT ittermates, which is different from previous observations in Ames dwarf mice (16).
Figure 1.
Percentage of tumor-bearing mice in GHR/BP KO group and their WT littermates. The percentage of mice with tumor(s) per group (the tumor-bearing mice) is shown. GHR/BP = growth hormone receptor/binding protein; KO = knockout; WT = wild type.
Figure 2.
Tumor burden in GHR/BP KO mice and their WT littermates. The average number of different types of tumors in mice (the tumor burden) from each group is shown. The values for the tumor burden represent the mean ± SEM for 19 GHR/BP KO and 30 WT mice. GHR/BP = growth hormone receptor/binding protein; KO = knockout; WT = wild type. *The value was significantly different (p < .05) from that of WT mice.
Probable Cause of Death
The probable causes of death for the GHR/BP KO and WT groups are shown in Table 1. Approximately 83% of WT littermates died from neoplastic diseases. The major, fatal neoplastic diseases observed in these WT mice were lymphoma, adenocarcinoma in lung, hepatocellular carcinoma, and hemangioma in the liver and spleen. And the neoplastic diseases were usually associated with metastasis to the other organs or other pathological lesions, for example, pleural effusion, ascites, hemorrhage in pleural and/or abdominal cavities, or severe congestion and edema in the lung. The GHR/BP KO mice showed a significantly lower incidence of fatal neoplasms (49% lower; p = .0002) than their WT littermates. The incidences of fatal adenocarcinoma of the lung and lymphoma were also significantly lower in GHR/BP mice (p < .05). The major, fatal nonneoplastic diseases observed in these mice were glomerulonephritis, thrombus in heart, hydronephrosis, and acidophilic macrophage pneumonia. These lesions were usually associated with other pathological lesions, for example, pleural effusion, ascites, and/or severe congestion and edema in the lung. Approximately 58% of GHR/BP KO mice died from nonneoplastic lesions, compared with approximately 17% for the WT littermates, mainly due to the high incidence of death cases in GHR/BP KO mice (approximately 47%) with no obvious evidence of lethal pathological changes.
Table 1.
Cause of Death in GHR/BP KO Mice
| Group | GHR/BP KO (N = 19) | WT (N = 30) |
| Neoplasm, n (%) | 8 (42.1)* | 25 (83.3) |
| Lymphoma | 3 (15.8)* | 9 (30.0) |
| Adenocarcinoma | 0* | 6 (20.0) |
| HCC | 3 (15.8) | 4 (13.3) |
| Other tumors | 2 | 6 |
| Nonneoplasm, n (%) | 11 (57.9) | 5 (16.7) |
| Glomerulonephritis | 1 | 0 |
| Thrombus | 1 | 1 |
| Hydronephrosis | 0 | 1 |
| Other | 9* | 3 |
Notes: GHR/BP = growth hormone receptor/binding protein; HCC = hepatocellular carcinoma; KO = knockout; WT = wild type.
*p < .05.
Age-Specific Distribution of Fatal Neoplasms
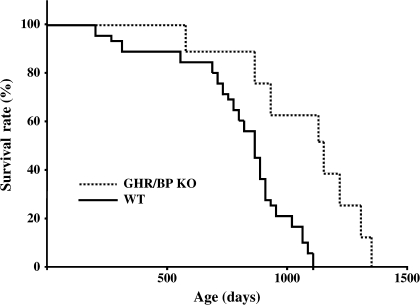
Because Ames dwarf mice showed delayed occurrences of fatal neoplastic lesions compared with their WT littermates (16), we compared Kaplan–Meier survival curves for fatal neoplastic disease in GHR/BP KO and their WT littermates. We found that the occurrence patterns of the GHR/BP KO mice tended to shift toward an older age compared with their WT littermates, as shown in Figure 3 (p = .0018). These data suggested that fatal neoplastic diseases occurred at an older age for GHR/BP KO mice than for their WT littermates. These results are similar to those found with Ames dwarf mice.
Figure 3.
Mortality by neoplastic lesions in GHR/BP KO mice and their WT littermates. Kaplan–Meier survival curves for fatal neoplastic lesions in GHR/BP KO mice and their WT littermates are shown. GHR/BP = growth hormone receptor/binding protein; KO = knockout; WT = wild type.
Severity of Diseases
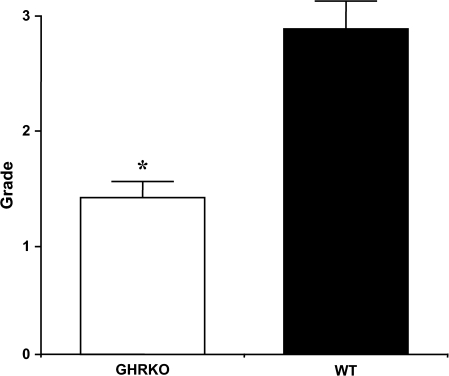
To find a possible explanation for the delayed occurrences of fatal neoplastic diseases in GHR/BP KO mice, we compared the severity of several neoplastic diseases using the grading system described in Methods section. Although GHR/BP KO mice did not have fatal pulmonary adenocarcinoma, three GHR/BP KO mice had incidental tumors. Because adenocarcinoma of the lung is the tumor Ames dwarf mice showed with less severity at death, we compared the severity of pulmonary adenocarcinoma (both fatal and incidental) in the GHR/BP KO and WT mice (Figure 4). We found the adenocarcinoma significantly less severe in GHR/BP KO mice than in their WT littermates (p = .0221). For other types of neoplasms, we generally found that the GHR/BP KO mice tended to have less severe neoplastic lesions compared with the WT control, but these apparent differences were not statistically significant.
Figure 4.
Severity of adenocarcinoma in GHR/BP KO mice and their WT littermates. The severity of adenocarcinoma was graded as follows: Grade 1, primary site only; Grade 2, primary site and intraorgan or one other organ metastasis; Grade 3, metastasis to two to three organs; and Grade 4, metastasis to more than four organs or Grade 3 + additional pathology, for example, pleural effusion, ascites, and subcutaneous edema. The average severity of adenocarcinoma in mice from each group is shown. GHR/BP = growth hormone receptor/binding protein; KO = knockout; WT = wild type. *The value was significantly different (p < .05) from that of WT mice.
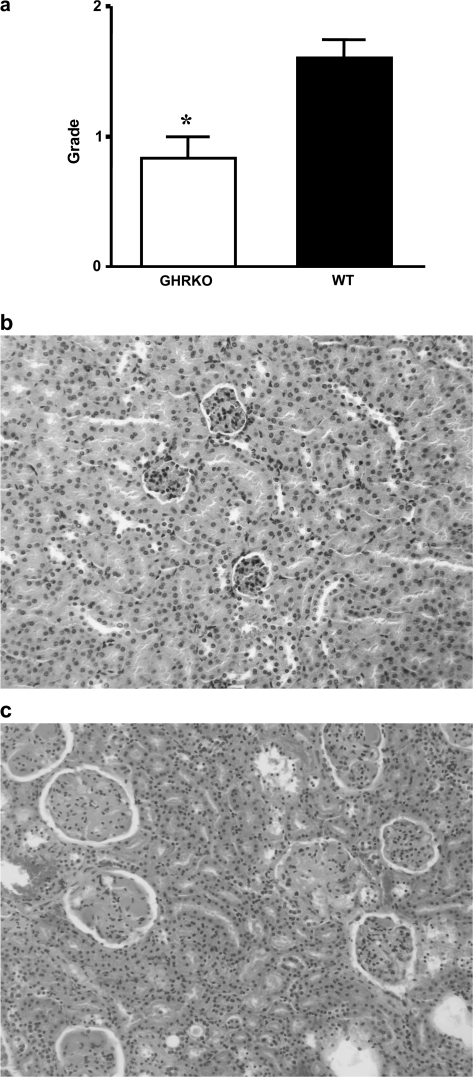
In addition to the severity of neoplastic disease, we compared the severity of nonneoplastic disease between the two groups. Because glomerulonephritis was the most common nonneoplastic lesion in these mice, we compared the severity of this lesion and found the severity of the lesions to be significantly less in GHR/BP KO mice than in their WT littermates (Figure 5a; p = .0110). Majority of GHR/BP KO mice had Grade 0 (Figure 5b) or 1 lesion, whereas many cases showed Grade 2 or 3 (Figure 5c) lesion in WT group.
Figure 5.
Severity of glomerulonephritis in GHR/BP KO mice and their WT littermates. The severity of the glomerulonephritis was scored using the criteria as follows: Glomerulonephritis was graded in the order of increasing severity: Grade 0, no lesions; Grade 1, minimal change in glomeruli (minimal glomerulosclerosis); Grade 2, Grade 1 with a few (less than 10) casts in renal tubules; Grade 3, Grade 1 with more than 10 casts in renal tubules; and Grade 4, Grade 3 with interstitial fibrosis. The average severity of glomerulonephritis in mice from each group is shown (a). *The value was significantly different (p < .05) from that of WT mice. (b) and (c) show the histopathology of kidney in GHR/BP KO (b: Grade 0) and WT (c: Grade 3) mice. GHR/BP = growth hormone receptor/binding protein; KO = knockout; WT = wild type.
Overall, these data indicated that GHR/BP KO mice had suppressed development of some neoplastic and nonneoplastic lesions, a finding that is similar to the results obtained in Ames dwarf mice (16).
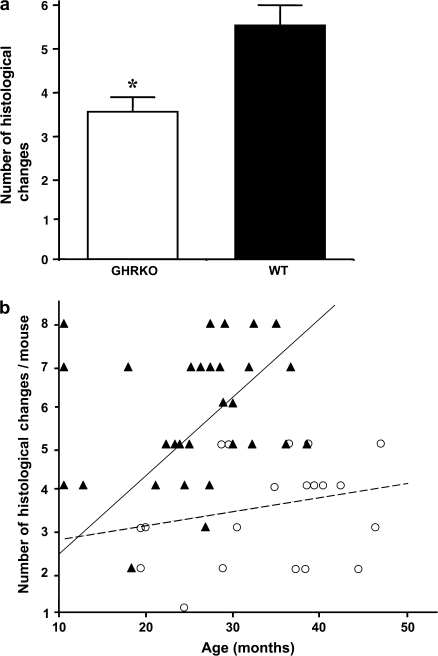
Disease Burden
The disease burden, defined as the total number of histopathological changes in a body, can serve as a good index of age-related accumulation of tissue and cell injury (17). Figure 6a shows a comparison of the disease burden between the GHR/BP KO group and their WT littermates, indicating that the GHR/BP KO group had a significantly lower (p = .0002) disease burden than their WT littermates. When the disease burden of each mouse at death was compared, GHR/BP KO mice seemed to have slower rates of accumulated histopathological changes than their WT littermates (Figure 6b), thereby showing that GHR/BP KO mice had a slower rate of tissue injury during aging, which possibly enabled them to maintain a better health status compared with their WT littermates.
Figure 6.
Disease burden in GHR/BP KO mice and their WT littermates. The average disease burden per mouse is the total number of pathological changes of any type found in individual mice from each group (a). The values for the disease burden represent the mean ± SEM for 19 GHR/BP KO and 30 WT mice (a). *The value was significantly different (p < .05) from that of WT mice. The disease burden of each mouse at death was also compared (b). The closed triangle and continuous line represent WT littermates, and the open circle and dashed line represent GHR/BP KO mice. The GHR/BP KO mice showed slower rates of accumulated histopathological changes than their WT littermates. GHR/BP = growth hormone receptor/binding protein; KO = knockout; WT = wild type.
DISCUSSION
The major aim of this study was to examine the pathological profile of GHR/BP KO mice to find possible explanations for their extended longevity. Because our previous report demonstrated that Ames dwarf mice show delayed occurrences of fatal neoplastic diseases with little change in the incidence of neoplastic diseases compared with their WT littermates (16), it is of interest to compare the pathological profiles of animals with primary hormonal change due to GH/IGF-1 deficiency. Our results demonstrated that the types of pathological changes were similar for both GHR/BP KO mice and their WT littermates in both sexes, that is, no extraordinary pathological changes were evident in the GHR/BP KO mice compared with their WT littermates. The major neoplastic lesions seen in GHR/BP KO mice and their WT littermates were lymphoma, adenocarcinoma in lung, hepatocellular carcinoma, and hemangioma in liver or spleen. The major nonneoplastic lesion found was glomerulonephritis, which develops as an animal ages. The percentage of tumor-bearing mice (the percentage of mice having neoplastic lesions in each group) was 22% lower for the GHR/BP KO mice than for their WT littermates. Also, the tumor burden (the number of different tumors found in a mouse) was significantly less (47.5% less) in GHR/BP KO mice compared with their WT littermates. GHR/BP KO mice also showed a significantly lower incidence of fatal neoplastic lesions compared with the WT littermates. In addition to the reduced incidence of total neoplastic lesions, incidences of fatal lymphoma and fatal adenocarcinoma in lung were significantly lower than in the WT littermates, which could indicate that tumorigenesis of lymphoma and pulmonary adenocarcinoma are GH/IGF-1 dependent. Thus, these data indicated that GHR/BP KO mice showed an overall suppressed occurrence of tumor development compared with their WT littermates. The changes found in the neoplastic lesions in GHR/BP KO mice were more similar to the effects of calorie restriction (CR) (17,20) than to the results from studies of Ames dwarf mice, which showed little influences on the percentage of tumor-bearing mice, tumor burden, and incidence of fatal neoplasms (16,21). In addition to the suppressed occurrence of neoplastic lesions, fatal neoplastic disease was shown to occur later in life in GHR/BP KO mice, and the severity of adenocarcinoma in these mice was significantly less compared with their WT littermates, indicating that the lack of GH action in GHR/BP KO mice seemed to delay the progression of fatal neoplastic disease.
GHR/BP KO mice also showed a delayed progression of nonneoplastic lesions. The severity of glomerulonephritis was significantly less in GHR/BP KO mice compared with their WT littermates, which also indicated that the progression of glomerulonephritis in GHR/BP KO mice is delayed, similar to that in Ames dwarf mice (16). Furthermore, compared with their WT littermates, GHR/BP KO mice showed a significantly lower disease burden and a slower age-related accumulation of various pathological changes, suggesting that GHR/BP KO mice maintained organ and whole-body integrity during aging. This implication is also supported by evidence showing that approximately 47% of GHR/BP KO mice had no obvious evidence of lethal pathology at death, which we commonly see in Ames and Snell dwarf mice (16,22) and CR C57BL/6 mice (23).
The pathological profile of the GHR/BP KO mice in this study showed more similarities to the effects of CR than to the pathological profile of Ames dwarf mice. Although Ames dwarf mice show the delayed occurrence of fatal neoplastic lesions, the incidence of neoplastic disease is affected little in these mice, which is different from the effects of CR in C57BL/6J mice (17,20) and from the GHR/BP KO mice studies here. The data obtained from studies of these three long-lived animals may provide us with an opportunity to dissect the possible common underlying anticancer and antiaging mechanism(s) of these animal models.
The common endocrine change among GHR/BP KO, Ames dwarf, and CR mice is suppressed levels of peripheral IGF-1 due to GH resistance or insensitivity (GHR/BP KO mice), lack of GH (Ames dwarf mice), or reduced plasma GH levels (CR mice, at least initially). The change in GH action, which is associated with reduced levels of peripheral IGF-1, seem to play important roles in the delayed occurrence of fatal neoplastic lesions as well as their retarded somatic growth and smaller adult body size (7,8) because IGF-1 is known for its potent mitogenic and antiapoptotic effects (24). This idea also could be supported by some epidemiological studies that indicate possible correlations between plasma IGF-1 levels and some tumors (25,26) and increased incidence and mortality from cancer by GH treatment during childhood (27).
Some nonneoplastic diseases seem to be affected by changes in GH levels or action, as was shown by the lessened severity of glomerulonephritis in both the GHR/BP KO and the Ames dwarf mice (16). Although studies of GH transgenic mice, which have pathologically high levels of GH, showed that the early death of these animals appears to be related primarily to pathological changes in the kidney [glomerulonephritis and glomerulosclerosis (24,28)], IGF-1 transgenic mice did not exhibit as severe pathological changes as the GH transgenic mice (29), indicating the important roles of GH in kidney pathology. Evidence also supports that CR rats have reduced GH levels (at least initially) (30) and that CR F344 rats show significantly reduced severity of kidney pathology compared with their ad libitum counterparts (31). Therefore, the reduced actions of GH/IGF-1 axis seem to play very important roles in the delayed progression of various age-related diseases and reduced disease burden in the body, which could be major contributing factors leading to the extended life span observed in these animals, including the GHR/BP KO mice.
GHR/BP KO mice showed significantly lower incidence of fatal neoplastic lesions compared with their WT littermates. This is similar to the effects of CR in mice (17,20,23) but different from Ames dwarf mice, which showed similar incidence of fatal neoplasms compared with their WT littermates (16). The reason(s) behind these interesting differences between GHR/BP KO, CR, and Ames dwarf mice remains to be identified. One possible explanation for these differences could be that primary changes in three major hormonal systems (somatotoropic, thyrotoropic, and lactotoropic axis), that is, the absence of three major pituitary hormones, in Ames dwarf mice may cause negative effects that counteract the anticancer actions commonly seen in long-lived animals. For example, GH and PRL have been shown to play important roles in intrathymic T-cell differentiation (32), and earlier studies show that the immune functions of Ames dwarf mice are at subnormal levels (33). Snell dwarf mice, another long-lived dwarf mouse due to hypopituitarism, also show suppressed B-cell development (34). Contrary to these two dwarf mice, GHR/BP KO mice show increased plasma PRL levels (10), which could be a compensatory mechanism because PRL and GH have some common actions. Thus, this elevated PRL level could play an important role in some of the immune functions in GHR/BP KO mice. Because cell-mediated cytotoxicity by natural killer cells is known for its destructive function of cancers cells, possible changes in immune functions could be the reason for the differences observed in the incidence of neoplastic diseases among the three long-lived animal models. Further studies are necessary to uncover the possible underlying mechanisms that could explain the differences in pathology of these animal models.
We concluded the following from this study: (i) GHR/BP KO mice had less overall incidence of neoplastic disease; (ii) GHR/BP KO mice had a significantly lower incidence of fatal neoplastic lesions, fatal lymphoma, and fatal pulmonary adenocarcinoma; (iii) GHR/BP KO mice exhibited a delayed occurrence of fatal neoplastic diseases; (iv) GHR/BP KO mice showed less severe lesions (both neoplastic and nonneoplastic) compared with their WT littermates at the time of death; (v) GHR/BP KO mice showed a reduced disease burden compared with their WT littermates at the time of death; and (vi) the pathological profiles of GHR/BP KO mice are very similar to those showing the effects of CR on age-related pathology.
We suggest that the reduced action of GH/IGF-1 axis and the subsequent pathophysiological changes could be a major underlying mechanism of life extension in long-lived animals, including GHR/BP KO, Ames dwarf, Snell dwarf, and CR mice.
FUNDING
This research was supported by National Institutes of Health grants AG13319 (Y.I.), AG19899 (A.B. and J.J.K.), CA099904-01 (J.J.K.), U24 DK059630 (J.J.K.), and DK064905 (D.E.B.); AICR grant (01A069) (Y.I.); VA Merit Review Grant (Y.I.) from Department of Veteran Affairs; grant from Glenn Foundation for Medical Research (Y.I.); the American Federation for Aging Research grant (Y.I.); The Ellison Medical Foundation grant (A.B.); Eminent Scholar Program (J.J.K.) from the State of Ohio; and the Diabetes Research Initiative grant (D.E.B. and J.J.K.) from Ohio University.
Acknowledgments
The authors thank Ms. Marisela Rodriguez for her technical support.
References
- 1.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 2.Miller RA. Kleemeier award lecture: are there genes for aging? J Gerontol A Biol Sci Med Sci. 1999;54:B297–B307. doi: 10.1093/gerona/54.7.b297. [DOI] [PubMed] [Google Scholar]
- 3.Bartke A, Coschigano K, Kopchick J, et al. Genes that prolong life: relationships of growth hormone and growth to aging and life span. J Gerontol A Biol Sci Med Sci. 2001;56:B340–B349. doi: 10.1093/gerona/56.8.b340. [DOI] [PubMed] [Google Scholar]
- 4.Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartke A. Histology of the anterior hypohysis, thyroid and gonads of two types of dwarf mice. Anat Rec. 1964;149:225–235. doi: 10.1002/ar.1091490206. [DOI] [PubMed] [Google Scholar]
- 6.Sornson MW, Wu W, Dasen JS, et al. Pituitary lineage determination by the prophet of pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- 7.Bartke A. The Molecular Genetics of Aging. Berlin, Germany: Springer-Verlab; 2000. Delayed aging in Ames dwarf mice. Relationships to endocrine function and body size; pp. 181–202. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y, Xu BC, Maheshwari HG, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci U S A. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin SC, Lin CR, Gukovsky I, Simmons DM, Swanson LW, Rosenfeld MG. Molecular basis of the little mouse phenotype and implications for cell type-specific growth. Nature. 1993;364:208–213. doi: 10.1038/364208a0. [DOI] [PubMed] [Google Scholar]
- 10.Chandrashekar V, Bartke A, Coschigano KT, Kopchick JJ. Pituitary and testicular function in growth hormone receptor gene knockout mice. Endocrinology. 1999;140:1082–1088. doi: 10.1210/endo.140.3.6557. [DOI] [PubMed] [Google Scholar]
- 11.Hauck SJ, Hunter WS, Danilovich N, Kopchick JJ, Bartke A. Reduced levels of thyroid hormones, insulin, and glucose, and lower body core temperature in the growth hormone receptor/binding protein knockout mouse. Exp Biol Med. 2001;226:552–558. doi: 10.1177/153537020122600607. [DOI] [PubMed] [Google Scholar]
- 12.Maiorano E, Ciampolillo A, Viale G, et al. Insulin-like growth factor I expression in thyroid tumors. Appl Immunohistochem Mol Morphol. 2000;8:110–119. doi: 10.1097/00129039-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Danilovich N, Wernsing D, Coschigano KT, Kopchick JJ, Bartke A. Deficits in female reproductive function in GH-R-KO mice; role of IGF-I. Endocrinology. 1999;140:2637–2640. doi: 10.1210/endo.140.6.6992. [DOI] [PubMed] [Google Scholar]
- 14.Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;14:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 15.Bartke A, Chandrashekar V, Bailey B, Zaczek D, Turyn D. Consequences of growth hormone (GH) overexpression and GH resistance. Neuropeptides. 2002;36:201–208. doi: 10.1054/npep.2002.0889. [DOI] [PubMed] [Google Scholar]
- 16.Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. The delayed occurrence of fatal neoplastic diseases in Ames dwarf mice: correlation to the extended longevity. J Gerontol A Biol Sci Med Sci. 2003;58:291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- 17.Bronson RT, Lipman RD. Reduction in rate of occurrence of age related lesions in dietary restricted laboratory mice. Growth Dev Aging. 1991;55:169–184. [PubMed] [Google Scholar]
- 18.Siegel S. Nonparametric Statistics for the Behavioral Sciences. New York: McGraw-Hill; 1956. [Google Scholar]
- 19.Lawless JF. Statistical Models and Methods for Lifetime Data. New York: John Wiley and Sons; 1990. [Google Scholar]
- 20.Volk MJ, Pugh TD, Kim M-J, Frith CH, Daynes RA, Ershler WB, Weindruch R. Dietary restriction from middle age attenuates age-associated lymphoma development and interleukin 6 dysregulation in C57BL/6 mice. Cancer Res. 1994;54:3054–3061. [PubMed] [Google Scholar]
- 21.Mattison JA, Wright C, Bronson RT, Roth GS, Ingram DK, Bartke A. Studies of aging in Ames dwarf mice: effects of caloric restriction. J Amer Aging Assoc. 2000;23:9–16. doi: 10.1007/s11357-000-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated Snell dwarf mice regain fertility but remain long lived and disease resistant. J Gerontol A Biol Sci Med Sci. 2004;59:1244–1250. doi: 10.1093/gerona/59.12.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeno Y, Hubbard GB, Lee S, et al. Housing density does not influence the longevity effect of calorie restriction. J Gerontol A Biol Sci Med Sci. 2005;60:1510–1517. doi: 10.1093/gerona/60.12.1510. [DOI] [PubMed] [Google Scholar]
- 24.Wanke R, Wolf E, Hermannns W, Folger S, Buchmuller T, Brem G. The GH-transgenic mouse as an experimental model for growth research: clinical and pathological studies. Horm Res. 1992;37:74–87. doi: 10.1159/000182406. [DOI] [PubMed] [Google Scholar]
- 25.Burroughs KD, Dunn SE, Barrett JC, Taylor JA. Insulin-like growth factor-1: a key regulator of human cancer risk? J Natl Cancer Inst. 1999;91:579–581. doi: 10.1093/jnci/91.7.579. [DOI] [PubMed] [Google Scholar]
- 26.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 27.Swerdlow AJ, Higgins CD, Adlard P, Preece MA. Risk of cancer in patients treated with human pituitary growth hormone in the UK, 1959–85: a cohort study. Lancet. 2002;60:273–277. doi: 10.1016/s0140-6736(02)09519-3. [DOI] [PubMed] [Google Scholar]
- 28.Yang CW, Striker LJ, Pesce C, et al. Glomerulosclerosis and body growth are mediated by different portions of bovine growth hormone: studies in transgenic mice. Lab Invest. 1993;68:62–70. [PubMed] [Google Scholar]
- 29.Doi T, Striker LJ, Quaife C, et al. Progressive glomerulosclerosis develops in transgenic mice chronically expressing growth hormone and growth hormone releasing factor but not in those expressing insulinlike growth factor-1. Am J Pathol. 1988;131:398–403. [PMC free article] [PubMed] [Google Scholar]
- 30.Sonntag WE, Xu X, Ingram RL, D'Costa A. Moderate caloric restriction alters the subcellular distribution of somatostatin mRNA and increases growth hormone pulse amplitude in aged animals. Neuroendocrinology. 1995;61:601–608. doi: 10.1159/000126885. [DOI] [PubMed] [Google Scholar]
- 31.Maeda H, Gleiser CA, Masoro EJ, Murata I, McMahan CA, Yu BP. Nutritional influences on aging of Fischer 344 rats: II. Pathology. J Gerontol. 1985;40:671–688. doi: 10.1093/geronj/40.6.671. [DOI] [PubMed] [Google Scholar]
- 32.De Mello-Coelho V, Savino W, Postel-Vinay MC, Dardenne M. Role of prolactin and growth hormone on thymus physiology. Dev Immunol. 1998;6:317–323. doi: 10.1155/1998/89782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esquifino AI, Villanúa MA, Szary A, Yau J, Bartke A. Ectopic pituitary transplants restore immunocompetence in Ames dwarf mice. Acta Endocrinol. 1991;125:67–72. doi: 10.1530/acta.0.1250067. [DOI] [PubMed] [Google Scholar]
- 34.Montecino-Rodriguez E, Clark R, Johnson A, Collins L, Dorshkind K. Defective B cell development in Snell dwarf (dw/dw) mice can be corrected by thyroxine treatment. J Immunol. 1996;157:3334–3340. [PubMed] [Google Scholar]