Abstract
Reduced insulin sensitivity and glucose intolerance have been long suspected of having important involvement in aging. Here we report that in studies of calorie restriction (CR) effects in mutant (Prop1df and growth hormone receptor knockout [GHRKO]) and normal mice, insulin sensitivity was strongly associated with longevity. Of particular interest was enhancement of the already increased insulin sensitivity in CR df/df mice in which longevity was also further extended and the lack of changes in insulin sensitivity in calorically restricted GHRKO mice in which there was no further increase in average life span. We suggest that enhanced insulin sensitivity, in conjunction with reduced insulin levels, may represent an important (although almost certainly not exclusive) mechanism of increased longevity in hypopituitary, growth hormone (GH)-resistant, and calorie-restricted animals. We also report that the effects of GH treatment on insulin sensitivity may be limited to the period of GH administration.
Keywords: Insulin, Longevity, GHRKO, Ames dwarf
MORE than 20 million people in the United States (approximately 7% of population) have diabetes mellitus. Its incidence increases to 20% of the population after age 75. The first study indicating impaired glucose metabolism in human beings older than 60 years was published in 1920 (1), and the progressive increase in glucose intolerance with advancing age has been recognized by scientists and health care professionals and studied throughout most of the 20th century. Numerous data show glucose tolerance declining as a function of age, typically beginning in the third decade of life span and continuing throughout the entire adult life span (2). In contrast, extreme human longevity seen in centenarians is associated with a low degree of insulin resistance (3). This could suggest that the exceptionally long-lived people are insulin sensitive throughout their life span and are genetically protected from an age-related decline of insulin action. Together with results obtained in different invertebrate species, these findings identify insulin signaling as one of the major mechanisms to be investigated in gerontology and aging research.
Evidence of the major involvement of growth hormone (GH) in regulating aging has been provided in studies demonstrating major alterations of life span in animals with GH deficiency, GH resistance, or GH excess. Hypopituitary Ames dwarf (Prop1df, df/df) mice that do not produce GH and mice with targeted disruption of the GH receptor gene (GH receptor knockout [GHRKO]) live much longer than their normal littermates (4,5). GH levels in rodents decline in response to reduced food intake (6), and calorie restriction (CR) has been well documented as the only intervention to consistently slow aging, prevent or delay age-related disease, and increase life span (7). However, the mechanisms of extended longevity in mutant and calorie-restricted mice are unknown. Importantly, long-lived GHRKO, Ames and Snell (Pit1dw) dwarfs, and normal calorie-restricted mice have reduced levels of both insulin and glucose in peripheral circulation (8–11).
Increased whole-animal insulin sensitivity, a characteristic that we suggest may represent one of the mechanisms of life extension, is implied by a concomitant reduction of insulin and glucose levels in these animals and was confirmed by measurements of “insulin tolerance,” that is, time course of changes in plasma glucose levels after insulin administration (8,10).
MATERIALS AND METHODS
Animals
Ames dwarf (Prop1df) homozygous recessive mice were produced by mating heterozygous females and homozygous mutant males in our breeding colony. GHRKO mice were also produced in our breeding colony derived from GHRKO animals kindly provided by J. J. Kopchick (Ohio University, Athens, OH). All animal protocols were approved by the Southern Illinois University Laboratory Animal Care Committee. Animals were maintained under temperature- and light-controlled conditions (20–23°C, 12-hour light/ dark cycle). The CR and longevity study protocols were described previously by Bartke and colleagues (12) and Bonkowski and colleagues (13).
Calorie Restriction
Ames dwarf mice were subjected to CR at the age of about 7 months old, and insulin tolerance test (ITT) was performed after 8 weeks of CR. GHRKO mice were calorically restricted starting at the age of 2 months for about 10 months, and ITT was performed when the animals were approximately 1 year old. Animals used for ITT and longevity were from different cohorts; however, all groups were maintained under the same environmental conditions and husbandry and were subjected to CR following the protocol used in the studies of the effects of CR on longevity as described previously (12,13).
GH Therapy
Starting at the age of 2 weeks, Ames dwarf mice were injected twice daily with bovine GH, 3 μg/g of body weight, from Monday to Friday and 6 μg/g of body weight once daily on Saturdays and Sundays. The treatment was continued for 6 weeks. ITT was performed 1 hour after last GH injection at the age of 2 months, and then, it was repeated 1 month after the last GH injection.
Insulin Tolerance Test
Because of the extremely small size of the animals (8–15 g of body weight), ITT instead of clamp study was chosen to measure insulin sensitivity in the present study. Animals subjected to ITT were injected intraperitoneally with 0.75 IU insulin per kilogram of body weight. Blood glucose levels were measured at 0, 15, 30, and 60 minutes using a glucometer (ONE Touch Ultra; LifeScan, Inc., Milpitas, CA).
Statistical Association Between Insulin Sensitivity and Longevity
Assessment of the association between insulin sensitivity, as measured by the ITT, and median life span was developed as follows: Glucose levels were measured at 15, 30, and 60 minutes subsequent to a baseline measure and expressed as a percentage of that baseline value. The area under the curve (AUC) for the time series was used as a measure of insulin sensitivity (smaller AUC = greater sensitivity). Repeated-measures analysis of variance was used to determine the interaction of the main effect variables, phenotype and diet. Longevity, as measured by median life span, was obtained from a separate set of animals on identical dietary protocol and being maintained under the same environmental conditions. Using these data sets imposes some limitations on the choice of statistical analysis and potential interpretations of the data; however, in the studies of longevity, animals should not be used for any experimental manipulations that could interfere with longevity outcome. All possible pairings of AUC and longevity were obtained within each treatment condition. The median AUC and longevity values derived from data for all treatment conditions were used to partition the pairs into quadrants (Q1, Q2, Q3, and Q4). Q1 consisted of all pairs for which the AUC was less than or equal to the median AUC value, and longevity was less than or equal to the median life span (AUC− and Long−). Q2 contained all pairs for which the AUC was less than or equal to the median AUC value, and longevity was greater than the median life span (AUC− and Long+). Q3 contained all the pairs in which both AUC and longevity exceeded the respective median values (AUC+ and Long+), and Q4 contained pairs for which AUC was greater than the median but longevity was less than or equal to the median (AUC+ and Long−). A chi-square test of independence was conducted on the quarter-sectioned data for the Ames dwarf (df/df), GHRKO, and their respective normal controls (Ns) on ad libitum (AL) or calorie-restricted diets.
RESULTS AND DISCUSSION
Association Between Median Life Span and Insulin Sensitivity in Ames Dwarf, GHRKO, and Calorie-Restricted Mice
In the present study, we have used the results of ITT in df/df and normal mice fed AL or subjected to 30% CR along with data previously reported by our laboratory (12,13) to relate insulin sensitivity to longevity in normal animals and in two types of long-lived mutant mice under different dietary conditions. ITT indicated that the remarkably long-lived df/df mice are more insulin sensitive than Ns (p < .0001; Figure 1) (4,8). Similar association of enhanced insulin sensitivity and increased life span was observed in GHRKO mice (Figure 2) (13). In the Ames dwarf mice study, CR increased both sensitivity to insulin and longevity of Ns (p < .0001) to levels characteristic of Ames dwarf mice (Figure 1). Moreover, CR produced further significant increases in both insulin sensitivity and life span measurements of Ames dwarf mice. Close relationship between the effects of CR on insulin sensitivity and on longevity was also observed in our study of GHRKO mice (Figure 2) (13). In this study, it was noteworthy that CR significantly increased both life span and insulin sensitivity in normal animals but had no or very little effect on the same parameters in GHRKO mice (13). In proposing insulin sensitivity as a potential mechanism or as a “biomarker” of longevity, or both, we would like to contrast the effects of CR in Ames dwarf and GHRKO mice (Figures 1 and 2) and emphasize the close correspondence of the effects of CR on longevity and insulin sensitivity. For this purpose, we have used the data from ITT collected in two different experiments, one with Ames dwarf mutants at the age of about 9 months and the other with approximately 1-year old GHRKO mice. Certainly, using only one age group places some limitations on the interpretation of the presented data; however, the data of ITT from Figure 4 and other unpublished data from Ames dwarf and GHRKO animals indicate that increased insulin sensitivity is present not only at one time point but also throughout the adult life in these animals.
Figure 1.
(A) Survival plots of Ames dwarf (Df) and normal (N) mice fed ad libitum (AL) or subjected to 30% calorie restriction (CR) starting at 2 months of age and maintained for the remainder of life span. From Bartke and colleagues (12). (B) Insulin tolerance test performed in N and Df mice indicated improved insulin sensitivity after CR in both genotypes. All groups were randomly fed (100% AL) overnight. Mice were injected intraperitoneally with insulin (0.75 U/kg of body weight). Glucose was measured in samples collected from the tail vein at specified time points. a, b, c—values that do not share the same letter are significantly different (p < .05).
Figure 2.
(A) Kaplan–Meier survival plot of normal (N) and growth hormone receptor knockout (GHRKO) mice that were fed ad libitum (AL) or subjected to 30% calorie restriction (CR) starting at 2 months of age and maintained for the remainder of life span. Data from males and females are combined. From Bonkowski and colleagues (13). (B) Results of insulin tolerance test in normal and GHRKO mice that were fed AL or subjected to 30% CR between 2 and 12 months of age. All groups were randomly fed (100% AL) overnight. Mice were injected intraperitoneally with insulin (0.75 U/kg of body weight). Glucose was measured in samples collected from the tail vein at specified time points. *p < .05 compared with AL controls within phenotype. From Bonkowski and colleagues (13).
Figure 4.
Insulin tolerance test performed in Ames dwarf mice after 6 weeks of growth hormone (GH) injections, and in control Ames dwarf and normal animals. (A) One day after the last injection of GH, insulin sensitivity of Ames dwarf mice was decreased to the level measured in normal mice. (B) One month after the last injection, insulin sensitivity of dwarf mice improved and returned to the level measured in control Ames dwarf mice. a, b—values that do not share the same letter in the superscript are significantly different (p < .05).
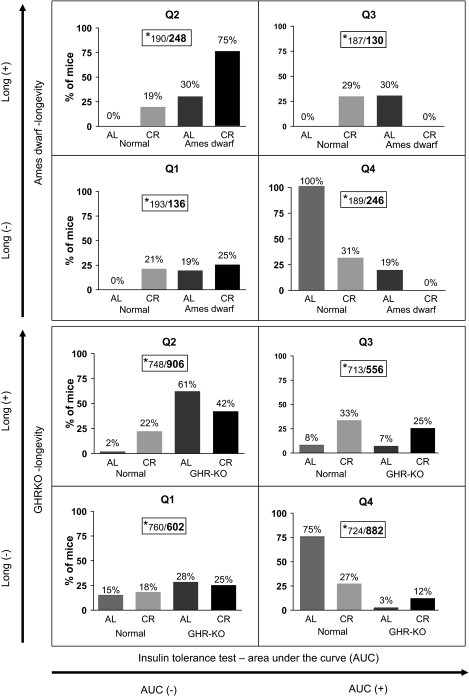
To assess the association between median life span and insulin sensitivity as measured by the AUC in ITT tests, a chi-square test of independence was conducted on the distribution of data for Ames dwarfs, GHRKOs, and their respective Ns fed AL or subjected to CR, and the results are presented in Figure 3. Chi-square test of association was chosen because the AUC and median life span data were collected form different animal cohorts. Quartiles were created that represent all possible combinations of long and short life span (Long+, Long−) and high and low insulin sensitivity (AUC−, AUC+; Figure 3). There was a significant (χ2 = 68.4, df = 1, p < .001) interaction between longevity and insulin sensitivity for df/df mice and controls on AL and calorie-restricted diets. There were greater than expected frequencies in quartiles 2 and 4 ([AUC−&Long+] and [AUC+&Long−]), indicating a positive association between longevity and insulin sensitivity. The same pattern of results was obtained for the GHRKO mice and controls on AL and calorie-restricted diets (χ2 = 135.0, df = 1, p < .001). Furthermore, the composition of the quadrants differed with respect to treatment conditions (diet and strain). A chi-square goodness of fit was computed in which 25% of observations in each of the treatment combinations served as the expected value for each of the quadrants. The analysis of the Ames dwarf study indicated that 100% of the N mice on the AL diet were in Q4 (χ2 = 390, df = 3, p < .001), whereas only 31% of N mice on the calorie-restricted diet were in Q4 and 29% in Q3 (χ2 = 11.2, df = 3, p < .05). The only problem with this pattern of distribution was noted among Ames dwarf mice on the AL diet, which were only moderately overrepresented in Q2 and Q4 (χ2 = 9.52, df = 3, p < .05). However, 75% of the df/df mice on the calorie-restricted diet were in Q2, with the remaining 25% in Q1 (χ2 = 288, df = 3, p < .001). In the GHRKO study, 75% of the N mice on the AL diet were in Q4 (χ2 = 985.6, df = 3, p < .001), whereas only 27% of the N mice on the calorie-restricted diet were in Q4 and 33% in Q3 (χ2 = 42.8, df = 3, p < .001). The GHRKO mice on the AL diet were overrepresented in Q2 (61%) and Q1 (28%; χ2 = 609.1, df = 3, p < .001). In addition, 42% of the GHRKOs on the calorie-restricted diet were in Q2 and 25% in Q3 (χ2 = 112.7, df = 3, p < .001).
Figure 3.
Chi-square test of independence representing the distribution of data for the Ames dwarf (df/df), growth hormone receptor knockout (GHRKO), and their respective normal controls on ad libitum (AL) or calorie-restricted diets into quartiles representing all possible combinations of long and short median longevity (Long+, Long−) and high (smaller area under the curve, AUC−) and low (bigger area under the curve, AUC+) insulin sensitivity (see Materials and Methods section). Notes. CR = calorie restriction. *Expected count/observed count.
To summarize, we have found that in normal, Ames dwarf, and GHRKO mice, changes in the whole-body insulin sensitivity as assessed by ITT are significantly associated with life span alterations, and we would like to propose enhanced insulin sensitivity as a potential biomarker of increased life expectancy.
The Effects of GH Treatment on Insulin Action
The proposal that increased insulin sensitivity produced by disruption of GH action in mutant mice and by CR in normal or Ames dwarf mice contributes to extended longevity of these animals appears to be inconsistent with the report that the life span of hypopituitary Snell dwarf mice treated with GH between the ages of 4 and 15 weeks was unaffected by this treatment (14). We injected df/df mice with GH starting at the age of 2 weeks, continuing for 6 weeks. One day after the last injection, we performed an ITT test that indicated a significant decrease of insulin sensitivity approaching the values measured in normal mice (Figure 4A). However, when we subjected the same GH-treated Ames dwarf mice to additional ITT tests 1 month after their last GH injections, we found that insulin sensitivity increased to the level characteristic of untreated dwarfs (Figure 4B). Presumably, Snell dwarf mice studied by Vergara and colleagues (14) had reduced insulin sensitivity only during the GH treatment, whereas during the remainder of their lives these animals most likely had high insulin sensitivity. This may have accounted for persistence of extended life span despite GH administration. In GH-deficient dwarf rats, plasma insulin and glucose levels do not differ from the levels measured in normal animals, and life span is unaltered (15). Curiously, GH replacement therapy between 4 and 15 weeks of age led to a significant increase in longevity of male dwarf rats (12). The mechanism responsible for increased longevity in this very complicated animal model is unknown, but the authors suggested that it may have been related to correction of developmental defects of pancreatic islets. Moreover, these animals exhibit impairments in tests of glucose tolerance that are resolved by short-term GH treatment (15).
The reported association of insulin resistance with extended life span in Klotho transgenic mice (16) and in mice with deletion of insulin receptor substrate 2 (IRS2) in the brain (17) likely represents benefits shielding the tissue from the effects of hyperinsulinemia, as suggested by Taguchi and colleagues (17) or, in the case of brain IRS2–deleted mice, a relationship peculiar to animals consuming a diet with relatively high fat content. However, findings in Klotho and brain IRS2–deficient mice somewhat complicate our interpretation. It is difficult to argue that insulin sensitivity could be considered as a longevity biomarker. However, all available findings could suggest that reduced strength of the insulin signal in some organs rather than whole-body insulin sensitivity could be crucial for longevity. It would be of obvious interest to measure insulin sensitivity and activation of insulin molecular signaling pathway in different target organs in animals and humans. Regardless of conflicting data derived from long-lived Ames dwarf, GHRKO, calorie-restricted, and Klotho mice, one common feature that emerges is a reduced strength of insulin signal (18). Long-living insulin-sensitive mice are characterized by severely decreased levels of insulin and insulin-like growth factor 1 (IGF1), which decrease the activation of these pathways. In Klotho transgenic mice, the levels of insulin and IGF1 were reported to be normal or elevated; however, cellular resistance to these molecules inhibits activation of insulin/IGF1 pathway (18) leading to similar suppression of these pathways in insulin-sensitive Ames dwarf, GHRKO, calorie-restricted, and insulin-resistant Klotho mice.
In summary, we propose that enhancement of insulin sensitivity, in conjunction with reduced insulin levels, may link reduced somatotropic signaling with increased longevity in long-lived mutant mice and in genetically normal (wild type) mice subjected to CR. The role of the concomitant reduction in circulating insulin levels in life span extension remains to be determined.
FUNDING
This work was supported by the National Institute on Aging (AG 19899 and U19 AG023122), Ellison Medical Foundation, Southern Illinois University Geriatrics Medicine and Research Initiative, and Central Research Committee of Southern Illinois University School of Medicine.
Acknowledgments
We thank Dr. George, S. Roth, and Dr. Donald K. Ingram for the consultation. We also thank Steve Sandstrom for helping with the editing of the manuscript.
References
- 1.Spence JW. Some observations on sugar tolerance, with special reference to variations found at different ages. QJM. 1920;14:314–326. [Google Scholar]
- 2.DeFronzo RA. Glucose intolerance and aging. Diabetes Care. 1981;4(4):493–501. doi: 10.2337/diacare.4.4.493. [DOI] [PubMed] [Google Scholar]
- 3.Barbieri M, Rizzo MR, Manzella D, et al. Glucose regulation and oxidative stress in healthy centenarians. Exp Gerontol. 2003;38(1–2):137–143. doi: 10.1016/s0531-5565(02)00153-5. [DOI] [PubMed] [Google Scholar]
- 4.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384(6604):33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 5.Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141(7):2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 6.Weindruch R, Real W. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: Thomas; 1988. [Google Scholar]
- 7.Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997;337(14):986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominici FP, Hauck S, Argentino DP, Bartke A, Turyn D. Increased insulin sensitivity and upregulation of insulin receptor, insulin receptor substrate (IRS)-1 and IRS-2 in liver of Ames dwarf mice. J Endocrinol. 2002;173(1):81–94. doi: 10.1677/joe.0.1730081. [DOI] [PubMed] [Google Scholar]
- 9.Hauck SJ, Hunter WS, Danilovich N, Kopchick JJ, Bartke A. Reduced levels of thyroid hormones, insulin, and glucose, and lower body core temperature in the growth hormone receptor/binding protein knockout mouse. Exp Biol Med (Maywood) 2001;226(6):552–558. doi: 10.1177/153537020122600607. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh CC, DeFord JH, Flurkey K, Harrison DE, Papaconstantinou J. Effects of the Pit1 mutation on the insulin signaling pathway: implications on the longevity of the long-lived Snell dwarf mouse. Mech Ageing Dev. 2002;123(9):1245–1255. doi: 10.1016/s0047-6374(02)00037-4. [DOI] [PubMed] [Google Scholar]
- 11.Liu JL, Coschigano KT, Robertson K, et al. Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. Am J Physiol Endocrinol Metab. 2004;287(3):E405–E413. doi: 10.1152/ajpendo.00423.2003. [DOI] [PubMed] [Google Scholar]
- 12.Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Extending the lifespan of long-lived mice. Nature. 2001;414(6862):412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- 13.Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. 2006;103(20):7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated snell dwarf mice regain fertility but remain long lived and disease resistant. J Gerontol A Biol Sci Med Sci. 2004;59(12):1244–1250. doi: 10.1093/gerona/59.12.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sonntag WE, Carter CS, Ikeno Y, et al. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146(7):2920–2932. doi: 10.1210/en.2005-0058. [DOI] [PubMed] [Google Scholar]
- 16.Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309(5742):1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317(5836):369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- 18.Bartke A. Long-lived Klotho mice: new insights into the roles of IGF-1 and insulin in aging. Trends Endocrinol Metab. 2006;17(2):33–35. doi: 10.1016/j.tem.2006.01.002. [DOI] [PubMed] [Google Scholar]