Abstract
Light scattering in biological tissues can be reduced by using optical clearing agents. Various physical methods in conjunction with agents have been studied to enhance the optical clearing efficacy of skin for diagnostic and therapeutic applications. In this study, we propose a new physical method to enhance the optical clearing potential of topically applied glycerol. A microneedle roller is used to easily create numerous transdermal microchannels prior to glycerol application. The optical clearing efficacy of skin is quantitatively evaluated with the use of a modulation transfer function target placed underneath ex vivo porcine skin samples. From cross-polarized images acquired at various time points after glycerol application, we find that samples treated with the microneedle roller resulted in an approximately two-fold increase in contrast compared to control samples 30 min after glycerol application. In conclusion, our data suggest that the microneedle roller can be a good physical method to enhance transdermal delivery of optical clearing agents, and hence their optical clearing potential over large regions of skin.
Keywords: optical clearing agent, microneedle, cross-polarization, quantitative evaluation, penetration enhancement, transdermal delivery
1 Introduction
Since Vargas et al.1 observed the optical clearing of skin using glycerol in 1999, numerous studies2-11 have been performed to study the reduction of light scattering in skin using optical clearing agents (OCAs). The in-vivo potential of optical skin clearing was demonstrated first with an experiment by Vargas et al., in which 0.1 mL of glycerol was injected directly into the dermal layer of hamster skin, resulting in a dramatic increase in visibility of subsurface blood vessels. Unfortunately, injection of glycerol has been associated with skin necrosis,6 thus motivating alternative strategies for transdermal delivery of prospective OCAs. The stratum corneum (SC) layer functions as a barrier that restricts the permeability of OCAs into deeper skin layers. A number of physical methods have been studied to accelerate the skin permeability of OCAs by altering temporarily the function of the SC barrier. Such methods include tape stripping, ultrasound, microneedles, iontophoresis, electroporation, microdermabrasion, laser ablation, needle-free injection guns, and photomechanical and chemical waves.12-20
We propose a new physical method to enhance the efficacy of optical skin clearing by an OCA, glycerol. The method uses a microneedle roller that is ISO 9001:2000 approved and widely and safely used in dermatology.21 The microneedle roller can easily create a number of transdermal microchannels by mechanically rolling it over the skin surface. The created microchannels close within hours and the skin returns to its original condition. The objective of this study is to assess the feasibility and efficacy of the microneedling method in conjunction with topically applied glycerol in optical skin clearing. With ex vivo porcine skin samples, we quantitatively analyzed the contrast of a modulation transfer function target placed under the samples. For the analysis, cross-polarization images of the samples were acquired as a function of time period of glycerol application.
2 Materials and Methods
2.1 Microneedling Method
Figure 1(a) shows the microneedle roller (Dermaroller, Horst Liebl, France). Each needle has a 70 μm diameter and 500 μm height.22,23 The roller has 192 needles (8 circular arrays of 24 needles each) on a cylindrical surface (diameter is 2 cm and width is 2 cm). Standard use of the microneedle roller typically results in a channel density of ~ 240 channels/cm2 after 10 to 15 applications over the same skin area.21,23 In this study, the microneedle roller was gently pressed down to minimize the change of sample thickness and rolled 50 times in horizontal, vertical, and diagonal directions over the skin samples, respectively. Figure 1(b) taken in our laboratory shows the microscopic image (10×) of microchannels formed on a porcine skin sample after microneedling.
Fig. 1.
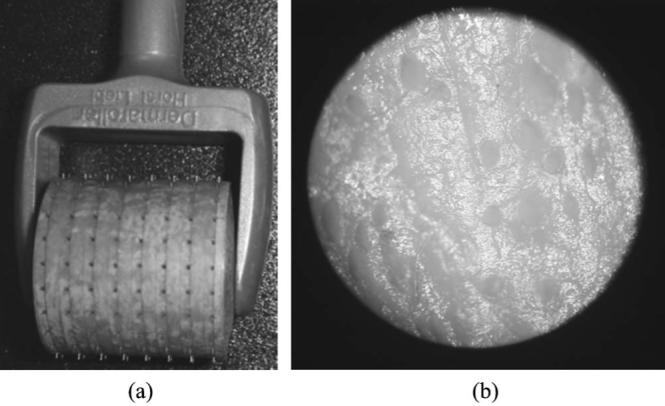
(a) Microneedle roller and (b) microscopic image (10×) of microchannels formed on a skin sample using a microneedle roller.
2.2 Skin Sample Preparation
An ex vivo abdominal porcine skin slab with a thickness of approximately 1.82 mm was used in this study. The subcutaneous fat layer was removed from the slab. The slab was cut into six samples, each with lateral dimension of 2× 2 cm2. The samples were divided into two groups: 1. control samples, which were not treated with the microneedle roller; and 2. test samples, which were treated with the microneedle roller. Each sample was placed directly over a customized modulation transfer function target (MTFT), and 0.1 ml of 95% glycerol was topically applied at the center of each sample. Both control and test groups involved three replicate experiments. The room temperature was maintained at 18 °C.
2.3 Cross-Polarization Image of Skin Samples
A polarization imaging system (MagVision, Optobiomed, Korea) shown in Fig. 2 was used to obtain cross-polarized images of the skin samples. The system consists of a fixed linearly polarized white LED ring light and a rotatible linear polarizer (analyzer) in front of a charge-coupled device (CCD) camera (Canon 350D, Canon, Japan) to control the polarization state of reflected light. The sample images were automatically taken in cross-polarization mode every 10 min for 90 min.
Fig. 2.
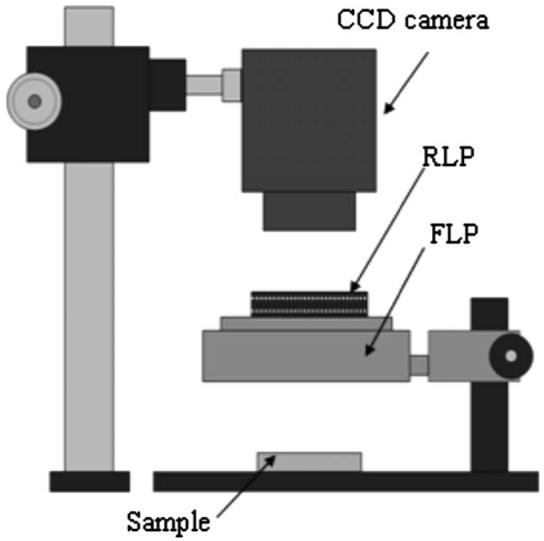
Polarization imaging system used to obtain cross-polarization images of skin samples. The RLP and FLP represent a rotatable and fixed linear polarizer, respectively. The white LED source is linearly polarized by the FLP.
2.4 Quantitative Evaluation of Optical Skin Clearing Efficacy
The efficacy of the microneedling method in optical skin clearing was quantitatively evaluated by calculating the contrast and relative contrast (RC) of the MTFT on both skin samples.24 A central region (100× 100 pixels) of glycerol application corresponding to the actual area of 1.5× 1.5 cm2 was extracted from each image and utilized for the evaluation. The contrast and RC were calculated at identical sites as a function of elapsed time over 90 min after glycerol application.
| (1) |
| (2) |
where MC0 and MCt are the mean contrasts of the modulation transfer function (MTF) immediately after glycerol application and at the elapsed time t, respectively. The MC was calculated as follows: 1. column pixels of the extracted image were added and their mean values calculated to obtain the MTF shown in Fig. 3; 2. the contrast of the MTF was calculated at five locations as marked in Figs. 3(a) and 3(b); and 3. finally, the mean contrast (MC) of the MTF was calculated by averaging the contrasts at the five locations. To compare the optical skin clearing efficacy between the control and test samples, we computed a relative optical clearing effect (%ROCE):
| (3) |
where RCcontrol and RCtest represent the RC of the control and test samples, respectively.
Fig. 3.
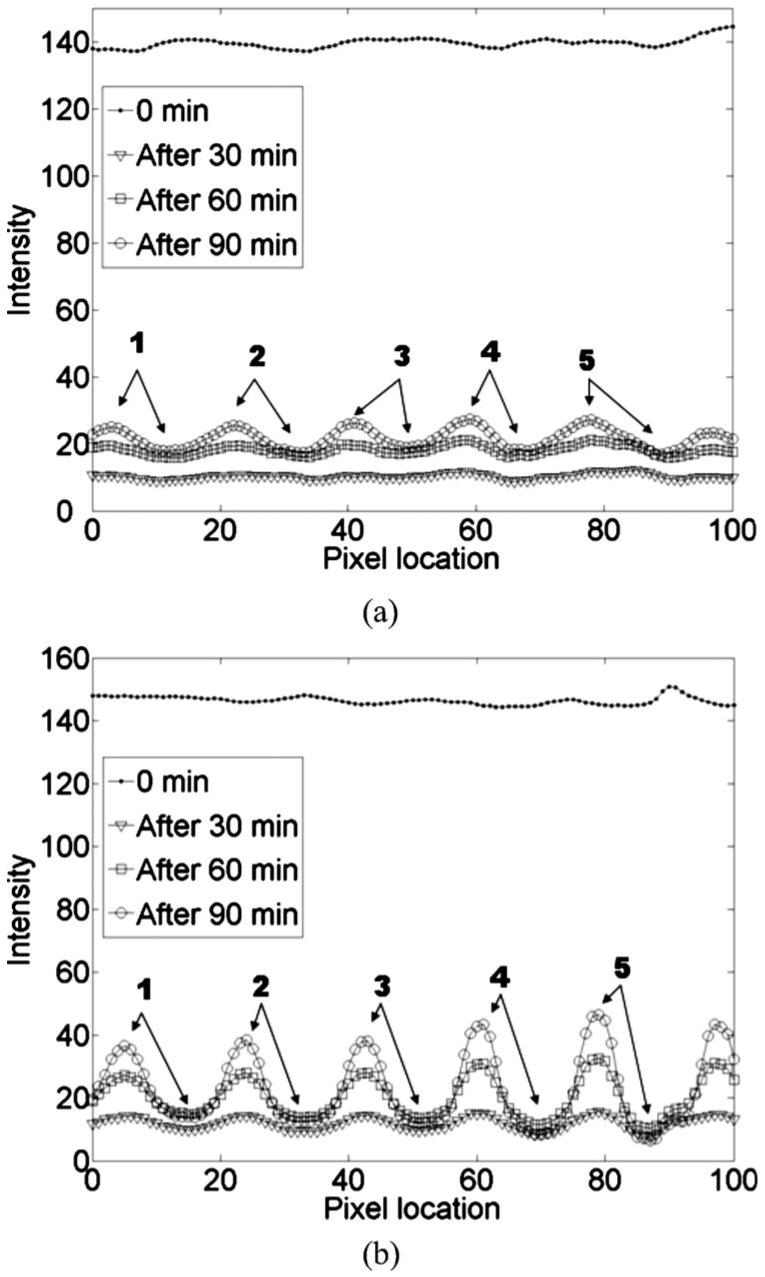
Modulation transfer functions (MTFs) on the (a) control and (b) test skin sample. Contrasts of the MTFs were calculated at five locations, as indicated in both figures.
3 Results
Our ex vivo experimental results demonstrated the potential of the microneedling method in the optical clearing of skin using glycerol. Figure 4 shows the dynamic change of samples without (left) and with (right) microneedling treatment at different time points (0, 30, 60, and 90 min) after glycerol application. During the 90-min duration of the experiments, the test samples compared to the control showed a greater optical clearing effect, as represented by the enhanced visualization of the MTFT.
Fig. 4.
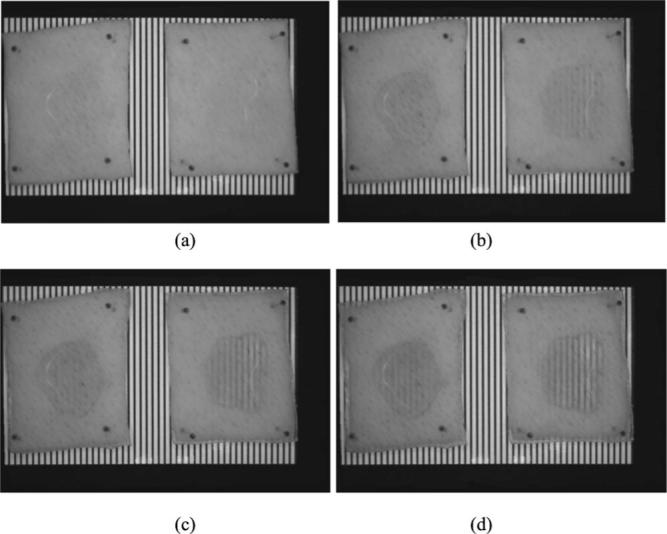
Photographs show the dynamic changes on the control (left) and test (right) skin sample at the elapsed times of (a) 0, (b) 30, (c) 60, and (d) 90 min after topically applying 0.1 ml of 95% glycerol.
MTFs were calculated every 10 min from the cross-polarization images of the samples. Figure 3 shows a representative example of the intensity variation of MTFs at 0, 30, 60, and 90 min after glycerol application on a control and test sample. The visibility of the MTF increased as a function of elapsed time in both samples. However, the intensity variation was much greater in the test sample than in the control samples (Fig. 3), demonstrating the potential of the microneedling method in the enhancement of optical skin clearing.
These differences were quantitatively compared by calculating the RCs for both samples. Figure 5 shows the RCs obtained from three replicate experiments. The change of RCs was greater in the test sample than in the control sample. The control (test) sample at 30, 60, and 90 min after glycerol application resulted in a 2.5 (5.0)-, 6.7 (13.1)-, and 13.2 (24.0)-fold increase in contrast, respectively. The quantitative comparison of the optical skin clearing efficacy was performed by computing the %ROCE [Eq. (3)]. Even though the difference in RC continuously increased, the maximum %ROCE (126.5 ± 45%) was observed after 50 min of glycerol application, as shown in Fig. 6.
Fig. 5.
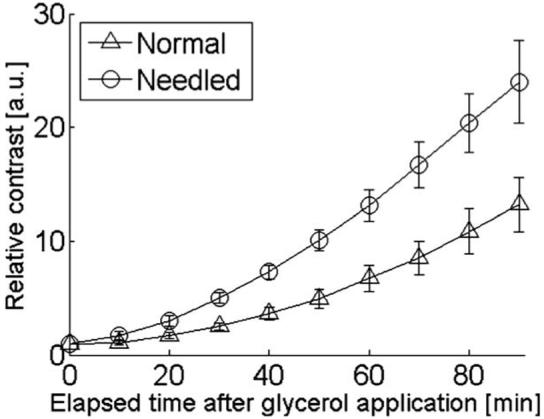
The average relative contrast of the control (Δ) and test ◯ skin sample.
Fig. 6.
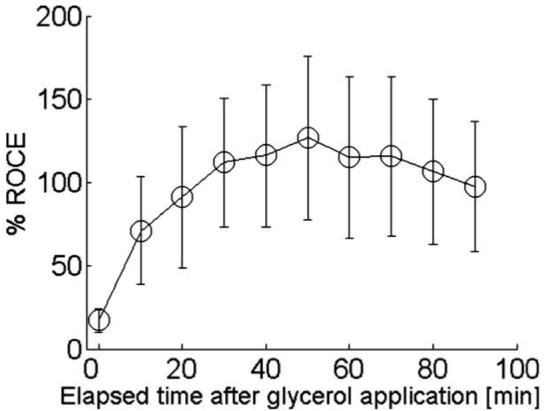
The average percent relative optical clearing effect (%ROCE) of the test skin sample as compared to the control skin sample.
4 Discussion
In this study, we have demonstrated the feasibility and efficacy of the microneedling method to enhance optical clearing of skin by topically applied glycerol. Previous studies have employed laser-based physical methods to enhance the transdermal delivery of OCAs, including selective ablation of stratum corneum with the use of topically applied absorbers17 and laser ablation of multiple spots to create a “lattice of islets of damage.”18 Although researchers have demonstrated the enhancement of optical skin clearing with these methods, the microneedling method is expected to be advantageous for the following reasons: 1. no exogenous absorber is required; 2. no laser light is required, reducing substantially the cost of a penetration enhancement method; 3. with the microneedling method, microchannels can be created rapidly over a large region of skin; and 4. the microneedling method is extremely simple to implement. Furthermore, the manufacturer says that the microneedle roller induces minimal pain and creates hundreds of physical microchannels in the skin [Fig. 1(b)], through which topically applied glycerol can diffuse to deeper skin layers. According to the study done by Henry et al., the transdermal drug delivery through microfabricated microneedles results in 10,000 times greater penetration as compared to a simple application.20 Analogously, we expect that the microneedling method might result in a similar outcome in the transdermal delivery of topically applied glycerol.21 However, it is necessary to experimentally investigate the efficacy of the microneedle method in transdermal glycerol delivery in future studies.
The efficacy of optical skin clearing was qualitatively assessed by visual observation of the MTFT under the samples (Fig. 4);. The optical skin clearing effect was observed after 30 min of glycerol application on the test sample [Fig. 4(b)]. However, at the same time point, no detectable optical clearing was observed on the control sample [Fig. 4(b)]. Such results can be attributed to the enhancement of skin permeability of glycerol through the transdermal microchannels [Fig. 1(b)] created by the microneedling method.
The optical clearing effect of skin was quantitatively evaluated by calculating the contrast of MTFs as a function of elapsed time after glycerol application (Fig. 3). The minor nonuniform intensity distribution of the MTFs (Fig. 3) might be due to a nonuniform glycerol distribution on the samples, or potential heterogeneities in skin structure. We instead studied the average optical clearing effect by calculating the RC (Fig. 5). The difference in the RC between the control and test samples gradually increased, representing much greater optical clearing efficacy in the test samples. We observed a difference of an approximately two-fold higher RC with the test samples 30 min after glycerol application (Figs. 5 and 6). It is important to note that this difference is not necessarily representative of a two-fold decrease in light scattering. A limit of the experimental protocol was that sample thickness was not quantified, and such information is required to separate the increased RC into scattering changes and sample thickness changes. Nevertheless, our data demonstrate an enhanced effect of glycerol on ex vivo skin pretreated with the microneedling roller.
The quantitative comparison of optical clearing efficacy between the control and test samples was performed by calculating the %ROCE [Eq. (3)]. The %ROCE begins to gradually increase from 10 min after glycerol application (Fig. 6). At 50 min after glycerol application, the test conditions led to a 126.5% greater RC compared with control conditions. Thereafter, the %ROCE began to decrease due to the increase of the RC in the control samples, even though the RC of the test samples continuously increased. In Fig. 6, the variation of error bars might be caused by experimental limitations such as: 1. residual fat layers of skin samples and 2. difference of the number of microchannels.
In our experiments, although we used a 0.5-mm microneedle roller to create microchannels, other microneedle rollers with 0.2, 0.5, 1, 1.5, and 2.2 mm height are also available from the manufacturer. By using various microneedle rollers, we expect to produce different optical clearing effects of skin, because the depth of microchannels depends on the height of the microneedle roller. To verify the expectation, we are performing ex vivo and in-vivo animal studies.
The concentration of solution is an important factor in drug delivery. In previous studies, various concentrations of glycerol were used for optical skin clearing. However, an optimal concentration was not investigated, probably because it might depend on the physical methods in conjunction with glycerol. In this study, 95% glycerol was topically applied on the skin samples pretreated with a microneedle roller. Other concentrations of glycerol might more effectively reduce the time period of optical skin clearing. The current work was focused on a feasibility study of the microneedling method, and we plan to include other concentrations and agents in a future study.
The dehydration and temperature of skin might affect the experimental results. For instance, our other experiment presented that the average hydration of ex vivo skin samples due to natural water evaporation decreased about 21% after 90 min in our laboratory conditions. In this study, such factors were not considered, because all experiments were performed in identical laboratory conditions, in which room temperature and humidity were constantly maintained. In future studies, it might be necessary to investigate the effect of such factors in optical skin clearing.
In conclusion, we demonstrate that the microneedling method can enhance the optical clearing efficacy of skin for topically applied glycerol. In addition, we present quantitative analysis methods to evaluate the optical clearing efficacy of skin using cross-polarization images. Finally, we anticipate that the skin permeability of OCAs can be further enhanced when the microneedling method is combined with other physical methods, such as superficial microdermabrasion or localized heating.
Acknowledgments
This research was supported by a grant from the Next Generation New Technology Development Program (10028424) provided by the Ministry of Commerce, Industry and Energy of the Korean Government. Author Nelson was supported by the following grants from the National Institutes of Health (AR47751 and EB 2495).
References
- 1.Vargas G, Chan EK, Barton JK, Rylander HG, III, Welch AJ. Use of an agent to reduce scattering in skin. Lasers Surg. Med. 1999;24:133–141. doi: 10.1002/(sici)1096-9101(1999)24:2<133::aid-lsm9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 2.Vargas G, Readinger A, Dozier SS, Welch AJ. Morphological changes in blood vessels produced by hyperosmotic agents and measured by optical coherence tomography. Photochem. Photobiol. 2003;77(5):541–549. doi: 10.1562/0031-8655(2003)077<0541:mcibvp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Khan MH, Choi B, Chess S, Kelly KM, Nelson JS. Can topically applied optical clearing agents increase the epidermal damage threshold and enhance therapeutic efficacy? Lasers Surg. Med. 2004;35:93–95. doi: 10.1002/lsm.20078. [DOI] [PubMed] [Google Scholar]
- 4.Khan MH, Choi B, Chess S, Kelly KM, McCullough J, Nelson JS. Optical clearing of in vivo human skin: implications for light-based diagnostic imaging and therapeutics. Lasers Surg. Med. 2004;34:83–85. doi: 10.1002/lsm.20014. [DOI] [PubMed] [Google Scholar]
- 5.Galanzha EI, Tuchin VV, Solovieva AV, Stepanova TV, Luo Q, Cheng H. Skin backreflectance and microvascular system functioning at the action of osmotic agents. J. Phys. D: Appl. Phys. 2003;36:1739–1746. [Google Scholar]
- 6.McNichols RJ, Fox MA, Gowda A, Tuya S, Bell B, Motamedi M. Temporary dermal scatter reduction: quantitative assessment and implications for improved laser tattoo removal. Lasers Surg. Med. 2005;36(4):289–296. doi: 10.1002/lsm.20152. [DOI] [PubMed] [Google Scholar]
- 7.Hirshburg J, Choi B, Nelson JS, Yeh AT. Collagen solubility correlates with skin optical clearing. J. Biomed. Opt. 2006;11(4):040501–040503. doi: 10.1117/1.2220527. [DOI] [PubMed] [Google Scholar]
- 8.Proskurin SG, Meglinski IV. Optical coherence tomography imaging depth enhancement by superficial skin optical clearing. Laser Phys. Lett. 2007;4(11):824–826. [Google Scholar]
- 9.Rylander CG, Stumpp OF, Milner TE, Kemp NJ, Mendenhall JM, Diller KR, Welch AJ. Dehydration mechanism of optical clearing in tissue. J. Biomed. Opt. 2006;11(4):041111–041117. doi: 10.1117/1.2343208. [DOI] [PubMed] [Google Scholar]
- 10.Yeh AT, Choi B, Nelson JS, Tromberg BJ. Reversible dissociation of collagen in tissue. J. Invest. Dermatol. 2003;121(6):1332–1335. doi: 10.1046/j.1523-1747.2003.12634.x. [DOI] [PubMed] [Google Scholar]
- 11.Choi B, Tsu L, Chen E, Ishak TS, Iskandar SM, Chess S, Nelson JS. Determination of chemical agent optical clearing potential using in vitro human skin. Lasers Surg. Med. 2005;36(2):72–75. doi: 10.1002/lsm.20116. [DOI] [PubMed] [Google Scholar]
- 12.Nelson JS, McCullough JL, Glenn TC, Wright WH, Liaw LH, Jacques SL. Mid-infrared laser ablation of stratum corneum enhances in vitro percutaneous transport of drugs. J. Invest. Dermatol. 1991;97(5):874–879. doi: 10.1111/1523-1747.ep12491600. [DOI] [PubMed] [Google Scholar]
- 13.Jakasa I, Verberk MM, Bunge AL, Kruse J, Kezic S. Increased permeability for polyethylene glycols through skin compromised by sodium lauryl sulphate. Exp. Dermatol. 2006;15(10):801–807. doi: 10.1111/j.1600-0625.2006.00478.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee WR, Tsai RY, Fang CL, Liu CJ, Hu CH, Fang JY. Microdermabrasion as a novel tool to enhance drug delivery via the skin: an animal study. J. Dermatol. Surg. 2006;32:1013–1022. doi: 10.1111/j.1524-4725.2006.32224.x. [DOI] [PubMed] [Google Scholar]
- 15.Pan Y, Zhao HY, Zheng JM. The enhancing effect of electroporation and iontophoresis on the permeation of insulin through human skin. Yao Xue Xue Bao. 2002;37(8):649–652. [PubMed] [Google Scholar]
- 16.Jin Y, Uchida M, Wang CF, Natsume H, Sugibayashi K, Morimoto Y. Transdermal microparticle delivery by a supersonic-helios gun system. Yao Xue Xue Bao. 2001;36:140–144. [PubMed] [Google Scholar]
- 17.Stumpp OF, Welch AJ, Milner TE, Neev J. Enhancement of transdermal skin clearing agent delivery using a 980 nm diode laser. Lasers Surg. Med. 2005;37:278–285. doi: 10.1002/lsm.20237. [DOI] [PubMed] [Google Scholar]
- 18.Tuchin VV, Altshuler GB, Gavrilova AA, Pravdin AB, Tabatadze D, Childs J, Yaroslavsky IY. Optical clearing of skin using flashlamp-induced enhancement of epidermal permeability. Lasers Surg. Med. 2006;38:824–836. doi: 10.1002/lsm.20392. [DOI] [PubMed] [Google Scholar]
- 19.Tezel A, Mitragotri S. Interactions of inertial cavitation bubbles with stratum corneum lipid bilayers during low-frequency sonophoresis. Biophys. J. 2003;85:3502–3512. doi: 10.1016/S0006-3495(03)74770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henry S, McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles: a novel approach to transdermal drug delivery. J. Pharm. Sci. 1998;87(8):922–925. doi: 10.1021/js980042+. [DOI] [PubMed] [Google Scholar]
- 21. See http://www.dermaroller.co.nz/about.html.
- 22. See http://www.dermaroller.co.nz/models.html.
- 23. See http://dermaroller.co.nz/research/dermaroller_series.htm.
- 24.Bravo-Zanoguera ME, Rivera-Castillo J, Vera-Perez M, Carranza M. A. Reyna. Use of the modulation transfer function to measure quality of digital cameras. Proc. 16th Intl. Conf. IEEE CONIELECOMP.2006. p. 52. [Google Scholar]


