Abstract
Recently, using the medial forebrain bundle (MFB) 6-hydroxydopmaine (6-OHDA) lesion rat model of Parkinson’s disease (PD), we have demonstrated that blockade of central IGF-1 receptors (IGF-1R) attenuated estrogen neuroprotection of substantia nigra pars compacta (SNpc) DA neurons, but exacerbated 6-OHDA lesions in IGF-1 only treated rats (Quesada and Micevych [2004]: J Neurosci Res 75:107–116). This suggested that the IGF-1 system is a central mechanism through which estrogen acts to protect the nigrostriatal DA system. Moreover, these results also suggest that IGF-1R-induced intracellular signaling pathways are involved in the estrogen mechanism that promotes neuronal survival. In vitro, two convergent intracellular signaling pathways used by estrogen and IGF-1, the mitogen-activated protein kinase (MAPK/ERK), and phosphatidylinositol-3-kinase/Akt (PI3K/Akt), have been demonstrated to be neuroprotective. Continuous central infusions of MAPK/ERK and PI3K/Akt inhibitors were used to test the hypothesis that one or both of these signal transduction pathways mediates estrogen and/or IGF-1 neuroprotection of SNpc DA neurons after a unilateral administration of 6-OHDA into the MFB of rats. Motor behavior tests and tyrosine hydroxylase immunoreactivity revealed that the inhibitor of the PI3K/Akt pathway (LY294002) blocked the survival effects of both estrogen and IGF-1, while an inhibitor of the MAPK/ERK signaling (PD98059) was ineffective. Western blot analyses showed that estrogen and IGF-1 treatments increased PI3K/Akt activation in the SN; however, MAPK/ERK activation was decreased in the SN. Indeed, continuous infusions of inhibitors blocked phosphorylation of PI3K/Akt and MAPK/ERK. These findings indicate that estrogen and IGF-1-mediated SNpc DA neuronal protection is dependent on PI3K/Akt signaling, but not on the MAPK/ERK pathway.
Keywords: estrogen, IGF-1, Akt, ERK, apoptosis, substantia nigra
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by a progressive and massive loss of midbrain dopaminergic (DA) neurons of the substantia nigra pars compacta (SNpc), the origin of the nigrostriatal pathway. This loss of SNpc DA neurons results in a degeneration of DA terminals and with DA depletion in the striatum, which is required for normal motor function. Epidemiological studies have indicated that PD is more common in men than women (Mayeux et al., 1992; Bower et al., 1999; Baldereschi et al., 2000; Wooten et al., 2004). This gender difference, in part, been attributed to the lack of estrogen, which acts as a neuroprotectant in females. In animal models of PD, 6-hydroxydopamine (6-OHDA), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxins, and methamphetamine toxicity have demonstrated that females are more resistant to neurotoxic injuries than males (Wagner et al., 1993; Disshon and Dluzen, 1997; Dluzen, 1997; Murray et al., 2003). Moreover, in females the natural variation in sex steroid levels during the estrous cycle influences the degree of neuroprotection (Datla et al., 2003). A novel mechanism for neuroprotection is the estrogen interaction with specific growth factors that are known to be neuroprotective (Toran-Allerand et al., 1999). One such factor is insulin-like growth factor-1 (IGF-1). Previously, we demonstrated that activation of IGF-1 receptors (IGF-1R) is a critical component in the protection of SNpc DA neurons after 6-OHDA lesions (Quesada and Micevych, 2004). Although IGF-1 clearly mediates neuroprotective responses, the intracellular signaling mechanisms underlying this protection remain elusive.
Biological actions of IGF-1 are mediated by the type I IGF-1R, which contains a tyrosine kinase domain responsible for the phosphorylation of intracellular signal transduction proteins (LeRoith et al., 1995). One signaling pathway activated by IGF-1R involves the formation of adapter complexes with Grb2, SOS, Ras, and Raf, which results in stimulation of the mitogen activated protein kinase (MAPK) pathway (Butler et al., 1998). A second signaling pathway that IGF-1R activates is phosphoinositide 3-kinase (PI3K/Akt/PKB) through tyrosine-phosphorylation of insulin receptor substrate molecules, which bind to the regulatory subunit (p85) of PI3K (Myers et al., 1994). In addition, parallel signal transduction pathways (MAPK/ERK and PI3K/Akt) are rapidly activated by estrogen in the adult brain (Razandi et al., 1999; Falkenstein et al., 2000). In the developing midbrain, estrogen enables the survival of and promotes the maturation and function of DA neurons (Beyer et al., 2002). These two convergent cell signaling pathways activated by estrogen and IGF-1 have been demonstrated to be neuroprotective in vitro (Singer et al., 1999; Singh et al., 2000; Zheng et al., 2002). We suggested that they might also be key molecular events underlying neuroprotection of SNpc DA neurons in vivo. The present study systemically examined downstream signal transduction pathways that underlie estrogen/IGF-1 neuroprotection of SNpc DA neurons in 6-OHDA-lesioned ovariectomized (OVX) rats. Continuous central infusions of MAPK/ERK and PI3K/Akt inhibitors were used to test the hypothesis that one or both of these signal transduction pathways mediates estrogen and/or IGF-1 protection of SNpc DA neurons.
MATERIALS AND METHODS
Animals
Adult Long-Evans female rats (Charles River, Portage, MI) weighing 225–250 g and ovariectomized bilaterally (OVX) by the distributor were used. At UCLA, all rats were housed two per cage prior to surgery and one per cage after 6-OHDA or vehicle. Animals were housed in a 12/12-h light/dark cycle (lights on at 6 am) and provided with food and water ad libitum, except during motor behavioral testing. The Chancellor’s Animal Research Committee at UCLA approved all procedures.
Steroid Treatment
Animals receiving steroid treatment were injected subcutaneously (s.c) with 20 μg of 17β-estradiol benzoate (EB; Steraloids, Wilton, NH). Control animals received safflower oil (vehicle) 24 h prior to 6-OHDA lesion and cannula placement.
6-OHDA Injected into the Medial Forebrain Bundle
Under isofluorane anesthesia and using normal stereotaxic procedures, rats were injected unilaterally with 6-OHDA using a 26-gauge needle that was lowered into the medial forebrain bundle (MFB, −2.2 mm anterior posterior, ± 1.5 mm medial lateral, −7.9 mm relative to the dura, and incisor bar set to −3.3 mm) (Paxinos et al., 1985). 6-OHDA (8 μg/2 μL) in normal saline containing 0.2 mg/mL ascorbic acid (Sigma, St. Louis, MO), was infused over a period of 4 min, at a rate of 0.5 mL/min, using a Hamilton syringe and infusion pump. Thirty minutes prior to surgery, rats received an intraperitoneal (ip) injection of despiramine and paragline (25 mg/kg; Sigma) in order to prevent noradrenergic terminal from taking up 6-OHDA (Carboni et al., 1990).
Cannula Placement into the Lateral Ventricle
After the 6-OHDA injection, a 28-gauge guide cannula (Alzet Brain Infusion kit II; DURECT Co., Cupertino, CA) was implanted into the lateral ventricle (−1.2 mm anterior posterior, ±1.4 mm medial lateral, −3.5 mm relative to the dura)(Paxinos et al., 1985). Using skull screws as anchors, the cannula was cemented in place with dental cement.
Drug Infusions
For intracerebroventricular (icv) infusions, Alzet osmotic minipumps (model 1007D; 0.5 μL/h)(Alzet DURECT Co., Cupertino, CA) with a reservoir volume of 96 ± 2 μL were used. Control safflower oil (vehicle) animals received osmotic minipumps filled with artificial cerebral spinal fluid (aCSF), a MAPK inhibitior (PD98059; 0.01, 0.10, and 1.0 μg/μL) (Calbiochem, USA) and a PI3K/Akt inhibitor (LY294002 0.01, 0.10 μg/μL)(Tocris, MO). These pharmacological inhibitors for MAPK/ERK and PI3K/Akt appear to be selective for their target kinases (Cuenda and Alessi 1999; Davies et al., 2000). 17β-estradiol benzoate (EB) treated group received osmotic minipumps filled with aCSF (EB alone), EB + PD98059 and/or EB + LY294002. IGF-1 treated group received osmotic minipumps filled with recombinant human IGF-1 (100 μg/mL, National Hormone and Peptide Program, NIDDK, Torrance, CA, USA), IGF-1 + LY294002 and/or IGF-1 + PD98059. Control animals received equal volumes of 0.4% dimethyl sulfoxide (DMSO) in aCSF. Each osmotic pump was primed 24 h prior to implantation, per manufacturer recommendations (Alzet DURECT Co., Cupertino, CA). The osmotic minipumps were implanted subcutaneously on the back over the latissimus dorsi muscles and connected by catheters to the infusion cannula. The pumps were implanted 5 min after the 6-OHDA injections and remained active for 7 days.
Motor Behavioral Assay
To assess motor deficits, a limb-use cylinder test was used (Schallert et al., 2000). Animals were placed in an enclosed cylinder. Forelimb-use to contacting the cylinder wall during vertical exploration was scored as described (Schallert et al., 2000; Quesada and Micevych, 2004). Briefly, animals were tested 24 h prior to 6-OHDA lesions and lateral ventricle cannula implantation (presurgery), and again 1-week post-surgery. The use of both, left and right forelimbs that contacted the cylinder wall was recorded with a video camera for 5 min per bout for each animal. An experimenter blind to the treatment protocol, examined and scored the behavior on video by using slow motion and frame-by-frame analysis. Behavior was expressed as the percentage use of forelimb relative to the total number of limb contacts with the cylinder wall.
Tissue Processing
One week following the 6-OHDA injections, animals were deeply anesthetized with sodium petabarbital (100 mg/kg) and transcardially perfused with saline, followed by 4% paraformaldahyde in 0.2 M Sorensons’s phosphate buffer. The brains were removed and post-fixed for 4 h and then transferred into 15% sucrose in 0.1 M phosphate buffer (pH 7.5) for cryoprotection, and stored at 4°C for 48 h. As described, 30 μm coronal sections through the striatum and ventral midbrain from each treatment group were obtained with a cryostate and processed for immunohistochemistry (Quesada and Micevych, 2004). Free-floating sections of the ventral midbrain were collected in 0.01 M phosphate buffered saline (PBS, pH 7.4). To quench endogenous peroxidase, all sections were preincubated with 10% methanol containing 1% H2O2 for 30 min before immunocytochemical procedures.
Tyrosine Hydroxylase Immunostaining
Free-floating sections were preincubated for 1 h at room temperature with 5% normal donkey serum (NDS). Next, a series of sections were incubated overnight at room temperature with primary antibody, polyclonal sheep-anti-tyrosine hydroxylase (TH); (1:6000, Chemicon, Temecula, CA). Sections were then washed with PBS, and incubated for 1 h at room temperature with biotinylated anti-sheep antiserum (1:2000, Vector Lab, UK). After washing again with PBS, sections were incubated for 1 h at room temperature with the avidin-biotin-peroxidase complex solution (Vector Laboratories). TH immunoreactivity was visualized by 3,3-diaminobenzidine tetrahydrochloride (DAB; Sigma). Sections were mounted on Superfrost Plus slides (Fisher Scientific), dehydrated through a series of graded alcohols and xylene and coverslipped using Permount mounting medium (Fisher Scientific).
Cell Counts and TH Fiber Density
Digital images of the immunostained tissue sections were obtained with an AxioCam mounted on a Zeiss Axioplan 2 microscope with Axiovision 3.1 software (Carl Zeiss, Thornwood, NY). An experimenter, blind to the treatment, counted the number of TH immunoreactive SNpc cell bodies per section. Because of the ease of identification of the SNpc DA neurons, the number of TH immunopositive cells in the SNpc on the contralateral (nonlesioned) side and ipsilateral (lesioned) were evaluated at four consecutive levels through the SNpc nuclei per animal, spanding the entire rostra-caudal extent of the nucleus [−4.8 mm, −5.1 mm, −5.4 mm, and −5.7 mm with respect to the bregma, (Paxinos et al., 1985)]. Survival was expressed as a percent of TH immunopositive SNpc cell bodies ipsilateral (lesioned) versus contralateral (nonlesioned) intact side. The design allow to express cell numbers as percentages of the contralateral side in the same section, thus avoiding methodological biases due to intersubject variation and has been used previously by others to assess the extent of 6-OHDA-induced lesions in the SNpc (Sauer and Oertel, 1994; Blandini et al., 2004; Paul et al., 2004; Armentero et al., 2006).
The optical densities (OD) of the TH immunoreactivity fibers in the striatum was measured in each animal at four different rostrocaudal levels, corresponding to +1.6 mm, +1.0 mm, +0.4 mm, −0.2 mm relative to the bregma (Paxinos et al., 1985). The images were analyzed using the NIH Image J 1.36 program (NIH). The corpus callosum OD background was subtracted from the OD values of the striatum. Tissue from all treatments were processed together to reduce variability because of differences in processing (Deumens et al., 2002; Mura et al., 2002). ODs were averaged from each animal and then expressed as a relative percentage of OD recorded from striata from control nonlesioned animals.
Western Blots
Following 7 days of drug infusions, groups of animals were deeply anesthetized with isofluorane and decapitated. Brains were immediately removed and 1-mm coronal sections were obtained using a chilled rodent brain matrix. Using a razor blade the substantia nigra was dissected from coronal sections of the midbrain. The dissected regions were pooled and homogenized with a Teflon piston in ice-cold 1% Triton X-100, 1 mM sodium vanadate, 1 mM phenylmethlsulfonyl fluoride, and 10 mM Tris-HCl, pH 7.4. After homogenization, samples were then centrifuged for 15 min at 14,000g, in order to remove insoluble material. Protein concentration was determined by the BCA method (BioRad; Hercules, CA). Fifty micrograms of protein was applied to a 12.5% SDS-polyacrylamide minigel and transferred onto polyvinylidenediflouride membranes as described in (Quesada and Etgen, 2002). Membranes were then incubated with an antibody for phosphorylated extracellular receptor-activated kinase (p-ERK1/2:1:1000; Santa Cruz Biotechnology, Santa Cruz, CA). After imaging, the blots were stripped and reprobed for nonphosphorylated ERK (1:300; Santa Cruz, Biotechnology, Santa Cruz, CA) for total ERK protein assessment. To assess PI3K activity, membranes were stained with antiphosphorylated Akt (p-Akt: 1:500; Santa Cruz, Biotechnology, Santa Cruz, CA), stripped and reprobed for nonphosphorylated Akt (1:200; Santa Cruz, Biotechnology, Santa Cruz, CA). Detection of primary antibody was done by anti-rabbit secondary antibodies conjugated to horseradish peroxidase (1:10,000 ERK and 1:2000 Akt) and visualized by chemiluminescence. The blots were then exposed to FUJI medical X-ray film (Fisher Scientific, Pittsburgh, PA). Band intensity was obtained by scanning autoradiograms and densitometry of bands analyzed with Image J 1.36 software (NIH).
Statistical Analysis
Relative percentages of survival of TH immunoreactive cells in the SNpc, OD of TH immunoreactivity in the striatum, and group comparisons of asymmetric forelimb use were converted by arcsin square root transformation. The transformation of percentages by arcsin square root transformation allows the analysis of data to be compared with one-way analysis of variance tests (ANOVA) and differences analyzed using a Student-Newman-Keuls post-hoc test with p < 0.05 considered significant. Values mean ± S.E.M of 5–7 animals per treatment group. For immunoblots, band OD was compared using a one-way ANOVA and differences analyzed using a Student-Newman-Keuls post-hoc test with p < 0.05 considered significant. All statistical analyses were performed using SigmaStat, v. 2.11 (Jandel Scientific, San Rafael, CA).
RESULTS
Inhibition of Akt Blocks Estrogen and IGF-1 Motor Improvement Against 6-OHDA Lesions
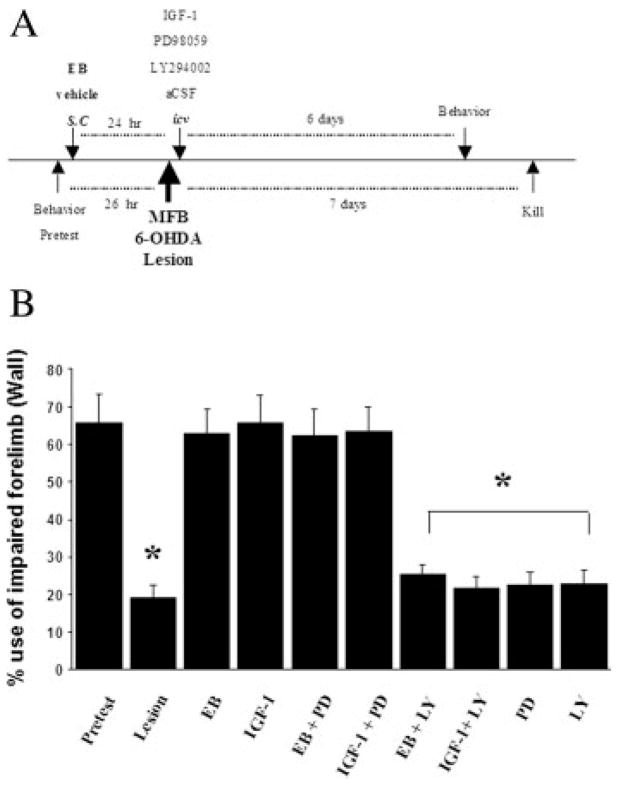
Animals tested for symmetric forelimb usage prior to 6-OHDA lesions (presurgery; pretest) demonstrated a symmetric use of both, left, and right forelimbs in the cylinder test for vertical exploration [Fig. 1(B)]. In contrast, 1 week after unilateral 6-OHDA lesions, resulted in severe asymmetrical limb use, reflected in a significant reduction of the contralateral (impaired) forelimb usage (from 66 to 20%; p < 0.001)[Fig 1(B)]. These behavioral symptoms are well characterized for the 6-OHDA lesion model of PD which is associated with SNpc DA neuronal loss which occurs 1 week following administration of 6-OHDA (Ungerstedt and Arbuthnott, 1970; Sauer and Oertel, 1994). As previously demonstrated, estrogen and IGF-1 treatments blocked motor impairment associated with 6-OHDA lesions to levels corresponding to pre-surgery [Fig. 1(B)] (Quesada and Micevych, 2004).
Figure 1.
A: Timeline of treatments; animals were given 17β-estradiol benzoate (EB; 20 μg, s.c) or vehicle 24 h before MFB 6-OHDA lesions. Lateral ventricle (icv) administration of IGF-1 (100 μg/mL) or aCSF were administered 5 min after 6-OHDA lesions. EB and IGF-1 treated groups received icv (0.1 μg/μL of PD98059; MAPK/ERK inhibitor or LY294002; PI3K/Akt inhibitor) 5 min after 6-OHDA lesions. Control lesion animals received icv (0.1 μg/μL of PD98059 or LY294002) 5 min after 6-OHDA lesions. IGF-1 and inhibitors were continuously infused for 7 days. B: Percent use of impaired (contralaral) forelimb, either alone or in combination with nonimpaired (ipsilateral) forelimb for contacting the wall of the cylinder (limb-use asymmetry cylinder test) during a rearing posture 26 h before lesion (pretest) and measurements were obtained again on the sixth day after 6-OHDA lesions. Values expressed as mean percentage ± S.E.M of impaired forelimb usage to the total number of limb contacts with the cylinder wall. Each value is calculated from 5 to 7 animals. * p < 0.05 compared to pretest, one-way ANOVA; Student Newman-Keuls posthoc.
To examine the pathway(s) associated with estrogen and IGF-1 actions, 1-week continuous central infusions with different doses of PI3K/Akt and MAPK/ERK inhibitors were used to block the effects of estrogen and IGF-1 on motor function, which served as a bioassay for the determination of the optimal tolerable dosage of inhibitors to be used [Fig. 1(A) depicts the timeline of treatments]. A low dose of the PI3K/Akt inhibitor, LY294002 (0.01 μg/μL), was ineffective (data not shown); but 0.10 μg/μL LY294002 abolished estrogen and IGF-1 protective effects on motor function [Fig. 1(B)]. Thus, the remaining experiments used 0.10 μg/μL LY294002. Similarly, the MAPK/ERK inhibitor, PD98059 at 0.01 μg/μL was ineffective in blocking either estrogens or IGF-1’s effects on motor function (data not shown). In contrast to the PI3K/Akt inhibitor, PD98059 (0.1 μg/μL) failed to block estrogen and IGF-1 effects on motor function [Fig. 1(B)]. Moreover, an ever greater dose of PD98059 (1.0 μg/μL), made the animals extremely ill, causing low general activity and weight loss after which they had to be euthanized. As with LY294002, the optimal tolerable dosage used for PD98059 was also 0.10 μg/μL. The dosages for PD98059 and LY294002 were chosen based on their reported ability to attenuate various forms of synaptic plasticity in the rat brain (LeRoith et al., 1995; Watters et al., 1997; Bi et al., 2001; Singh, 2001; Cardona-Gomez et al., 2002), inhibition of estrogen-dependent female sexual receptivity (Etgen and Acosta-Martinez, 2003), and inhibition of in vivo neuroprotection (D’Onofrio et al., 2001; Mori et al., 2002). Furthermore, as previously reported, PD98059 and LY294002 at 0.10 μg/μL administered alone did not produce any observable effects on motor behavior, general activity (Etgen and Acosta-Martinez, 2003) or cellular toxicity (D’Onofrio et al., 2001; Mori et al., 2002) nor altered the impaired fore-limb usage in 6-OHDA lesioned animals [Fig. 1(B)].
Estrogen and IGF-1 Protective Effects on SNpc DA Neurons is Mediated via Akt
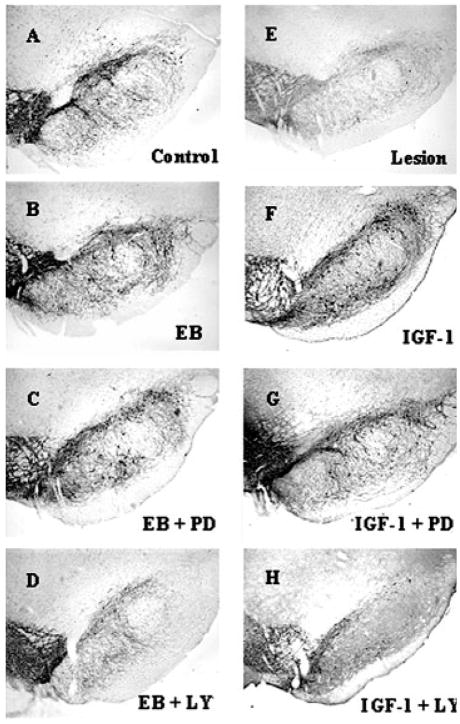
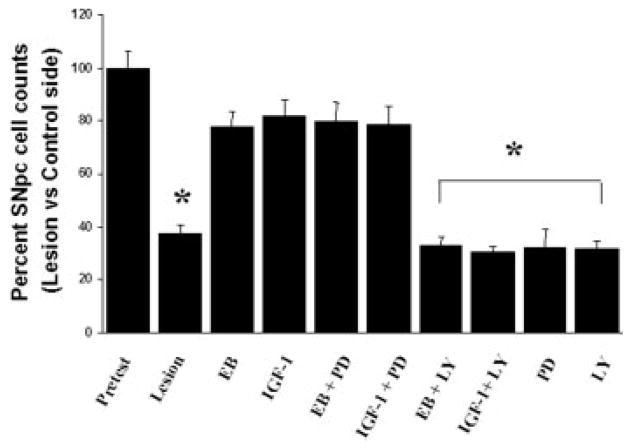
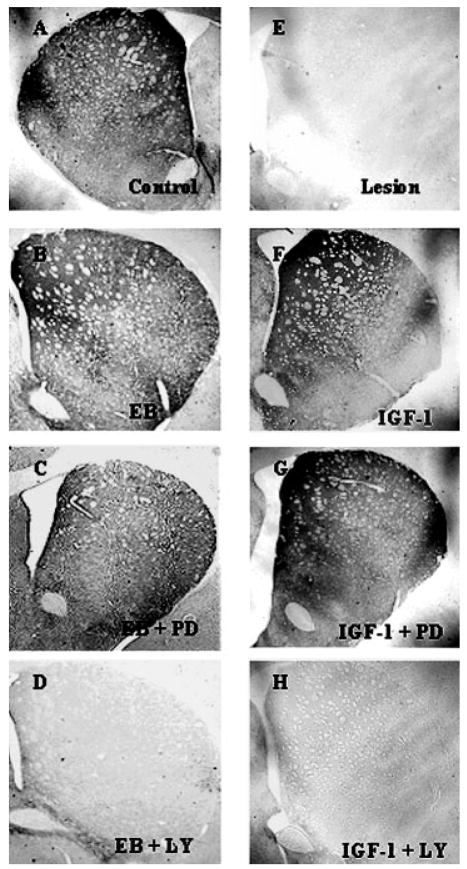
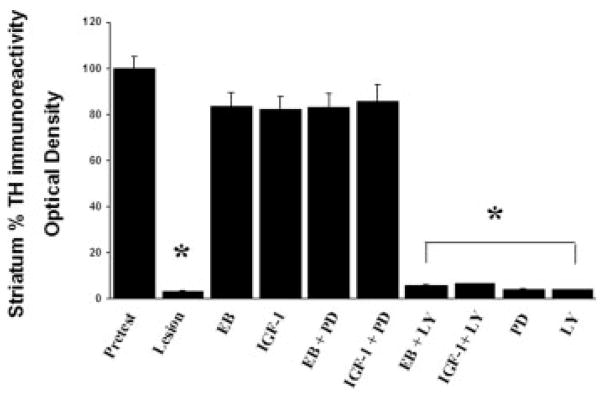
As previously reported (Quesada and Micevych, 2004), intracerebral injection of 6-OHDA into the MFB induced a >60% loss of SNpc TH immunoreactive neurons on the ipsilateral (lesioned) side compared with the contralateral (nonlesioned) control side [Figs. 2(E) and 3). As previously characterized with TH immunoreactivity and Flourogold-labeled nigral neurons, the time course (1 week after 6-OHDA lesions) and amount of 6-OHDA (8 μg) administered reflects true loss of structural integrity and cell death (Zuch et al., 2000). This loss of TH immunoreactive cell bodies is accompanied by a reduction in >95% of TH immunoreactivity in the striatum [Figs. 4(E) and 5) as compared with control animals. As expected, estrogen and IGF-1 treatments result in a significant (p < 0.05) increase in the survival of SNpc TH immunoreactive neurons (>80.0%) after 6-OHDA lesions in animals treated with estrogen or IGF-1 [Figs. 2(B,F) and 3]. Continuous infusion of PI3K/Akt inhibitor, LY294002, blocked estrogen and IGF-1 neuroprotective effects on SNpc DA neurons [p < 0.05; Figs. 2(D,H) and 3] and striatal TH immunoreactivity [Figs. 4(D,H) and 5]. Whereas, treatment with PD98059 (MAPK inhibitor) did not block estrogens and IGF-1-induced survival of TH immunoreactive neurons in the SNpc or TH immunoreactivity in the striatum [Figs. 2(C,G), 3, 4(C,G) and 5].
Figure 2.
Photomicrographs of TH immunoreactivity in the SNpc. PI3K and MAPK inhibitors were used to study estrogen and IGF-1 protection of DA neurons 7 days following unilateral injection of 6-hyroxydopamine (6-OHDA, 2.0 μL of 4 μg/μL) into the medial forebrain bundle. A: Nonlesion control SNpc, (E) Lesion side of SNpc. B: 17β-estradiol benzoate (EB; 20 μg, s.c) was given 24 h prior to lesion, (C) EB + PD98059 (MAPK/ERK inhibitor; 0.1 μg/μL) and (D) EB + LY294002 (PI3K/Akt inhibitor; 0.10 μg/μL). For IGF-1 treated groups (F–H). F: Central infusion of IGF-1 (100 μg/mL), (G) IGF-1 + PD98059 (0.10 μg/μL) and H) IGF-1 + LY294002 (0.10 μg/μL). IGF-1 and inhibitors were continuously infused for 7 days.
Figure 3.
Histographs of SNpc DA neuronal survival following estrogen, IGF-1, PD98059 (MAPK/ERK inhibitor) and LY294002 (PI3K/Akt inhibitor) treatment. Timeline of treatments in Fig. 1(A). Values are mean percentage ± S.E.M of SNpc DA neuronal survival (lesion) ipsilateral vs. (control) contralateral intact side. Each value is calculated from 5 to 7 animals. * p < 0.05 compared to lesion, one-way ANOVA, Student Newman-Keuls posthoc.
Figure 4.
Photomicrographs of striatal TH immunoreactivity 7 days after unilateral injection of 6-OHDA (2.0 μL of 4 μg/μL) into the medial forebrain bundle. A: Nonlesion control, (E) Lesion side. B: 17β-estradiol benzoate (EB; 20 μg) was given 24 h prior to lesion, (C) EB + PD98059 (MAPK/ERK inhibitor; 0.10 μg/μL) and (D) EB + LY294002 (PI3K/Akt inhibitor; 0.10 μg/μL). For IGF-1 treated groups (F–H). F: Central infusion of IGF-1 (100 μg/mL), (G) IGF-1 + PD98059 (0.10 μg/μL) and H) IGF-1 + LY294002 (0.10 μg/μL). IGF-1 and inhibitors were continuously infused for 7 days.
Figure 5.
Histographs of striatal TH immunoreactivity following estrogen, IGF-1, PD98059 (MAPK/ERK inhibitor) and LY294002 (PI3K/Akt inhibitor) treatment. Values are mean percentage ± S.E.M of optical density values of TH immunostaining from (lesion) ipsilateral striata vs. (control) contralateral striata. Each value is calculated from 5 to 7 animals. * p < 0.001 compared to pretest, one-way ANOVA, Student Newman-Keuls posthoc.
Effects of Estrogen and IGF-1 on Substantia Nigra Akt and ERK
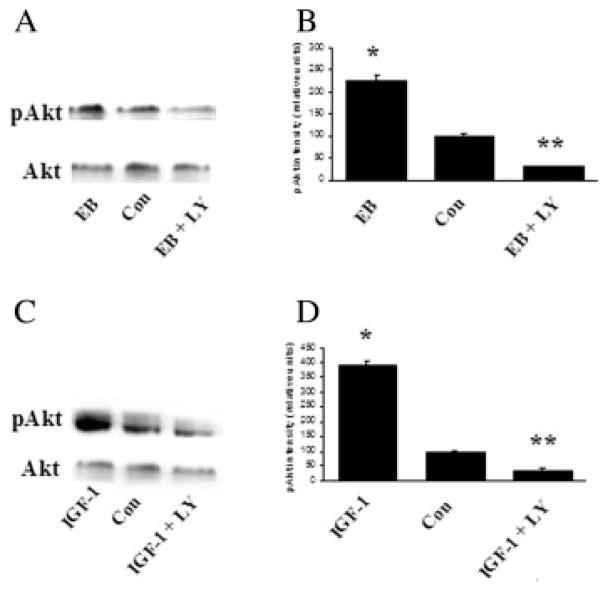
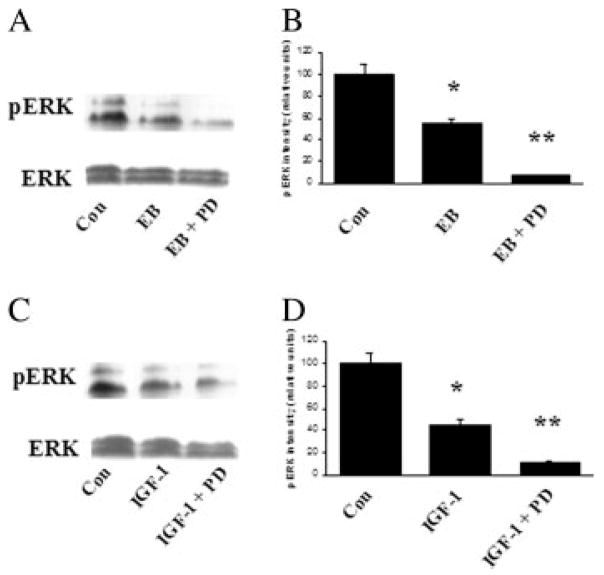
To investigate whether estrogen and IGF-1 activate Akt and ERK in the SN, Western blots of SN tissue were stained using phospho-Akt and phospho-ERK antibodies. All SN tissue samples for Western blot analysis were taken on the 7th day following continuous icv administration of PD98059 and LY294002. Results demonstrate that both estrogen and IGF-1 caused a significant increase in phospho-Akt (twofold and fourfold, respectively; p < 0.001) compared with vehicle-treated animals. Total Akt immunoreactive levels were not affected (see Fig. 6). In contrast, both estrogen and IGF-1 resulted in a significant reduction (twofold; p < 0.001) of phospho-ERK 1/2 compared with control, without affecting total ERK 1/2 at day 7 (see Fig. 7). Thus, estrogen and IGF-1 both activate PI3K/Akt pathway, while inhibiting MAPK/ERK signaling in the SN. Moreover, administration of the MAPK/ERK inhibitor, PD98059, resulted in a significant reduction of ERK 1/2 phosphorylation (fivefold below control levels; p < 0.0001) in the SN compared with controls at day 7 [Fig. 7(B,D)]. Furthermore, the PI3K/Akt inhibitor, LY294002 resulted in a significant inhibition of Akt phosphorylation (twofold; p < 0.0001) in the SN compared with controls at day 7 [Fig. 6(B,D)]. As a control for inhibitor stability, incubation of inhibitors for 7 days at 37°C (body temperature) diluted in 0.4% dimethyl sulfoxide (DMSO) did not affect the stability of inhibitors to inhibit PI3K/Akt and MAPK/ERK activation (data not shown), demonstrating that inhibitors remain stable under these conditions.
Figure 6.
Western blot analysis of estrogen and IGF-1 effects on total Akt and phospho-Akt in the SN (A and B). Rats were killed 7 days after continuous inhibitor administration and total proteins were extracted from SN and assayed. A: Effect of 17β-estradiol benzoate (EB; 20 μg) and EB + LY294002 (PI3K/Akt inhibitor; 0.10 μg/μL) on phospho-Akt. C: Effect of central infusion of IGF-1 (100 μg/mL) and IGF-1 + LY294002 (PI3K/Akt inhibitor; 0.10 μg/μL) on phospho-Akt. B, D: O.D. are expressed as change relative to that of vehicle-treated group (control). *p > 0.05 compared to control; **p < 0.05 compared to control, one-way ANOVA, Student Newman-Keuls posthoc.
Figure 7.
Western blot analysis of estrogen and IGF-1 effects on total ERK and phospho-ERK in the SN (A and B). Rats were killed 7 days after continuous inhibitor administration and total proteins were extracted from SN and assayed. A: Effect of 17β-estradiol benzoate (EB; 20 μg) and EB + PD98059 (MAPK/ERK inhibitor; 0.10 μg/μL) on phospho-ERK (p-ERK 1/2). C: Effect of central infusion of IGF-1 (100 μg/mL) and IGF-1 + PD98059 (MAPK/ERK inhibitor; 0.10 μg/μL) on phospho-ERK. B, D: O.D. are expressed as change relative to that of vehicle-treated group (control). *p < 0.05 compared to control; **p < 0.001 compared to control, one-way ANOVA, Student Newman-Keuls posthoc.
DISCUSSION
In the present study, we demonstrate that estrogen and IGF-1 treatment activated PI3K/Akt and decreased MAPK/ERK signaling in the SN. However, maintenance of motor behavior and SNpc DA neurons revealed that estrogen and IGF-1 neuroprotection is dependent on PI3K/Akt signaling and not MAPK/ERK. The present in vivo study validates several in vitro findings demonstrating PI3K/Akt, but not MAPK/ERK pathway, are critical protective mechanisms for neuronal survival (Russell et al., 1998; Brunet et al., 1999; Zheng and Quirion, 2004; Signore et al., 2006). The role of Akt kinases in cell survival was first suggested by Dudek et al. (1997) who found it to be the key kinase involved in the survival-promoting effects of a broad range of survival factors in various cell types (Dudek et al., 1997; Datta et al., 1999; Zheng et al., 2002). Moreover, decreased PI3K/Akt signaling associated with cell death has been demonstrated in drosophila models of PD (Yang et al., 2005). In terms of estrogen and IGF-1 treatment, inhibition of PI3K/Akt pathway was shown to block IGF-1 neuroprotection of retinal ganglion cells after axotomy (Kermer et al., 2000), and mediated the neuroprotective actions of estrogen in the striatum of MPTP- treated mice (D’Astous et al., 2006).
The mechanism of cell death for 6-OHDA enhances oxidative stress that generates reactive oxygen species (ROS) and mitochondrial defects resulting in neuronal apoptosis (Junn and Mouradian, 2002). Therefore, Akt downstream effectors such as glycogen synthase kinase-3β (GSK-3β), cyclic AMP response element binding protein (CREB), and fork-head (Leinninger et al., 2004) may mediate inhibition of apoptosis in SNpc DA neurons. Among the downstream effectors of Akt signaling, CREB may be critical (Walton et al., 1999). Loss of CREB impairs axonal growth and leads to excessive apoptosis of peripheral sensory and sympathetic neurons (Lonze and Ginty, 2002). Although the targets of estrogen and IGF-1 induced CREB transcription are not currently known in SNpc DA neurons, several CRE-containing survival genes are known, and these include BDNF and IGF-1 itself (Thomas et al., 1996; Mayr and Montminy, 2001). It is plausible that CREB activation may amplify the IGF-1 neuroprotective response by increasing growth factor expression to mediate long-term survival.
Additionally, by promoting the expression of anti-apoptotic signals, the PI3K/Akt pathway may also inhibit proapoptotic gene expression (Barber et al., 2001; Nakamura et al., 2001). For instance, Akt phosphorylates and inactivates the caspase-9 (Cardone et al., 1998) and pro-apoptotic protein BAD (Datta et al., 1997; del Peso et al., 1997). Because caspase-9 is a major activator of the downstream effector caspase-3 (Li et al., 1997), reduced activity of the latter might account for the neuroprotection mediated by Akt, which is congruous with studies demonstrating that IGF-1 effectively blocked caspase-3 activity in hippocampal neurons in vitro (Suzuki et al., 1998; Tamatani et al., 1998; Kermer et al., 2000) and retinal ganglion cells in vivo. Thus, by shifting the balance between the levels of expression of pro-apoptotic and anti-apoptotic genes, estrogen and IGF-1 can act as a protective agent for neural cells. The exact mechanism(s) by which Akt mediates the survival effects of IGF-1 and estrogen on SNpc DA neurons remains to be elucidated.
Recent in vitro studies have demonstrated that inhibition of PI3K/Akt and MAPK/ERK pathways decrease the expression and function of dopamine transporter (DAT). Phosphorylation of DAT via both Akt and MAPK pathways restores the expression and function of this tranporter (Rothman et al., 2002; Lin et al., 2003; Moron et al., 2003; Garcia et al., 2005; Kahlig et al., 2006; Wei et al., 2007). DAT regulates the extracellular clearance of dopamine by an uptake-mediated mechanism, and is the route through which 6-OHDA enters DA neurons. Although not examined in this study it seems unlikely that LY294002 and PD98059 interfere with the toxicokinetics of 6-OHDA uptake through DAT directly for the following reasons. (1) Inhibitor-induced decrease in DAT expression and/or function would result in a decrease in 6-OHDA uptake by the DA neuron therefore resulting in higher levels of SNpc DA neuronal survival. (2) Conversely, the enhanced PI3K/Akt activity in the SN induced by IGF-1 and estrogen should have resulted in enhanced 6-OHDA neurotoxicity due to enhanced expression and/or function of DAT. The present results are not consistent with such outcomes, indicating that IGF-1 and estrogen neuroprotective actions appear to act directly through the PI3K/Akt intracellular signaling pathway and not through DAT regulation of 6-OHDA uptake.
Western blot analysis revealed that 1-week after a single injection of estrogen or continuous central infusion of IGF-1, Akt activation was enhanced in the SN, whereas ERK activation was decreased by both estrogen and IGF-1 treatments. The 1-week continuous infusion of IGF-1 resulted in a twofold increase in Akt activation compared to single injection of estrogen. In contrast, ERK activation is further reduced (>33%) by IGF-1 compared with estrogen treatment. These results are not surprising since estrogens effects may be wearing off after 7 days compared to IGF-1 that is still actively present after 7 days. Although the temporal paradigm of estrogen and IGF-1 treatments differs, both treatments result in similar effects in ERK and Akt activity in the SN. These results are suggestive of estrogen regulating the IGF-1 signaling system in neural and glial cells (Fernandez-Galaz et al., 1997; Cardona-Gomez et al., 2001; Zhang et al., 2004), which may explain the temporal effects of estrogen and IGF-1.
There is need for caution interpreting the LY294002 results since besides being a selective inhibitor for PI3K/Akt, LY294002 may also bind to estrogen receptors and have direct anti-estrogenic actions (Pasapera Limon et al., 2003). Thus, based on the antagonism studies alone, PI3K/Akt pathway may not necessarily participate in the protective effects of estrogen. However, estrogen and IGF-1 induce Akt activation in the SN and previous results indicate that estrogen neuroprotection of SNpc DA neurons is dependent on IGF-1 signaling through the IGF-1R (Quesada and Micevych, 2004) which are abundantly expressed on SNpc DA neurons and glial cells in the SN compared with estrogen receptors (Quesada et al., 2007). Furthermore, the importance of the IGF-1 system in protecting SNpc DA neurons is further supported by the result that central blockade of IGF-1R exacerbates SNpc DA cell death (Quesada and Micevych, 2004). These results provide anatomical support for estrogen regulation of the IGF-1 system in the SN (e.g., enhanced expression of IGF-1 and/or IGF-1R) to stimulate IGF-1R signaling through the PI3K/Akt pathway mediating neuroprotection. In addition, others have indicated a direct role of PI3K/Akt-induced by estrogen and IGF-1 in protecting nigral DA neurons (Mannella and Brinton, 2006) and indirectly via glial cells (Ye et al., 2004). Evidence for a direct action of Akt in SNpc DA neurons, was the protection of a DA cell line (MN9D) against 6-OHDA-induced cell death by expression of a constitutive active Akt (Signore et al., 2006).
In the case of ERK, the finding that IGF-1 protects cultured neurons by inhibiting ERK activation via the PI3K/PKA/Raf pathway (Subramaniam et al., 2005) may be a possible neuroprotective mechanism for SNpc DA neurons. Others have reported that sustained activation of MAPK/ERK is deleterious and promotes neuronal death. For example, sustained activation of MAPK/ERK pathway exacerbates hyperexcitation and focal ischemic injury (Murray et al., 1998; Alessandrini et al., 1999; Stanciu et al., 2000) and that inhibition of MAPK/ERK activation prevents loss of cortical volume after traumatic brain injury (Mori et al., 2002). Although, we did not examine the rapid signaling of estrogen and IGF-1 in the SN, other investigators have reported that estrogen causes a rapid enhancement of MAPK/ERK in rodent brain within minutes of estrogen treatment (Cardona-Gomez et al., 2002; Bryant et al., 2005) and rapid activation of PI3K/Akt and MAPK/ERK within the same population of cortical neurons (Mannella and Brinton, 2006). Thus, it is possible that estrogen and IGF-1 may cause a rapid increase of MAPK/ERK activity in SNpc DA neurons that, in turn, is inhibited by sustained PI3K/Akt kinase activity, suggesting that sustained MAPK/ERK activity may be deleterious for SNpc DA neuronal survival. On the basis of these results, we expected that blocking MAPK/ERK activity would improve forelimb usage after 6-OHDA lesions. No such improvement or survival of SNpc DA neurons was observed in the present studies, suggesting that MAPK/ERK activity in the SN may involve other neuronal functions (e.g. neuronal plasticity and/or maintenance), but its role in neuroprotection is dubious.
The limitations of the 6-OHDA-model are not unlike many other animal model of PD. Although we are modeling a neurodegenerative disease that has a late-onset, animals are young and healthy prior to insult and the rapid neuronal loss does not recapitulate the usual course of neurodegenerative diseases (Brinton, 2005). Although transgenic mouse models of human PD genes have been generated, none show relatively selective DA neuronal degeneration typically found in clinical PD. Thus far, among the various experimental PD models, neurotoxins such as 6-OHDA and MPTP remain the most popular experimental tools to produce selective DA neuronal death in both in vitro and in vivo systems, and provokes molecular alterations comparable to those seen in PD (Blum et al., 2001). Genetic and environment factors certainly contribute to PD vulnerability, but one of the most important risk factors is age, which underlies an important caveat to the present studies. Although estrogen replacement in young animals is beneficial, it may be deleterious in aged animals. For example, estrogen protects against excitotoxic injury to the forebrain in young animals, but it exacerbates neural injury in aging animals (Nordell et al., 2003). Moreover, a recent in vitro study demonstrates that estrogen exposure prior to neurodegenerative insult promotes neuron survival whereas estrogen exposure following insult does not, and can in instances, exacerbate such degeneration (Chen et al., 2006). The clinical data regarding the role of estrogen are conflicting. Most support a role of estrogen in improving parkinsonism, whereas some refute a positive effect (Shulman, 2002; Ragonese et al., 2006). Thus, there may be important differences concerning estrogen neuroprotective effects on the nigrostriatal DA system in aging animals, i.e., whether estrogen effects in the aging brain are beneficial or not.
In summary, this report provides the first evidence that PI3K/Akt pathway is critical in the estrogen and IGF-1 neuroprotection of SNpc DA neurons in vivo. Moreover, understanding these intracellular signaling pathways action in protecting cells from dying will have far-reaching implications for understanding disease mechanisms and developing therapeutic strategies.
Acknowledgments
Contract grant sponsor: NIH; contract grant number: DA013185.
Contract grant sponsor: American Parkinson’ disease Association.
Footnotes
This manuscript is dedicated to the loving memory of Becky Y. Lee B.S., MPH who was instrumental in this work
References
- Alessandrini A, Namura S, Moskowitz MA, Bonventre JV. MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proc Natl Acad Sci USA. 1999;96:12866–12869. doi: 10.1073/pnas.96.22.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentero MT, Fancellu R, Nappi G, Bramanti P, Blandini F. Prolonged blockade of NMDA or mGluR5 glutamate receptors reduces nigrostriatal degeneration while inducing selective metabolic changes in the basal ganglia circuitry in a rodent model of Parkinson’s disease. Neurobiol Dis. 2006;22:1–9. doi: 10.1016/j.nbd.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Baldereschi M, Di Carlo A, Rocca WA, Vanni P, Maggi S, Perissinotto E, Grigoletto F, et al. Parkinson’s disease and parkinsonism in a longitudinal study: Twofold higher incidence in men. ILSA Working Group. Italian Longitudinal Study on Aging. Neurology. 2000;55:1358–1363. doi: 10.1212/wnl.55.9.1358. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Nakamura M, Wolpert EB, Reiter CE, Seigel GM, Antonetti DA, Gardner TW. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J Biol Chem. 2001;276:32814–32821. doi: 10.1074/jbc.M104738200. [DOI] [PubMed] [Google Scholar]
- Beyer C, Ivanova T, Karolczak M, Kuppers E. Cell type-specificity of nonclassical estrogen signaling in the developing midbrain. J Steroid Biochem Mol Biol. 2002;81:319–325. doi: 10.1016/s0960-0760(02)00119-x. [DOI] [PubMed] [Google Scholar]
- Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci USA. 2001;98:13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandini F, Armentero MT, Fancellu R, Blaugrund E, Nappi G. Neuroprotective effect of rasagiline in a rodent model of Parkinson’s disease. Exp Neurol. 2004;187:455–459. doi: 10.1016/j.expneurol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, Verna JM. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol. 2001;65:135–172. doi: 10.1016/s0301-0082(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976–1990. Neurology. 1999;52:1214–1220. doi: 10.1212/wnl.52.6.1214. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: Evidence in support of a healthy cell bias of estrogen action. Ann NY Acad Sci. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Bryant DN, Bosch MA, Ronnekleiv OK, Dorsa DM. 17-β estradiol rapidly enhances extracellular signal-regulated kinase 2 phosphorylation in the rat brain. Neuroscience. 2005;133:343–352. doi: 10.1016/j.neuroscience.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Butler AA, Yakar S, Gewolb IH, Karas M, Okubo Y, LeR-oith D. Insulin-like growth factor-I receptor signal transduction: At the interface between physiology and cell biology. Comp Biochem Physiol B Biochem Mol Biol. 1998;121:19–26. doi: 10.1016/s0305-0491(98)10106-2. [DOI] [PubMed] [Google Scholar]
- Carboni E, Tanda GL, Frau R, Di Chiara G. Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: Evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem. 1990;55:1067–1070. doi: 10.1111/j.1471-4159.1990.tb04599.x. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogens and insulin-like growth factor-I in the brain: Implications for neuroprotection. Brain Res Brain Res Rev. 2001;37:320–334. doi: 10.1016/s0165-0173(01)00137-0. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, Mendez P, Garcia-Segura LM. Synergistic interaction of estradiol and insulin-like growth factor-I in the activation of PI3K/Akt signaling in the adult rat hypothalamus. Brain Res Mol Brain Res. 2002;107:80–88. doi: 10.1016/s0169-328x(02)00449-7. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Chen S, Nilsen J, Brinton RD. Dose and temporal pattern of estrogen exposure determines neuroprotective outcome in hippocampal neurons: Therapeutic implications. Endocrinology. 2006;147:5303–5313. doi: 10.1210/en.2006-0495. [DOI] [PubMed] [Google Scholar]
- Cuenda A, Alessi DR. Use of Kinase Inhibitors to Dissect Signaling Pathways. Totowa, NJ: Humana Press, Inc; 1999. [DOI] [PubMed] [Google Scholar]
- D’Astous M, Mendez P, Morissette M, Garcia-Segura LM, Di Paolo T. Implication of the PI3K/Akt signaling pathway in the neuroprotective effect of estradiol in the striatum of MPTP mice. Mol Pharmacol. 2006;69:1492–1498. doi: 10.1124/mol.105.018671. [DOI] [PubMed] [Google Scholar]
- D’Onofrio M, Cuomo L, Battaglia G, Ngomba RT, Storto M, Kingston AE, Orzi F, et al. Neuroprotection mediated by glial group-II metabotropic glutamate receptors requires the activation of the MAP kinase and the phosphatidylinositol-3-kinase pathways. J Neurochem. 2001;78:435–445. doi: 10.1046/j.1471-4159.2001.00435.x. [DOI] [PubMed] [Google Scholar]
- Datla KP, Murray HE, Pillai AV, Gillies GE, Dexter DT. Differences in dopaminergic neuroprotective effects of estrogen during estrous cycle. Neuroreport. 2003;14:47–50. doi: 10.1097/00001756-200301200-00009. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: A play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- Deumens R, Blokland A, Prickaerts J. Modeling Parkinson’s disease in rats: An evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 2002;175:303–317. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- Disshon KA, Dluzen DE. Estrogen as a neuromodulator of MPTP-induced neurotoxicity: Effects upon striatal dopamine release. Brain Res. 1997;764:9–16. doi: 10.1016/s0006-8993(97)00418-6. [DOI] [PubMed] [Google Scholar]
- Dluzen D. Estrogen decreases corpus striatal neurotoxicity in response to 6-hydroxydopamine. Brain Res. 1997;767:340–344. doi: 10.1016/s0006-8993(97)00630-6. [DOI] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Acosta-Martinez M. Participation of growth factor signal transduction pathways in estradiol facilitation of female reproductive behavior. Endocrinology. 2003;144:3828–3835. doi: 10.1210/en.2003-0157. [DOI] [PubMed] [Google Scholar]
- Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones—A focus on rapid, nongenomic effects. Pharmacol Rev. 2000;52:513–556. [PubMed] [Google Scholar]
- Fernandez-Galaz MC, Morschl E, Chowen JA, Torres-Aleman I, Naftolin F, Garcia-Segura LM. Role of astroglia and insulin-like growth factor-I in gonadal hormone-dependent synaptic plasticity. Brain Res Bull. 1997;44:525–531. doi: 10.1016/s0361-9230(97)00238-4. [DOI] [PubMed] [Google Scholar]
- Garcia BG, Wei Y, Moron JA, Lin RZ, Javitch JA, Galli A. Akt is essential for insulin modulation of amphetamine-induced human dopamine transporter cell-surface redistribution. Mol Pharmacol. 2005;68:102–109. doi: 10.1124/mol.104.009092. [DOI] [PubMed] [Google Scholar]
- Junn E, Mouradian MM. Human α-synuclein over-expression increases intracellular reactive oxygen species levels and susceptibility to dopamine. Neurosci Lett. 2002;320:146–150. doi: 10.1016/s0304-3940(02)00016-2. [DOI] [PubMed] [Google Scholar]
- Kahlig KM, Lute BJ, Wei Y, Loland CJ, Gether U, Javitch JA, Galli A. Regulation of dopamine transporter trafficking by intracellular amphetamine. Mol Pharmacol. 2006;70:542–548. doi: 10.1124/mol.106.023952. [DOI] [PubMed] [Google Scholar]
- Kermer P, Klocker N, Labes M, Bahr M. Insulin-like growth factor-I protects axotomized rat retinal ganglion cells from secondary death via PI3-K-dependent Akt phosphorylation and inhibition of caspase-3 in vivo. J Neurosci. 2000;20:2–8. [PubMed] [Google Scholar]
- Leinninger GM, Backus C, Uhler MD, Lentz SI, Feldman EL. Phosphatidylinositol 3-kinase and Akt effectors mediate insulin-like growth factor-I neuroprotection in dorsal root ganglia neurons. FASEB J. 2004;18:1544–1546. doi: 10.1096/fj.04-1581fje. [DOI] [PubMed] [Google Scholar]
- LeRoith D, Werner H, Beitner-Johnson D, Roberts CT., Jr Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Lin Z, Zhang PW, Zhu X, Melgari JM, Huff R, Spieldoch RL, Uhl GR. Phosphatidylinositol 3-kinase, protein kinase C, and MEK1/2 kinase regulation of dopamine transporters (DAT) require N-terminal DAT phosphoacceptor sites. J Biol Chem. 2003;278:20162–20170. doi: 10.1074/jbc.M209584200. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Mannella P, Brinton RD. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: A unified mechanism of estrogen action. J Neurosci. 2006;26:9439–9447. doi: 10.1523/JNEUROSCI.1443-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux R, Denaro J, Hemenegildo N, Marder K, Tang MX, Cote LJ, Stern Y. A population-based investigation of Parkinson’s disease with and without dementia. Relationship to age and gender. Arch Neurol. 1992;49:492–497. doi: 10.1001/archneur.1992.00530290076015. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- Mori T, Wang X, Jung JC, Sumii T, Singhal AB, Fini ME, Dixon CE, et al. Mitogen-activated protein kinase inhibition in traumatic brain injury: In vitro and in vivo effects. J Cereb Blood Flow Metab. 2002;22:444–452. doi: 10.1097/00004647-200204000-00008. [DOI] [PubMed] [Google Scholar]
- Moron JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, Lin ZC, et al. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci. 2003;23:8480–8488. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mura A, Mintz M, Feldon J. Behavioral and anatomical effects of long-term L-dihydroxyphenylalanine (L-DOPA) administration in rats with unilateral lesions of the nigrostriatal system. Exp Neurol. 2002;177:252–264. doi: 10.1006/exnr.2002.7976. [DOI] [PubMed] [Google Scholar]
- Murray B, Alessandrini A, Cole AJ, Yee AG, Furshpan EJ. Inhibition of the p44/42 MAP kinase pathway protects hippocampal neurons in a cell-culture model of seizure activity. Proc Natl Acad Sci USA. 1998;95:11975–11980. doi: 10.1073/pnas.95.20.11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray HE, Pillai AV, McArthur SR, Razvi N, Datla KP, Dexter DT, Gillies GE. Dose- and sex-dependent effects of the neurotoxin 6-hydroxydopamine on the nigrostriatal dopaminergic pathway of adult rats: Differential actions of estrogen in males and females. Neuroscience. 2003;116:213–222. doi: 10.1016/s0306-4522(02)00578-x. [DOI] [PubMed] [Google Scholar]
- Myers MG, Jr, Grammer TC, Wang LM, Sun XJ, Pierce JH, Blenis J, White MF. Insulin receptor substrate-1 mediates phosphatidylinositol 3′-kinase and p70S6k signaling during insulin, insulin-like growth factor-1, and interleukin-4 stimulation. J Biol Chem. 1994;269:28783–28789. [PubMed] [Google Scholar]
- Nakamura M, Barber AJ, Antonetti DA, LaNoue KF, Robinson KA, Buse MG, Gardner TW. Excessive hexosamines block the neuroprotective effect of insulin and induce apoptosis in retinal neurons. J Biol Chem. 2001;276:43748–43755. doi: 10.1074/jbc.M108594200. [DOI] [PubMed] [Google Scholar]
- Nordell VL, Scarborough MM, Buchanan AK, Sohrabji F. Differential effects of estrogen in the injured forebrain of young adult and reproductive senescent animals. Neurobiol Aging. 2003;24:733–743. doi: 10.1016/s0197-4580(02)00193-8. [DOI] [PubMed] [Google Scholar]
- Pasapera Limon AM, Herrera-Munoz J, Gutierrez-Sagal R, Ulloa-Aguirre A. The phosphatidylinositol 3-kinase inhibitor LY294002 binds the estrogen receptor and inhibits 17β-estradiol-induced transcriptional activity of an estrogen sensitive reporter gene. Mol Cell Endocrinol. 2003;200:199–202. doi: 10.1016/s0303-7207(02)00421-5. [DOI] [PubMed] [Google Scholar]
- Paul G, Meissner W, Rein S, Harnack D, Winter C, Hosmann K, Morgenstern R, et al. Ablation of the sub-thalamic nucleus protects dopaminergic phenotype but not cell survival in a rat model of Parkinson’s disease. Exp Neurol. 2004;185:272–280. doi: 10.1016/s0014-4886(03)00363-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, Pennisi M, Topple A. Bregma, lambda, and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods. 1985;13:139–143. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- Quesada A, Etgen AM. Functional interactions between estrogen and insulin-like growth factor-I in the regulation of α 1B-adrenoceptors and female reproductive function. J Neurosci. 2002;22:2401–2408. doi: 10.1523/JNEUROSCI.22-06-02401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A, Micevych PE. Estrogen interacts with the IGF-1 system to protect nigrostriatal dopamine and maintain motoric behavior after 6-hydroxdopamine lesions. J Neurosci Res. 2004;75:107–116. doi: 10.1002/jnr.10833. [DOI] [PubMed] [Google Scholar]
- Quesada A, Romeo HE, Micevych P. Distribution and localization patterns of estrogen receptor-β and insulin-like growth factor-1 receptors in neurons and glial cells of the female rat substantia nigra: Localization of ERβ and IGF-1R in substantia nigra. J Comp Neurol. 2007;503:198–208. doi: 10.1002/cne.21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragonese P, D’Amelio M, Savettieri G. Implications for estrogens in Parkinson’s disease: An epidemiological approach. Ann NY Acad Sci. 2006;1089:373–382. doi: 10.1196/annals.1386.004. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: Studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Dersch CM, Carroll FI, Ananthan S. Studies of the biogenic amine transporters. VIII. Identification of a novel partial inhibitor of dopamine uptake and dopamine transporter binding. Synapse. 2002;43:268–274. doi: 10.1002/syn.10046. [DOI] [PubMed] [Google Scholar]
- Russell JW, Windebank AJ, Schenone A, Feldman EL. Insulin-like growth factor-I prevents apoptosis in neurons after nerve growth factor withdrawal. J Neurobiol. 1998;36:455–467. doi: 10.1002/(sici)1097-4695(19980915)36:4<455::aid-neu1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: A combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59:401–415. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Shulman LM. Is there a connection between estrogen and Parkinson’s disease? Parkinsonism Relat Disord. 2002;8:289–295. doi: 10.1016/s1353-8020(02)00014-7. [DOI] [PubMed] [Google Scholar]
- Signore AP, Weng Z, Hastings T, Van Laar AD, Liang Q, Lee YJ, Chen J. Erythropoietin protects against 6-hydroxydopamine-induced dopaminergic cell death. J Neurochem. 2006;96:428–443. doi: 10.1111/j.1471-4159.2005.03587.x. [DOI] [PubMed] [Google Scholar]
- Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J Neurosci. 1999;19:2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. Ovarian hormones elicit phosphorylation of Akt and extracellular-signal regulated kinase in explants of the cerebral cortex. Endocrine. 2001;14:407–415. doi: 10.1385/ENDO:14:3:407. [DOI] [PubMed] [Google Scholar]
- Singh M, Setalo G, Jr, Guan X, Frail DE, Toran-Allerand CD. Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-alpha knock-out mice. J Neurosci. 2000;20:1694–1700. doi: 10.1523/JNEUROSCI.20-05-01694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanciu M, Wang Y, Kentor R, Burke N, Watkins S, Kress G, Reynolds I, et al. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J Biol Chem. 2000;275:12200–12206. doi: 10.1074/jbc.275.16.12200. [DOI] [PubMed] [Google Scholar]
- Subramaniam S, Shahani N, Strelau J, Laliberte C, Brandt R, Kaplan D, Unsicker K. Insulin-like growth factor 1 inhibits extracellular signal-regulated kinase to promote neuronal survival via the phosphatidylinositol 3-kinase/protein kinase A/c-Raf pathway. J Neurosci. 2005;25:2838–2852. doi: 10.1523/JNEUROSCI.5060-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Kaziro Y, Koide H. Synergistic action of R-Ras and IGF-1 on Bcl-xL expression and caspase-3 inhibition in BaF3 cells: R-Ras and IGF-1 control distinct anti-apoptotic kinase pathways. FEBS Lett. 1998;437:112–116. doi: 10.1016/s0014-5793(98)01213-7. [DOI] [PubMed] [Google Scholar]
- Tamatani M, Ogawa S, Tohyama M. Roles of Bcl-2 and caspases in hypoxia-induced neuronal cell death: a possible neuroprotective mechanism of peptide growth factors. Brain Res Mol Brain Res. 1998;58:27–39. doi: 10.1016/s0169-328x(98)00095-3. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Umayahara Y, Shu H, Centrella M, Rotwein P, McCarthy TL. Identification of the cAMP response element that controls transcriptional activation of the insulin-like growth factor-I gene by prostaglandin E2 in osteoblasts. J Biol Chem. 1996;271:21835–21841. doi: 10.1074/jbc.271.36.21835. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Singh M, Setalo G., Jr Novel mechanisms of estrogen action in the brain: New players in an old story. Front Neuroendocrinol. 1999;20:97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U, Arbuthnott GW. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970;24:485–493. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Tekirian TL, Cheo CT. Sexual differences in sensitivity to methamphetamine toxicity. J Neural Transm Gen Sect. 1993;93:67–70. doi: 10.1007/BF01244939. [DOI] [PubMed] [Google Scholar]
- Walton M, Woodgate AM, Muravlev A, Xu R, During MJ, Dragunow M. CREB phosphorylation promotes nerve cell survival. J Neurochem. 1999;73:1836–1842. [PubMed] [Google Scholar]
- Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells: Effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- Wei Y, Williams JM, Dipace C, Sung U, Javitch JA, Galli A, Saunders C. Dopamine transporter activity mediates amphetamine-induced inhibition of Akt through a Ca2+/calmodulin-dependent kinase II-dependent mechanism. Mol Pharmacol. 2007;71:835–842. doi: 10.1124/mol.106.026351. [DOI] [PubMed] [Google Scholar]
- Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson’s disease than women? J Neurol Neurosurg Psychiatry. 2004;75:637–639. doi: 10.1136/jnnp.2003.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Haque ME, Imai Y, Kosek J, Yang L, Beal MF, et al. Inactivation of drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci USA. 2005;102:13670–13675. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Popken GJ, Kemper A, McCarthy K, Popko B, D’Ercole AJ. Astrocyte-specific overexpression of insulin-like growth factor-I promotes brain overgrowth and glial fibrillary acidic protein expression. J Neurosci Res. 2004;78:472–484. doi: 10.1002/jnr.20288. [DOI] [PubMed] [Google Scholar]
- Zhang L, Nair A, Krady K, Corpe C, Bonneau RH, Simpson IA, Vannucci SJ. Estrogen stimulates microglia and brain recovery from hypoxia-ischemia in normoglycemic but not diabetic female mice. J Clin Invest. 2004;113:85–95. doi: 10.1172/JCI200418336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng WH, Kar S, Quirion R. Insulin-like growth factor-1-induced phosphorylation of transcription factor FKHRL1 is mediated by phosphatidylinositol 3-kinase/Akt kinase and role of this pathway in insulin-like growth factor-1-induced survival of cultured hippocampal neurons. Mol Pharmacol. 2002;62:225–233. doi: 10.1124/mol.62.2.225. [DOI] [PubMed] [Google Scholar]
- Zheng WH, Quirion R. Comparative signaling pathways of insulin-like growth factor-1 and brain-derived neurotrophic factor in hippocampal neurons and the role of the PI3 kinase pathway in cell survival. J Neurochem. 2004;89:844–852. doi: 10.1111/j.1471-4159.2004.02350.x. [DOI] [PubMed] [Google Scholar]
- Zuch CL, Nordstroem VK, Briedrick LA, Hoernig GR, Granholm AC, Bickford PC. Time course of degenerative alterations in nigral dopaminergic neurons following a 6-hydroxydopamine lesion. J Comp Neurol. 2000;427:440–454. doi: 10.1002/1096-9861(20001120)427:3<440::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]