Abstract
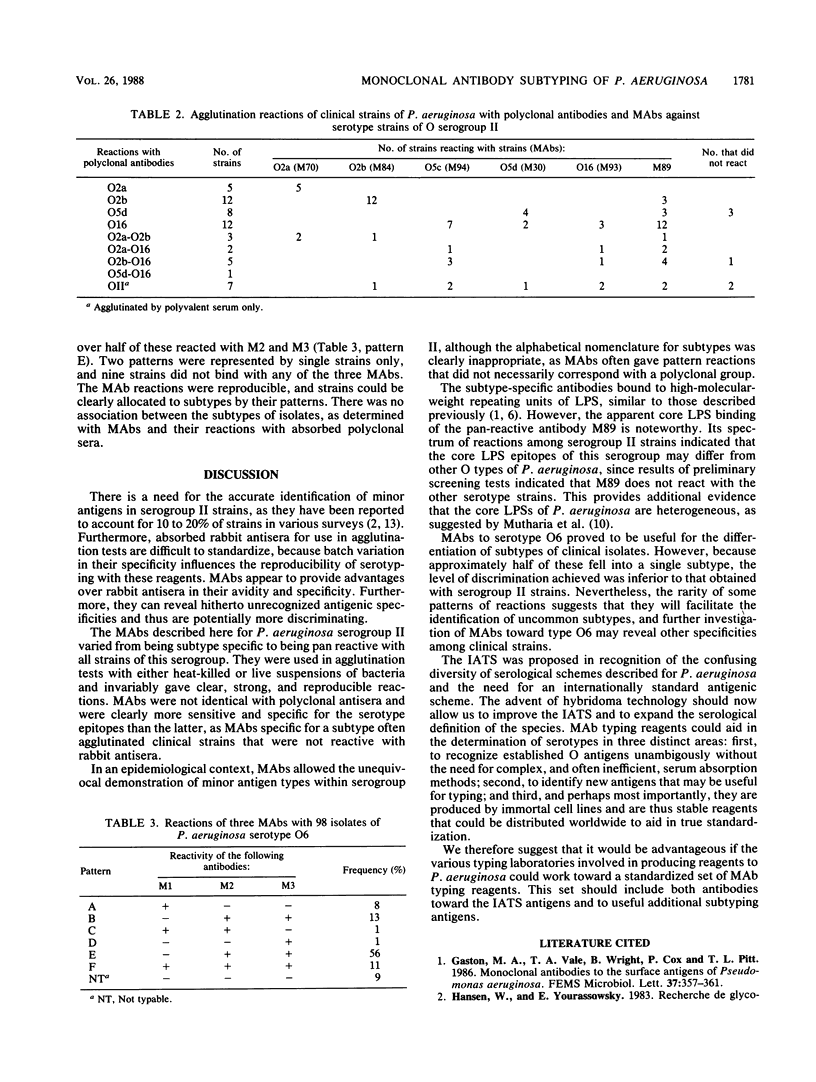
Sixteen murine monoclonal antibodies (MAbs) against serotypes O2, O5, and O16 (serogroup II) and subtypes O2b and O5d of Pseudomonas aeruginosa were evaluated by agglutination and enzyme-linked immunosorbent assay. Six MAbs that exhibited different specificities were compared with absorbed rabbit O-type antisera for the serotyping of 55 clinical isolates of serogroup II. There was good agreement between the antibodies for strains of serotypes O2 and O2b, but MAbs revealed reproducible differences between strains that were indistinguishable with rabbit antisera. The greater serotype specificity of MAbs was illustrated by the fact that only 5 of 20 strains which were agglutinated by rabbit antiserum O16 reacted with the MAb to that serotype. One antibody, M89, that reacted with 27 of 55 serogroup II strains, apparently bound to core lipopolysaccharide epitopes. Three MAbs to the frequent serotype O6 identified six subtypes, one of which accounted for over half of the clinical strains, while two subtypes were represented by single strains only. Overall, MAbs provided a greater discrimination between strains of P. aeruginosa of the same serotype than did absorbed polyclonal antisera.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam J. S., MacDonald L. A., Lam M. Y., Duchesne L. G., Southam G. G. Production and characterization of monoclonal antibodies against serotype strains of Pseudomonas aeruginosa. Infect Immun. 1987 May;55(5):1051–1057. doi: 10.1128/iai.55.5.1051-1057.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutharia L. M., Hancock R. E. Surface localization of Pseudomonas aeruginosa outer membrane porin protein F by using monoclonal antibodies. Infect Immun. 1983 Dec;42(3):1027–1033. doi: 10.1128/iai.42.3.1027-1033.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips I. Identification of Pseudomonas aeruginosa in the clinical laboratory. J Med Microbiol. 1969 Feb;2(1):9–16. doi: 10.1099/00222615-2-1-9. [DOI] [PubMed] [Google Scholar]
- Pitt T. L. A comparison of flagellar typing and phage typing as means of subdividing the O groups of Pseudomonas aeruginosa. J Med Microbiol. 1981 Aug;14(3):261–270. doi: 10.1099/00222615-14-3-261. [DOI] [PubMed] [Google Scholar]
- Pitt T. L., Erdman Y. J. The specificity of agglutination reactions of Pseudomonas aeruginosa with O antisera. J Med Microbiol. 1978 Feb;11(1):15–23. doi: 10.1099/00222615-11-1-15. [DOI] [PubMed] [Google Scholar]
- Pitt T. L. State of the art: typing Pseudomonas aeruginosa. J Hosp Infect. 1980 Sep;1(3):193–199. doi: 10.1016/0195-6701(80)90056-0. [DOI] [PubMed] [Google Scholar]
- Sadoff J. C., Wright D. C., Futrovsky S., Sidberry H., Collins H., Kaufmann B. Characterization of mouse monoclonal antibodies directed against Pseudomonas aeruginosa lipopolysaccharides. Antibiot Chemother (1971) 1985;36:134–146. doi: 10.1159/000410478. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]




