Summary
Accurate retinotectal axon pathfinding depends on the correct establishment of dorsalventral retinal polarity. We show that dorsal retinal gene expression is regulated by Wnt signaling in the dorsal retinal pigment epithelium (RPE). We find that a Wnt reporter transgene and Wnt pathway components are expressed in the dorsal RPE beginning 14–16 hours post-fertilization. In the absence of Wnt signaling, tbx5 and bmp genes initiate normal dorsal retinal expression, but are not maintained. Expression of these genes is rescued by downstream activation of Wnt signaling, and tbx5 is rescued by Bmp signaling. Furthermore, activation of Wnt signaling cannot rescue tbx5 in the absence of Bmp signaling, suggesting that Wnt signaling maintains dorsal retinal gene expression by regulating Bmp signaling. We present a model in which dorsal RPE-derived Wnt activity maintains the expression of Bmp ligands in the dorsal retina, thus coordinating the patterning of these two ocular tissues.
Keywords: Wnt, Bmp, dorsal retina, RPE, zebrafish
Introduction
Vertebrate retinal development is a complex process that involves the coordination of morphogenetic tissue movements with gene expression. During this process, domains of gene expression are maintained despite large-scale changes in the size and shape of the retina and associated tissues. A key early step in eye development is the establishment of dorsal-ventral (D-V) and nasal-temporal (N-T) retinal polarity, manifested by the expression of specific genes in discrete retinal domains, leading to accurate retinotopic targeting of retinal ganglion cell (RGC) axons to their targets in the brain. For example, in zebrafish, tbx5 is expressed continuously in the presumptive dorsal retina starting from the early optic vesicle stage at 12 hours post-fertilization (hpf; shown in Figure 2M–P), while vax2 is expressed in the ventral retina and optic stalk starting from 12 hpf (Take-uchi et al., 2003). The activity of the transcription factors encoded by these and other genes ultimately leads to the correct D-V topographical mapping of RGC axons to the optic tectum in anamniotes and avians, or superior colliculus in mammals, through the regulated expression of guidance molecules (reviewed in McLaughlin and O'Leary, 2005).
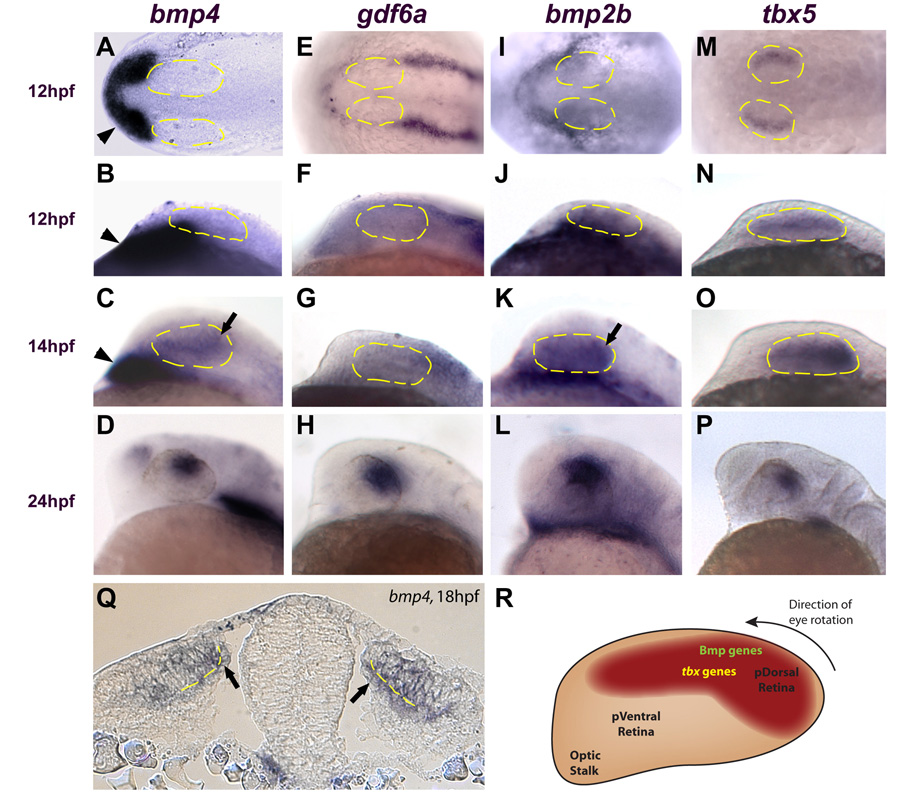
Figure 2. Multiple bmp genes and tbx5 are expressed in the retina before canonical Wnt activity.
A, E I, M: Dorsal views, anterior left. B–D, F–H, J–L, N–P: Lateral views, dorsal up, anterior left. A–D: bmp4 is expressed in the prechordal mesoderm at 12 and 14 hpf (arrowheads in A–C) but is not expressed in the optic vesicle until 14 hpf (arrow in C). At 24 hpf, bmp4 expression is restricted to the dorsal retina (D). E–L: gdf6a and bmp2b are not expressed in the optic vesicle at 12 hpf (expression of these genes is restricted to the surface ectoderm). Expression of bmp2b is present in the retina at 14 hpf (arrow in K), but gdf6a does not appear in the optic vesicle until 16 hpf (not shown). Both genes are expressed in the dorsal retina at 24 hpf (H, L). M–P: tbx5 expression begins in the optic vesicle at 12 hpf and becomes progressively restricted to the dorsal retina by 24 hpf. Q: Transverse section through the midbrain at 18 hpf. bmp4 is expressed in the presumptive dorsal neural retina and RPE (arrows) Broken yellow lines indicate the interface between the neural retina and RPE. R: Diagram of zebrafish retina at approximately 14 hpf, showing expression domains of bmp genes and tbx5 at this timepoint. At approximately 22 hpf, the entire eye rotates 90° in the direction indicated. Anterior left, dorsal up.
The sequence of events leading to ventral retinal identity is initiated when Sonic hedgehog (Shh) from the ventral midline triggers the expression of ventral retinal transcription factors, including Vax2 (Ekker et al., 1995; Macdonald et al., 1995; Take-uchi et al., 2003; Zhang and Yang, 2001). Vax2 can exclude the expression of dorsal retinal genes from the ventral retina and also induce the graded expression of EphB2 and EphB3 (Schulte et al., 1999). This process leads to retinal progenitor cells that have been “coded” with ventral positional identity in the form of EphB receptor tyrosine kinase expression (Barbieri et al., 2002; Mui et al., 2002; Schulte et al., 1999).
The establishment of dorsal retinal identity appears to be controlled by another family of growth factors. A current model of dorsal retinal patterning posits that Bmp4, expressed in the dorsal retina, triggers the graded dorsal expression of tbx5, which in turn leads to graded expression of EphrinB molecules (McLaughlin et al., 2003). Genetic inactivation of Bmp receptors and Bmp4 demonstrate the requirement of Bmp signaling for dorsal retinal identity in mouse (Murali et al., 2005), and misexpression of Bmp4 can dorsalize the ventral retina in chick and Xenopus (Koshiba-Takeuchi et al., 2000; Sasagawa et al., 2002). In zebrafish, multiple bmp genes as well as tbx5 are expressed in the dorsal retina (Rissi et al., 1995; Thisse and Thisse, 2005). Furthermore, at least one Bmp family member, Gdf6a, has been implicated in controlling expression of dorsal retina markers including tbx5 in multiple vertebrate organisms (Asai-Coakwell et al., 2007; Delot et al., 1999; French et al., 2007; Hanel and Hensey, 2006). However, current models do not address whether Bmps or tbx genes might act in distinct steps of dorsal patterning, such as initiation, maintenance, or refinement, and leave open the possibility that other factors may also play key roles.
We were interested in whether canonical Wnt signaling acts in D-V retinal patterning, based on several previous observations. The canonical Wnt pathway plays key roles in many important aspects of vertebrate CNS development, including the patterning of CNS structures (Bonner et al., 2008; Dorsky et al., 2003; Ille et al., 2007; Kapsimali et al., 2004; Kudoh et al., 2002; Muroyama et al., 2002). Several components of the canonical Wnt signaling pathway have also been shown to be expressed in developing vertebrate eye structures (reviewed in Van Raay and Vetter, 2004), suggesting their involvement in eye formation. Both Wnt reporter transgenes and Wnts themselves are expressed in the dorsal retinal pigmented epithelium (RPE) as well as surrounding tissues during early eye development (Burns et al., 2008; Cho and Cepko, 2006; Fokina and Frolova, 2006; Liu et al., 2006). In the developing brain and spinal cord, both Bmp and Wnt signaling are required for proper dorsal patterning, and Shh induces ventral identities (see reviews by Briscoe and Novitch, 2008; Ulloa and Briscoe, 2007; Zhuang and Sockanathan, 2006). The similarity in functions of Bmp and Shh in patterning both the neural tube and the retina raises the possibility that Wnt signaling may also have a conserved function in patterning the dorsal retina. To date, we know of only one report suggesting a role for canonical Wnt signaling in D-V retinal patterning. In analyzing Lrp6−/− mice, which lack expression of the Wnt reporter BAT-gal (Maretto et al., 2003), the authors observed that Tbx5 is not expressed in the dorsal retina at E10.5, but did not assay additional timepoints or other D-V markers.
Here we test the hypothesis that canonical Wnt signaling plays a role in the establishment of dorsal retinal identity. Through a combination of precisely timed in situ hybridization analyses and conditional misexpression experiments, we show that dorsal retinal identity in zebrafish is initiated at 12 hpf, very early in eye development, and then enters a maintenance phase between 14–16 hpf. We then find that Wnt signaling is required for the maintenance of dorsal-specific retinal genes during this second phase, likely through the activation of Bmp signaling. We show that inhibition of the Wnt pathway leads to the loss of dorsal-specific retinal genes, with the concomitant expansion of ventral retinal genes. The loss of dorsal genes reflects a requirement for Wnt signaling in their maintenance, since they initiate their expression normally before Wnt signaling is active in the eye field. Finally, we show that Bmp signaling can rescue dorsal markers in the absence of Wnt signaling, but activation of Wnt signaling cannot rescue dorsal markers in the absence of Bmp signaling, demonstrating that Wnts signal through Bmps to maintain the dorsal retinal domain.
Materials and Methods
Animals
Zebrafish (Danio rerio) were maintained in a laboratory breeding colony on a 14/10 h light/dark cycle. Embryos were maintained at 28.5°C and staged as described previously (Kimmel et al., 1995). The Tg(TOP:GFP)w25 stable transgenic line was generated by Dorsky et al. (2002); the Tg(hsp70l:dkk1-GFP)w32 line was generated by Stoick-Cooper et al. (2007); the Tg(hsp70l:Tcf3-GFP)w26 line was generated by Lewis et al. (2004); and the Tg(hsp70l:nog3)fr14 line was generated by Chocron et al. (2007). Wild-type fish and background of all transgenic lines were of the AB strain.
In situ hybridization
Digoxigenin—UTP-labeled riboprobes for tbx5, bmp4, bmp2b, gdf6a, vax2, pax6a, pax6b, vsx2, egfp, efnb2a, and ephb2, and fluorescein—UTP-labeled riboprobe for rx3 were synthesized by in vitro transcription. Probes for vsx2 and egfp were synthesized in our laboratory. References for other probes are as follows: tbx5 (Ruvinsky et al., 2000), bmp4 (gift from M. Mullins), bmp2b (Nikaido et al., 1997), gdf6a (Open Biosystems EDR1052-524137; Genbank BI475848), vax2 (Take-uchi et al., 2003), pax6a (Puschel et al., 1992), pax6b (Krauss et al., 1991), efnb2a (Durbin et al., 1998), ephb2 (IMAGE Consortium clone 3714371). Whole-mount in situ hybridization and double in situ hybridization were performed as previously described (Jowett and Lettice, 1994). For histological analysis, embryos were stained for 40 h in BM Purple (Roche Applied Sciences) and refixed for 4 h in 4% PFA in phosphate buffer, dehydrated, embedded in plastic, and sectioned.
Transgenic heat shock experiments
Adults heterozygous for the ΔTcf and Dkk1 transgenes were outcrossed to AB strain fish, and Noggin transgenic fish were outcrossed to TL strain fish. The resulting clutches were heat-shocked at various times for 1 h at 39°C (2 h at 39°C for hs:Dkk1 and hs:Noggin), sorted for GFP expression under a fluorescent dissecting microscope, and fixed in 4% PFA at the required stages. Since the hs:Noggin transgene is untagged, these embryos were not sorted for GFP.
Lithium chloride treatment experiments
For Dkk1 rescue, embryos were transferred to embryo water containing 150 mM LiCl at 11 hpf and removed to fresh water at 14 hpf. Heat-shock was performed at 12 hpf and embryos fixed at 24 hpf. For hs:Noggin rescue, embryos were transferred to embryo water containing 200 mM LiCl at 18 hpf until 24 hpf. Heat shock was performed at 18–20 hpf and embryos fixed at 24 hpf.
Bmp rescue experiments
The DNA construct pDestTol2pA2;hsp70l:bmp4-IRES-GFP was generated using the Tol2kit (Kwan et al., 2007). 25 pg of the construct, along with 15 pg tol2 transposase mRNA, was injected into one-cell stage embryos. Embryos were heat-shocked at 12 hpf and fixed at 24 hpf.
Results
Wnt signaling becomes active in the developing eye field between 14–16 hpf
Reasoning that the spatial and temporal domains of expression of Wnt pathway components in and around the developing eye field can provide clues to the function of Wnt signaling in establishing D-V polarity, we sought to determine where and when the Wnt reporter TOP:dGFP is expressed. The Tg(TOP:GFP)w25 transgenic line expresses this reporter, which carries four LEF/TCF binding sites driving destabilized EGFP, and has been shown to be a reliable readout of active Wnt signaling (Dorsky et al., 2002). To increase sensitivity, we detected the reporter using in situ hybridization for gfp mRNA (Figure 1A-F).
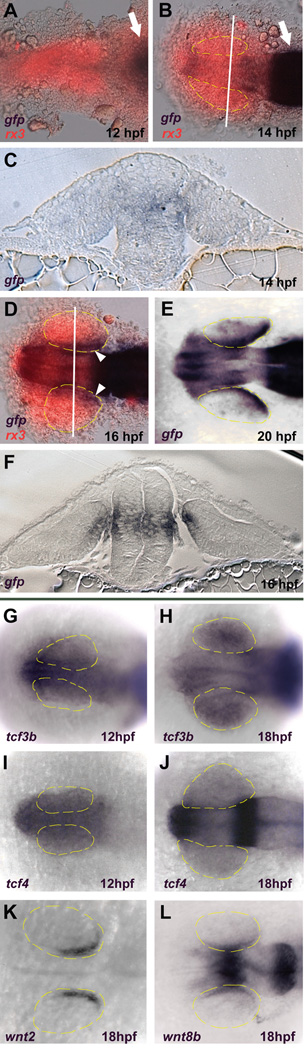
Figure 1. Wnt signaling becomes active in the dorso-posterior retinal pigmented epithelium (RPE) between 14–16 hpf.
A–F: Expression of the TOP:dGFP Wnt reporter which was detected using in situ hybridization for gfp (blue). In A, B, and D, the embryos were also probed for rx3 expression which marks the eye field (red). A, B: Dorsal views, anterior left. Active Wnt signaling does not extend rostrally past the midbrain-hindbrain boundary (arrows) at 12 hpf and approaches but does not enter the eye field at 14 hpf. C, F: Coronal sections through caudal midbrain/posterior optic vesicles, dorsal up. The lines in B and D indicate the planes of section in C and F, respectively. Active Wnt signaling is seen in the dorso-posterior RPE at 16 hpf, but not at 14 hpf. D, E: Dorsal views, anterior left. Active Wnt signaling is clearly present in the dorso-posterior eye field at 16 and 20 hpf. G–L: Dorsal views, anterior left. G–J: Expression of tcf3b and tcf4 is present in the early eye-field during optic vesicle evagination (12 hpf) and throughout the eye at 18 hpf. K: The Wnt ligand wnt2 is expressed in the dorsal RPE at 18 hpf. L: Expression of wnt8b in the midbrain and RPE at 18 hpf.
The eyes in zebrafish develop as a bilateral evagination of the anterior neural keel beginning at approximately 11hpf to form the optic vesicles. At this stage, the optic stalk is located at the anterior of the optic vesicle, and the future dorsal retina is located posteriorly. At 16hpf, the optic vesicle begins to invaginate to form the optic cup, and the lens placode forms from the surface ectoderm in contact with the presumptive neural retina. Finally, at about 22hpf, the entire optic cup rotates approximately 90° so that the posterior part of the optic cup becomes dorsal. At 12 hpf [6 somite stage (ss)], during early optic vesicle evagination, the rostral limit of active Wnt signaling is at the midbrain-hindbrain boundary (Figure 1A), several cell diameters caudal to the eye field. At 14 hpf (10 ss), the gfp signal has extended rostrally along the neural tube to the presumptive telencephalon, but still appears to be excluded from the optic vesicles (Figure 1B, C). By 16 hpf (14 ss), Wnt signaling activity is clearly evident in the optic vesicles and is restricted to the dorso-posterior presumptive RPE (Figure 1D, F). In embryos sectioned coronally through the midbrain, TOP:dGFP expression is absent from the optic vesicles at 14 hpf, and is present in the presumptive RPE but not in the neural retina at 16 hpf (Figure 1C, F). As development proceeds, TOP:dGFP expression becomes stronger in the developing eye, remaining in the dorsal RPE (Figure 1E). By 24 hpf, TOP:dGFP is expressed throughout the entire RPE and ciliary marginal zone (Dorsky et al., 2002). This expression analysis shows that Wnt signaling becomes active in the dorso-posterior RPE between 14–16 hpf. Thus, any role played by Wnt signaling in the establishment of D-V retinal polarity likely begins at this time. Furthermore, it suggests that the reception of Wnt signaling is localized to the presumptive RPE and excluded from the neural retina at optic vesicle stages.
We next analyzed the expression of Tcf transcription factors and Wnt ligands by in situ hybridization at 12 hpf (6 ss) and 18 hpf (18 ss). There are 5 Tcf transcription factor family members in zebrafish: Tcf7, Lef1, Tcf3a (Headless; Tcf7l1a), Tcf3b (Tcf7l1b), and Tcf4 (Tcf7l2) (Dorsky et al., 1999; Kim et al., 2000; Veien et al., 2005). At 12 hpf, during optic vesicle evagination, tcf3a, tcf3b, and tcf4 are expressed throughout the anterior neural tube and optic vesicle primordia, while the other family members are not expressed in this region (Figure 1G-J and data not shown). By 18 hpf, tcf3a and tcf3b are expressed at high levels throughout the optic vesicles, and expression of tcf4 is present at somewhat lower levels in the same region. These expression patterns persist through 24 hpf (not shown). The expression of tcf7 initiates at 16 hpf specifically in the dorsal retina and is maintained in this region through 36 hpf (Veien et al., 2005). We found no lef1 expression in the optic vesicles at any stage examined (not shown). Of the approximately 20 Wnt ligands present in zebrafish, at least two, Wnt2 and Wnt8b, are expressed in or around developing eye structures. Expression of wnt8b has been previously observed in the dorsal RPE as early as 16 hpf (Kelly et al., 1995). We observed expression of both wnt2 and wnt8b in the dorsal RPE at 18 hpf (Figure 1K, L). Therefore, multiple Wnt ligands and Lef/Tcf factors are expressed in the right place and at the right time to mediate Wnt activation in the dorsal RPE during mid-somitogenesis stages.
Bmp ligands are expressed in developing eye structures (Behesti et al., 2006; Delot et al., 1999; French et al., 2007; Hocking and McFarlane, 2007; Liu et al., 2003; Lupo et al., 2005; Murali et al., 2005; Sakuta et al., 2006; Sasagawa et al., 2002). Because Bmp signaling can control D-V retinal polarity in other vertebrates (Behesti et al., 2006; Liu et al., 2003; Murali et al., 2005; Plas et al., 2008), we wanted to determine which Bmp ligands might play a role in zebrafish dorsal retinal patterning. We therefore examined the expression patterns of the Bmp ligands bmp4, gdf6a, and bmp2b during the initial stages of zebrafish eye development. bmp4 does not appear in the optic vesicle until 14 hpf, when it begins to be expressed most strongly in the presumptive dorsal retina (Figure 2A-C). At 24 hpf, bmp4 expression is restricted to the dorsal retina (Figure 2D). While gdf6a and bmp2b are expressed in the ectoderm overlying the anterior neural plate at 12 hpf, they are not expressed in the retina until 16 and 14 hpf, respectively (Figure 2 E-G, I-K). These ligands also become restricted to the dorsal retina by 24 hpf (Figure 2H,L). Interestingly, the putative Bmp target tbx5 begins its expression in the retina earlier than these Bmp ligands, initially in a lateral ocular domain at 12 hpf, immediately after optic vesicle evagination (Figure 2M, N). As the eye undergoes morphogenetic changes, the tbx5 domain becomes reoriented, coming to occupy the presumptive dorsal retina at 14 hpf (Figure 2O) and eventually the dorsal retina at 24 hpf (Figure 2P). A cross-section through the midbrain and optic vesicles reveals that bmp4 is expressed in both the presumptive RPE and retina at 18 hpf (arrows in Figure 2Q). Thus, multiple Bmp ligands and tbx5 are expressed in and around in the optic vesicle during mid-somitogenesis, including in the presumptive dorsal retina (Figure 2R). These data suggest that Bmp factors in either the RPE or retina could act to maintain tbx5 expression in the dorsal retina, and that both bmp and tbx5 expression in the retina precede Wnt activity.
Repression of Wnt target genes leads to the loss of tbx5
To examine the role of canonical Wnt signaling in the establishment of D-V retinal polarity, we used a zebrafish line that expresses a dominant-repressor form of Tcf3 (ΔTcf) fused to GFP under the control of the hsp70 promoter [Tg(hsp70l:Tcf3-GFP)w26]. This transgene has been shown to reliably repress Wnt target genes in an inducible manner (Lewis et al., 2004). A heterozygous outcross of these fish was heat-shocked and embryos were sorted for GFP fluorescence to examine the effect of ΔTcf expression on retinal patterning. Activation of the ΔTcf transgene at any of multiple timepoints resulted in the abolition of tbx5 expression, with no effect on tbx5 expression in nontransgenic embryos (Figure 3A-H). When the transgene was activated at 10 hpf and embryos fixed at 18 hpf (HS10; F18), tbx5 expression was strongly downregulated in 100% (n=37) of embryos. For HS10; F24 and HS18; F24, 100% (n=40, 49) of embryos showed a similarly strong reduction of tbx5 expression. At these later timepoints, tbx5 expression was maintained in non-ocular areas such as the heart and pectoral fin buds (data not shown). When the transgene was activated as late as 24 hpf (HS24; F30), tbx5 expression was still strongly reduced in 93% (n=109) of embryos. Thus, regardless of when the heat-shock was performed or when the embryos were fixed, activation of the ΔTcf transgene eliminated tbx5 expression in the dorsal retina, suggesting that tbx5 is downstream of Wnt signaling in this region.
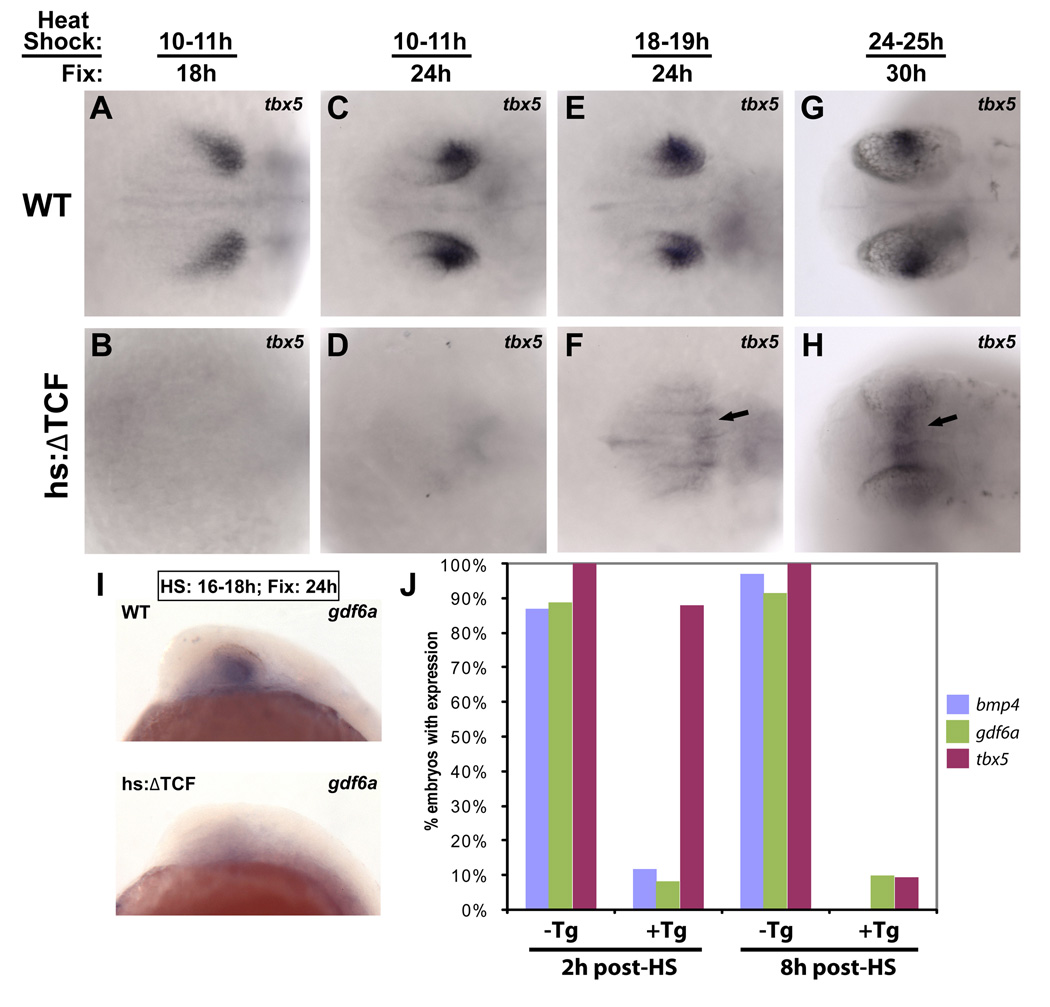
Figure 3. Expression of dorsal retinal genes is lost following repression of Wnt signaling.
The Tg(hsp70l:Tcf3-GFP)w26 transgenic zebrafish line, which expresses a dominant-repressor form of Tcf3 (ΔTcf-GFP) upon heat-shock, was used for these experiments. Dorsal views, anterior left. A–H: Embryos were heat-shocked and fixed at the indicated times, and sorted for GFP expression. Repression of Wnt targets led to the downregulation of tbx5 in the dorsal retina at every timepoint. tbx5 expression was upregulated in the dorsal diencephalon at later timepoints (arrows in F, H). I: Expression of gdf6a was also eliminated in embryos expressing ΔTcf at 18 hpf and fixed at 24 hpf. J: To determine the times at which bmp4, gdf6a, and tbx5 are lost in the dorsal retina following repression of Wnt targets, embryos were heat-shocked at 16 hpf and fixed 2 and 8 hours later. bmp4 and gdf6a expression were strongly reduced at the 2 hour timepoint, while tbx5 was still expressed. By 8 hours, expression of all three genes was lost.
At later heat-shock timepoints, we noticed that tbx5 was ectopically expressed in the dorsal diencephalon (Figure 3F, H), perhaps because ΔTcf represses a gene that normally represses tbx5 in this region. Together with maintained tbx5 expression in non-ocular areas, this result suggests that tbx5 may be an indirect transcriptional target of Wnt signaling. In the course of examining other dorsal markers, we observed that gdf6a and bmp4 expression were also reduced following ΔTcf expression (Fig. 3I and not shown). This suggested that down-regulation of tbx5 could be a result of decreased Bmp signaling. To further investigate this possibility, we examined the expression of bmps and tbx5 in more detail, focusing on the time course of downregulation of these genes following ΔTcf expression. Following heat shock at 16 hpf, we found that bmp4 expression was present in 12% (n=17) of optic vesicles at 2 hours post-HS (18 hpf) and 0% (n=44) at 8 hours post-HS (24 hpf). gdf6a was present in 9% (n=11) at 2 hours post-HS and 11% (n=9) at 8 hours post-HS. By contrast tbx5 expression was present in 88% (n=25) of embryos at 2 hours post-HS, and 9% (n=67) at 8 hours post-HS (Figure 3J), indicating that bmp genes are downregulated before tbx5. These data are consistent with a model in which tbx5 is indirectly regulated by Wnt signaling through Bmp activity.
Wnt signaling is required for the maintenance of dorsal retinal genes
A potential concern with the ΔTcf transgene is that it may repress targets that contain Tcf-binding sites, but are not controlled by endogenous Wnt activity. Thus, we used a second transgenic fish line that expresses a secreted inhibitor of Wnt signaling, Dickkopf-1 (Dkk1), upon heat-shock stimulation [Tg(hsp70l:dkk1-GFP)w32] (Stoick-Cooper et al., 2007). This transgene inhibits Wnt signaling at the receptor level instead of the transcriptional level, and thus is expected to block only active Wnt signaling. Embryos in which Dkk1 is activated early (9 hpf) display an enlarged head and a truncated tail (not shown), phenotypes associated with the loss of Wnt signaling, and downregulation of the Wnt reporter TOP:dGFP (Stoick-Cooper et al., 2007). When Dkk1 expression was activated at 9 hpf and embryos fixed at 15 hpf, the dorsal marker tbx5 was expressed normally (Figure 4A, B). This result was consistent with our findings that active Wnt signaling begins in the eye field between 14–16 hpf, after tbx5 expression has been initiated at 12 hpf. However, Dkk1 misexpression resulted in the strong down-regulation of tbx5 in the dorsal retina at 18 hpf and 24 hpf, similar to the results obtained using ΔTcf; at later timepoints, tbx5 was reduced, though not completely absent (Figure 4C-J). For HS10; F18, 96% (n=71) of embryos had strongly reduced tbx5 expression. For HS10; F24, and HS18; F24, tbx5 expression was strongly reduced in 88% (n=65) and 83% (n=71) of embryos, respectively. By contrast, at the last timepoint (HS24; F30), only 26% (n=19) of embryos showed reduced tbx5 expression in the dorsal retina. This may indicate that Wnt signaling is required for the expression of dorsal retinal genes during a time window of approximately 14–24 hpf, a developmental period in which the eye goes through dramatic morphological changes (C-B. Chien and K. Kwan, unpublished data), and when genes initially expressed in a broad retinal domain refine their expression to the dorsal retina. The finding that tbx5 expression in Dkk1-expressing embryos initiates normally then later disappears suggests that Wnt signaling is necessary for the maintenance of tbx5, but not for its initiation.
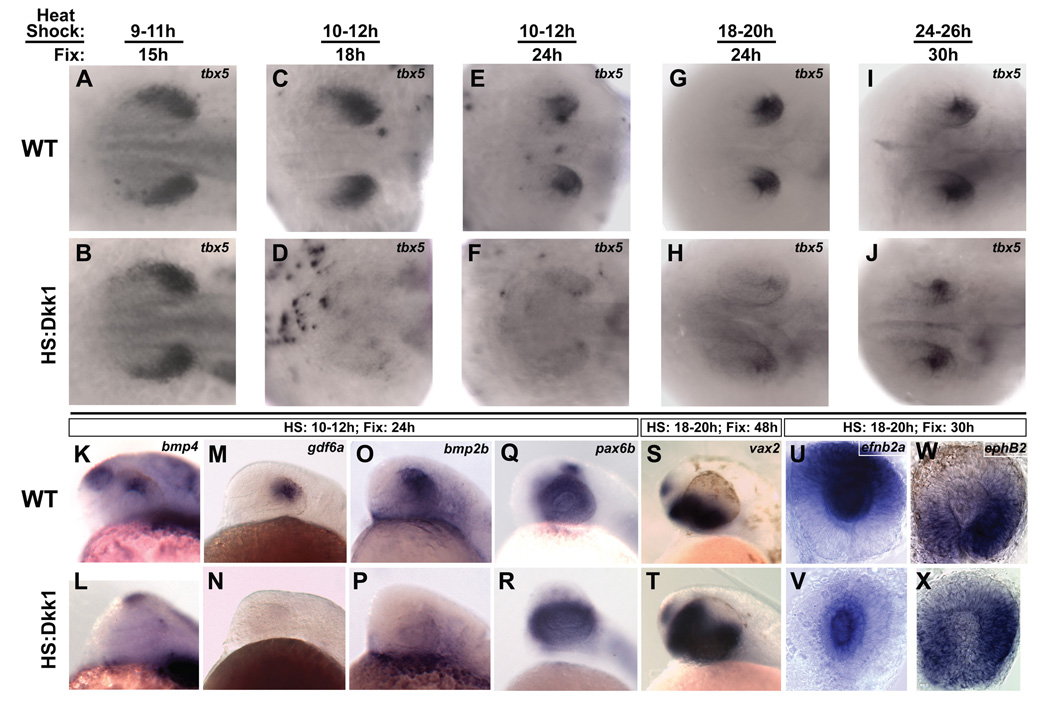
Figure 4. Wnt signaling is required for the maintenance of dorsal retinal identity.
The Tg(hsp70l:dkk1-GFP)w32 transgenic zebrafish line, which expresses the secreted Wnt pathway inhibitor Dkk1 upon heat-shock, was used for these experiments. A–J: Dorsal views, anterior left. Embryos were heat-shocked and fixed at the indicated times, and sorted for GFP expression. A, B: Embryos fixed just before Wnt signaling becomes active in dorsal RPE express tbx5 normally, showing that tbx5 expression initiates properly in the absence of Wnt signaling. C–J: Inhibition of Wnt signaling caused a strong downregulation of tbx5 at the early timepoints, with a weaker effect at the last timepoint. This demonstrates a requirement for Wnt signaling in the maintenance of tbx5. K–T: Lateral views, dorsal up, anterior left. K–P: Expression of the Bmp ligands bmp4, gdf6a, and bmp2b are lost from the dorsal retina following Wnt inhibition, suggesting loss of dorsal character. Q–T: pax6b is expressed normally and vax2 expands dorsally, suggesting a ventralized retina. U–X: Whole eyes, dorsal up. Expression of ephrinB2a is downregulated in the dorsal retina, but maintained in the lens, and ephB2 expands dorsally following Wnt inhibition.
We next examined whether expression of Bmp ligands was affected by Dkk1 misexpression. Similar to tbx5, bmp4 (91%, n=46), gdf6a (95%, n=58), and bmp2b (96%, n=25) were all reduced when the transgene was activated at 10 hpf and embryos fixed at 24 hpf (Figure 4K-P). To rule out the possibility that blocking Wnt signaling grossly perturbs eye development, we looked at the pan-retinal markers, pax6a, pax6b and vsx2 in embryos heat-shocked at 10 hpf and fixed at 24 hpf. These markers were unaffected in transgenic embryos (Figure 4R and not shown), indicating that the retina is specified correctly and that Wnt signaling specifically acts on dorsally restricted retinal markers. Recent studies have shown that the loss or inhibition of dorsal-specific retinal genes such as bmp4 and tbx5 correlates with a concomitant expansion of ventral genes into the dorsal retinal domain (Behesti et al., 2006; Koshiba-Takeuchi et al., 2000; Murali et al., 2005; Sasagawa et al., 2002); thus, we examined the expression of the ventral retinal gene vax2 in embryos induced to express Dkk1 at 18 hpf and fixed at 48 hpf. Expression of vax2 expanded significantly into the dorsal retina in 76% (n=13) of these embryos (Figure 4J). These results demonstrate that in the absence of Wnt signaling, the retina forms correctly but is ventralized.
Since experimental manipulations of Bmp and Tbx5 levels have been shown to perturb the expression of the EphrinB and EphB axon guidance molecules (Koshiba-Takeuchi et al., 2000; Murali et al., 2005), we examined the dorsal gene ephrinB2a and the ventral gene ephB2 in Dkk1-expressing embryos heat-shocked at 18 hpf and fixed at 30 hpf. In accord with the observed reduction in tbx5 expression and expansion of vax2 expression, ephrinB2a was strongly reduced in 93% (n=29) of embryos, and ephB2 was modestly expanded dorsally in 84% (n=19) of embryos (Figure 4U-X). ephrinB2a is also expressed in the lens, and this domain of expression was still present after Dkk1 misexpression, again demonstrating the specific requirement of Wnt signaling for dorsal retinal gene expression. An obvious prediction from these results is that the retinotectal map will be perturbed in a predictable way. However, Dkk1-expressing embryos did not survive until 5 dpf, when retinotectal pathfinding could be assayed, precluding such an analysis. In addition, multiple Wnt pathway components, including Wnt3 and Sfrp5, are expressed in the tectum and Wnt signaling is also required for axon guidance in this target tissue (Schmitt et al., 2006; Tendeng and Houart, 2006). Tissue-specific modulation of Wnt signaling in the eye is thus required to determine the ultimate role of this pathway in pathfinding. At this point, our data suggest that Wnt signaling is specifically required for the maintenance of dorsal retinal genes, the loss of which results in a dorsal expansion of ventral retinal genes.
Activation of Wnt signaling downstream of Dkk1 rescues the hs:Dkk1-GFP phenotype
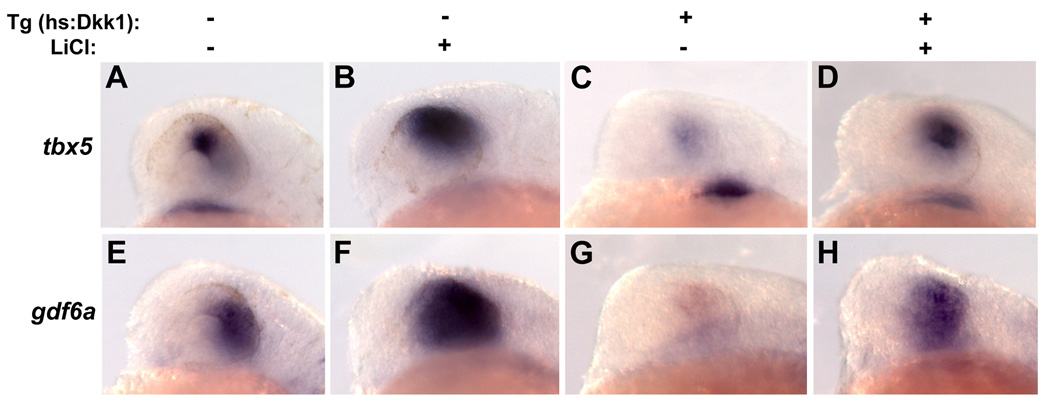
If Wnt signaling is required for dorsal retinal identity, Wnt pathway activation downstream of the Dkk1-Lrp6 interaction should rescue the expression of dorsal retinal genes lost in embryos misexpressing Dkk1. To test this hypothesis in a temporally controlled manner, we used LiCl, which is known to inhibit glycogen synthase kinase-3β (GSK-3β), resulting in the accumulation of unphosphorylated â-catenin and amplified transcription of Wnt target genes (Hedgepeth et al., 1997). 150 mM LiCl was applied to embryos at 11 hpf, followed by Dkk1 transgene activation at 12 hpf. LiCl was then removed at 14 hpf, and embryos were fixed at 24 hpf. We found that expression of both gdf6a (30%, n=47) and tbx5 (33%, n=108) were expanded in wild-type embryos treated with LiCl (Figure 5B, F). Importantly, application of LiCl rescued tbx5 expression to wild-type levels in 26% (n=69) of Dkk1-expressing embryos, and gdf6a to wild-type levels in 51% (n=63) of Dkk1-expressing embryos (Figure 5D, H). While LiCl application at 11 hpf resulted in a highly variable phenotype, this rescue was significant because wild-type expression levels of tbx5 and gdf6a were never seen in untreated Dkk1-expressing embryos. These results confirm a specific role for canonical Wnt signaling in the maintenance of dorsal retinal gene expression.
Figure 5. Activation of Wnt signaling rescues loss of dorsal eye markers in Dkk1-expressing embryos.
Dkk1-expressing embryos were treated with the Wnt pathway activator LiCl (150 mM) from 11–14 hpf, heat-shocked at 12 hpf, and fixed at 24 hpf. Expression of tbx5 (A–D) and gdf6a (E–H) were analyzed by in situ hybridization. LiCl led to an expansion of tbx5 and gdf6a expression in embryos not expressing Dkk1 (B, F), and a rescue of tbx5 and gdf6a in embryos expressing Dkk1 (D, H). Lateral views, dorsal up, anterior left.
Loss of dorsal identity downstream of Wnt inactivation can be rescued by Bmp signaling
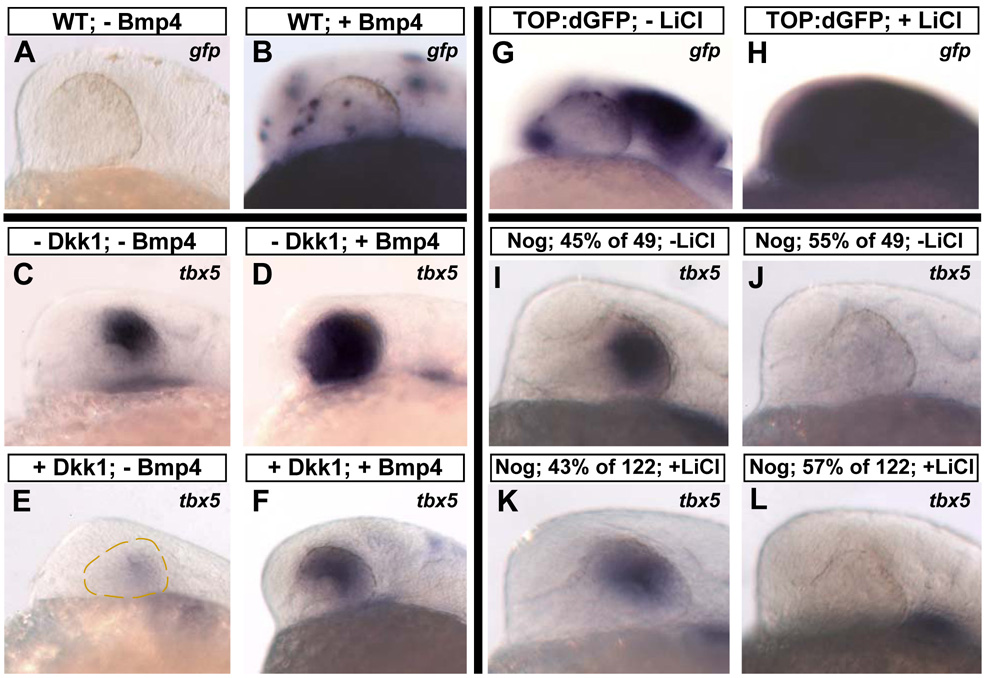
In order to examine the relationship between Wnt and Bmp signaling during the establishment of D-V retinal polarity, we asked whether activation of Bmp signaling could substitute for the loss of Wnt signaling. We injected one-cell stage embryos with DNA for a Bmp4 construct driven by the hsp70 promoter (pDestTol2pA2;hsp70l:bmp4-IRES-GFP). When injected into WT embryos heat-shocked at 12 hpf, this construct led to the widespread clonal expression of bmp4 and gfp throughout the embryos, and gfp-expressing clones were found within the retina in 85% (n=54) of these embryos, as assayed by in situ hybridization (Figure 6A, B). We next injected this construct into Tg(hsp70l:dkk1-GFP)w32 embryos at the one-cell stage and heat-shocked them at 12 hpf, which simultaneously blocked Wnt signaling and stimulated the clonal expression of Bmp4. In WT embryos, Bmp4 expression led to an expansion of tbx5 into the ventral retinal domain in 38% (n=46) of embryos (Figure 6D), showing that Bmps can upregulate tbx5 in the eye. This result is consistent with a recent study which showed that implantation of Bmp4-soaked beads into the mouse eye caused a ventral expansion of tbx2, tbx3, and tbx5 (Behesti et al., 2006). In embryos expressing Dkk1, activation of Bmp4 rescued tbx5 expression in 44% (n=62) of embryos (Figure 6F). This rescue was specific to the eye, since no other part of the embryo displayed ectopic tbx5 staining. We next examined whether the loss of gdf6a expression in embryos expressing Dkk1 could be rescued by activation of Bmp4. No significant rescue was seen in this case (n=49; data not shown). Together with the rescue of dorsal genes by Wnt pathway activation described previously, these results show that 1) tbx5 is downstream of both Wnt and Bmp signaling; and 2) activation of Bmp signaling rescues tbx5 but not gdf6a. This supports a model in which Wnt signaling maintains dorsal retinal identity through regulation of Bmp signaling.
Figure 6. Bmp signaling is downstream of Wnt signaling in the maintenance of dorsal retinal markers.
A–F: Bmp4 can rescue dorsal retinal markers in the absence of Wnt signaling. hs:Dkk1 and control WT embryos at the one-cell stage were injected with a construct that expresses Bmp4 upon heat-shock (pDestTol2pA2;hsp70l:bmp4-IRES-GFP), heat-shocked at 12 hpf, and fixed at 24 hpf. A, B: To illustrate transgene expression following heat-shock, in situ hybridization was performed for gfp. Widespread clonal expression was observed in the retinas in 85% of embryos. C–F: Expression of Bmp4 caused a clear expansion of tbx5 in embryos not expressing Dkk1 (D) and rescue of tbx5 in embryos expressing Dkk1 (F). G–L: Activation of Wnt signaling does not rescue dorsal markers in the absence of Bmp signaling. Embryos heterozygous for the Tg(hsp70l:nog3)fr14 transgene, which express the Bmp pathway inhibitor Noggin upon heat-shock, were outcrossed to TL strain fish and placed in 200 mM LiCl at 18 hpf. A 2 hour heat-shock was performed at 18 hpf, and embryos were fixed at 24 hpf. To illustrate Wnt pathway activation, TOP:dGFP embryos were similarly treated with LiCl from 18–24 hpf and gfp detected by in situ hybridization (G, H). For hs:Noggin embryos untreated with LiCl, 55% of 49 embryos lost expression of tbx5 (J), and for embryos treated with LiCl, 57% of 122 embryos lost expression of tbx5 (L), showing that activation of Wnt signaling cannot rescue tbx5 in the absence of Bmp signaling. Lateral views, dorsal up, anterior left.
Activation of Wnt signaling does not rescue dorsal identity lost from Bmp inhibition
Our results suggest that Wnt signaling maintains dorsal retinal markers by activating Bmp signaling, but another formal possibility is that Wnts and Bmps act in parallel. To address this point, we first confirmed that tbx5 expression is lost following Bmp inhibition, then tried to rescue tbx5 expression by activating Wnt signaling. Implantation of beads soaked with the Bmp inhibitor Noggin just dorsal to the optic vesicle was recently shown to abolish tbx5 expression (Behesti et al., 2006). We used the transgenic fish line Tg(hsp70l:nog3)fr14, which expresses Noggin upon heat-shock stimulation (Chocron et al., 2007). To achieve robust activation of Wnt signaling, embryos were placed in 200mM LiCl from 18–24 hpf, and then heat-shocked at 18 hpf for 2 hours to activate Noggin expression. Embryos were fixed at 24 hpf and processed for tbx5 expression by in situ hybridization. To confirm that these embryos had increased Wnt signaling, we treated the TOP:dGFP reporter line with LiCl from 18–24 hpf, which revealed strongly upregulated reporter expression (Figure 6G, H). Because these embryos were obtained from a heterozygous outcross, we expected 50% of them to express Noggin. For embryos untreated with LiCl, 55% (n=49) embryos lost tbx5 expression, while for embryos treated with LiCl, 57% (n=122) embryos lost tbx5 expression (Figure 6 I-L). No significant rescue was seen; thus, inhibiting Bmp signaling indeed abolishes dorsal identity, and this effect is downstream of the dorsal-promoting effect of Wnt activation. Taken together, these experiments reveal a linear pathway in which Wnt signaling in the RPE maintains the identity of the dorsal retinal domain through the activation of Bmp signaling in the RPE and retina.
Discussion
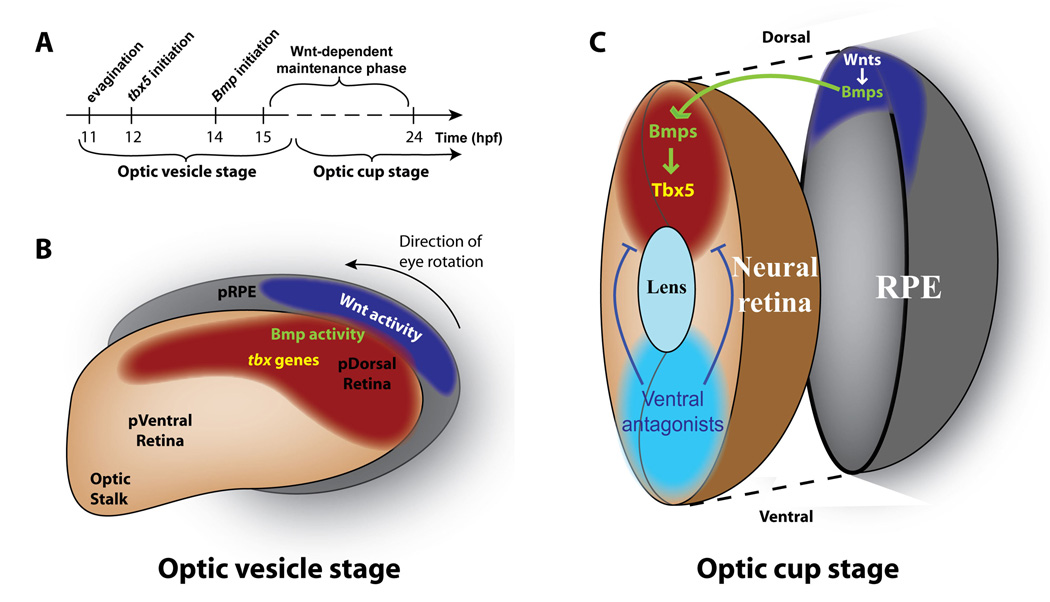
The activation of region-specific genetic cascades during early retinal development is thought to lead to accurate topographic targeting of RGC axons to the optic tectum. In this study, we have shown for the first time that Wnt signaling is required for the proper development of D-V retinal polarity. Our expression analysis suggests that Wnt signaling functions in the RPE, while Bmp ligands are expressed in both the RPE and retina (Figure 1, Figure 2). Our results demonstrate that dorsal retinal genes initiate their expression normally at around 12 hpf in the absence of Wnt signaling, but soon thereafter require Wnt signaling for their maintained expression in the dorsal retinal domain (Figure 3, Figure 4). Expression of Bmp ligands in the dorsal retina is dependent on Wnt signaling, and following Wnt inhibition the loss of at least one Bmp ligand, gdf6a, can be rescued by activation of Wnt signaling (Figure 5). In addition, tbx5, an early marker of dorsal identity, is rescued by activation of either Wnt or Bmp signaling following Wnt inhibition. By contrast, tbx5 cannot be rescued by activation of Wnt signaling in the absence of Bmp signaling (Figure 5, Figure 6). These data together suggest a model for the maintenance of D-V retinal identity in which Wnt signaling in the dorsal RPE transcriptionally maintains Bmp expression in the dorsal RPE and retina, which regulates the expression of downstream D-V axis genes, including tbx5 and ephrinB axon guidance molecules (Figure 7). This mechanism provides a conduit through which a Wnt signal in the RPE can exert its effects in the neural retina. It is likely that this mechanism functions to maintain the integrity of the dorsal retinal domain by coordinating its patterning with the dorsal RPE, but detailed fate-mapping in the developing retina and RPE is needed to show this conclusively.
Figure 7. Model for Wnt-dependent maintenance of dorsal identity.
A: Timeline of dorsal identity establishment. Optic vesicles evaginate from the anterior neural tube at 11 hpf. Soon thereafter, at 12 hpf, the first dorsally restricted marker, tbx5, begins to be expressed. Expression of Bmp ligands within the dorsal retina and RPE begins at 14 hpf, and a phase of Wnt-dependent dorsal identity maintenance begins between 14–16 hpf. B: Diagram of the optic vesicle at approximately 14 hpf. Wnt signaling becomes active in the dorso-posterior presumptive RPE at this point, Bmp signaling is active in the presumptive dorsal retina and presumptive RPE, and tbx5 is expressed in the presumptive dorsal retina. At about 22 hpf, the entire eye undergoes an approximately 90° rotation so that the posterior eye assumes its final dorsal position. C: Model of Wnt-mediated maintenance of dorsal retinal identity. A Wnt signaling center in the dorsal RPE maintains bmp expression in the dorsal RPE and retina. Bmp signaling then maintains tbx5 expression in the dorsal retina. Simultaneously, inhibitors such as Bmp antagonists and transcription factors like Vax2 act to limit the extent of dorsal identity. Abbreviations: pRPE: presumptive retinal pigmented epithelium; pDorsal Retina: presumptive dorsal retina; p Ventral Retina: presumptive ventral retina.
Our expression analysis revealed that several Wnt pathway components are expressed in and around the developing eyes from optic vesicle stage through 24 hpf. Of the five Tcfs present in zebrafish, only tcf3a, tcf3b, and tcf4 are present in or around the optic vesicles at the stage when Wnt signaling becomes active in the dorsal RPE (14–16 hpf). tcf3a and tcf3b are both expressed at high levels in the evaginating optic vesicles, and tcf4 is expressed in the same domain at a slightly lower level (Figure 1G-J). Although Tcf3 is usually referred to as a “repressor” in the literature (see review by Arce et al., 2006), it may also function as an activator under conditions where β-catenin is stabilized, and therefore Tcf3 and/or Tcf4 are likely the transcription factors through which Wnt signaling maintains the expression of dorsal retinal genes. A practical difficulty in testing this idea stems from the fact that Tcf3 loss of function results in embryos lacking anterior forebrain structures including eyes (Kim et al., 2000), while fish mutant for tcf4 do not have a retinal phenotype on their own (Muncan et al., 2007, and our unpublished observations). Thus, the unique contribution of Tcf3a, Tcf3b, and/or Tcf4 in mediating Wnt signaling relevant to the expression of dorsal retinal genes is still unknown. tcf7 is also expressed in the dorsal retina, but it appears after the initial onset of Wnt signaling in the dorsal neural retina, a domain slightly different from the Wnt reporter (Veien et al., 2005). Therefore we believe that tcf7, rather than mediating Wnt function in the dorsal RPE, may in fact be a downstream target of the pathway, possibly regulated through Bmp activity.
We identified Wnt2b and Wnt8b as being expressed in the dorsal RPE at 18hpf, which suggested that Wnt activity from these two ligands might be responsible for maintenance of the dorsal retinal domain. We thus used morpholino oligonucleotides targeted against these genes, both alone and in combination, to knock down their expression. Although the embryos were strongly affected by these manipulations, tbx5 expression was still seen in the dorsal retina, although at lower levels (data not shown). These results point to the difficulty in studying Wnt ligands during development: they are often expressed in highly redundant, overlapping patterns. Other Wnts have been identified in the RPE of mouse and chick including Wnt5a and Wnt5b (Fokina and Frolova, 2006; Rossi et al., 2007; Van Raay and Vetter, 2004). Further work is necessary to identify other members of the Wnt family that are expressed in the RPE and their individual contributions to D-V retinal patterning.
Both the ΔTcf and Dkk1-expressing zebrafish lines are powerful tools to study the loss of Wnt signaling in a temporally-controlled manner, acting through distinct mechanisms. ΔTcf directly represses target genes containing Wnt response elements (WREs) within their promoters, and Dkk1 specifically inhibits Wnts from signaling through the canonical pathway by competing for Lrp receptor occupancy (reviewed in Arce et al., 2006). Early activation of either transgene resulted in the loss of tbx5 and bmp genes in the dorsal retina, providing evidence that the observed phenotype is not a result of ectopic repression of genes that are not normally responsive to Wnt signaling. The downregulation of multiple Bmp ligands suggests two possible nonexclusive mechanisms: Wnt signaling may transcriptionally regulate multiple bmp genes, or there may be a positive feedback mechanism through which one Wnt-dependent Bmp molecule positively regulates the expression of other bmp genes. At later heat shock timepoints, activation of ΔTcf still led to the loss of dorsal markers, but tbx5 expression was seen ectopically in the dorsal diencephalon. This suggests that factor(s) present in the diencephalon normally repress tbx5 in this region, and are themselves repressed by ΔTcf. In addition, this finding, together with maintained nonocular tbx5 expression and downregulation of bmp genes before tbx5 in ΔTcf embryos, suggests that tbx5 is an indirect target of Wnt signaling and supports our hypothesis in which Wnt signaling maintains the dorsal retinal domain through the regulation of Bmp signaling. However we cannot completely rule out the possibility that tbx5 is also a direct target of Wnt signaling in the retina, and direct analysis of tbx5 regulatory elements is required to further address this issue. Activation of Dkk1 at 24 hpf led to a modest downregulation of tbx5 in the dorsal retina at 30 hpf, suggesting that Wnt signaling is required during a specific time window, approximately 14–24 hpf, as the dorsal retinal domain is being established.
Wnt and Bmp signaling are known to co-regulate gene expression in several parts of the developing vertebrate embryo. For example, in zebrafish, Wnt8 and Bmp2b have recently been shown be required for the establishment of ventrolateral mesoderm through their cooperative regulation of vent, vox, and ved (Ramel et al., 2005), and Wnt and Bmp signals also function cooperatively in the formation of posterior structures through their regulation of genes such as tbx6 (Szeto and Kimelman, 2004). Wnt and Bmp signals coordinately control the specification of dorsal spinal cord neurons by regulating Olig3 transcription in mice (Zechner et al., 2007). Conditional ablation of the Bmp receptor 1a and β-catenin in the mouse heart revealed that Bmp signaling is required for the expression of Tbx5 and specification of the first heart field, and Wnt signaling is required for the expression of Bmp4 and specification of the second heart field (Klaus et al., 2007). Thus, Wnt/Bmp co-regulation of gene expression and pattern formation is a general mechanism used in multiple places and times in developing embryos. Our results show that this mechanism is also used during maintenance of the dorsal retinal domain, and that Wnt signaling is itself required for Bmp pathway activity in this region. In addition to the possibility that bmp genes may be direct targets of Wnt signaling, another possible mechanism by which Wnt signaling could regulate the Bmp pathway is through GSK3-dependent phosphorylation of Smad proteins (Fuentealba et al., 2007). The presence of differential Smad phosphorylation in the developing optic vesicle, particularly in the dorsal vs. ventral presumptive RPE, would support such a model. The use of multiple signaling pathways for patterning a complex organ such as the eye has obvious advantages. If Bmp signaling alone regulated the expression of dorsal retinal genes, it would be difficult to maintain this expression in a static domain during morphogenetic tissue movements. Localized to the dorsal RPE, Wnt signaling could stabilize dorsal retinal identity by coordinating the development of these two tissues during eye patterning.
Acknowledgments
We thank Sabine Fuhrmann, David Grunwald, and Corinne Houart for helpful discussions, and the Centralized Zebrafish Animal Resource (CZAR) for fish care. This work was supported by NIH T32 HD007491 (E.V.), NIH T32 DC008553-03 (R. K-B.), and by NIH P01 HD048886 (R.I.D. and C.B.C.).
References
- Arce L, Yokoyama NN, Waterman ML. Diversity of LEF/TCF action in development and disease. Oncogene. 2006;25:7492–7504. doi: 10.1038/sj.onc.1210056. [DOI] [PubMed] [Google Scholar]
- Asai-Coakwell M, French CR, Berry KM, Ye M, Koss R, Somerville M, Mueller R, van Heyningen V, Waskiewicz AJ, Lehmann OJ. GDF6, a novel locus for a spectrum of ocular developmental anomalies. Am J Hum Genet. 2007;80:306–315. doi: 10.1086/511280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri AM, Broccoli V, Bovolenta P, Alfano G, Marchitiello A, Mocchetti C, Crippa L, Bulfone A, Marigo V, Ballabio A, et al. Vax2 inactivation in mouse determines alteration of the eye dorsal-ventral axis, misrouting of the optic fibres and eye coloboma. Development. 2002;129:805–813. doi: 10.1242/dev.129.3.805. [DOI] [PubMed] [Google Scholar]
- Behesti H, Holt JK, Sowden JC. The level of BMP4 signaling is critical for the regulation of distinct T-box gene expression domains and growth along the dorso-ventral axis of the optic cup. BMC Dev Biol. 2006;6:62. doi: 10.1186/1471-213X-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J, Gribble SL, Veien ES, Nikolaus OB, Weidinger G, Dorsky RI. Proliferation and patterning are mediated independently in the dorsal spinal cord downstream of canonical Wnt signaling. Dev Biol. 2008;313:398–407. doi: 10.1016/j.ydbio.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, Novitch BG. Regulatory pathways linking progenitor patterning, cell fates and neurogenesis in the ventral neural tube. Philos Trans R Soc Lond B Biol Sci. 2008;363:57–70. doi: 10.1098/rstb.2006.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns C, Zhang J, Brown E, Van Bibber A, Van Es J, Clevers H, Ishikawa T, Taketo M, Vetter M, Fuhrmann S. Investigation of Firzzled-5 during embryonic neural development in mouse. Developmental Dynamics. 2008 doi: 10.1002/dvdy.21565. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Cepko CL. Wnt2b/beta-catenin-mediated canonical Wnt signaling determines the peripheral fates of the chick eye. Development. 2006;133:3167–3177. doi: 10.1242/dev.02474. [DOI] [PubMed] [Google Scholar]
- Chocron S, Verhoeven MC, Rentzsch F, Hammerschmidt M, Bakkers J. Zebrafish Bmp4 regulates left-right asymmetry at two distinct developmental time points. Dev Biol. 2007;305:577–588. doi: 10.1016/j.ydbio.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Delot E, Kataoka H, Goutel C, Yan YL, Postlethwait J, Wittbrodt J, Rosa FM. The BMP-related protein radar: a maintenance factor for dorsal neuroectoderm cells? Mech Dev. 1999;85:15–25. doi: 10.1016/s0925-4773(99)00026-x. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Itoh M, Moon RT, Chitnis A. Two tcf3 genes cooperate to pattern the zebrafish brain. Development. 2003;130:1937–1947. doi: 10.1242/dev.00402. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Sheldahl LC, Moon RT. A transgenic Lef1/beta-catenin-dependent reporter is expressed in spatially restricted domains throughout zebrafish development. Dev Biol. 2002;241:229–237. doi: 10.1006/dbio.2001.0515. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Snyder A, Cretekos CJ, Grunwald DJ, Geisler R, Haffter P, Moon RT, Raible DW. Maternal and embryonic expression of zebrafish lef1. Mech Dev. 1999;86:147–150. doi: 10.1016/s0925-4773(99)00101-x. [DOI] [PubMed] [Google Scholar]
- Durbin L, Brennan C, Shiomi K, Cooke J, Barrios A, Shanmugalingam S, Guthrie B, Lindberg R, Holder N. Eph signaling is required for segmentation and differentiation of the somites. Genes Dev. 1998;12:3096–3109. doi: 10.1101/gad.12.19.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekker SC, Ungar AR, Greenstein P, von Kessler DP, Porter JA, Moon RT, Beachy PA. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr Biol. 1995;5:944–955. doi: 10.1016/s0960-9822(95)00185-0. [DOI] [PubMed] [Google Scholar]
- Fokina VM, Frolova EI. Expression patterns of Wnt genes during development of an anterior part of the chicken eye. Dev Dyn. 2006;235:496–505. doi: 10.1002/dvdy.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French CR, Erickson T, Callander D, Berry KM, Koss R, Hagey DW, Stout J, Wuennenberg-Stapleton K, Ngai J, Moens CB, et al. Pbx homeodomain proteins pattern both the zebrafish retina and tectum. BMC Dev Biol. 2007;7:85. doi: 10.1186/1471-213X-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanel ML, Hensey C. Eye and neural defects associated with loss of GDF6. BMC Dev Biol. 2006;6:43. doi: 10.1186/1471-213X-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgepeth CM, Conrad LJ, Zhang J, Huang HC, Lee VM, Klein PS. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev Biol. 1997;185:82–91. doi: 10.1006/dbio.1997.8552. [DOI] [PubMed] [Google Scholar]
- Hocking JC, McFarlane S. Expression of Bmp ligands and receptors in the developing Xenopus retina. Int J Dev Biol. 2007;51:161–165. doi: 10.1387/ijdb.062185jh. [DOI] [PubMed] [Google Scholar]
- Ille F, Atanasoski S, Falk S, Ittner LM, Marki D, Buchmann-Moller S, Wurdak H, Suter U, Taketo MM, Sommer L. Wnt/BMP signal integration regulates the balance between proliferation and differentiation of neuroepithelial cells in the dorsal spinal cord. Dev Biol. 2007;304:394–408. doi: 10.1016/j.ydbio.2006.12.045. [DOI] [PubMed] [Google Scholar]
- Jowett T, Lettice L. Whole-mount in situ hybridizations on zebrafish embryos using a mixture of digoxigenin- and fluorescein-labelled probes. Trends Genet. 1994;10:73–74. doi: 10.1016/0168-9525(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Kapsimali M, Caneparo L, Houart C, Wilson SW. Inhibition of Wnt/Axin/beta-catenin pathway activity promotes ventral CNS midline tissue to adopt hypothalamic rather than floorplate identity. Development. 2004;131:5923–5933. doi: 10.1242/dev.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly GM, Greenstein P, Erezyilmaz DF, Moon RT. Zebrafish wnt8 and wnt8b share a common activity but are involved in distinct developmental pathways. Development. 1995;121:1787–1799. doi: 10.1242/dev.121.6.1787. [DOI] [PubMed] [Google Scholar]
- Kim CH, Oda T, Itoh M, Jiang D, Artinger KB, Chandrasekharappa SC, Driever W, Chitnis AB. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407:913–916. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci U S A. 2007;104:18531–18536. doi: 10.1073/pnas.0703113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba-Takeuchi K, Takeuchi JK, Matsumoto K, Momose T, Uno K, Hoepker V, Ogura K, Takahashi N, Nakamura H, Yasuda K, et al. Tbx5 and the retinotectum projection. Science. 2000;287:134–137. doi: 10.1126/science.287.5450.134. [DOI] [PubMed] [Google Scholar]
- Krauss S, Johansen T, Korzh V, Moens U, Ericson JU, Fjose A. Zebrafish pax[zf-a]: a paired box-containing gene expressed in the neural tube. Embo J. 1991;10:3609–3619. doi: 10.1002/j.1460-2075.1991.tb04927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoh T, Wilson SW, Dawid IB. Distinct roles for Fgf, Wnt and retinoic acid in posteriorizing the neural ectoderm. Development. 2002;129:4335–4346. doi: 10.1242/dev.129.18.4335. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- Lewis JL, Bonner J, Modrell M, Ragland JW, Moon RT, Dorsky RI, Raible DW. Reiterated Wnt signaling during zebrafish neural crest development. Development. 2004;131:1299–1308. doi: 10.1242/dev.01007. [DOI] [PubMed] [Google Scholar]
- Liu H, Thurig S, Mohamed O, Dufort D, Wallace VA. Mapping canonical Wnt signaling in the developing and adult retina. Invest Ophthalmol Vis Sci. 2006;47:5088–5097. doi: 10.1167/iovs.06-0403. [DOI] [PubMed] [Google Scholar]
- Liu J, Wilson S, Reh T. BMP receptor 1b is required for axon guidance and cell survival in the developing retina. Dev Biol. 2003;256:34–48. doi: 10.1016/s0012-1606(02)00115-x. [DOI] [PubMed] [Google Scholar]
- Lupo G, Liu Y, Qiu R, Chandraratna RA, Barsacchi G, He RQ, Harris WA. Dorsoventral patterning of the Xenopus eye: a collaboration of Retinoid, Hedgehog and FGF receptor signaling. Development. 2005;132:1737–1748. doi: 10.1242/dev.01726. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Barth KA, Xu Q, Holder N, Mikkola I, Wilson SW. Midline signalling is required for Pax gene regulation and patterning of the eyes. Development. 1995;121:3267–3278. doi: 10.1242/dev.121.10.3267. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T, Hindges R, O'Leary DD. Regulation of axial patterning of the retina and its topographic mapping in the brain. Curr Opin Neurobiol. 2003;13:57–69. doi: 10.1016/s0959-4388(03)00014-x. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, O'Leary DD. Molecular gradients and development of retinotopic maps. Annu Rev Neurosci. 2005;28:327–355. doi: 10.1146/annurev.neuro.28.061604.135714. [DOI] [PubMed] [Google Scholar]
- Mui SH, Hindges R, O'Leary DD, Lemke G, Bertuzzi S. The homeodomain protein Vax2 patterns the dorsoventral and nasotemporal axes of the eye. Development. 2002;129:797–804. doi: 10.1242/dev.129.3.797. [DOI] [PubMed] [Google Scholar]
- Muncan V, Faro A, Haramis AP, Hurlstone AF, Wienholds E, van Es J, Korving J, Begthel H, Zivkovic D, Clevers H. T-cell factor 4 (Tcf7l2) maintains proliferative compartments in zebrafish intestine. EMBO Rep. 2007;8:966–973. doi: 10.1038/sj.embor.7401071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali D, Yoshikawa S, Corrigan RR, Plas DJ, Crair MC, Oliver G, Lyons KM, Mishina Y, Furuta Y. Distinct developmental programs require different levels of Bmp signaling during mouse retinal development. Development. 2005;132:913–923. doi: 10.1242/dev.01673. [DOI] [PubMed] [Google Scholar]
- Muroyama Y, Fujihara M, Ikeya M, Kondoh H, Takada S. Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev. 2002;16:548–553. doi: 10.1101/gad.937102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido M, Tada M, Saji T, Ueno N. Conservation of BMP signaling in zebrafish mesoderm patterning. Mech Dev. 1997;61:75–88. doi: 10.1016/s0925-4773(96)00625-9. [DOI] [PubMed] [Google Scholar]
- Plas DT, Dhande OS, Lopez JE, Murali D, Thaller C, Henkemeyer M, Furuta Y, Overbeek P, Crair MC. Bone morphogenetic proteins, eye patterning, and retinocollicular map formation in the mouse. J Neurosci. 2008;28:7057–7067. doi: 10.1523/JNEUROSCI.3598-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puschel AW, Gruss P, Westerfield M. Sequence and expression pattern of pax-6 are highly conserved between zebrafish and mice. Development. 1992;114:643–651. doi: 10.1242/dev.114.3.643. [DOI] [PubMed] [Google Scholar]
- Ramel MC, Buckles GR, Baker KD, Lekven AC. WNT8 and BMP2B co-regulate non-axial mesoderm patterning during zebrafish gastrulation. Dev Biol. 2005;287:237–248. doi: 10.1016/j.ydbio.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Rissi M, Wittbrodt J, Delot E, Naegeli M, Rosa FM. Zebrafish Radar: a new member of the TGF-beta superfamily defines dorsal regions of the neural plate and the embryonic retina. Mech Dev. 1995;49:223–234. doi: 10.1016/0925-4773(94)00320-m. [DOI] [PubMed] [Google Scholar]
- Rossi E, Siwiec F, Yan CY. Pattern of Wnt ligand expression during chick eye development. Braz J Med Biol Res. 2007;40:1333–1338. doi: 10.1590/s0100-879x2006005000155. [DOI] [PubMed] [Google Scholar]
- Ruvinsky I, Oates AC, Silver LM, Ho RK. The evolution of paired appendages in vertebrates: T-box genes in the zebrafish. Dev Genes Evol. 2000;210:82–91. doi: 10.1007/s004270050014. [DOI] [PubMed] [Google Scholar]
- Sakuta H, Takahashi H, Shintani T, Etani K, Aoshima A, Noda M. Role of bone morphogenic protein 2 in retinal patterning and retinotectal projection. J Neurosci. 2006;26:10868–10878. doi: 10.1523/JNEUROSCI.3027-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasagawa S, Takabatake T, Takabatake Y, Muramatsu T, Takeshima K. Axes establishment during eye morphogenesis in Xenopus by coordinate and antagonistic actions of BMP4, Shh, and RA. Genesis. 2002;33:86–96. doi: 10.1002/gene.10095. [DOI] [PubMed] [Google Scholar]
- Schmitt AM, Shi J, Wolf AM, Lu CC, King LA, Zou Y. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature. 2006;439:31–37. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- Schulte D, Furukawa T, Peters MA, Kozak CA, Cepko CL. Misexpression of the Emx-related homeobox genes cVax and mVax2 ventralizes the retina and perturbs the retinotectal map. Neuron. 1999;24:541–553. doi: 10.1016/s0896-6273(00)81111-3. [DOI] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- Szeto DP, Kimelman D. Combinatorial gene regulation by Bmp and Wnt in zebrafish posterior mesoderm formation. Development. 2004;131:3751–3760. doi: 10.1242/dev.01236. [DOI] [PubMed] [Google Scholar]
- Take-uchi M, Clarke JD, Wilson SW. Hedgehog signalling maintains the optic stalk-retinal interface through the regulation of Vax gene activity. Development. 2003;130:955–968. doi: 10.1242/dev.00305. [DOI] [PubMed] [Google Scholar]
- Tendeng C, Houart C. Cloning and embryonic expression of five distinct sfrp genes in the zebrafish Danio rerio. Gene Expr Patterns. 2006;6:761–771. doi: 10.1016/j.modgep.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B. High Throughput Expression Analysis of ZF-Models Consortium Clones. ZFIN Direct Data Submission. 2005 [Google Scholar]
- Ulloa F, Briscoe J. Morphogens and the control of cell proliferation and patterning in the spinal cord. Cell Cycle. 2007;6:2640–2649. doi: 10.4161/cc.6.21.4822. [DOI] [PubMed] [Google Scholar]
- Van Raay TJ, Vetter ML. Wnt/frizzled signaling during vertebrate retinal development. Dev Neurosci. 2004;26:352–358. doi: 10.1159/000082277. [DOI] [PubMed] [Google Scholar]
- Veien ES, Grierson MJ, Saund RS, Dorsky RI. Expression pattern of zebrafish tcf7 suggests unexplored domains of Wnt/beta-catenin activity. Dev Dyn. 2005;233:233–239. doi: 10.1002/dvdy.20330. [DOI] [PubMed] [Google Scholar]
- Zechner D, Muller T, Wende H, Walther I, Taketo MM, Crenshaw EB, 3rd, Treier M, Birchmeier W, Birchmeier C. Bmp and Wnt/beta-catenin signals control expression of the transcription factor Olig3 and the specification of spinal cord neurons. Dev Biol. 2007;303:181–190. doi: 10.1016/j.ydbio.2006.10.045. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Yang XJ. Temporal and spatial effects of Sonic hedgehog signaling in chick eye morphogenesis. Dev Biol. 2001;233:271–290. doi: 10.1006/dbio.2000.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang B, Sockanathan S. Dorsal-ventral patterning: a view from the top. Curr Opin Neurobiol. 2006;16:20–24. doi: 10.1016/j.conb.2005.11.001. [DOI] [PubMed] [Google Scholar]