Abstract
Caffeine is a well described and characterized ryanodine receptor (RyR) activator. Previous evidence from independent research studies also indicate caffeine inhibits InsP3 receptor functionality, which is important to activation of capacitative Ca2+ entry (CCE) in some cell types. In addition, RyR activation elicits excitatory-coupled Ca2+ entry (ECCE) in skeletal muscle myotubes. Recent studies by our group show that canine pulmonary arterial smooth muscle cells (PASMCs) have functional InsP3 receptors as well as RyRs, and that CCE is dependent on InsP3 receptor activity. The potential for caffeine to activate ECCE as well as inhibit InsP3 receptor function and CCE was examined using fura-2 fluorescent imaging in canine PASMCs. The data show caffeine causes transient as well as sustained cytosolic Ca2+ increases, though this is not due to CCE or ECCE activity as evidenced by a lack of an increase in Mn2+ quench of fura-2. The experiments also show caffeine reversibly inhibits 5-HT elicited – InsP3 mediated Ca2+ responses with an IC50 of 6.87 × 10−4 M and 10 mM caffeine fully inhibits CCE. These studies provide the first evidence that caffeine is an inhibitor of InsP3 generated Ca2+ signals and CCE in PASMCs.
Keywords: Fura-2, pulmonary arterial smooth muscle, intracellular calcium, ryanodine receptor, InsP3 receptor
1.1 Introduction
The sarcoplasmic reticulum of smooth muscle cells have two types of Ca2+ release channels that can be differentiated on their functionality and pharmacology. These include inositol 1,4,5-triphosphate (InsP3) sensitive Ca2+ channels that are activated by InsP3 and ryanodine sensitive channels (RyRs) that are activated by cytosolic Ca2+ increases. Functionally, InsP3 receptors are activated downstream of neural or humoral stimulation of G-protein coupled or tyrosine coupled membrane bound receptors (Bootman and Berridge, 1995). We find 5-Hydroxytryptamine (5-HT) is a potent activator of InsP3 receptor mediated Ca2+ responses in canine pulmonary arterial smooth muscle cells (PASMCs) (Wilson et al., 2005) with responses being mediated through 5-HT2A receptors. Caffeine elicits Ca2+ responses with similar temporal characteristics; however it selectively activates RyRs as evidenced by the inhibition of responses by ryanodine, dantrolene, and FLA 365 (Janiak et al., 2001;Ng et al., 2007;Ostrovskaya et al., 2007;Wilson et al., 2002).
Pharmacological discrimination of ryanodine and InsP3 receptor activity is important because a number of reports show these receptors influence the activity of one another (Lamont and Wier, 2004;MacMillan et al., 2005;McCarron et al., 2003;Ng et al., 2007;Zhang et al., 2003;Zhang et al., 2004;Zheng et al., 2005). Determining the extent of communication between InsP3 receptors and RyRs is significant because these Ca2+ release channels are important mediators of excitation-contraction coupling in smooth muscle. Unfortunately, common blockers of InsP3 and RyRs have non-selective effects on intracellular Ca2+ signaling, which makes interpreting their roles and activities difficult. For example, 2-Amino-biphenyl borate (2-APB) is a commonly used and commercially available InsP3 receptor antagonist (Birnbaumer et al., 2000;Boulay et al., 1999;Ma et al., 2000;Wu et al., 2000), and yet it also inhibits non-selective cation channels important to SR Ca2+ store refilling (Boulay et al., 1999;Ma et al., 2000;Prakriya and Lewis, 2001;Wilson et al., 2005). 2-APB therefore not only directly blocks InsP3 generated Ca2+ responses but also may limit RyR or InsP3 Ca2+ signals by reducing SR store refilling. Ryanodine is a widely used RyR antagonist, however, its binding characteristics are time and dose dependent. What is more, ryanodine binding often locks the RyR into a sub-conductance state, which ultimately depletes the SR Ca2+ store (Janiak et al., 2001;Wilson et al., 2002;Zucchi and Ronca-Testoni, 1997). Related to this, ryanodine administration and subsequent SR Ca2+ stores depletion often activates capacitative Ca2+ entry, which elevates the cytosolic [Ca2+] (Ng et al., 2007;Wilson et al., 2002).
RyR stimulation activates excitation-coupled calcium entry pathways (ECCE) in skeletal muscle myotubes, which share common pharmacological and functional properties with CCE (Cherednichenko et al., 2004;Hurne et al., 2005). Like CCE, ECCE may also be important to repletion of emptied SR Ca2+ stores, and yet they are activated through a distinctive coupling mechanism. ECCE in skeletal muscle myotubes is dependent on functional L-type Ca2+ channels and RyRs while CCE is reliant on release or depletion of the SR (Cherednichenko et al., 2004; Putney, Jr., 2005;Wilson et al., 2002). This novel excitatory-coupled Ca2+ entry pathway in skeletal muscle is therefore likely to be important to skeletal muscle excitation-contraction coupling. Given the expression and importance of RyRs to smooth muscle excitability, it is plausible ECCE also exists in arterial myocytes. Even still, there is no information regarding the presence of ECCE in smooth muscle.
Previous contractile and Ca2+ imaging studies from our laboratory demonstrated that structural and functional differences exist in the SR Ca2+ stores of pulmonary arterial smooth muscle cells (Jabr et al, 1997; Janiak et al, 2001; Wilson et al, 2002; Wilson et al, 2005; Ng et al, 2007). Phenylephrine contracted pulmonary arterial rings through release of InsP3 sensitive stores (Jabr et al, 1997). This initial contraction could be inhibited without affecting subsequent contraction due to release of ryanodine sensitive stores with caffeine, when the InsP3 stores were depleted in the presence of the sarcoplasmic-endoplasmic reticulum Ca2+ ATPase (SERCA) blocker cyclopiazonic acid. Similarly, stimulating ryanodine-treated cells with caffeine depleted the ryanodine Ca2+ stores, but did not affect subsequent contraction due to release of the InsP3 sensitive stores (Jabr et al, 1997). These contractile experiments were substantiated with Ca2+ imaging experiments on isolated pulmonary arterial myocytes (Janiak et al, 2001; Wilson et al, 2002). These experiments provided good evidence that in canine pulmonary arterial smooth muscle cells (PASMCs) the InsP3 and ryanodine sensitive Ca2+ stores were functionally independent. Additional experiments performed in isolated PASMCs determined that capacitative Ca2+ entry could only be activated through simultaneous depletion of the caffeine-ryanodine and InsP3-related Ca2+ stores (Wilson et al, 2002), and that active InsP3 receptors were important to this process (Ng et al, 2007).
Caffeine is a widely used RyR activator, but previous research indicates it blocks InsP3 receptor functionality at concentrations commonly used to activate RyRs (Bezprozvanny et al., 1994;Parker and Ivorra, 1991). Because caffeine is used extensively in vascular biology we wished to examine the potential for caffeine to activate ECCE or CCE, and its ability to inhibit InsP3 receptor function. The functional separation of the caffeine-ryanodine and InsP3 related Ca2+ stores, the well characterized Ca2+ responses to caffeine and to agonists that generate InsP3, and the influence that RyR and InsP3R activation has to CCE makes canine PASMCs an ideal preparation to test the hypotheses that caffeine inhibits InsP3 related Ca2+ responses, and modulates the activity of CCE and ECCE (Janiak et al., 2001;Ng et al, 2007; Ostrovskaya et al., 2007;Wilson et al., 2005;Wilson et al., 2002).
2.1 Experimental/Materials and methods
2.2 Cell isolation
Smooth muscle cells were isolated from high resistance canine pulmonary arteries as previously described (Janiak et al., 2001;Wilson et al., 2002). Mongrel dogs of either sex were sacrificed with pentobarbital sodium (45 mg kg−1 I.V.) and ketamine (15 mg kg−1 I.V.), as approved by the University of Nevada at Reno Institutional Animal Care and Use Committee. The heart and lungs were excised en bloc. The third and fourth branches of pulmonary arteries were dissected at 5° C to decrease cellular metabolic activity. Pulmonary artery isolations and smooth muscle cell dispersions were made in a low-Ca2+ physiological saline solution (PSS) containing in mM: 125 NaCl; 5.36 KCl; 0.336 Na2HPO4; 0.44 K2HPO4; 11 HEPES; 1.2 MgCl2; 0.05 CaCl2; 10 glucose; pH 7.4 (adjusted with Tris), osmolarity 300 mOsm. Arteries were cleaned of connective tissue, cut into small pieces and placed in a tube containing fresh PSS. Tissue was immediately digested or cold stored in the refrigerator (5° C) up to 24 hours. To disperse cells, tissue was placed in low-Ca2+ PSS containing enzymes containing (in mg ml−1): 0.5 collagenase type XI; 0.03 elastase type IV, and 0.5 bovine serum albumin (fat-free) for 14–16 hours at 5° C. The tissue was then washed several times with 5° C low-Ca2+ PSS solution and triturated with a fire-polished Pasteur pipette. The resulting dispersed PASMCs were cold stored at 5° C up to 8 hours until experiments were performed.
2.3 Cell Culture
HEK 293 cells (ATCC, Manassas, VA) were obtained and cultured in low glucose Dubelco’s modified Eagles media (Invitrogen, San Diego, CA) with 10% fetal bovine serum. Cells were passed weekly and fresh media applied every 2–3 days.
2.4 Fluorescence Imaging
2.4.1 Global Ca2+ measurements
The cytosolic [Ca2+] was measured in canine PASMCs and HEK 293 cells loaded with the ratiometric Ca2+ sensitive dye fura-2 AM (Molecular Probes, Eugene, OR) using a dual excitation digital Ca2+ imaging system (IonOptix Inc., Milton, MA) equipped with an intensified CCD as previously described (Janiak et al., 2001;Wilson et al., 2005;Wilson et al., 2002). The imaging system was mounted on an inverted microscope (Nikon) outfitted with a 40X (NA 1.3, Nikon Inc., Melville, NY) oil immersion objective. Fura-2 AM was dissolved in DMSO and added from a 1 mM stock to the cell suspension at a final concentration of 10 μM. Cells were loaded with fura-2 AM for 20–30 min in a perfusion chamber (Warner Instruments, Hamden, CT) at room temperature in the dark. Cells were then washed for 30 min to allow for dye esterification at 2 ml min−1 with a balanced salt solution of the following composition (mM): 126 NaCl; 5 KCl; 0.3 NaH2PO4; 10 HEPES; 1 MgCl2; 2 CaCl2; 10 glucose; pH 7.4 (adjusted with NaOH) 285 – 305 mOsm. Measurements of cytosolic [Ca2+] before and during capacitative Ca2+ entry and pharmacological manipulation were made once the fura-2 fluorescence ratio stabilized. The Ca2+ free balanced salt solution was prepared by substituting MgCl2 for CaCl2 and adding 1 mM EGTA. Cells were illuminated with a xenon arc lamp at 340 ± 15 and 380 ± 12 nm (Omega Optical, Brattleboro, VT) and emitted light was collected from regions that encompassed single cells with a CCD at 510 nm (Nikon Inc., Melville, NY). In most experiments, images were acquired at 1 Hz and stored on either compact disk or magnetic media for later analysis.
Although it is difficult to precisely measure the intracellular calcium concentration ([Ca2+]I) (Baylor and Hollingworth, 2000) estimates were made from the ratio of fluorescence excited at 340 and 380 nm (R) as described by Grynkiewicz et al (1985) based on the relation [Ca2+]I= Kd * (Sf2/Sb2) * (R−Rmin)/(Rmax−R), where the KD for Ca2+ binding to fura-2 was assumed to be 224 nM (Grynkiewicz et al., 1985) and the values for F380 in the absence of extracellular Ca2+ (Sf2), F380 in the presence of 10 mM extracellular Ca2+ (Sb2), minimum ratio (Rmin), and maximum ratio (Rmax) were determined from in situ calibrations of fura-2 for each cell after applying 1 μM ionomycin. To determine the maximum ratio, Rmax, cells were perfused with a balanced salt solution that contained 10 mM Ca2+, while the minimum ratio, Rmin, was obtained after applying balanced salt solution that did not have any added Ca2+ and contained 10 mM EGTA. These calibration procedures are based on those described previously by us (Ng et al, 2005; Ng et al, 2007; Wilson et al, 2002; Wilson et al, 2005). During the Ca2+ calibration, 5 mM 2,3 butane dione monoxime was added to the bathing solution to inhibit smooth muscle contraction (Waurick et al., 1999).
2.4.2 Mn2+ quench
Mn2+ is a commonly used probe for studying Ca2+ influx changes because many Ca2+ selective channels are permeable to Mn2+ and because it quenches the fura-2 fluorescence emission (Missiaen et al., 1990). Also, Mn2+ cannot be transported out of the cytosol into intracellular compartments or extruded from the cell (Gomes and Madeira, 1986). The rate of Mn2+ quench of fura-2 was determined by regression analysis of fluorescent intensity over time for canine PASMCs excited at 357 ± 10 nm as previously described (Janiak et al., 2001;Wilson et al., 2005;Wilson et al., 2002). Cells were analyzed only if the rate of fura-2 quench by Mn2+ in the presence of ionomycin was at least 4 fold greater than the basal rate. Background fluorescence was collected automatically and subtracted from the acquired fluorescence video images during each experiment. The Ca2+ free balanced salt solution used in the Mn2+ quench experiments did not have any added Ca2+ or EGTA. Experimental temperature was 22–25° C.
2.5 Chemicals and drugs
Ionomycin free acid was purchased from Calbiochem (San Diego, CA), Fura-2 from molecular probes (Eugene, OR) and all other chemicals were purchased from Sigma (St. Louis, MO).
2.6 Statistical analysis
All data are presented as mean ± S.E.M. Statistical difference between groups was assessed with a One-Way Analysis of Variance (ANOVA) with a Newman-Keuls multiple comparison procedure or a Friedman repeated measures ANOVA on ranks with SNK multiple comparison procedure. The specific test used for each data set is noted in the legend for each figure. A P value < 0.05 was accepted as statistically significant. A Hill equation (eq. 1)
| (1) |
was used to determine the half-maximum inhibition of agonist mediated Ca2+ increases by pharmacological blockers, where A1 = bottom asymptote, A2 = top asymptote, Log xo = IC50, p = hill slope. The n values reported reflect the total number of cells tested. Multiple trials were performed on cells isolated from multiple dogs for most experimental paradigms with the specific number of cells being listed in the figure legends.
3.1 Results
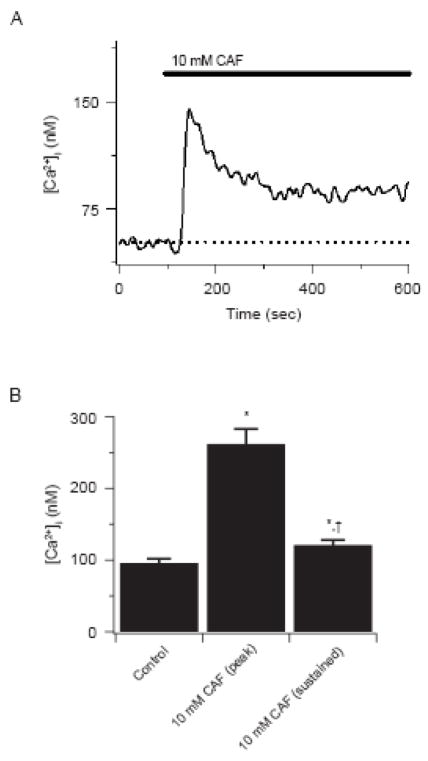
Figure 1 shows the influence of 10 mM caffeine on estimated cytosolic [Ca2+] in canine PASMCs. Figure 1A shows that 10 mM caffeine elicited a rapid increase in cytosolic [Ca2+] of 93 nM, which then relaxed and stabilized ~ 40 nM above basal values in the continued presence of the agonist. This caffeine-mediated increase in cytosolic [Ca2+] is somewhat lower than the average response of 166 ± 21 nM above resting levels shown in Figure 1B, but well within the normal range of variability for caffeine-elicited Ca2+ responses in canine PASMCs (Janiak et al, 2001; Ng et al, 2007; Ostrovskaya et al; 2007; Wilson et al, 2002; Wilson et al, 2005). In the continued presence of 10 mM caffeine, cytosolic [Ca2+] was substantially lower but remained 26 ± 3 nM above basal values in these same cells.
Figure 1.
Caffeine elicits cytosolic [Ca2+] ncreases in PASMCs. (A) Caffeine induced Ca2+ transient. Caffeine was present at times shown by the horizontal bar. Dashed line shows resting cytosolic [Ca2+]. (B) Bars indicate the cytosolic [Ca2+] before and during caffeine. Error bars represent ± S.E.M for 53 cells * Denotes significant difference to control while † denotes difference as compared to peak caffeine conditions using Friedman repeated measures ANOVA on ranks with SNK multiple comparison procedures (P<0.05).
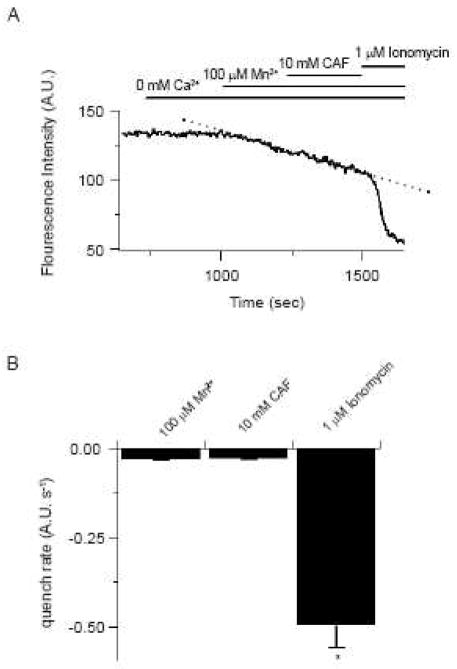
Previous reports show that activation of ECCE or CCE pathways enhances the rate of Mn2+ quench of Fura-2 (Cherednichenko et al., 2004;Hurne et al., 2005;Ng et al., 2005;Wilson et al., 2005;Wilson et al., 2002). The potential for caffeine activation of ECCE pathways was therefore examined in canine PASMCs by measuring the rate of Mn2+ quench of fura-2. Figure 2 shows the results of these studies. Figure 2A shows the fluorescence intensity over time measured at 510 nm at an excitation wavelength of 357 nm in a single PASMC. Removal of extracellular Ca2+ did not cause any decline in the fluorescence intensity. However, 100 μM Mn2+ caused the fluorescence intensity to decrease at a rate of −0.065 s−1. The quench rate by Mn2+ was not appreciably influenced by 10 mM caffeine remaining at −0.055 s−1. Figure 2B summarizes these results showing that 10 mM caffeine does not alter Mn2+ permeability. Exposure to 10 mM caffeine did not significantly alter the Mn2+ quench of fura-2, which was −0.029 ± 0.003 s−1 before and −0.029 ± 0.004 s−1 during caffeine. Subsequent exposure to 1 μM ionomycin shows these cells were viable as it caused a 19-fold increase in the quench rate. This lack of an influence of caffeine on the Mn2+ quench rate is similar to our finding that 5-HT stimulation also does not increase Mn2+ entry across the plasma membrane (Wilson et al, 2005). In comparison to the lack of effect of caffeine, our previous studies show the Mn2+ quench rate doubles when the intracellular Ca2+ stores are depleted (Wilson et al, 2002; Ng et al, 2005; Ng et al, 2007).
Figure 2.
Caffeine does not enhance Mn2+ quench of fura-2 in PASMCs. (A) 10 mM caffeine effect on the rate of fura-2 quench by 100 μM Mn2+. Agonists were present at times shown by the horizontal bars. Dashed line shows the resting quench rate. (B) Bars show the fura-2 quench rate. Error bars represent ± S.E.M. for 23 cells. * Denotes significant difference to 100 μM Mn2+ and 100 μM Mn2+ + 10 mM CAF conditions using Friedman repeated measures ANOVA on ranks with SNK multiple comparison procedures (P<0.05).
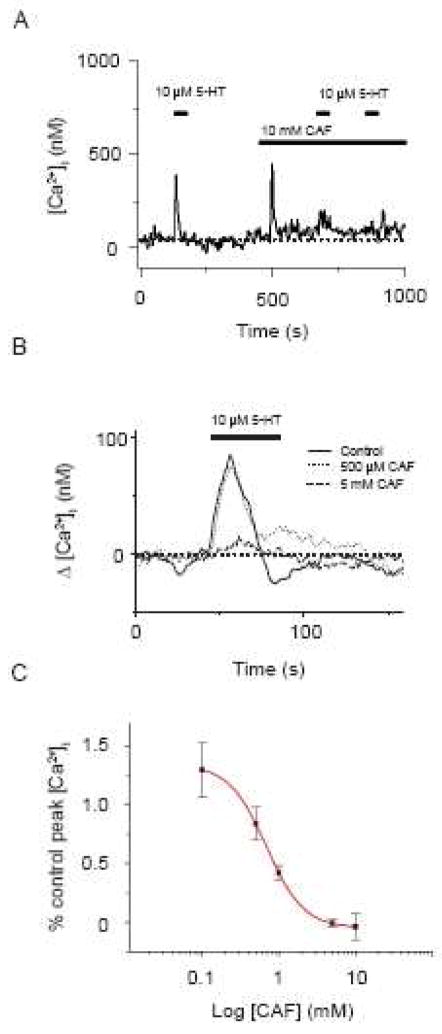
Given the previous reports of caffeine inhibition of InsP3 receptors, we examined the effects of sustained caffeine exposure on 5-HT elicited cytosolic [Ca2+] responses. Figure 3 shows the results of these studies. Figure 3A shows that a 30 s 10 μM 5-HT exposure caused a rapid, transient elevation in the cytosolic [Ca2+] of 247 nM that fell back to baseline with agonist washout. 10 mM caffeine caused a rapid transient increase in cytosolic [Ca2+] of 404 nM that fell toward baseline in the continuous presence of agonist, stabilizing 35 nM above basal values. After ~3 minutes in the continued presence of 10 mM caffeine, a 30 s 10 μM 5-HT exposure elicited a reduced cytosolic [Ca2+] increase of 80 nM.
Figure 3.
Caffeine blocks 5-HT elicited cytosolic [Ca2+] increases in PASMCs. (A) 10 μM 5-HT induced Ca2+ transient in the absence then presence of 10 mM caffeine. Agonists were present at times shown by the horizontal bars. Dashed line shows the resting cytosolic [Ca2+]. (B) Dose dependent inhibition of 10 μM 5-HT induced Ca2+ transients by caffeine. Solid line is the resultant dose-response curve fitted to the data with a simple binding equation. Values are means of % peak Ca2+. Error bars represent ± S.E.M for 8 to 10 cells at each concentration of caffeine evaluated.
Figure 3B shows the change in estimated cytosolic [Ca2+] from baseline for a cell stimulated with 10 μM 5-HT in the absence and presence of 500 μM and then 5 mM caffeine. Ten-micromolar 5-HT elicited a cytosolic [Ca2+] increase that was minimally affected by 500 μM caffeine. However, Figure 3B also shows increasing the caffeine concentration to 5 mM markedly reduced the 5-HT mediated [Ca2+] response.
Figure 3C shows a normalized dose-response curve for caffeine inhibition of 5-HT elicited cytosolic [Ca2+] increases for cells that were stimulated with 10 μM 5-HT in the absence and then presence of different concentrations of caffeine. This concentration of 5-HT was used as it elicits the maximal cytosolic [Ca2+] response (Wilson et al, 2005). 5-HT-mediated cytosolic [Ca2+] increases were not affected by 100 μM caffeine, which also illustrates the Ca2+-responses to 5-HT stimulation do not desensitize. However, the cytosolic [Ca2+] increases to 5-HT were fully blocked by 5–10 mM caffeine. These data were fit to a simple binding equation (eq. 1, solid line) with an estimated IC50 = 6.87 × 10−4 M.
Caffeine inhibition of 5-HT elicited cytosolic [Ca2+] increases is comparable to the effects of known InsP3 receptor antagonists (Wilson et al, 2005). Wilson et al, 2005 (Figure 2) shows the InsP3 receptor inhibitors 2-APB and xestospongin C inhibited 5-HT-mediated Ca2+ responses. Importantly, Ng et al, 2007 (Figure 1) shows 2-APB and xestospongin C did not reduce caffeine elicited Ca2+ transients. Thus, the caffeine-induced decrements in the 5-HT elicited Ca2+ responses shown in Figure 3 are not because caffeine caused prior activation of InsP3 receptors and release or depletion of the InsP3-portion of the sarcoplasmic reticulum.
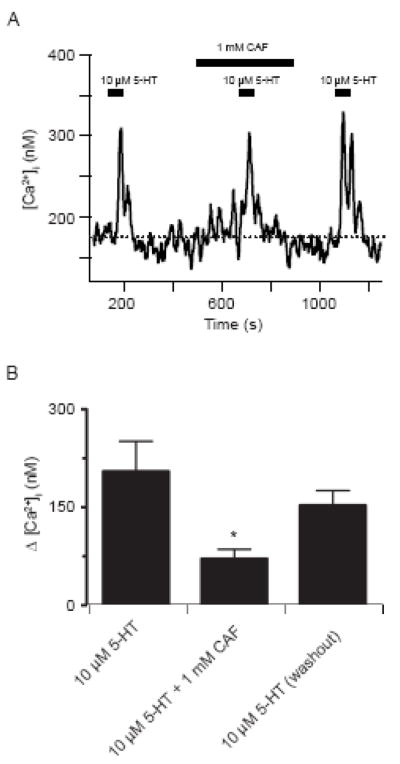
One important pharmacological consideration is the ability to recover from antagonism. This property was examined by stimulating cells with 10 μM 5-HT in the absence as well as presence of 1 mM caffeine, which was chosen as it is close to the IC50 value. Figure 4A shows the estimated cytosolic [Ca2+] in a single myocyte. Exposing the cell to 10 μM 5-HT for 30 s caused a rapid transient increase in cytosolic [Ca2+] of 134 nM, which decayed back to basal values. Exposure of this cell to 1 mM caffeine for ~ 3 min caused basal cytosolic [Ca2+] to increase roughly 30 nM, in accordance with ryanodine receptor activation (Janiak et al, 2001). 1 mM caffeine reduced the cytosolic [Ca2+] increase due to 10 μM 5-HT approximately one-third, being 99 nM. When caffeine was removed from the bathing solution the cytosolic [Ca2+] relaxed to baseline values, showing further evidence for RyR activation by 1 mM caffeine. The cytosolic [Ca2+] increase due to 10 μM 5-HT also returned to control levels following caffeine washout in this cell, being 163 nM. Figure 4B summarizes the findings showing caffeine reversibly inhibits 5-HT elicited cytosolic [Ca2+] increases in pulmonary arterial myocytes. Before 1 mM caffeine, 10 μM 5-HT caused a 205 ± 16 nM cytosolic [Ca2+] increase. In the presence of 1 mM caffeine, however, the response was reduced nearly two-thirds, being 72 ± 14 nM in these same cells. Once caffeine was washed from the bath, 10 μM 5-HT caused a cytosolic [Ca2+] elevation of 153 ± 23 nM, which was not significantly different than the control value.
Figure 4.
Caffeine reversibly inhibits 5-HT mediated cytosolic [Ca2+] increases in PASMCs. (A) 10 μM 5-HT induced cytosolic [Ca2+] transient in the absence and presence of 1 mM caffeine. Agonists were present at times shown by the horizontal bars. Dashed line shows the resting cytosolic [Ca2+]. (B) Bars indicate the change in the cytosolic [Ca2+] from basal values in the presence and absence of caffeine. Error bars represent ± S.E.M for 16 myocytes. * Denotes significant difference to 10 μM 5-HT and 10 μM 5-HT during washout conditions using a repeated measures ANOVA with a SNK multiple comparison procedure (P<0.05).
Even though caffeine appears to reduce the 5-HT mediated Ca2+ response it is important to know that 5-HT mediated Ca2+ responses do not desensitize. Figures 3 and 4 as well as our previous studies provide evidence that 5-HT can repeatedly cause Ca2+ responses of similar magnitude (Wilson et al, 2005; Ostrovskaya et al, 2007). The overlaid traces in Figure 3B clearly show that 10 μM 5-HT cause repeated Ca2+ increases of similar magnitude, even in the presence of a low caffeine concentration. The dose-response curve shown in Figure 3C substantiates this finding, and shows summary data that also indicates 10 μM 5-HT can cause multiple cytosolic Ca2+ increases of similar magnitude in the presence of low caffeine concentrations. Figure 4 provides additional support as it shows recovery of the 5-HT-mediated Ca2+ response once caffeine is washed from the bathing solution. These findings are similar to Ostrovskaya et al, 2007 (Figures 6C and 7A), which show results from specific control experiments; where canine PASMCs were repeatedly stimulated with 10 μM 5-HT without any decrement in the elicited Ca2+-response. In Wilson et al, 2005 (Figure 5A), we also show that continuous 5-HT application caused robust and maintained Ca2+ signals in canine PASMCs.
Figure 6.
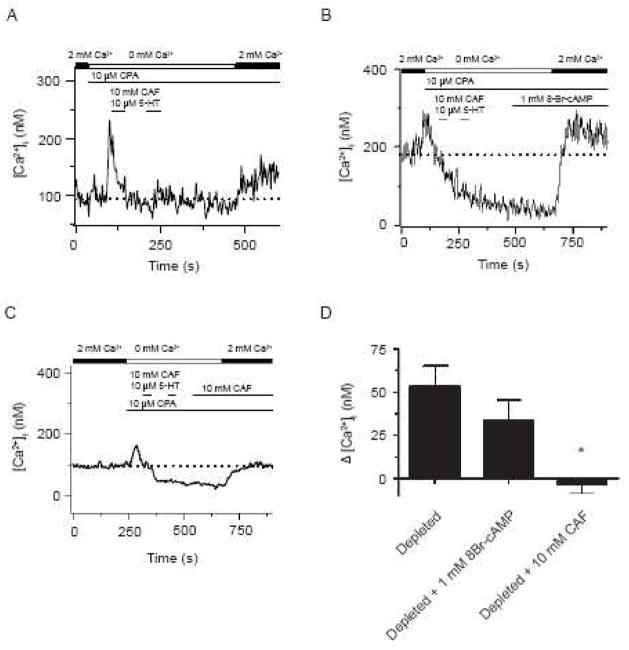
Caffeine but not 8-Br-cAMP inhibits CCE in canine PASMCs. (A) Effect of extracellular Ca2+ removal, 10 μM CPA, sequential exposure to 10 mM caffeine and 10 μM 5-HT on cytosolic [Ca2+] with extracellular Ca2+ re-addition. Effects of (B) 1 mM 8-Br-cAMP and (C) 10 mM caffeine on the cytosolic [Ca2+] with depletion of the sarcoplasmic reticulum Ca2+ stores. Agonists were present at times shown by the horizontal bars. Dashed line shows the resting cytosolic [Ca2+]. (D) Bars indicate the change in cytosolic [Ca2+] from resting levels for myocytes that were store depleted in the absence (n=9) or presence of 8-Br-cAMP (n=6) or caffeine (n=10). Error bars represent ± S.E.M. Mean value significantly different from other groups by a One-Way ANOVA with a Newman-Keuls Multiple Comparison Test (P<0.05).
Figure 5.
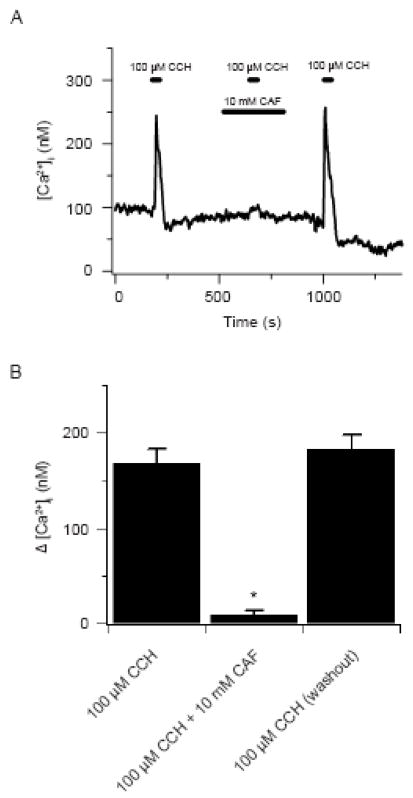
Caffeine blocks carbachol elicited cytosolic [Ca2+] increases in HEK 293 cells. (A) 100 μM carbachol induced Ca2+ transient in the absence and presence of 10 mM caffeine. Agonists were present at times shown by the horizontal bars. (B) Bars indicate the change in the cytosolic [Ca2+] from basal values in the absence and presence of caffeine. Error bars represent ± S.E.M for 65 cells. * Denotes significant difference to 100 μM carbachol and 100 μM carbachol during washout conditions using a Friedman repeated measures ANOVA on ranks with a SNK multiple comparison procedure (P<0.05).
To test if sustained caffeine exposure causes a general inhibition of InsP3 receptor induced cytosolic [Ca2+] increases or whether the elevation in cytosolic 6[Ca2+] negatively influences InsP3 activation analogous experiments were performed on HEK 293 cells. HEK 293 cells are known to express functional InsP3 receptors (Luo et al., 2000), while functional ryanodine receptors, such as can be activated by caffeine, are diminutive (Querfurth et al., 1998) or non-existent (Ostrovskaya et al., 2007;Rossi et al., 2002). Figure 5A shows the estimated cytosolic [Ca2+] in a single HEK 293 cell. Exposing the cell to 100 μM carbachol for 30 s, which releases Ca2+ from the endoplasmic reticulum stores in these cells, caused a rapid transient increase in cytosolic [Ca2+] of 149 nM. Following the rapid increase, the cytosolic [Ca2+] decayed back to basal values. Exposure of this cell to 10 mM caffeine for ~ 3 min did not alter basal cytosolic [Ca2+] from the control values of 85–95 nM. Although this does not conclusively show a lack of molecular expression, it shows these cells lack functional RyRs as caffeine activates all known RyR isoforms (Zucchi and Ronca-Testoni, 1997). Subsequently, 100 μM carbachol for 30 s in the continued presence of 10 mM caffeine elicited a 14 nM cytosolic [Ca2+] increase. The cytosolic [Ca2+] responses to 100 μM carbachol recovered after removing caffeine, being 170 nM. Figure 5B summarizes the data showing caffeine reversibly inhibits carbachol induced cytosolic [Ca2+] increases in HEK 293 cells. Prior to 10 mM caffeine, 100 μM carbachol elicited a 169 ± 15 nM increase in cytosolic [Ca2+]. In the presence of 10 mM caffeine, however, 100 μM carbachol caused a small, 9 ± 4 nM, yet significant (P<0.05, paired t-test) increase in cytosolic [Ca2+] in these same cells. Following caffeine washout, 100 μM carbachol caused cytosolic [Ca2+] elevations equivalent to control, being 184 ± 15 nM.
We recently described InsP3 receptor inhibition blocks CCE in canine PASMCs (Ng et al., 2007). This finding led us to examine whether or not caffeine inhibits CCE, and to compare these findings with our earlier studies (Ng et al., 2007). We also performed separate control experiments as caffeine exposure for significant periods inhibits phosphodiesterase function and increases cAMP. This is an important consideration as cAMP dependent kinase activity has recently been shown to inhibit CCE (Ay et al., 2006, Smani et al., 2007, Zhang et al., 2007), and store operated channels (Liu et al., 2005). In these control experiments, cells were exposed to the membrane permeable cAMP analogue 8-Br-cAMP (1 mM) for similar lengths of time as those treated with caffeine, which was a total of 5 – 10 minutes, and thus sufficiently long enough to increase cytosolic cAMP levels. This concentration of 8-Br-cAMP was chosen because it should provide a saturating level of intracellular cAMP (Wilson et al, 2000), and thus provide an adequate measure for the influence of cAMP dependent kinases on CCE.
Figure 6 summarizes the effects of caffeine and 8-Br-cAMP on CCE. Figure 6A shows that capacitative Ca2+ entry was activated by depleting the SR Ca2+ stores, and then reintroducing extracellular Ca2+ as we have done previously (Ng et al., 2005;Ng et al., 2007;Wilson et al., 2002). These experiments were performed by perfusing the cells with a Ca2+ free bathing solution in the continuous presence of 10 μM CPA and exposing the cells twice for 30 s to 10 mM caffeine and 10 μM 5-HT. Subsequent to this, 2 mM-extracellular Ca2+ was added to the bathing solution in the continued presence of 10 μM CPA. This procedure elevates cytosolic Ca2+ above basal values, and is due to activation of CCE pathways (Ng et al., 2005;Ng et al., 2007;Wilson et al., 2002). Figure 6A shows a representative recording of the estimated cytosolic [Ca2+] from a canine pulmonary arterial myocyte following depletion of the intracellular Ca2+ stores and during re-addition of extracellular Ca2+. Following Ca2+ readdition, the cytosolic [Ca2+] increased 43 nM above basal levels, which is consistent with activation of CCE in canine PASMCs (Ng et al., 2005;Ng et al., 2007;Wilson et al., 2002). Figure 6B shows the influence of 1 mM 8-Br-cAMP on the cytosolic [Ca2+] with Ca2+ readdition following depletion of the sarcoplasmic reticulum Ca2+ stores. Following Ca2+ readdition, the cytosolic [Ca2+] increased 31 nM above basal levels in this cell, which is similar to the control cell shown in Figure 6A and also indicative of CCE. Figure 6D provides a summary of the results, showing that incubation with 1 mM 8-Br-cAMP did not prevent CCE activation. In the 8-Br-cAMP treated cells, the cytosolic [Ca2+] increased 33 ± 12 nM, which was equivalent to the development of CCE in untreated cells (54 ± 12 nM) from the same animals.
In comparison to the lack of effect of 8-Br-cAMP on CCE, Figure 6C shows sustained exposure to 10 mM caffeine prevented CCE activation, which builds on the finding that it fully inhibits 5-HT elicited cytosolic [Ca2+] responses in canine PASMCs (Figure 3). Figure 6D summarizes the CCE mediated cytosolic [Ca2+] responses in the presence of 10 mM caffeine, which were 4 ± 5 nM below basal Ca2+ levels. The decrement in the CCE response by 10 mM caffeine is similar to the depression in CCE due to pharmacological inhibition of the store-operated channels responsible for CCE as shown in Wilson et al., 2002 (Figures 1, 2, 7 and 8), or due to InsP3 receptor inhibition as shown in Ng et al, 2007(Figures 3 and 5). In Wilson et al., 2002, CCE was diminished by reducing extracellular Ca2+ entry through extracellular Ca2+ removal (6 ± 6 nM below basal [Ca2+] values) or by 10 mM Ni2+ (70 ± 14 nM below basal [Ca2+] values). Similarly CCE was reduced in Ng et al., 2007 through InsP3R inhibition with 50 μM 2-APB (19 ± 15 nM below basal [Ca2+] values), 20 μM xestospongin C (4 ± 10 nM below basal [Ca2+] values), or 50 μM dantrolene (0 ± 13 nM).
The inhibition of CCE by caffeine is markedly dissimilar from modifiers of RyR activity used in Ng et al., 2007 (Figures 4 and 5). In these previous studies, we showed CCE was not reduced by 10 or 300 μM ryanodine or by 10 μM dantrolene. This is important because it indicates that the influence of caffeine on CCE is not due to ryanodine receptor activation.
4.1 Discussion
Our results show the widely used RyR activator caffeine is also a rapid, yet reversible, inhibitor of 5-HT elicited and presumably InsP3 receptor mediated cytosolic [Ca2+] increases in both acutely isolated smooth muscle cells and cultured mammalian cells. Caffeine inhibited 5-HT mediated Ca2+ increases with an IC50 similar to its direct effects on InsP3 receptors (Bezprozvanny et al., 1994). Further to this, the antagonism of 5-HT elicited Ca2+ responses by caffeine in canine PASMCs was akin to the actions of the InsP3 receptor inhibitors 2-APB and xestospongin C (Wilson et al, 2005) and similar to caffeine antagonism of InsP3 related Ca2+ responses in Xenopous oocytes (Parker and Ivorra, 1991). Capacitative calcium entry was also reduced by caffeine concentrations that fully inhibit InsP3 receptors (Bezprozvanny et al., 1994) but not by 8-Br-cAMP.
Caffeine-dependent depression of 5-HT mediated Ca2+ responses may be due to either direct or indirect inhibition of InsP3 receptor signaling. The basis of the present studies was to assess if caffeine inhibits InsP3-related Ca2+ signaling events, and the experiments accomplished this goal. The simplest explanation is that caffeine reduces 5-HT elicited Ca2+ responses by directly inhibiting InsP3 receptor activation as has been previously observed (Bezprozvanny et al., 1994;Parker and Ivorra, 1991). Support for this is the observation that the IC50 for caffeine-inhibition of the 5-HT dependent Ca2+ responses is similar to its antagonism of InsP3 receptor function (Bezprozvanny et al., 1994;Parker and Ivorra, 1991). However, as a methylxanthine, caffeine also enhances cAMP formation through phosphodiesterase inhibition, and this cAMP elevation can antagonize InsP3 receptor activation (Pauvert et al, 2003; Pauvert et al, 2004; Bai and Sanderson, 2006). We attempted to minimize this cAMP generation by only applying caffeine for short periods prior to 5-HT stimulation. The reversibility of caffeine inhibition of 5-HT elicited Ca2+ responses also suggests there was not long-lived cAMP accumulation. Even still, this cannot refute the potential that caffeine-mediated cAMP formation reduces 5-HT dependent Ca2+ responses. Elucidating the specific mechanism of caffeine inhibition of 5-HT mediated Ca2+ responses is an important consideration, and thus warrants future investigation.
Caffeine- dependent inhibition of 5-HT mediated Ca2+ responses is not likely due to a direct effect on L-type Ca2+ channels. Although L-type Ca2+ channels are inhibited by millimolar caffeine (Zholos et al., 1991), the IC50 is roughly 20-fold higher (Martin et al., 1989) than its ability to inhibit 5-HT mediated Ca2+ increases. Further support is that dihydropyridine inhibition of L-type Ca2+ channels does not reduce the magnitude of 5-HT-dependent Ca2+ responses in canine PASMCs (Wilson et al, 2005; Ostrovskaya et al, 2007).
Caffeine does not likely directly activate CCE or ECCE pathways in canine PASMCs. Evidence for this is that caffeine failed to increase the fura-2 quench rate by Mn2+, which compares with the increase in Mn2+ quench due to depletion of the intracellular Ca2+ stores (Ng et al., 2005;Ng et al., 2007;Wilson et al., 2002). This inability of caffeine to enhance Mn2+-permeability mimics that of 5-HT, which also does not activate CCE (Wilson et al., 2005). The lack of ECCE was anticipated as the coupling between L-type Ca2+ channels and RyRs in skeletal and smooth muscle are distinct from one another. In skeletal muscle, these proteins are physically associated and allow for classic conformational-coupling (Hurne et al., 2005). Comparatively, RyR activity in smooth muscle is loosely coupled to L-type Ca2+ channels, where extracellular Ca2+ influx excites RyRs (Collier et al., 2000). Importantly, ECCE activity in skeletal muscle is dependent on L-type Ca2+ channel and RyR expression, where RyR mutations alter ECCE gating (Hurne et al., 2005). This suggests there is a physical association between the RyR and the channel responsible for ECCE in skeletal muscle, an association that may not exist in smooth muscle.
Caffeine inhibition of CCE was not surprising as we recently showed InsP3 receptors but not RyRs are important to CCE activity in canine PASMCs (Ng et al., 2007). The finding that caffeine inhibits CCE also builds on several previous studies showing InsP3 receptor activity is important to CCE (Birnbaumer et al., 2000;Ma et al., 2000;Wang et al., 2001). However, as caffeine can directly inhibit InsP3 receptors (Bezprozvanny et al., 1994;Parker and Ivorra, 1991) as well as L-type Ca2+ channels (Zholos et al., 1991) it is possible that caffeine may also block other Ca2+ permeable ion channels, such as store-operated channels. The potential for caffeine inhibition of store-operated channels will require additional studies, as we were unable to find any reports supporting or refuting this alternative.
The finding that 8-Br-cAMP did not influence capacitative calcium entry is revealing as several recent reports indicate cAMP inhibits store-operated Ca2+ entry in airway smooth muscle (Ay et al., 2006), coronary arterial myocytes (Smani et al., 2007), and human intrapulmonary arterial smooth muscle cells (Zhang et al., 2007), as well as store-operated channel currents in portal vein myocytes (Liu et al., 2005). We are unsure of why our findings in canine PASMCs are distinct from the results found in other myocytes. One possibility is that the magnitude of the CCE response in the canine cells is relatively small and the heterogeneity between individual cells may be sufficiently high to mask a small change in capacitative calcium entry due to cAMP, or it may be the result of differences in the experimental conditions. For example, Zhang et al., 2007 preincubated their cells for 30 minutes with agents that increased cyclic nucleotide levels while we treated our cells for only 5–10 minutes. On closer examination of the data presented by Zhang and co-workers they observed that forskolin and IBMX only partially reduced the capacitative calcium entry response, and that this effect was dependent on the administration time, which was 30 min or 4 hours. Based on the data we present in Figure 6, there may be a trend for cAMP to reduce capacitative calcium entry, however this depression in cytosolic calcium is small and within the observed variability. The potential modulation of capactiative calcium entry by cAMP is important as this pathway is essential during the development of hypoxic induced pulmonary vasoconstriction (Ng et al., 2005; Ng et al., 2007), a process that regulates blood flow distribution and gas exchange in the lung, and because these pathways are important for cell growth and proliferation (Sweeney et al., 2002). This may also be therapeutically relevant as agents that increase cyclic nucleotides, such as the PDE5 inhibitor sildenafil and the prostacylin analogue iloprost cause pulmonary arterial vasorelaxation and thus are useful in the treatment of pulmonary hypertension (Gessler et al., 2008; Wilkins et al., 2008).
5.1 Conclusions
This manuscript shows the following:
Caffeine inhibits 5-HT elicited - InsP3 – mediated Ca2+ responses and CCE in smooth muscle.
The data provide correlative support for our previous studies that suggest InsP3 receptors are important to the activation of CCE in canine PASMCs.
ECCE pathways may not exist in canine PASMCs.
The role of InsP3 receptor activity may be difficult to assess when caffeine is used in tandem to activate RyRs.
There is a fundamental need for the development and characterization of RyR activators that do not interfere with InsP3 receptor activity.
Acknowledgments
We would like to thank Phillip Keller and Shen Xiao-Ming for technical assistance. This work was supported by NIH grants HL49254 and P20RR15518 from NCRR (JRH), HL10476 and AI55462 (SMW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6.1 References
- Ay B, Iyanoye A, Sieck GC, Prakash YS, Pabelick CM. Cyclic nucleotide regulation of store-operated ca2+ influx in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;290:L278–L283. doi: 10.1152/ajplung.00188.2005. [DOI] [PubMed] [Google Scholar]
- Bai Y, Sanderson MJ. Airway smooth muscle relaxation results from a reduction in the frequency of ca2+ oscillations induced by a camp-mediated inhibition of the ip3 receptor. Respir Res. 2006;7:34–53. doi: 10.1186/1465-9921-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor SM, Hollingworth S. Measurement and Interpretation of Cytoplasmic [Ca2+] Signals From Calcium-Indicator Dyes. NIPS. 2000;15:19–26. [PubMed] [Google Scholar]
- Bezprozvanny I, Bezprozvannaya S, Ehrlich BE. Caffeine-induced inhibition of inositol(1,4,5)-trisphosphate-gated calcium channels from cerebellum. Mol Biol Cell. 1994;5:97–103. doi: 10.1091/mbc.5.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L, Boulay G, Brown D, Jiang M, Dietrich A, Mikoshiba K, Zhu X, Qin N. Mechanism of capacitative Ca2+ entry (CCE): interaction between IP3 receptor and TRP links the internal calcium storage compartment to plasma membrane CCE channels. Recent Prog Horm Res. 2000;55:127–161. [PubMed] [Google Scholar]
- Bootman MD, Berridge MJ. The elemental principles of calcium signaling. Cell. 1995;83:675–678. doi: 10.1016/0092-8674(95)90179-5. [DOI] [PubMed] [Google Scholar]
- Boulay G, Brown DM, Qin N, Jiang M, Dietrich A, Zhu MX, Chen Z, Birnbaumer M, Mikoshiba K, Birnbaumer L. Modulation of Ca(2+) entry by polypeptides of the inositol 1,4, 5- trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion-activated Ca(2+) entry. Proc Natl Acad Sci USA. 1999;96:14955–14960. doi: 10.1073/pnas.96.26.14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherednichenko G, Hurne AM, Fessenden JD, Lee EH, Allen PD, Beam KG, Pessah IN. Conformational activation of Ca2+ entry by depolarization of skeletal myotubes. Proc Natl Acad Sci USA. 2004;101:15793–15798. doi: 10.1073/pnas.0403485101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier ML, Ji G, Wang Y, Kotlikoff MI. Calcium-induced calcium release in smooth muscle: loose coupling between the action potential and calcium release. J Gen Physiol. 2000;115:653–662. doi: 10.1085/jgp.115.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessler T, Seeger W, Schmehl T. Inhaled prostanoids in the therapy of pulmonary hypertension. J Aerosol Med Pulm Drug Deliv. 2008;21:1–12. doi: 10.1089/jamp.2007.0657. [DOI] [PubMed] [Google Scholar]
- Gomes dC, Madeira VM. Magnesium and manganese ions modulate Ca2+ uptake and its energetic coupling in sarcoplasmic reticulum. Arch Biochem Biophys. 1986;249:199–206. doi: 10.1016/0003-9861(86)90575-8. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hurne AM, O’Brien JJ, Wingrove D, Cherednichenko G, Allen PD, Beam KG, Pessah IN. Ryanodine receptor type 1 (RyR1) mutations C4958S and C4961S reveal excitation-coupled calcium entry (ECCE) is independent of sarcoplasmic reticulum store depletion. J Biol Chem. 2005;280:36994–37004. doi: 10.1074/jbc.M506441200. [DOI] [PubMed] [Google Scholar]
- Jabr RI, Toland H, Gelband CH, Wang XX, Hume JR. Prominent role of intracellular ca2+ release in hypoxic vasoconstriction of canine pulmonary artery. British Journal of Pharmacology. 1997;122:21–30. doi: 10.1038/sj.bjp.0701326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiak R, Wilson SM, Montague S, Hume JR. Heterogeneity of calcium stores and elementary release events in canine pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol. 2001;280:C22–C33. doi: 10.1152/ajpcell.2001.280.1.C22. [DOI] [PubMed] [Google Scholar]
- Lamont C, Wier WG. Different roles of ryanodine receptors and inositol (1,4,5)-trisphosphate receptors in adrenergically stimulated contractions of small arteries. Am J Physiol Heart Circ Physiol. 2004;287:H617–H625. doi: 10.1152/ajpheart.00708.2003. [DOI] [PubMed] [Google Scholar]
- Liu M, Large WA, Albert AP. Stimulation of beta-adrenoceptors inhibits store-operated channel currents via a camp-dependent protein kinase mechanism in rabbit portal vein myocytes. J Physiol. 2005;562:395–406. doi: 10.1113/jphysiol.2004.077602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Broad LM, Bird GS, Putney J. Signaling Pathways Underlying Muscarinic Receptor-induced [Ca2+]i Oscillations in HEK293 Cells. J Biol Chem. 2000;276:20186–9. doi: 10.1074/jbc.M007524200. [DOI] [PubMed] [Google Scholar]
- Ma HT, Patterson RL, van Rossum DB, Birnbaumer L, Mikoshiba K, Gill DL. Requirement of the Inositol Trisphosphate Receptor for Activation of Store-Operated Ca(2+) Channels. Science. 2000;287:1647–1651. doi: 10.1126/science.287.5458.1647. [DOI] [PubMed] [Google Scholar]
- MacMillan D, Chalmers S, Muir TC, McCarron JG. IP3-mediated Ca2+ increases do not involve the ryanodine receptor, but ryanodine receptor antagonists reduce IP3-mediated Ca2+ increases in guinea-pig colonic smooth muscle cells. J Physiol. 2005;569:533–544. doi: 10.1113/jphysiol.2005.096529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Dacquet C, Mironneau C, Mironneau J. Caffeine-induced inhibition of calcium channel current in cultured smooth cells from pregnant rat myometrium. Br J Pharmacol. 1989;98:493–498. doi: 10.1111/j.1476-5381.1989.tb12622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron JG, Bradley KN, MacMillan D, Muir TC. Sarcolemma agonist-induced interactions between InsP3 and ryanodine receptors in Ca2+ oscillations and waves in smooth muscle. Biochem Soc Trans. 2003;31:920–924. doi: 10.1042/bst0310920. [DOI] [PubMed] [Google Scholar]
- Missiaen L, Declerck I, Droogmans G, Plessers L, De Smedt H, Raeymaekers L, Casteels R. Agonist-dependent Ca2+ and Mn2+ entry dependent on state of filling of Ca2+ stores in aortic smooth muscle cells of the rat. J Physiol. 1990;427:171–186. doi: 10.1113/jphysiol.1990.sp018166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng LC, Wilson SM, Hume JR. Mobilization of sarcoplasmic reticulum stores by hypoxia leads to consequent activation of capacitative Ca2+ entry in isolated canine pulmonary arterial smooth muscle cells. J Physiol. 2005;563:409–419. doi: 10.1113/jphysiol.2004.078311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng LC, Wilson SM, McAllister CE, Hume JR. Role of InsP(3) and ryanodine receptors in the activation of capacitative Ca(2+) entry by store depletion or hypoxia in canine pulmonary arterial smooth muscle cells. Br J Pharmacol. 2007;152:101–111. doi: 10.1038/sj.bjp.0707357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrovskaya O, Goyal R, Osman N, McAllister CE, Pessah IN, Hume JR, Wilson SM. Inhibition of ryanodine receptors by 4-(2-aminopropyl)-3,5-dichloro-N,N-dimethylaniline (FLA 365) in canine pulmonary arterial smooth muscle cells. J Pharmacol Exp Ther. 2007;323:381–390. doi: 10.1124/jpet.107.122119. [DOI] [PubMed] [Google Scholar]
- Parker I, Ivorra I. Caffeine inhibits inositol trisphosphate-mediated liberation of intracellular calcium in Xenopus oocytes. J Physiol. 1991;433:229–240. doi: 10.1113/jphysiol.1991.sp018423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauvert O, Lugnier C, Keravis T, Marthan R, Rousseau E, Savineau JP. Effect of sildenafil on cyclic nucleotide phosphodiesterase activity, vascular tone and calcium signaling in rat pulmonary artery. Br J Pharmacol. 2003;139:513–522. doi: 10.1038/sj.bjp.0705277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauvert O, Bonnet S, Rousseau E, Marthan R, Savineau JP. Sildenafil alters calcium signaling and vascular tone in pulmonary arteries from chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol. 2004;287:L577–L583. doi: 10.1152/ajplung.00449.2003. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. Potentiation and inhibition of Ca(2+) release-activated Ca(2+) channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP(3) receptors. J Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW., Jr Capacitative calcium entry: sensing the calcium stores. J Cell Biol. 2005;169:381–382. doi: 10.1083/jcb.200503161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfurth HW, Haughey NJ, Greenway SC, Yacono PW, Golan DE, Geiger JD. Expression of ryanodine receptors in human embryonic kidney (HEK293) cells. Biochem J. 1998;334:79–86. doi: 10.1042/bj3340079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D, Simeoni I, Micheli M, Bootman M, Lipp P, Allen PD, Sorrentino V. RyR1 and RyR3 isoforms provide distinct intracellular Ca2+ signals in HEK 293 cells. J Cell Sci. 2002;115:2497. doi: 10.1242/jcs.115.12.2497. [DOI] [PubMed] [Google Scholar]
- Smani T, Dominguez-Rodriguez A, Hmadcha A, Calderon-Sanchez E, Horrillo-Ledesma A, Ordonez A. Role of ca2+-independent phospholipase a2 and store-operated pathway in urocortin-induced vasodilatation of rat coronary artery. Circ Res. 2007;101:1194–1203. doi: 10.1161/CIRCRESAHA.107.159053. [DOI] [PubMed] [Google Scholar]
- Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous trp1 decreases capacitative ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L144–L155. doi: 10.1152/ajplung.00412.2001. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen J, Wang Y, Taylor CW, Hirata Y, Hagiwara H, Mikoshiba K, Toyo-oka T, Omata M, Sakaki Y. Crucial role of type 1, but not type 3, inositol 1,4,5-trisphosphate (IP(3)) receptors in IP(3)-induced Ca(2+) release, capacitative Ca(2+) entry, and proliferation of A7r5 vascular smooth muscle cells. Circ Res. 2001;88:202–209. doi: 10.1161/01.res.88.2.202. [DOI] [PubMed] [Google Scholar]
- Waurick R, Knapp J, Van Aken H, Boknik P, Neumann J, Schmitz W. Effect of 2,3-butanedione monoxime on force of contraction and protein phosphorylation in bovine smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:484–492. doi: 10.1007/pl00005380. [DOI] [PubMed] [Google Scholar]
- Wilkins MR, Wharton J, Grimminger F, Ghofrani HA. Phosphodiesterase inhibitors for the treatment of pulmonary hypertension. Eur Respir J. 2008;32:198–209. doi: 10.1183/09031936.00124007. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Lee SC, Shook S, Pappone PA. Atp and beta-adrenergic stimulation enhance voltage-gated k current inactivation in brown adipocytes. Am J Physiol Cell Physiol. 2000;279:C1847–C1858. doi: 10.1152/ajpcell.2000.279.6.C1847. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Mason HS, Ng LC, Montague S, Johnston L, Nicholson N, Mansfield S, Hume JR. Role of basal extracellular Ca(2+) entry during 5-HT-induced vasoconstriction of canine pulmonary arteries. Br J Pharmacol. 2005;144:252–264. doi: 10.1038/sj.bjp.0706077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Mason HS, Smith GD, Nicholson N, Johnston L, Janiak R, Hume JR. Comparative capacitative calcium entry mechanisms in canine pulmonary and renal arterial smooth muscle cells. J Physiol. 2002;543:917–931. doi: 10.1113/jphysiol.2002.021998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Kamimura N, Takeo T, Suga S, Wakui M, Maruyama T, Mikoshiba K. 2-Aminoethoxydiphenyl borate modulates kinetics of intracellular Ca(2+) signals mediated by inositol 1,4,5-trisphosphate-sensitive Ca(2+) stores in single pancreatic acinar cells of mouse. Mol Pharmacol. 2000;58:1368–1374. doi: 10.1124/mol.58.6.1368. [DOI] [PubMed] [Google Scholar]
- Zhang WM, Lin MJ, Sham JS. Endothelin-1 and IP3 Induced Ca2+ Sparks in Pulmonary Arterial Smooth Muscle Cells. J Cardiovasc Pharmacol. 2004;44:S121–S124. doi: 10.1097/01.fjc.0000166226.03712.4f. [DOI] [PubMed] [Google Scholar]
- Zhang WM, Yip KP, Lin MJ, Shimoda LA, Li WH, Sham JS. ET-1 activates Ca2+ sparks in PASMC: local Ca2+ signaling between inositol trisphosphate and ryanodine receptors. Am J Physiol Lung Cell Mol Physiol. 2003;285:L680–L690. doi: 10.1152/ajplung.00067.2003. [DOI] [PubMed] [Google Scholar]
- Zheng YM, Wang QS, Rathore R, Zhang WH, Mazurkiewicz JE, Sorrentino V, Singer HA, Kotlikoff MI, Wang YX. Type-3 ryanodine receptors mediate hypoxia-, but not neurotransmitter-induced calcium release and contraction in pulmonary artery smooth muscle cells. J Gen Physiol. 2005;125:427–440. doi: 10.1085/jgp.200409232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholos AV, Baidan LV, Shuba MF. The inhibitory action of caffeine on calcium currents in isolated intestinal smooth muscle cells. Pflugers Arch. 1991;419:267–273. doi: 10.1007/BF00371106. [DOI] [PubMed] [Google Scholar]
- Zucchi R, Ronca-Testoni S. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: modulation by endogenous effectors, drugs and disease states. Pharmacol Rev. 1997;49:1–51. [PubMed] [Google Scholar]