Abstract
Helicobacter pylori is a Gram-negative bacterium that infects over 50% of the world's population. This organism causes various gastric diseases such as chronic gastritis, peptic ulcer, and gastric cancer. H. pylori possesses lipopolysaccharides that share structural similarity to Lewis blood group antigens in gastric mucosa. Such antigenic mimicry could result in immune tolerance against antigens of this pathogen. On the other hand, H. pylori colonizes gastric mucosa by utilizing adhesins that bind Lewis blood group antigen-related carbohydrates expressed on gastric epithelial cells. After colonization, H. pylori induces acute inflammatory responses mainly by neutrophils. This acute phase is gradually replaced by a chronic inflammatory response. In chronic gastritis, lymphocytes infiltrate the lamina propria, and such infiltration is facilitated by the interaction between L-selectin on lymphocytes and peripheral lymph node addressin (PNAd), which contains 6-sulfo sialyl Lewis X-capped O-glycans, on high endothelial venule (HEV)-like vessels. H. pylori barely colonizes gland mucous cell-derived mucin where α1,4-GlcNAc-capped O-glycans exist. In vitro experiments show that α1,4-GlcNAc-capped O-glycans function as a natural antibiotic to inhibit H. pylori growth. These findings show that distinct sets of carbohydrates expressed in the stomach are closely associated with pathogenesis and prevention of H. pylori-related diseases, providing therapeutic potentialities based on specific carbohydrate modulation.
Keywords: 6-sulfo sialyl Lewis X-capped O-glycan; α1, 4-GlcNAc-capped O-glycan; cholesterol α-glucoside; cholesterol α-glucosyltransferase; Helicobacter pylori
Introduction
Impact of Helicobacter pylori discovery
Spiral microorganisms in the stomach had been observed in the 1930s and 1940s (Doenges 1938; Freedburg and Barron 1940), but little attention was paid to gastric microorganisms. In 1983, Marshall and Warren first isolated and succeeded in culturing the bacterium Helicobacter pylori from the gastric mucosa of patients with chronic gastritis (Warren and Marshall 1983; Marshall and Warren 1984). As a heroic act, Marshall himself drank a culture of H. pylori to prove that the bacteria could infect a healthy person and cause gastritis (Marshall et al. 1985). Their epoch-making discovery revealed that H. pylori is associated with various gastric diseases such as chronic gastritis, peptic ulcer, and malignant tumors including gastric carcinoma and malignant lymphoma, and the eradication of this microorganism likely prevents such gastric disorders (Rauws and Tytgat 1990; Montalban et al. 1995; Fukase et al. 2008). For their achievement, Marshall and Warren won the Nobel Prize in Physiology or Medicine in 2005 (Megraud 2005).
Specialized traits of H. pylori
H. pylori is a spiral-shaped, Gram-negative, and microaerophilic bacterium, measuring approximately 2.5–5.0 μm in length. H. pylori is a member of a genus of bacteria that have adapted to the ecological niche provided by gastric mucus, where there is little competition from other microorganisms (Liu and Crawford 2005). Many specialized traits allow this organism to flourish in the harsh environment of the stomach. First, H. pylori elaborates a large amount of urease which produces ammonia and carbon dioxide resulting from hydrolysis of endogenous urea, thereby buffering gastric acid in the immediate vicinity of the organism. H. pylori also possesses numerous long flagella, and the flailing movements of the flagella allow them to swim through viscous gastric mucus. Finally, H. pylori have adhesins that enhance adhesion with gastric epithelial cells by recognizing specific carbohydrate structures, such as the Lewis b blood group antigen and glycolipids having sialyl dimeric Lewis X (Ilver et al. 1998).
Epidemiology of H. pylori infection
H. pylori infection occurs worldwide and affects over 50% of the world's population, but the prevalence of infection varies greatly from country to country. The overall prevalence is highly correlated with socioeconomic status measured by household crowding and parental income (Hopkins et al. 1990; The EUROGAST Study Group 1993). Prevalence among adults is 70–90% in many developing countries and 25–50% in industrialized countries (Farinha and Gascoyne 2005).
The mode of transmission has not yet been fully defined; however, it is widely believed that the organism is transmitted directly from person to person by premasticated foods (oral–oral spread) or gastric contents (gastric–oral spread) (Dunn et al. 1997). It is now generally accepted that most individuals acquire H. pylori infection in childhood (Kumagai et al. 1998). Once the stomach is colonized by H. pylori and left untreated, the organism persists for decades, if not for a lifetime (Everhart 2000).
H. pylori-associated gastric diseases
Chronic Gastritis
Following H. pylori infection, a chronic, usually lifelong mucosal inflammation (gastritis), develops with concomitant appearance of serological responses against the bacterium. However, H. pylori is resistant to innate and acquired immune responses, and the immune system fails to remove the organism effectively (Sipponen and Hyvarinen 1993). Chronic gastritis leads eventually to mucosal atrophy characterized by a decrease in the proper gastric glands.
Peptic Ulcer
Peptic ulcers are chronic, often solitary lesions that occur in gastroduodenal mucosa exposed to aggressive action of acid-peptic juices. These lesions appear to be produced by an imbalance between mucosal defense mechanisms and damaging forces. The pathogenesis of peptic ulcers appears to be multifactorial, and the apparent role of H. pylori in peptic ulcers cannot be overemphasized. However, H. pylori infection is present in virtually all patients with duodenal ulcers and about 70% of those with gastric ulcers. Furthermore, antibiotic treatment of H. pylori infection promotes healing of ulcers and tends to prevent their recurrence (Liu and Crawford 2005).
Gastric Adenocarcinoma
Gastric adenocarcinoma is the fourth most common cancer and the second leading cause of cancer-related death worldwide (Parkin 2001). Gastric adenocarcinoma can be divided into two distinct histological subtypes (Lauren 1965), each with different epidemiological and clinicopathological features. One subtype is intestinal-type adenocarcinoma, which usually occurs at a later age, and progresses through a relatively well-defined series of histological steps, namely, chronic gastritis (associated with H. pylori infection), atrophy of pyloric glands, intestinal metaplasia, and dysplasia (Sipponen and Marshall 2000). The other subtype is diffuse-type adenocarcinoma, which more commonly affects younger people and is not associated with intestinal metaplasia (Sipponen and Marshall 2000).
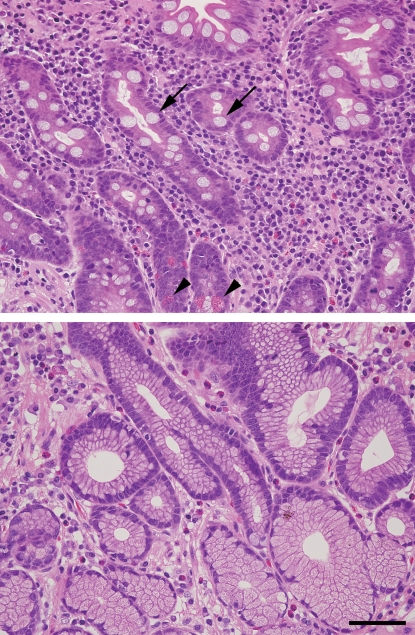
Intestinal metaplasia, which is marked by the replacement of gastric epithelial cells with other epithelial cells such as columnar absorptive cells and goblet cells of intestinal morphology (Sipponen et al. 1985) (Figure 1), has been categorized into two major types: one is the complete type, which is characterized by the presence of absorptive cells, Paneth cells, and goblet cells secreting sialomucins and corresponds to the small intestine phenotype, and the other is the incomplete type, which is characterized by the presence of columnar and goblet cells secreting sialo and/or sulfomucins (Reis et al. 1999). These two types of intestinal metaplasia can also be distinguished by altered mucin expression patterns. While the intestinal mucin MUC2 is expressed in goblet cells of both types of intestinal metaplasia (normal gastric mucosa does not express MUC2 (Reis et al. 1997)), MUC5AC and/or MUC6 are expressed in the incomplete type but not in the complete type (Reis et al. 2000).
Fig. 1.
Photomicrograph of gastric mucosa with (upper panel) or without (lower panel) intestinal metaplasia. (A) Complete-type intestinal metaplasia of gastric epithelial cells observed in chronically inflamed gastric mucosa. Gastric epithelial cells are replaced with absorptive cells, mucin-secreting goblet cells (shown by arrows), and Paneth cells having bright eosinophilic granules (shown by arrowheads). (B) Almost normal gastric epithelial cells in the pyloric gland area without intestinal metaplasia. These were stained by hematoxylin and eosin. Bar = 100 μm.
In spite of the fact that H. pylori has been categorized as a carcinogen, screening for and treatment of infected individuals to prevent gastric adenocarcinoma is not generally accepted (Fock et al. 2008). However, recently, Fukase and colleagues (2008) from the Japan Gast Study Group revealed that eradication of H. pylori reduces the risk of subsequent gastric adenocarcinoma irrespective of age, despite preexisting severe gastric atrophy or intestinal metaplasia. Population screening and treatment should now be pursued by governments in populations at very high risk and by WHO (Talley 2008).
In the course of inflammation to gastric cancer formation, chronic superficial gastritis leads to atrophic gastritis, intestinal metaplasia, dysplasia, and adenocarcinoma. In this sequence, an increased IL-1β secretion caused by inflammatory response facilitates the conversion from superficial gastritis to atrophic gastritis. Further mutation in RAS and loss of deleted in colorectal cancer (DCC) eventually results in gastric adenocarcinoma (Peek and Blaser 2002). As described below, this inflammation–carcinoma sequence is significantly facilitated by the CagA protein and a subtype of VacA. These proteins unique to H. pylori play important roles in gastric carcinoma formation.
MALT Lymphoma
Most lymphomas of the stomach are mucosa-associated lymphoid tissue (MALT) lymphoma, a low-grade B-cell lymphoma. This type of lymphoma arises in MALT, from which the name was derived. B cells that give rise to MALT lymphomas normally reside in the marginal zones of lymphoid follicles and are increased in response to various types of chronic inflammation, including chronic gastritis due to H. pylori infection (Isaacson and Wright 1984). Chronic infection with H. pylori leads to generation of H. pylori-reactive T cells, which, in turn, activate a polyclonal population of B cells by secreting soluble factors (Hussell et al. 1996). In time, a monoclonal but T-cell-dependent population of proliferating B cells emerges. Presumably, such monoclonal B-cell proliferation subsides when the antigenic stimulus for T cells is removed by antibiotic treatment. However, if untreated, genetic mutations accumulate in these proliferating B cells, and they eventually become T cell independent (Kumar et al. 2005).
Virulence factors
CagA
In the industrialized world, 60–70% of H. pylori strains express the cytotoxin-associated antigen A (CagA), a 120– 145 kDa protein (Covacci et al. 1993). The cagA gene is localized at one end of the cag pathogenicity island (cag PAI), a 37 kb genomic fragment containing putative 31 genes (Tomb et al. 1997; Alm et al. 1999). Several of these are homologous to genes encoding the type IV secretion apparatus (Covacci et al. 1999). Upon direct contact of H. pylori with gastric epithelial cells, CagA is injected from the bacterium into the host cell via the type IV secretion system (Segal et al. 1999; Asahi et al. 2000; Odenbreit et al. 2000; Stein et al. 2000). After entering an epithelial cell, CagA is phosphorylated and binds to Src homology 2 domain-containing tyrosine phosphatase 2 (SHP-2), leading to a growth factor-like cellular response and cytokine production (Higashi et al. 2002). Deregulation of SHP-2 by CagA is an important mechanism by which CagA promotes gastric epithelial carcinogenesis (Ohnishi et al. 2008).
Recently, it was reported that CagA-positive strains induce the expression of several genes involved in glycan biosynthesis in gastric epithelial cells, in particular encoding β1,3-N-acetylglucosaminyltransferase 5 (β3GnT5) (Marcos et al. 2008), which is essential for the biosynthesis of Lewis antigens on glycolipids (Togayachi et al. 2001). This induction is dependent on CagA and CagE, most probably through the TNF/NF-κB pathway. The study identified a novel mechanism by which H. pylori modulates the biosynthesis of the sialic acid-binding adhesin (SabA) ligand in gastric epithelial cells, thereby increasing the epithelial attachment necessary to achieve successful colonization. Details of SabA are described below.
VacA
Vacuolating toxin (VacA) is a major virulence factor secreted by H. pylori and is a key component in the pathogenesis of gastric diseases (Cover and Blaser 1992). Approximately 50% of H. pylori strains express the VacA protein, and that expression is correlated with the expression of CagA. The most established activity of VacA is cellular vacuolation in mammalian cells (Catrenich and Chestnut 1992; Cover and Blaser 1992; Papini et al. 1993). Although the precise mechanism of VacA-induced vacuole formation is not fully understood, it involves binding and internalization of toxin. It has been proposed that vacuolation is a consequence of anion-selective channel formation in late endosomal compartments (Czajkowsky et al. 1999; Szabo et al. 1999; Tombola et al. 1999; Papini et al. 2001). In addition to its vacuole formation activity, VacA causes numerous cellular events, including depolarization of the membrane (Szabo et al. 1999; Schraw et al. 2002), apoptosis (Peek et al. 1999; Galmiche et al. 2000; Kuck et al. 2001; Willhite et al. 2003), interference with epithelial cell attachment (Fujikawa et al. 2003), and inhibition of T-lymphocyte activation (Gebert et al. 2003). These effects collectively contributed to the pathogenesis of H. pylori.
Glycoconjugates associated with H. pylori
Putative role of the Lewis blood group antigen in LPS of H. pylori
The cell wall of all Gram-negative bacteria is composed of two phospholipid bilayers with a peptidoglycan layer sandwiched between them. Lipopolysaccharide (LPS) is a structural component of the outer cell wall. LPS is composed of a long-chain fatty acid anchor called lipid A, a core sugar chain, and a variable carbohydrate chain designated O antigen, which is attached to the core sugar (McAdam and Sharpe 2005). Thus, the O antigen has the potential to exhibit enormous structural variability and is the domain determining the serological specificity of LPS (Moran and Prendergast 2001).
Clinical isolates of H. pylori produce the O antigen of a relatively constant chain length (Moran 1995). It is this region of H. pylori LPS that shares structural homology with Lewis blood group antigens in the gastric mucosa, predominantly Lewis X and Lewis Y antigens bearing type 2 blood group determinants. Serologically, 80–90% of H. pylori strains have been found to contain Lewis X and/or Lewis Y epitopes. Lewis blood group antigens are present in normal human gastric mucosa, and the expression of these antigens on H. pylori LPS has important biological implications. Molecular mimicry mediated by H. pylori LPS has been suggested to camouflage the bacterium and facilitate initial colonization (Edwards et al. 2000). However, immunogenicity of LPS is rather weak and a recent report showed that gastric H+, K+-ATPase is a major autoantigen in chronic H. pylori gastritis with body mucosa atrophy (Claeys et al. 1998).
Additionally, H. pylori Lewis antigens undergo phase variation: specifically, random, reversible high-frequency switching of phenotype contributes to virulence. The molecular mechanisms involved in phase variation are slipped-strand mispairing in poly-C tracts and translational frameshifting by ribosomal slippage (Wang et al. 2000). At least five glycosyltransferase genes are involved in generating phase variants: the genes encoding α3-fucosyltransferase (of which there are two similar but nonidentical copies), α2-fucosyltransferase, β3-galactosyltransferase, and β3-N-acetyl-d-glucosaminyltransferase (Appelmelk et al. 2000). Each of these genes can be either “on” or “off,” and thus, in any H. pylori cell population, at least 32 different glycosyltransferase gene “on–off” combinations and potentially the same number of LPS phenotypes are present (Appelmelk et al. 2000). Thus, different H. pylori strains can potentially express different LPS Lewis phenotypes.
This antigenic mimicry may result in immune tolerance against antigens of the pathogen or in the induction of autoantibodies that recognize gastric epithelial cells, which are frequently observed in patients with chronic active gastritis.
Adhesion of H. pylori to gastric epithelial cells
Attachment is a prerequisite for microbial colonization of epithelial surfaces and is mediated by molecules on the bacterial surface, adhesins, which recognize proteins or glycoconjugates on the surface of eukaryotic cells. The specificity of this interaction and the limited distribution of receptors often result in a restricted range of hosts and tissues utilized for colonization, a phenomenon known as tropism. Bacteria, which are unable to adhere to epithelia, tend to be rapidly removed by shedding from surface cells and the mucus layer.
H. pylori expresses adhesins that confer intimate adherence to the gastric epithelium where the bacteria can gain easy access to nutrients from host tissues (Aspholm-Hurtig et al. 2004). These adherence properties protect the bacteria from the extreme acidity of the gastric lumen and displacement from the stomach by forces such as those generated by peristalsis and gastric emptying (Ilver et al. 1998). Two carbohydrate structures in surface mucous cells serve as specific ligands for H. pylori adhesins: Lewis b, which binds to blood group antigen-binding adhesin (BabA), and sialyl dimeric Lewis X-bearing glycosphingolipid, which binds to sialic acid-binding adhesin (SabA). In addition, attachment of H. pylori to gastric epithelial cells can induce pedestal formation (Segal et al. 1996). Pedestal formation describes the creation of an upright support, constructed of host cell material, beneath an attached bacterium.
BabA
The best defined H. pylori adhesin–receptor interaction characterized to date is that between BabA, a member of a family of H. pylori outer membrane proteins, and Lewis b, H, and related ABO antigens (Ilver et al. 1998). These fucose-containing blood group antigens are found on red blood cells and in the gastrointestinal mucosa. Blood group O individuals suffer disproportionately from peptic ulcer disease (Ikehara et al. 2001), suggesting that bacterial adherence to H and Lewis b antigens influences severity of infection. The human population of South American Amerindians dominantly expresses the blood group O antigen. Interestingly, BabA from this population binds the blood group O antigen more efficiently than other blood group antigens (Aspholm-Hurtig et al. 2004). These findings suggest that H. pylori that binds to the blood group O antigen is preferentially increased during infection, and those bacteria gradually dominate among different H. pylori. BabA has two isoforms: babA1 and babA2. The product of the babA1 gene, in contrast to that encoded by the babA2 gene, cannot interact with Lewis b; thus it does not enhance H. pylori colonization of the surface epithelium (Ilver et al. 1998; Gerhard et al. 1999).
SabA
The sabA gene encodes a 651-amino-acid protein of 70 kDa and belongs to the large hop family of H. pylori outer membrane protein genes, which also includes the babA gene (Mahdavi et al. 2002). Sialyl dimeric Lewis X glycolipid is rarely expressed in normal gastric mucosa. However, the gastric mucosa infected by H. pylori, particularly CagA-positive strains, newly expresses this unique glycolipid in surface mucous cells partly facilitated by the increased expression of β3GnT5 (Marcos et al. 2008), which is essential for poly-N-acetyllactosamine synthesis in glycolipids (Togayachi et al. 2001). The adhesion mediated by SabA binding to sialyl dimeric Lewis X glycolipid thus contributes to persistent H. pylori infection established after the initial infection. Since βGnT5 expression levels are increased as inflammation progresses, H. pylori facilitates further infection by increasing ligands for the SabA adhesion molecule. Sialyl dimeric Lewis X is also expressed in leukocytes, but an “on–off” frameshift mutation of the SabA gene allows H. pylori to escape intimate contact with these inflammatory cells. Such adaptive mechanisms play an important role in the extraordinary chronicity of H. pylori infection in human gastric mucosa.
Induction of PNAd in gastric mucosa infected by H. pylori
In chronic inflammatory states, L-selectin and its ligands are implicated in lymphocyte recruitment in those diseases in which peripheral lymph node addressin (PNAd) is induced on high endothelial venule (HEV)-like vessels (von Andrian and Mackay 2000; Rosen 2004). Such HEV-like vessels have been observed in rheumatoid arthritis, lymphocytic thyroiditis, and inflammatory bowel diseases (Duijvestijn et al. 1987; Kabel et al. 1989; van Dinther-Janssen et al. 1990; Salmi et al. 1994; Suzawa et al. 2007). In these studies, the induction of PNAd is detected by the MECA-79 antibody (Streeter et al. 1988), which decorates PNAd on HEV-like vessels. MECA-79-positive HEVs in secondary lymphoid organs play a major role in lymphocyte homing (Rosen 2004). The MECA-79 epitope has been shown to be 6-sulfo N-acetyllactosamine attached to extended core 1 O-glycans, Galβ1→4(SO3→6)GlcNAcβ1→ 3Galβ1→3GalNAcα1→Ser/Thr (Yeh et al. 2001). Moreover, the MECA-79 antibody can also bind to its sialylated and fucosylated form that constitutes PNAd (Yeh et al. 2001). Structural studies also show that 6-sulfo sialyl Lewis X on core 2 branched O-glycans, sialic acidα2→3Galβ1→4[Fucα1→3 (SO3→6)]GlcNAcβ1→6(Galβ1→3)GalNAcα1→Ser/Thr, is present as a major L-selectin ligand on HEVs (Hemmerich et al. 1995; Yeh et al. 2001). This structure is recognized by the NCC-ST-439 antibody (Kumamoto et al. 1998; Kobayashi et al. 2004).
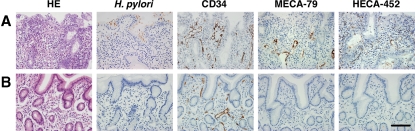
In H. pylori-infected gastric mucosa, HEV-like vessels can be stained by MECA-79 and NCC-ST-439 antibodies. This indicates that PNAd in gastric mucosa formed by H. pylori infection is similar, if not identical, to PNAd present in secondary lymphoid organs, which is formed under normal condition (Yeh et al. 2001). The number of HEV-like vessels, as detected by MECA-79 and HECA-452 antibodies, correlates positive progression of chronic inflammation in H. pylori-infected gastric mucosa. Surprisingly, the gastric mucosa no longer displayed HEV-like vessels after the eradication of H. pylori (Kobayashi et al. 2004) (Figure 2). These findings indicate that the infection by and the presence of H. pylori is a primary cause to induce and maintain PNAd on HEV-like vessels in infected gastric mucosa, thereby facilitating inflammatory response. Recently, it was reported that PNAd presented by N-glycans is almost as an efficient ligand as that on O-glycans (Mitoma et al. 2007). These findings suggest that PNAd carried by N-glycans on HEV-like vessels also functions in facilitating inflammatory response in H. pylori-infected gastric mucosa.
Fig. 2.
Disappearance of HEV-like vessels in the gastric mucosa after the eradication of H. pylori. Gastric mucosa infected with H. pylori was examined before and 2 months after treatment to eradicate H. pylori. (A) Before treatment, HEV-like vessels detected by MECA-79 and HECA-452 antibodies were abundant, and large numbers of mononuclear cells (lymphocytes) were present around these vessels. (B) After the eradication of H. pylori, HEV-like vessels were no longer present and very few mononuclear cells were present. CD34 was used for a marker of vascular endothelial cells. HE, hematoxylin and eosin, Bar = 100 μm. Adapted with permission from Kobayashi et al. (2004).
It is tempting to speculate that bacterial components such as LPS acting through Toll-like receptor-dependent pathways in the gastric epithelium stimulate the release of cytokines, i.e., lymphotoxin α (Pablos et al. 2005). This effect might in turn modulate gene expression in postcapillary venules in a way that could cause their biochemical, functional, and morphological transformation by upregulating chemokines, such as CCL19 and CCL21 that act on CCR7 receptors (Drayton et al. 2003).
Roles of gastric mucin in H. pylori infection
Gastric mucins are classified into two types based upon their histochemical properties (Ota et al. 1991): one is a surface mucous cell-derived mucin displayed on the MUC5AC core protein (Reis et al. 1999) and the other is a mucin displayed on the MUC6 core protein secreted by gland mucous cells, including cardiac gland cells, mucous neck cells, and pyloric gland cells (Reis et al. 2000). These two mucins form the surface mucus gel layer (SMGL), which shows an alternately laminated array. The thicker layer mostly consists of MUC5AC and MUC6 (Ho et al. 2004). However, the most recent report showed that MUC6 is negligible in SMGL (Phillipson et al. 2008), in supporting the conclusion that SMGL mostly consists of MUC5AC.
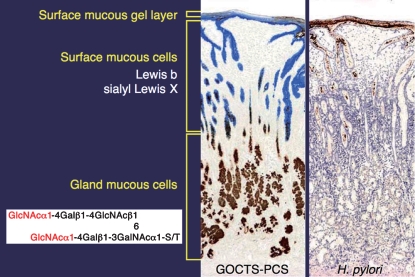
H. pylori is exclusively associated with surface mucous cell-derived mucins and rarely colonizes deeper portions (Figure 3). H. pylori density correlates with the Lewis b antigen that is presented on MUC5AC glycoproteins (Van de Bovenkamp et al. 2003). As MUC5AC constitutes a major component of surface mucosa mucins (Nordman et al. 2002), H. pylori mostly resides in surface mucosa.
Fig. 3.
Two distinct mucins present in gastric mucosa. Surface mucous cell-derived mucins containing Lewis b and sialyl Lewis X (in blue), and gland mucous cell-derived mucins containing α1.4-GlcNAc-capped O-glycans (in brown) can be distinguished by galactose oxidase-cold thionin Schiff-paradoxical concanavalin A staining (GOCTS-PCS) (left panel). H. pylori in brown in the right panel is almost exclusively present in the surface mucous-cell derived mucin.
In contrast to MUC5AC, MUC6 is expressed in deeper portion of the mucosa, and its mucin-type O-glycans are capped by α1,4-linked N-acetylglucosamine (α1,4-GlcNAc) (Nakayama et al. 1999). MUC6 rather excludes the colonization of H. pylori.
These findings suggest that mucin-type O-glycans on MUC6 may inhibit H. pylori growth. Indeed, H. pylori growth was inhibited by the presence of mucin proteins such as CD43, which express α1,4-GlcNAc-capped O-glycans with the concomitant decrease in cholesterol α-glucoside in H. pylori cell wall (Kawakubo et al. 2004). Cholesterol α-glucoside and its derivatives, synthesized by cholesterol α-glucosyltransferase, constitute 25% of H. pylori cell wall lipids (Hirai et al. 1995; Haque et al. 1996). Morphological abnormality of H. pylori after incubation with those recombinant mucin proteins expressing α1,4-GlcNAc-capped O-glycans may be due to the decrease in cholesterol α-glucoside.
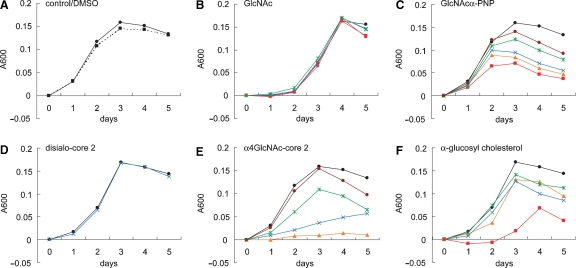
Significantly, the decrease in cholesterol α-glucoside either by inactivation of the enzyme or increase in cholesterol resulted in higher susceptibility to macrophage and increased response by T lymphocytes (Wunder et al. 2006). Similarly, synthetic oligosaccharides containing the α1,4-GlcNAc-capping structure inhibit in vitro activity of cholesterol α-glucosyltransferase from H. pylori (Lee et al. 2006) and H. pylori growth (Lee et al. 2008) (Figure 4). In parallel to these findings, porcine mucin inhibits H. pylori growth most likely due to the binding of H. pylori to the mucin, which expresses blood group antigens (Gustafsson et al. 2006). It is also possible that this inhibition shares a similar or the same mechanism where mucins containing the α1,4-GlcNAc-capping structure inhibit H. pylori growth. The mechanism how mucins expressing α1,4-GlcNAc-capping structures can inhibit H. pylori growth needs to be elucidated. In a separate study, H. pylori infection was treated with sialic acidα2→ 3Galβ1→4Glc (Miller-Podraza et al. 2005) with a modest success (Mysore et al. 1999). As this oligosaccharide inhibits H. pylori adhesion mostly in gastritis patients, Lewis b-containing glycans may be useful in preventing BabA-mediated adhesion that also functions in early stages of H. pylori infection.
Fig. 4.
Inhibition of H. pylori growth by synthetic oligosaccharides and monosaccharides. H. pylori was cultured for 5 days in Mueller–Hinton broth supplemented with 5.5% horse serum containing various amounts of synthetic oligosaccharides and monosaccharides. Bacterial growth was measured at O.D. 600 nm, and the absorbance for control experiments at time 0 was subtracted from absorbance at later time points. Oligosaccharide and monosaccharide concentrations are 1 mM (red), 0.75 mM (orange), 0.5 mM (blue), 0.25 mM (green), 0.125 mM (brown), and control (closed circle). Two mM GlcNAc were also added in B (magenta). Oligosaccharides and monosaccharides were initially dissolved in dimethyl sulfoxide (DMSO), and the final DMSO concentration in the culture medium was 1%. The growth curve in the absence of DMSO is shown as a dotted line (A) Adapted from Lee et al. (2008).
These findings would point to a possibility that α1,4-GlcNAc-capped O-glycans may be useful for treatment of H. pylori infection. This possibility becomes more reasonable if oligosaccharides with multiple α1,4-GlcNAc residues are synthesized. However, the effective concentration of inhibitory oligosaccharide containing a monovalent α1,4-GlcNAc residue is very high (∼0.5 mM) (Lee et al. 2008), and it is likely that the oligosaccharide approach may not be practical. On the other hand, the inhibition of cholesterol α-glucosyltransferase should lead to increased susceptibility to immune response by innate immunity and T-cell response. Identifying the cholesterol α-glucosyltransferase inhibitor of low molecular weight is thus an important task yet to be explored.
Conclusion
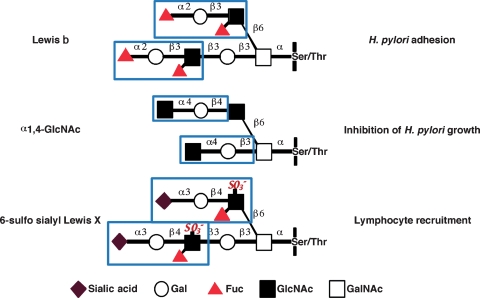
In this review, we have shown that a battery of O-glycans such as 6-sulfo sialyl Lewis X and α1,4-GlcNAc-capped O-glycans expressed in the HEV-like vessels and gland mucous cells, respectively, plays pivotal roles on pathogenesis of chronic active gastritis and on protection of the gastric mucosa from H. pylori, respectively (Figure 5). Moreover, Lewis b and sialyl dimeric Lewis X on mucus cells serve as counterreceptors for adhesins coded by BabA and SabA, respectively. In the healthy gastric mucus layer, BabA and acid charge affect binding to mucin, while in gastritis patients, BabA/Lewis b-dependent binding to MUC5AC remains, but SabA and low pH binding increases (Mahdavi et al. 2002; Linden et al. 2008). After H. pylori established its infection, the inflammatory response to H. pylori infection leads to the formation of PNAd in HEV-like vessels, which facilitate lymphocyte recruitment to inflammatory sites, enhancing inflammatory response. These findings demonstrate that distinct sets of carbohydrates play critical roles in adhesion of H. pylori to epithelial cells (Lewis b, blood group O antigens, sialyl dimeric Lewis X), attenuation of H. pylori colonization (α1,4-GlcNAc-capped O-glycans), and facilitation of inflammatory response following H. pylori infection (6-sulfo sialyl Lewis X). These discoveries allow us to not only understand the pathogenesis of H. pylori-associated diseases but also to develop new therapy or prevention toward H. pylori infection by inhibiting these enzymes that are important for infection and colonization of H. pylori.
Fig. 5.
Carbohydrates critical for H. pylori infection and pathogenesis. Distinct sets of carbohydrates on gastric mucosa play critical roles in H. pylori adhesion, inhibition of H. pylori growth, and recruitment of lymphocytes and facilitation of inflammatory response following H. pylori infection. BabA adhesin on H. pylori binds to Lewis b blood group antigen, thus facilitating H. pylori colonization (Ilver et al. 1998). This colonization is counteracted by α1,4-GlcNAc-capped O-glycans present in the deeper portion of gastric mucosa (Kawakubo et al. 2004). Once H. pylori infects the stomach a series of inflammatory responses is initiated, and this response is facilitated by the de novo expression of 6-sulfo sialyl Lewis X on HEV-like vessels that recruit lymphocytes to inflammatory sites (Kobayashi et al. 2004).
Funding
National Cancer Institute; the National Institutes of Health (CA33000 and CA71932 to M.F.); and the Ministry of Education, Culture, Sports, Science and Technology of Japan (14082201 to J.N. and B-18790240 to M.K.).
Acknowledgments
We thank the members of the Fukuda's, Nakayama's, and Peter Seeberger's laboratories for their critical contribution to the studies and useful discussion. We also thank Dr. Elise Lamar for critical reading of the manuscript and Aleli Morse for organizing the manuscript.
Glossary
Abbreviations
- β3GnT5
β1,3-N-acetylglucosaminyltransferase 5
- BabA
blood group antigen-binding adhesin
- CagA
cytotoxin-associated antigen A
- DCC
deleted in colorectal cancer
- HEV
high endothelial venule
- LPS
lipopolysaccharide
- MALT
mucosa-associated lymphoid tissue
- PNAd
peripheral lymph node addressin
- SabA
sialic acid-binding adhesin
- SHP-2
Src homology 2 domain-containing tyrosine phosphatase 2
- SMGL
surface mucous gel layer
- VacA
vacuolating toxin
Conflict of interest statement
None declared.
References
- Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, deJonge BL, et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- Appelmelk BJ, Monteiro MA, Martin SL, Moran AP, Vandenbroucke- Grauls CM. Why Helicobacter pylori has Lewis antigens? Trends Microbiol. 2000;8:565–570. doi: 10.1016/s0966-842x(00)01875-8. [DOI] [PubMed] [Google Scholar]
- Asahi M, Azuma T, Ito S, Ito Y, Suto H, Nagai Y, Tsubokawa M, Tohyama Y, Maeda S, Omata M, et al. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J Exp Med. 2000;191:593–602. doi: 10.1084/jem.191.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspholm-Hurtig M, Dailide G, Lahmann M, Kalia A, Ilver D, Roche N, Vikstrom S, Sjostrom R, Linden S, Backstrom A, et al. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science. 2004;305:519–522. doi: 10.1126/science.1098801. [DOI] [PubMed] [Google Scholar]
- Catrenich CE, Chestnut MH. Character and origin of vacuoles induced in mammalian cells by the cytotoxin of Helicobacter pylori. J Med Microbiol. 1992;37:389–395. doi: 10.1099/00222615-37-6-389. [DOI] [PubMed] [Google Scholar]
- Claeys D, Faller G, Appelmelk BJ, Negrini R, Kirchner T. The gastric H+,K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology. 1998;115:340–347. doi: 10.1016/s0016-5085(98)70200-8. [DOI] [PubMed] [Google Scholar]
- Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N, et al. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covacci A, Telford JL, Giudice GD, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- Cover TL, Blaser MJ. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267:10570–10575. [PubMed] [Google Scholar]
- Czajkowsky DM, Iwamoto H, Cover TL, Shao Z. The vacuolating toxin from Helicobacter pylori forms hexameric pores in lipid bilayers at low pH. Proc Natl Acad Sci USA. 1999;96:2001–2006. doi: 10.1073/pnas.96.5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doenges JL. Spirochaetes in gastric glands of Macacus rhesus and humans without definite history of related disease. Proc Soc Exp Biol Med. 1938;38:536–538. [Google Scholar]
- Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH. Ectopic LTαβ directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med. 2003;197:1153–1163. doi: 10.1084/jem.20021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijvestijn AM, Kerkhove M, Bargatze RF, Butcher EC. Lymphoid tissue- and inflammation-specific endothelial cell differentiation defined by monoclonal antibodies. J Immunol. 1987;138:713–719. [PubMed] [Google Scholar]
- Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards NJ, Monteiro MA, Faller G, Walsh EJ, Moran AP, Roberts IS, High NJ. Lewis X structures in the O antigen side-chain promote adhesion of Helicobacter pylori to the gastric epithelium. Mol Microbiol. 2000;35:1530–1539. doi: 10.1046/j.1365-2958.2000.01823.x. [DOI] [PubMed] [Google Scholar]
- Everhart JE. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin North Am. 2000;29:559–578. doi: 10.1016/s0889-8553(05)70130-8. [DOI] [PubMed] [Google Scholar]
- Farinha P, Gascoyne RD. Helicobacter pylori and MALT lymphoma. Gastroenterology. 2005;128:1579–1605. doi: 10.1053/j.gastro.2005.03.083. [DOI] [PubMed] [Google Scholar]
- Fock KM, Talley NJ, Moayyedi P, Hunt R, Azuma T, Sugano K, Xiao SD, Lam SK, Goh KL, Chiba T, et al. Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol. 2008;23:351–365. doi: 10.1111/j.1440-1746.2008.05314.x. [DOI] [PubMed] [Google Scholar]
- Freedburg AS, Barron LE. The presence of spirochaetes in human gastric mucosa. Am J Dig Dis. 1940;7:443–445. [Google Scholar]
- Fujikawa A, Shirasaka D, Yamamoto S, Ota H, Yahiro K, Fukada M, Shintani T, Wada A, Aoyama N, Hirayama T, et al. Mice deficient in protein tyrosine phosphatase receptor type Z are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nat Genet. 2003;33:375–381. doi: 10.1038/ng1112. [DOI] [PubMed] [Google Scholar]
- Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An open-label, randomized controlled trial. Lancet. 2008;372:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- Galmiche A, Rassow J, Doye A, Cagnol S, Chambard JC, Contamin S, de Thillot V, Just I, Ricci V, Solcia E, et al. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 2000;19:6361–6370. doi: 10.1093/emboj/19.23.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebert B, Fischer W, Weiss E, Hoffmann R, Haas R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science. 2003;301:1099–1102. doi: 10.1126/science.1086871. [DOI] [PubMed] [Google Scholar]
- Gerhard M, Lehn N, Neumayer N, Boren T, Rad R, Schepp W, Miehlke S, Classen M, Prinz C. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci USA. 1999;96:12778–12783. doi: 10.1073/pnas.96.22.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson A, Hultberg A, Sjostrom R, Kacskovics I, Breimer ME, Boren T, Hammarstrom L, Holgersson J. Carbohydrate-dependent inhibition of Helicobacter pylori colonization using porcine milk. Glycobiology. 2006;16:1–10. doi: 10.1093/glycob/cwj031. [DOI] [PubMed] [Google Scholar]
- Haque M, Hirai Y, Yokota K, Mori N, Jahan I, Ito H, Hotta H, Yano I, Kanemasa Y, Oguma K. Lipid profile of Helicobacter spp.: Presence of cholesteryl glucoside as a characteristic feature. J Bacteriol. 1996;178:2065–2070. doi: 10.1128/jb.178.7.2065-2070.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerich S, Leffler H, Rosen SD. Structure of the O-glycans in GlyCAM-1, an endothelial-derived ligand for l-selectin. J Biol Chem. 1995;270:12035–12047. doi: 10.1074/jbc.270.20.12035. [DOI] [PubMed] [Google Scholar]
- Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, Hatakeyama M. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295:683–686. doi: 10.1126/science.1067147. [DOI] [PubMed] [Google Scholar]
- Hirai Y, Haque M, Yoshida T, Yokota K, Yasuda T, Oguma K. Unique cholesteryl glucosides in Helicobacter pylori: Composition and structural analysis. J Bacteriol. 1995;177:5327–5333. doi: 10.1128/jb.177.18.5327-5333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SB, Takamura K, Anway R, Shekels LL, Toribara NW, Ota H. The adherent gastric mucous layer is composed of alternating layers of MUC5AC and MUC6 mucin proteins. Digest Dis Sci. 2004;49:1598–1606. doi: 10.1023/b:ddas.0000043371.12671.98. [DOI] [PubMed] [Google Scholar]
- Hopkins RJ, Russell RG, O’Donnoghue JM, Wasserman SS, Lefkowitz A, Morris JG Jr. 7th ed. Seroprevalence of Helicobacter pylori in Seventh-Day Adventists and other groups in Maryland. Lack of association with diet. Arch Intern Med. 1990;150:2347–2348. [PubMed] [Google Scholar]
- Hussell T, Isaacson PG, Crabtree JE, Spencer J. Helicobacter pylori-specific tumour-infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. J Pathol. 1996;178:111–112. doi: 10.1002/(SICI)1096-9896(199602)178:2<122::AID-PATH486>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Ikehara Y, Nishihara S, Yasutomi H, Kitamura T, Matsuo K, Shimizu N, Inada K, Kodera Y, Yamamura Y, Narimatsu H, et al. Polymorphisms of two fucosyltransferase genes (Lewis and Secretor genes) involving type I Lewis antigens are associated with the presence of anti-Helicobacter pylori IgG antibody. Cancer Epidemiol Biomarkers Prev. 2001;10:971–977. [PubMed] [Google Scholar]
- Ilver D, Arnqvist A, Ogren J, Frick I, Kersulyte D, Incecik ET, Berg DE, Covacci A, Engstrand L, Boren T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- Isaacson P, Wright DH. Extranodal malignant lymphoma arising from mucosa-associated lymphoid tissue. Cancer. 1984;53:2515–2524. doi: 10.1002/1097-0142(19840601)53:11<2515::aid-cncr2820531125>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Kabel PJ, Voorbij HA, de Haan-Meulman M, Pals ST, Drexhage HA. High endothelial venules present in lymphoid cell accumulations in thyroids affected by autoimmune disease: A study in men and BB rats of functional activity and development. Clin Endocrinol Metab. 1989;68:744–751. doi: 10.1210/jcem-68-4-744. [DOI] [PubMed] [Google Scholar]
- Kawakubo M, Ito Y, Okimura Y, Kobayashi M, Sakura K, Kasama S, Fukuda MN, Fukuda M, Katsuyama T, Nakayama J. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science. 2004;305:1003–1006. doi: 10.1126/science.1099250. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Mitoma J, Nakamura N, Katsuyama T, Nakayama J, Fukuda M. Induction of peripheral lymph node addressin in human gastric mucosa infected by Helicobacter pylori. Proc Natl Acad Sci USA. 2004;101:17807–17812. doi: 10.1073/pnas.0407503101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuck D, Kolmerer B, Iking-Konert C, Krammer PH, Stremmel W, Rudi J. Vacuolating cytotoxin of Helicobacter pylori induces apoptosis in the human gastric epithelial cell line AGS. Infect Immun. 2001;69:5080–5087. doi: 10.1128/IAI.69.8.5080-5087.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai T, Malaty HM, Graham DY, Hosogaya S, Misawa K, Furihata K, Ota H, Sei C, Tanaka E, Akamatsu T, et al. Acquisition versus loss of Helicobacter pylori infection in Japan: Results from an 8-year birth cohort study. J Infect Dis. 1998;178:717–721. doi: 10.1086/515376. [DOI] [PubMed] [Google Scholar]
- Kumamoto K, Mitsuoka C, Izawa M, Kimura N, Otsubo N, Ishida H, Kiso M, Yamada T, Hirohashi S, Kannagi R. Specific detection of sialyl Lewis X determinant carried on the mucin GlcNAcβ1→6GalNAcα core structure as a tumor-associated antigen. Biochem Biophys Res Commun. 1998;247:514–517. doi: 10.1006/bbrc.1998.8824. [DOI] [PubMed] [Google Scholar]
- Kumar V, Abbas A, Fausto N, editors. 7th ed. Philadelphia: Elsevier Saunders; 2005. Robbins and Cortran Pathologic Basis of Disease; pp. 269–342. [Google Scholar]
- Lauren P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- Lee H, Kobayashi M, Wang P, Nakayama J, Seeberger PH, Fukuda M. Expression cloning of cholesterol α-glucosyltransferase, a unique enzyme that can be inhibited by natural antibiotic gastric mucin O-glycans, from Helicobacter pylori. Biochem Biophys Res Commun. 2006;349:1235–1241. doi: 10.1016/j.bbrc.2006.08.145. [DOI] [PubMed] [Google Scholar]
- Lee H, Wang P, Hoshino H, Ito Y, Kobayashi M, Nakayama J, Seeberger PH, Fukuda M. α1,4GlcNAc-capped mucin-type O-glycan inhibits cholesterol α-glucosyltransferase from Helicobacter pylori and suppresses H. pylori growth. Glycobiology. 2008;18:549–558. doi: 10.1093/glycob/cwn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden SK, Wickstrom C, Lindell G, Gilshenan K, Carlstedt I. Four modes of adhesion are used during Helicobacter pylori binding to human mucins in the oral and gastric niches. Helicobacter. 2008;13:81–93. doi: 10.1111/j.1523-5378.2008.00587.x. [DOI] [PubMed] [Google Scholar]
- Liu C, Crawford JM. The gastrointestinal tract. In: Kumar V, Abbas AK, Fausto N, editors. 7th ed. Philadelphia: Elsevier Saunders; 2005. pp. 797–875. Robbins and Cortran Pathologic Basis of Disease. [Google Scholar]
- Mahdavi J, Sonden B, Hurtig M, Olfat FO, Forsberg L, Roche N, Angstrom J, Larsson T, Teneberg S, Karlsson KA, et al. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297:573–578. doi: 10.1126/science.1069076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos NT, Magalhaes A, Ferreira B, Oliveira MJ, Carvalho AS, Mendes N, Gilmartin T, Head SR, Figueiredo C, David L, et al. Helicobacter pylori induces β3GnT5 in human gastric cell lines, modulating expression of the SabA ligand sialyl-Lewis x. J Clin Invest. 2008;118:2325–2336. doi: 10.1172/JCI34324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall BJ, Armstrong JA, McGechie DB, Glancy RJ. Attempt to fulfil Koch's postulates for pyloric Campylobacter. Med J Aust. 1985;142:436–439. doi: 10.5694/j.1326-5377.1985.tb113443.x. [DOI] [PubMed] [Google Scholar]
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;i:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- McAdam AJ, Sharpe AH. Infectious diseases. In: Kumar V, Abbas AK, Fausto N, editors. 7th ed. Philadelphia: Elsevier Saunders; 2005. pp. 343–414. Robbins and Cortran Pathologic Basis of Disease. [Google Scholar]
- Megraud F. A humble bacterium sweeps this year's Nobel Prize. Cell. 2005;123:975–976. doi: 10.1016/j.cell.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Miller-Podraza H, Lanne B, Angstrom J, Teneberg S, Milh MA, Jovall PA, Karlsson H, Karlsson KA. Novel binding epitope for Helicobacter pylori found in neolacto carbohydrate chains: Structure and cross-binding properties. J Biol Chem. 2005;280:19695–19703. doi: 10.1074/jbc.M412688200. [DOI] [PubMed] [Google Scholar]
- Mitoma J, Bao X, Petryanik B, Schaerli P, Gauguet JM, Yu SY, Kawashima H, Saito H, Ohtsubo K, Marth JD, et al. Critical functions of N-glycans in l-selectin-mediated lymphocyte homing and recruitment. Nat Immunol. 2007;8:409–418. doi: 10.1038/ni1442. [DOI] [PubMed] [Google Scholar]
- Montalban C, Manzanal A, Boixeda D, Redondo C, Bellas C. Treatment of low-grade gastric MALT lymphoma with Helicobacter pylori eradication. Lancet. 1995;345:798–799. doi: 10.1016/s0140-6736(95)90679-7. [DOI] [PubMed] [Google Scholar]
- Moran AP. Cell surface characteristics of Helicobacter pylori. FEMS Immunol Med Microbiol. 1995;10:271–280. doi: 10.1111/j.1574-695X.1995.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Moran AP, Prendergast MM. Molecular mimicry in Campylobacter jejuni and Helicobacter pylori lipopolysaccharides: Contribution of gastrointestinal infections to autoimmunity. J Autoimmun. 2001;16:241–256. doi: 10.1006/jaut.2000.0490. [DOI] [PubMed] [Google Scholar]
- Mysore JV, Wigginton T, Simon PM, Zopf D, Heman-Ackah LM, Dubois A. Treatment of Helicobacter pylori infection in rhesus monkeys using a novel antiadhesion compound. Gastroenterology. 1999;117:1316–1325. doi: 10.1016/s0016-5085(99)70282-9. [DOI] [PubMed] [Google Scholar]
- Nakayama J, Yeh JC, Misra AK, Ito S, Katsuyama T, Fukuda M. Expression cloning of a human α1,4-N-acetylglucosaminyltransferase that forms GlcNAcα1→4Galβ→R, a glycan specifically expressed in the gastric gland mucous cell-type mucin. Proc Natl Acad Sci USA. 1999;96:8991–8996. doi: 10.1073/pnas.96.16.8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordman H, Davies JR, Lindell G, de Bolos C, Real F, Carlstedt I. Gastric MUC5AC and MUC6 are large oligomeric mucins that differ in size, glycosylation and tissue distribution. Biochem J. 2002;364:191–200. doi: 10.1042/bj3640191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenbreit S, Puls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- Ohnishi N, Yuasa H, Tanaka S, Sawa H, Miura M, Matsui A, Higashi H, Musashi M, Iwabuchi K, Suzuki M, et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci USA. 2008;105:1003–1008. doi: 10.1073/pnas.0711183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota H, Katsuyama T, Ishii K, Nakayama J, Shiozawa T, Tsukahara Y. A dual staining method for identifying mucins of different gastric epithelial mucous cells. Histochem J. 1991;23:22–28. doi: 10.1007/BF01886504. [DOI] [PubMed] [Google Scholar]
- Pablos JL, Santiago B, Tsay D, Singer MS, Palao G, Galindo M, Rosen SD. A HEV-restricted sulfotransferase is expressed in rheumatoid arthritis synovium and is induced by lymphotoxin-α/β and TNF-α in cultured endothelial cells. BMC Immunol. 2005;6:6. doi: 10.1186/1471-2172-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papini E, Bugnoli M, de Bernard M, Figura N, Rappuoli R, Montecucco C. Bafilomycin A1 inhibits Helicobacter pylori-induced vacuolization of HeLa cells. Mol Microbiol. 1993;7:323–327. doi: 10.1111/j.1365-2958.1993.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Papini E, Zoratti M, Cover TL. In search of the Helicobacter pylori VacA mechanism of action. Toxicon. 2001;39:1757–1767. doi: 10.1016/s0041-0101(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- Peek RM, Jr, Blaser MJ, Mays DJ, Forsyth MH, Cover TL, Song SY, Krishna U, Pietenpol JA. Helicobacter pylori strain-specific genotypes and modulation of gastric epithelial cell cycle. Cancer Res. 1999;59:6124–6131. [PubMed] [Google Scholar]
- Phillipson M, Johansson ME, Henriksnas J, Petersson J, Gendler SJ, Sandler S, Persson AE, Hansson GC, Holm L. The gastric mucus layers: Constituents and regulation of accumulation. Am J Physiol Gastrointest Liver Physiol. 2008;295:G806–G812. doi: 10.1152/ajpgi.90252.2008. [DOI] [PubMed] [Google Scholar]
- Rauws EA, Tytgat GN. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet. 1990;335:1233–1235. doi: 10.1016/0140-6736(90)91301-p. [DOI] [PubMed] [Google Scholar]
- Reis CA, David L, Carvalho F, Mandel U, de Bolos C, Mirgorodskaya E, Clausen H, Sobrinho-Simoes M. Immunohistochemical study of the expression of MUC6 mucin and co-expression of other secreted mucins (MUC5AC and MUC2) in human gastric carcinomas. J Histochem Cytochem. 2000;48:377–388. doi: 10.1177/002215540004800307. [DOI] [PubMed] [Google Scholar]
- Reis CA, David L, Correa P, Carneiro F, de Bolos C, Garcia E, Mandel U, Clausen H, Sobrinho-Simoes M. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res. 1999;59:1003–1007. [PubMed] [Google Scholar]
- Reis CA, David L, Nielsen PA, Clausen H, Mirgorodskaya K, Roepstorff P, Sobrinho-Simoes M. Immunohistochemical study of MUC5AC expression in human gastric carcinomas using a novel monoclonal antibody. Int J Cancer. 1997;20:112–121. doi: 10.1002/(sici)1097-0215(19970220)74:1<112::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Rosen SD. Ligand for l-selectin: Homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- Salmi M, Granfors K, MacDermott R, Jalkanen S. Aberrant binding of lamina propria lymphocytes to vascular endothelium in inflammatory bowel diseases. Gastroenterology. 1994;106:596–605. doi: 10.1016/0016-5085(94)90691-2. [DOI] [PubMed] [Google Scholar]
- Schraw W, Li Y, McClain MS, Van Der Goot FG, Cover TL. Association of Helicobacter pylori vacuolating toxin (VacA) with lipid rafts. J Biol Chem. 2002;277:34642–34650. doi: 10.1074/jbc.M203466200. [DOI] [PubMed] [Google Scholar]
- Segal ED, Cha J, Lo J, Falkow S, Tompkins LS. Altered states: Involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc Natl Acad Sci USA. 1999;96:14559–14564. doi: 10.1073/pnas.96.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal ED, Falkow S, Tompkins LS. Helicobacter pylori attachment to gastric cells induces cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins. Proc Natl Acad Sci USA. 1996;93:1259–1264. doi: 10.1073/pnas.93.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipponen P, Hyvarinen H. Role of Helicobacter pylori in the pathogenesis of gastritis, peptic ulcer and gastric cancer. Scand J Gastroenterol Suppl. 1993;196:3–6. doi: 10.3109/00365529309098333. [DOI] [PubMed] [Google Scholar]
- Sipponen P, Kekki M, Haapakoski J, Ihamaki T, Siurala M. Gastric cancer risk in chronic atrophic gastritis: Statistical calculations of cross-sectional data. Int J Cancer. 1985;35:173–178. doi: 10.1002/ijc.2910350206. [DOI] [PubMed] [Google Scholar]
- Sipponen P, Marshall BJ. Gastritis and gastric cancer. Western countries. Gastroenterol Clin North Am. 2000;29:579–592. doi: 10.1016/s0889-8553(05)70131-x. [DOI] [PubMed] [Google Scholar]
- Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci USA. 2000;97:1263–1268. doi: 10.1073/pnas.97.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter PR, Rouse BT, Butcher EC. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzawa K, Kobayashi M, Sakai Y, Hoshino H, Watanabe M, Harada O, Ohtani H, Fukuda M, Nakayama J. Preferential induction of peripheral lymph node addressin on high endothelial venule-like vessels in the active phase of ulcerative colitis. Am J Gastroenterol. 2007;102:1499–1509. doi: 10.1111/j.1572-0241.2007.01189.x. [DOI] [PubMed] [Google Scholar]
- Szabo I, Brutsche S, Tombola F, Moschioni M, Satin B, Telford JL, Rappuoli R, Montecucco C, Papini E, Zoratti M. Formation of anion-selective channels in the cell plasma membrane by the toxin VacA of Helicobacter pylori is required for its biological activity. EMBO J. 1999;18:5517–5527. doi: 10.1093/emboj/18.20.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley NJ. Is it time to screen and treat H. pylori to prevent gastric cancer? Lancet. 2008;372:350–352. doi: 10.1016/S0140-6736(08)61136-8. [DOI] [PubMed] [Google Scholar]
- The EUROGAST Study Group Epidemiology of, and risk factors for, Helicobacter pylori infection among 3194 asymptomatic subjects in 17 populations. Gut. 1993;34:1672–1676. doi: 10.1136/gut.34.12.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togayachi A, Akashima T, Ookubo R, Kudo T, Nishihara S, Iwasaki H, Natsume A, Mio H, Inokuchi J, Irimura T, et al. Molecular cloning and characterization of UDP-GlcNAc:lactosylceramide β1,3-N-acetylglucosaminyltransferase (β3Gn-T5), an essential enzyme for the expression of HNK-1 and Lewis X epitopes on glycolipids. J Biol Chem. 2001;276:22032–22040. doi: 10.1074/jbc.M011369200. [DOI] [PubMed] [Google Scholar]
- Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- Tombola F, Carlesso C, Szabo I, de Bernard M, Reyrat JM, Telford JL, Rappuoli R, Montecucco C, Papini E, Zoratti M. Helicobacter pylori vacuolating toxin forms anion-selective channels in planar lipid bilayers: Possible implications for the mechanism of cellular vacuolation. Biophys J. 1999;76:1401–1409. doi: 10.1016/S0006-3495(99)77301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Bovenkamp JH, Mahdavi J, Korteland-Van Male AM, Buller HA, Einerhand AW, Boren T, Dekker J. The MUC5AC glycoprotein is the primary receptor for Helicobacter pylori in the human stomach. Helicobacter. 2003;8:521–532. doi: 10.1046/j.1523-5378.2003.00173.x. [DOI] [PubMed] [Google Scholar]
- van Dinther-Janssen AC, Pals ST, Scheper R, Breedveld F, Meijer CJ. Dendritic cells and high endothelial venules in the rheumatoid synovial membrane. J Rheumatol. 1990;17:11–17. [PubMed] [Google Scholar]
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- Wang G, Ge Z, Rasko DA, Taylor DE. Lewis antigens in Helicobacter pylori: Biosynthesis and phase variation. Mol Microbiol. 2000;36:1187–1196. doi: 10.1046/j.1365-2958.2000.01934.x. [DOI] [PubMed] [Google Scholar]
- Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;i:1273–1275. [PubMed] [Google Scholar]
- Willhite DC, Cover TL, Blanke SR. Cellular vacuolation and mitochondrial cytochrome c release are independent outcomes of Helicobacter pylori vacuolating cytotoxin activity that are each dependent on membrane channel formation. J Biol Chem. 2003;278:48204–48209. doi: 10.1074/jbc.M304131200. [DOI] [PubMed] [Google Scholar]
- Wunder C, Churin Y, Winau F, Warnecke D, Vieth M, Lindner B, Zahringer U, Mollenkopf HJ, Heinz E, Meyer TF. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nat Med. 2006;12:1030–1038. doi: 10.1038/nm1480. [DOI] [PubMed] [Google Scholar]
- Yeh JC, Hiraoka N, Petryniak B, Nakayama J, Ellies LG, Rabuka D, Hindsgaul O, Marth JD, Lowe JB, Fukuda M. Novel sulfated lymphocyte homing receptors and their control by a core1 extension β1,3-N-acetylglucosaminyltransferase. Cell. 2001;105:957–969. doi: 10.1016/s0092-8674(01)00394-4. [DOI] [PubMed] [Google Scholar]