Abstract
Intelectin is an extracellular animal lectin found in chordata. Although human and mouse intelectin-1 recognize galactofuranosyl residues included in cell walls of various microorganisms, the physiological function of mammalian intelectin had been unclear. In this study, we found that human intelectin-1 was a serum protein and bound to Mycobacterium bovis bacillus Calmette-Guérin (BCG). Human intelectin-1-binding to BCG was inhibited by Ca2+-depletion, galactofuranosyl disaccharide, ribose, or xylose, and was dependent on the trimeric structure of human intelectin-1. Although monomeric, mouse intelectin-1 bound to BCG, with its C-terminal region contributing to efficient binding. Human intelectin-1-transfected cells not only secreted intelectin-1 into culture supernatant but also expressed intelectin-1 on the cell surface. The cell surface intelectin-1 was not a glycosylphosphatidylinositol-anchored membrane protein. Intelectin-1-transfected cells captured BCG more than untransfected cells, and the BCG adherence was inhibited by an inhibitory saccharide of intelectin-1. Intelectin-1-preincubated cells took up BCG more than untreated cells, but the adhesion of intelectin-1-bound BCG was the same as that of untreated BCG. Mouse macrophages phagocytosed BCG more efficiently in medium containing mouse intelectin-1 than in control medium. These results indicate that intelectin is a host defense lectin that assists phagocytic clearance of microorganisms.
Keywords: galactofuranose, innate immunity, intelectin, lectin, mycobacteria
Introduction
Animal lectins in extracellular fluids function as host defense lectins, which can induce agglutination, opsonization, or complement activation. Mannan-binding lectin, a typical collectin, is associated with mannan-binding-lectin-associated serine proteases (MASPs) in serum and is an initiator of the lectin pathway of the complement system (Endo et al. 2006). Pulmonary surfactant protein A, a collectin, functions as an opsonin using the C1q receptor or the surfactant protein A receptor SP-R210 (Kuroki et al. 2007). l-Ficolin in serum is also an opsonin (Matsushita et al. 1996) and a complement activator associated with MASPs (Endo et al. 2006). These observations suggest that many animal lectins in extracellular fluid can enhance phagocytosis and contribute to innate immunity against pathogenic microorganisms.
Intelectin is a soluble protein secreted into extracellular fluid (Tsuji et al. 2001). Mammalian intelectin is present in the intestinal lumen (Wrackmeyer et al. 2006), and intelectin homologs in ascidian (Abe et al. 1999), lamprey (GenBank database accession number AB055981), fish (Russell et al. 2008), and frog (AB061238, AB061239) (Roberson et al. 1985) are found in serum. Human intelectin-1 is a disulfide-linked homotrimer and a Ca2+-dependent lectin with affinity for galactofuranosyl residues and ribose, but it is not a C-type lectin or a ficolin (Tsuji et al. 2007). Human intelectin-1 binds to arabinogalactan of Nocardia rubra containing galactofuranosyl residues (Tsuji et al. 2001). Galactofuranosyl residues, which are not found on mammalian tissues, are contained in the cell walls of various microorganisms, including Nocardia rubra (Daffe et al. 1993), Mycobacterium tuberculosis (Pedersen and Turco 2003), Streptococcus oralis (Abeygunawardana et al. 1991), Aspergillus fumigatus (Leitao et al. 2003), Leishmania major, and Trypanosoma cruzi (Suzuki et al. 1997). Furthermore, mRNA expression of intelectin increases during immune responses, such as in infections (Pemberton et al. 2004; Datta et al. 2005; Chang and Nie 2007; French et al. 2008; Takano et al. 2008) and asthma (Kuperman et al. 2005). On the basis of these observations, it is proposed that intelectin plays a role in host defense against invading pathogenic microorganisms.
In the present study, we found that human intelectin-1 is a serum protein that binds to Mycobacterium bovis bacillus Calmette-Guérin (BCG). Secreted intelectin-1 appears to deposit on mammalian cell surfaces through an autocrine and/or paracrine mechanism. The deposition of intelectin-1 on epithelial cell lines assists in the capture of BCG. Mouse macrophages phagocytosed BCG more efficiently in the medium containing mouse intelectin-1 than in the control medium. These results suggest that intelectin is a host defense lectin that assists in phagocytic clearance of microorganisms.
Results
Binding of intelectin-1 to BCG
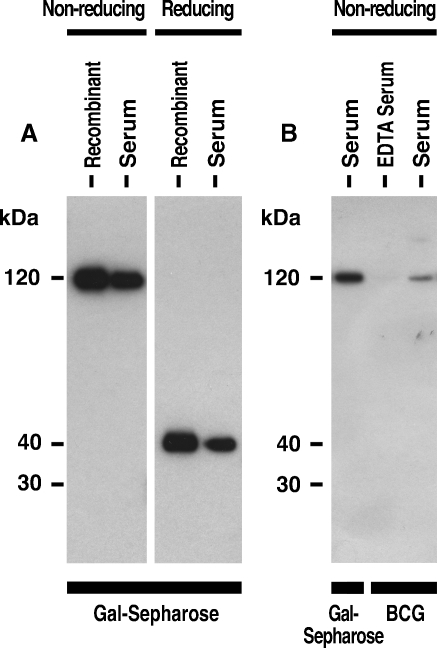
Human intelectin-1 was purified from serum by using galactose–Sepharose. Serum intelectin-1 showed a similar band to recombinant intelectin-1 on Western blotting under nonreducing (Figure 1A, left panel) and reducing conditions (Figure 1A, right panel). Recombinant human intelectin-1 is a 120-kDa disulfide-linked homotrimer (Tsuji et al. 2007). Thus, this result indicates that intelectin-1 is present in human serum as a trimer. The concentration of intelectin-1 in human plasma was measured by an enzyme-linked immunosorbent assay (ELISA) and was found to be 95.5 ± 41.4 ng/mL (mean ± SD) in a cohort of normal, healthy adult donors (n = 17, 40.8 ± 7.2 years old).
Fig. 1.
Binding of human serum intelectin-1 to BCG. Recombinant human intelectin-1 (Recombinant) or human intelectin-1 in serum (Serum) was purified by using galactose–Sepharose (Gal-Sepharose) or ultraviolet-killed BCG (BCG), and then eluted with 100 mM ribose, resolved by 10% SDS–PAGE under nonreducing (A and B) or reducing conditions (A), and detected by Western blotting, as described in Material and methods. Human intelectin-1 was not precipitated with BCG from serum containing 10 mM EDTA (EDTA serum).
To determine whether serum intelectin-1 binds to bacteria whose cell walls contain galactofuranosyl residues, ultraviolet-killed BCG with intact membranes was used for the binding studies. Intelectin-1 was precipitated from serum in the presence of Ca2+ by using ultraviolet-killed BCG (Figure 1B).
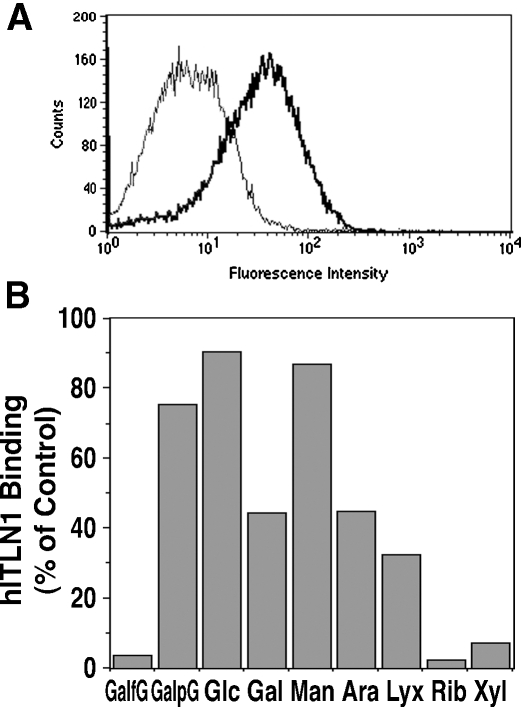
The binding of intelectin-1 to BCG was further confirmed cytometrically by using anti-intelectin-1 antibodies. For biological safety, heat-killed BCG (HK-BCG) was used in the following studies. Recombinant human intelectin-1 bound to HK-BCG in the presence of Ca2+ (Figure 2A). This binding was completely inhibited by a disaccharide containing galactofuranosyl residue, 2-acetamido-2-deoxy-4-O-beta-d-galactofuranosyl-d-glucopyranose (GalfG) but not a galactopyranoside isomer, 2-acetamido-2-deoxy-4-O-beta-d-galactopyranosyl-d-glucopyranose (Figure 2B). In addition, the binding of intelectin-1 to HK-BCG was completely inhibited by ribose and xylose; partially inhibited by galactose, arabinose, or lyxose; and not inhibited by glucose or mannose (Fig- ure 2B).These results are similar to previous observations of the competitive inhibition of intelectin-1 binding to arabinogalactan of Nocardia rubra by saccharides (Tsuji et al. 2001). Thus, intelectin-1 likely binds to arabinogalactan on BCG as well.
Fig. 2.
Flow cytometric analysis of intelectin-1-binding to BCG. HK-BCG was incubated in culture supernatant of human intelectin-1-transfected RK-13 cells with (thin line in A) or without (bold line in A) 10 mM EDTA, or with 100 mM saccharide (B). The bacteria were washed, incubated with antibodies, and analyzed by flow cytometry. Human intelectin-1 (hITLN1) binding with each saccharide was determined from mean fluorescence intensity and is expressed as the percentage of intelectin-1 binding to BCG without saccharide. GalfG, 2-acetamido-2-deoxy-4-O-beta-d-galactofuranosyl-d-glucopyranose; GalpG, 2-acetamido-2-deoxy-4-O-beta-d-galactopyranosyl-d-glucopyranose; Glc, glucose; Gal, galactose; Man, mannose; Ara, arabinose; Lyx, lyxose; Rib, ribose; Xyl, xylose.
Structure of human intelectin-1 required for binding to BCG
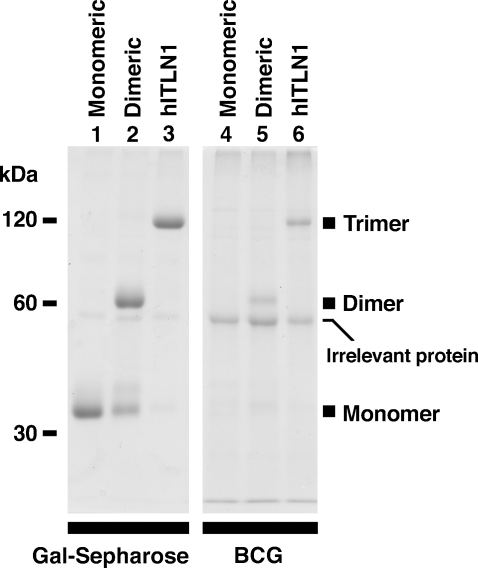
To investigate whether the trimeric structure of human intelectin-1 is required to bind BCG, we precipitated point-mutated intelectin-1 with HK-BCG from culture supernatants containing monomeric, dimeric, or trimeric intelectin-1. Monomeric intelectin-1 bound to galactose–Sepharose but not to HK-BCG (Figure 3, lanes 1 and 4). Dimeric intelectin-1 and trimeric native intelectin-1 bound to both galactose–Sepharose and HK-BCG; however, more intelectin-1 bound to galactose–Sepharose than to HK-BCG (Figure 3, lanes 2, 3, 5, and 6). These results suggest that an oligomerized structure is required for human intelectin-1 for binding to HK-BCG, unlike binding to galactose–Sepharose.
Fig. 3.
The requirement of the oligomeric structure of human intelectin-1 for binding to BCG. As described in Material and methods, monomeric, dimeric, or trimeric recombinant human intelectin-1 was precipitated with galactose–Sepharose (Gal-Sepharose) or HK-BCG (BCG), eluted with 10 mM EDTA, resolved by nonreducing SDS–PAGE, and stained with Coomassie blue. Recombinant human intelectin-1 with Ser substituted for both Cys-31 and Cys-48 was monomeric, recombinant human intelectin-1 with Ser substituted for Cys-48 was mainly dimeric, and intact human intelectin-1 (hITLN1) was trimeric.
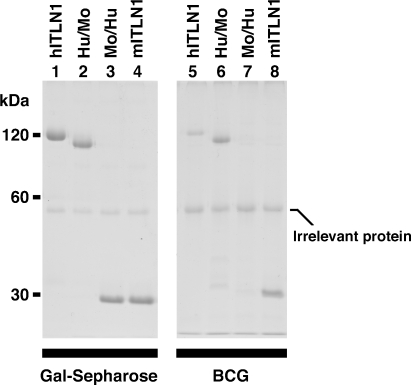
To investigate whether another mammalian intelectin binds to BCG, mouse intelectin-1 was tested. Although mouse intelectin-1 is monomeric (Tsuji et al. 2007), mouse intelectin-1 bound to both HK-BCG and galactose–Sepharose in a similar proportion (Figure 4, lanes 4 and 8). Thus, mouse intelectin-1 does not require an oligomerized structure for the binding to HK-BCG.
Fig. 4.
The binding of mouse intelectin-1 to BCG. Recombinant human intelectin-1 was precipitated with galactose–Sepharose (Gal-Sepharose) or HK-BCG (BCG), eluted with 10 mM EDTA, resolved by nonreducing SDS–PAGE, and stained with Coomassie blue. hITLN-1, human intelectin-1; Hu/Mo, a chimeric molecule consisting of N-terminus of human intelectin-1 and C-terminus of mouse intelectin-1; Mo/Hu, a chimeric molecule consisting of N-terminus of mouse intelectin-1 and C-terminus of human intelectin-1; mITLN1, mouse intelectin-1.
To determine the regions of human and mouse intelectin-1 that are associated with binding to HK-BCG, chimeras formed between human and mouse intelectin-1 were used. A chimeric molecule consisting of the N-terminus of human intelectin-1 and the C-terminus of mouse intelectin-1 (Hu/Mo) was a trimer, and this molecule bound to HK-BCG in a proportion similar to that of mouse intelectin-1 (Figure 4, lanes 2 and 6). A chimeric molecule consisting of the N-terminus of mouse intelectin-1 and the C-terminus of human intelectin-1 (Mo/Hu) was a monomer and did not bind to HK-BCG (Figure 4, lanes 3 and 7). These results suggest that the C-terminal region of mouse intelectin-1 contributes to efficient binding to BCG.
Accumulation of intelectin-1 on cell surfaces
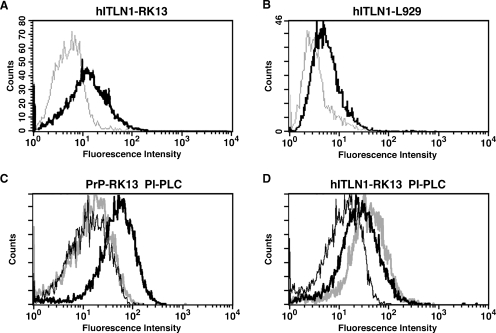
Although intelectin is a serum protein (Figure 1), swine intelectin is found on the cellular membrane of epithelial cells (Wrackmeyer et al. 2006). Thus, secreted intelectin-1 may accumulate on the cell surface through an autocrine and/or paracrine mechanism. By flow cytometric analysis, intelectin-1 was detected on the cell surfaces of RK-13 rabbit kidney epithelial cells and L929 mouse fibroblastic cells that were stably transfected with human intelectin-1 (Figure 5A and B). Intelectin-1 has a similar, although incomplete, C-terminal region to the signal sequence for the glycosylphosphatidylinositol (GPI) anchor. To investigate whether cell surface intelectin-1 is a GPI-anchored membrane protein, we attempted to release cell surface intelectin-1 by phosphatidylinositol-specific phospholipase C (PI-PLC) digestion. Prion protein, a GPI-anchored protein, expressed on RK-13 cells was removed from the cell surface by PI-PLC treatment (Figure 5C). However, intelectin-1 was not released by PI-PLC (Figure 5D). These results indicate that the cell surface intelectin-1 is not a GPI-anchored membrane protein.
Fig. 5.
Flow cytometric analysis of intelectin-1 on the cell surface. The cells were treated with PI-PLC, incubated with antibodies, and analyzed by flow cytometry. (A and B) Untransfected RK-13 cells (gray thin line in A), untransfected L929 cells (gray thin line in B), human intelectin-1-transfected RK-13 cells (hITLN1-RK13) (black bold line in A), or human intelectin-1-transfected L929 cells (hITLN1-L929) (black bold line in B) were treated with anti-intelectin antibodies. The fluorescence level of cells treated with unimmunized rabbit IgG was similar to that of untransfected cells treated with anti-intelectin antibodies (data not shown). (C and D) Untransfected RK-13 cells (black thin line in C and D), untreated prion protein-transfected RK-13 cells (PrP-RK13) (black bold line in C), PI-PLC-treated PrP-RK13 cells (gray bold line in C), untreated hITLN1-RK13 cells (black bold line in D), or PI-PLC-treated hITLN1-RK13 cells (gray bold line in D) were treated with anti-prion protein (C) or anti-intelectin (D) antibodies.
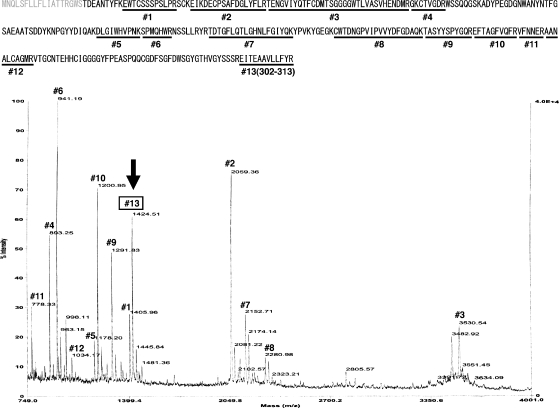
We further investigated whether secreted intelectin-1 was a GPI-anchored protein that was released or proteolytically cleaved from the cell membrane. Since a GPI-anchored protein has its C-terminal signal peptide cleaved and replaced by a GPI anchor, a mature GPI-anchored protein does not have the C-terminal amino acid sequences in the extracellular space. Tryptic peptides of purified secreted intelectin-1 were analyzed by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF), and 13 peptides were identified (Figure 6). The calculated molecular weight of peptide 13, the C-terminal peptide of human intelectin-1 (residues 302–313), is 1424.64, which is almost identical to the molecular mass of the ion identified at 1424.51 (Figure 6, arrow). This result indicates that secreted intelectin-1 includes the C-terminal peptide of intelectin-1. Secreted intelectin-1 is the full-length protein; it is neither a GPI-anchored protein nor a proteolytic fragment.
Fig. 6.
MALDI-TOF spectrum of tryptic peptides of human intelectin-1. The amino acid sequences identified on the mass spectrum are indicated by underlining in human intelectin-1 sequence at the top of the figure. The gray characters indicate the signal peptide.
Taken together, these results indicate that secreted intelectin-1 in culture supernatant deposits on the cell surface through an autocrine and/or paracrine mechanism.
Capture of BCG by intelectin-1 on the cell membrane
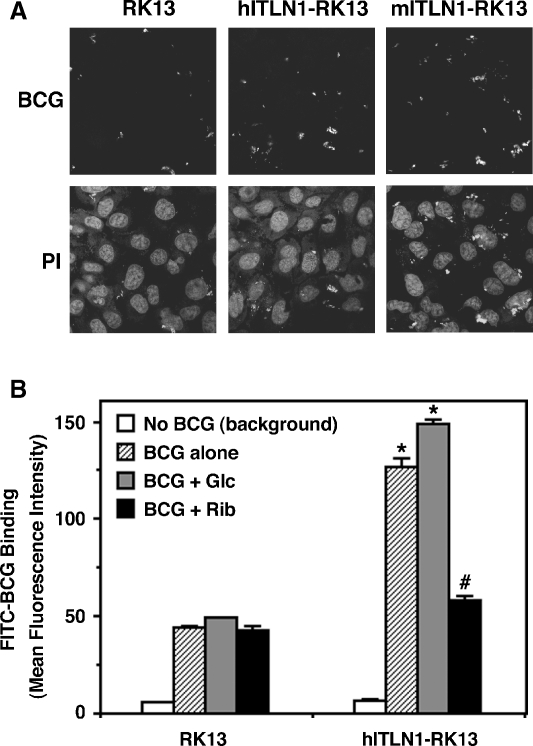
To determine whether intelectin-1 on the cellular membranes enhances the capture of bacteria, RK-13 cells transfected with human intelectin-1 were incubated with fluorescein isothiocyanate-labeled HK-BCG (FITC-BCG). As shown in Figure 7A, human intelectin-1-transfected RK-13 cells captured more FITC-BCG than untransfected RK-13 cells. Human intelectin-1-transfected RK-13 cells bound twice the amount of FITC-BCG as compared to untransfected RK-13 cells (Figure 7B, BCG alone). As shown in Figure 7B, the increased FITC-BCG binding to human intelectin-1-transfected RK-13 cells was reduced by ribose, which competitively inhibited the binding of human intelectin-1 to HK-BCG (Figure 2B). FITC-BCG binding to untransfected RK-13 cells was not affected by saccharides. These results indicate that human intelectin-1 on the cell surface enhances the capture of BCG. GalfG, the inhibitory saccharide containing a galactofuranosyl residue, could not be used in these experiments because it decreased the survival rate of cells at the inhibitory concentration of binding (data not shown).
Fig. 7.
Binding of BCG to cells through intelectin-1. (A) Untransfected RK-13 cells (RK13), human intelectin-1-transfected RK-13 cells (hITLN1-RK13), or mouse intelectin-1-transfected RK-13 cells (mITLN1-RK13) were cultured with FITC-BCG. These cells were washed, fixed with paraformaldehyde, and stained with propidium iodide (PI). The figures are representative photographs. (B) RK13 cells or hITLN1-RK13 cells were cultured with FITC-BCG in the medium containing 100 mM glucose (BCG + Glc), 100 mM ribose (BCG + Rib), or no added carbohydrate (BCG alone). Mean fluorescence intensity of FITC-BCG-bound cells was measured by flow cytometry. Values represent the means ± SD of triplicate determinations. Differences between mean values for the FITC-BCG binding were analyzed by Student's t-test. *, P < 0.001 compared with the corresponding treatment in the RK13. #, P < 0.001 compared with the treatment with BCG alone in hITLN1-RK13.
In these experiments, most captured FITC-BCG was not internalized, since the fluorescence of the captured FITC-BCG was quenched with trypan blue (data not shown). Thus, intelectin-1 enhances the adhesion of BCG to cell surfaces but not the ingestion of BCG into nonphagocytic cells such as RK-13 cells.
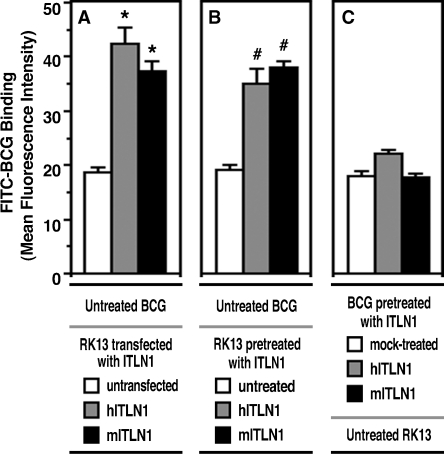
We investigated whether the exogenous intelectin-1 deposited on the cell surface functioned as an acceptor for BCG on cells and/or whether soluble intelectin-1 had an opsonic effect. As shown in Figure 8B, RK-13 cells preincubated with intelectin-1 took up more FITC-BCG than untreated RK-13 cells. Their binding level was comparable to that of intelectin-1-transfected RK-13 cells (Figure 8A). Surprisingly, pretreatment of FITC-BCG with intelectin-1 caused little increase in binding of BCG to untreated RK-13 cells (Figure 8C). These results suggest that secreted intelectin-1 is deposited on the cellular membranes in an autocrine and/or paracrine manner and functions as a receptor for pathogens rather than as an opsonin.
Fig. 8.
Capture of BCG by exogenous intelectin-1. Mean fluorescence intensity of FITC-BCG-bound cells was measured by flow cytometry. Values represent the means ± SD of triplicate determinations. Differences between mean values for the FITC-BCG binding were analyzed by Student's t-test. (A) Untransfected RK-13 cells (open column), human intelectin-1 (hITLN1)-transfected RK13 cells (gray closed column), or mouse intelectin-1 (mITLN1)-transfected RK-13 cells (closed column) were cultured with untreated FITC-BCG. *, P < 0.005 compared with the untransfected RK13 cells. (B) Untreated RK-13 cells (open column), RK-13 cells preincubated with culture supernatant containing hITLN1 (gray closed column), or RK-13 cells preincubated with culture supernatant containing mITLN1 (closed column) were cultured with untreated FITC-BCG. #, P < 0.005 compared with the untreated RK13 cells. (C) Untreated RK-13 cells were cultured with FITC-BCG preincubated with mock culture supernatant (open column) or culture supernatant containing hITLN1 (gray closed column) or mITLN1 (closed column).
For comparative studies, we examined whether mouse intelectin-1-transfected RK-13 cells or mouse intelectin-1-pretreated RK-13 cells enhanced binding to FITC-BCG. As shown in Figures 7A and Figure 8, RK-13 cells either transfected or pretreated with mouse intelectin-1 demonstrate increased BCG binding to cell surfaces compared to untreated RK-13 cells. The effects of mouse intelectin-1 on binding were comparable to those of human intelectin-1 (Figure 8A and B). Similarly to its human homolog, soluble mouse intelectin-1 did not show any opsonic activity (Figure 8C). These results indicate that mouse intelectin-1 also enhances the capture of BCG on cell surfaces.
Phagocytosis of BCG by intelectin-1
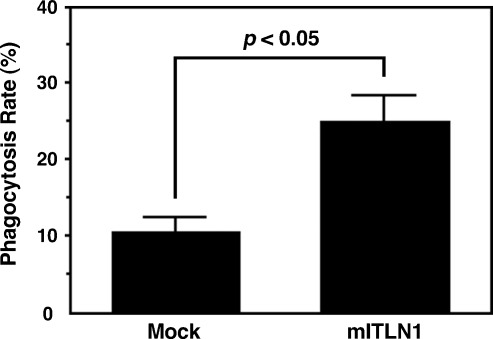
To investigate the effects of intelectin-1 on macrophage phagocytosis, mouse alveolar macrophages were incubated with HK-BCG in culture supernatant containing recombinant mouse intelectin-1. Approximately 25% of the macrophages that were cultured with intelectin-1 phagocytosed HK-BCG, and approximately 10% of the macrophages phagocytosed HK-BCG in the medium without intelectin-1 (Figure 9). These results suggest that exogenous intelectin-1 enhances phagocytosis of BCG by macrophages. However, when untreated macrophages were incubated with mouse intelectin-1-bound HK-BCG, no increase in phagocytosis was observed, indicating that mouse intelectin-1 has no opsonic effects (data not shown).
Fig. 9.
Phagocytosis of BCG using mouse intelectin-1. Mouse alveolar macrophages were incubated with HK-BCG in culture supernatants of untransfected RK-13 cells (Mock) or mouse intelectin-1-transfected RK13 cells (mITLN1). The percentage of BCG-ingesting macrophages (Phagocytosis Rate) was determined by counting at least 500 macrophages from each independent culture. Values represent the means ± SD of triplicate determinations. Differences between mean values were analyzed by Student's t-test.
Discussion
Some homologs of intelectin have been found in chordates. Xenopus laevis cortical granule lectin has a role in forming the fertilization envelope (Gerton and Hedrick 1986; Quill and Hedrick 1996). Ascidian galactose-specific lectin (Abe et al. 1999), lamprey serum lectin (accession number AB055981), rainbow trout plasma intelectin (Russell et al. 2008), Xenopus laevis serum lectin types 1 and 2 (Roberson et al. 1985), and human intelectin-1 (Figure 1A) have been found to be present in serum. In this study, we demonstrated that human serum intelectin-1 binds to BCG and that BCG adheres to intelectin-1-secreting cells. In addition, exogenous mouse intelectin-1 enhances the phagocytosis of BCG by macrophages. These results suggest that mammalian intelectin enhances phagocytic clearance of invading organisms. The phagocytosis-enhancing function of intelectin may be a basic function in chordates.
All intelectin homologs lack transmembrane domains, and human intelectin-1 is present in serum and in the culture supernatants of intelectin-1-transfected cells (Figure 1A). Although these observations suggest that intelectin-1 is a secretory protein, some reports have indicated that intelectin and its homologs are also present on cell surfaces (Nomura et al. 1998; Suzuki et al. 2001; Wrackmeyer et al. 2006). In this study, we confirmed that human intelectin-1 is present on the cell surface (Figure 5). It has been proposed that intelectin-1 is GPI anchored by its C-terminal peptide, and it has been reported that the human intelectin-1/intestinal lactoferrin receptor is a GPI-anchored protein on intestinal epithelial cells (Suzuki et al. 2001). However, the cell surface intelectin-1 on intelectin-1-transfected RK-13 cells was resistant to digestion by PI-PLC, although the prion protein, which is known to be GPI anchored, expressed on RK-13 cells was removed by PI-PLC (Figure 5). These results indicate that the cell surface intelectin-1 is not a GPI-anchored protein but is a secretory protein that binds to the cell surface. Intelectin-1 found on normotopic tissues may include an alternative form that is GPI anchored to the cell membranes. However, measurements of serum intelectin-1 (Figure 1A), the C-terminal peptide of secreted intelectin-1 (Figure 6), and the C-terminal peptide of intelectin-1 in intestinal mucosa (Wrackmeyer et al. 2006) indicate that the major form of intelectin-1 is a secretory protein.
Because the amount of intelectin-1 bound to cell surfaces is not affected by Ca2+-depletion with ethylenediamine tetra-acetic acid (EDTA) (data not shown), intelectin-1 does not bind via saccharide chains on cell surfaces. The receptor for intelectin has not been identified, but intelectin has been reported to deposit on epithelial cell rafts (Wrackmeyer et al. 2006). Galectin-4, a soluble galactose-binding lectin, interacts with sulfatides in lipid rafts in intestinal epithelial cell lines (Delacour et al. 2005). The similarity between galectin-4 and intelectin-1 in their affinity for galactose and tissue distribution reminds that cell surface intelectin-1 may also bind to glycolipids that have accumulated in rafts. Their deposition on lipid rafts could be advantageous in trapping pathogens in the intestinal lumen, since lipid rafts accumulate on the apical membrane of epithelial cells. The receptors for intelectin-1 require further characterization.
The cells that initially encounter mycobacteria in the alveolar space are macrophages and lung epithelial cells. Invading mycobacteria are internalized by macrophages, and then multiply within macrophages and in type II alveolar epithelial cells, which are not professional phagocytes (Bermudez and Goodman 1996). Because human airway epithelial cells express intelectin-1 during inflammation (Kuperman et al. 2005), it is possible that cell surface intelectin-1 mediates mycobacterial infection of lung epithelial cells. Similarly, intestinal epithelial cells, which can be invaded by mycobacteria (Bermudez et al. 1997; Sangari et al. 2001), have intelectin-1 on their surfaces (Wrackmeyer et al. 2006). The intelectin-1-mediated capture of mycobacteria by epithelial cells may play a unique role in host responses to mycobacterial infection. The internalization of mycobacteria by epithelial cells results in the production of soluble factors including tumor necrosis factor-α and granulocyte macrophage colony-stimulating factor, which potentiate macrophage anti-mycobacterial activities (Sato et al. 2002). Alternatively, mycobacteria internalized by epithelial cells may not be attacked by activated macrophages (Bermudez and Goodman 1996; Bermudez et al. 1997; Sangari et al. 2001).
Mouse intelectin-1 is monomeric. Mouse intelectin-1 migrated farther than trimeric or monomeric human intelectin-1 using native polyacrylamide gel electrophoresis (supplementary data). Furthermore, mouse intelectin-1 was detected as a monomer using MALDI-TOF analysis (Tsuji et al. 2007). Monomeric mouse intelectin-1 binds to BCG, although an oligomerized structure is required in order for human intelectin-1 binding to BCG to occur (Figures 3 and 4). These results suggest that mouse intelectin-1 binds to BCG more efficiently than human intelectin-1. Mouse intelectin-1 might have evolved as a defense lectin against mycobacteria. There are marked pathological differences in the response to mycobacterial infections in humans and mice with respect to control of initial infection, delayed-type hypersensitivity response, and granuloma formation (Orme et al. 2001; Flynn 2006). The granulomatous reaction in mice does not result in typical caseous necrosis and cavity formation, and the murine immune response to mycobacteria may be more robust than necessary to control the infection (Flynn 2006). It has been proposed that these differences result from differences in the innate immune system between humans and mice. For example, mice lack CD1b and CD1c, which present mycobacterial lipids (Orme 2003; Sköld and Behar 2003). Our results further suggest that the molecular and biochemical distinctions between mouse and human intelectin-1 may also contribute to the differential immune responses against mycobacteria between the two species. A recent report indicates no significant modification of immune responses or clearance of Mycobacterium tuberculosis in mouse intelectin-1 transgenic mice (Voehringer et al. 2007). An excessive level of endogenous mouse intelectin-1 does not influence the host defense against mycobacteria. We observed that exogenous mouse intelectin-1 enhanced phagocytosis of BCG by macrophages (Figure 9). However, investigations of mouse intelectin-1 are limited due to the lack of anti-mouse intelectin antibodies with sufficient affinity for use in flow cytometric analysis or Western blotting. Moreover, mouse intelectin-1 has little affinity for monosaccharides that do not influence cell viability at the inhibitory concentration of binding (Tsuji et al. 2007). It is therefore necessary to further study the roles of endogenous intelectin-1 by employing intelectin-1-deficient conditions.
Although intelectin-1 deposited on cells enhanced the adhesion of BCG to cell surfaces, intelectin-1 subjected to BCG-prebinding did not function as an opsonin (Figure 8). Intelectin-1 affinity purified by galactose–Sepharose tends to become insoluble and inactive in the presence of Ca2+ (Tsuji et al. 2001). Thus, the conformation of intelectin-1 may change after binding to ligands, and intelectin-1 may subsequently lose the structure required for binding to the cell surface. Although this change decreases the opsonic effect of intelectin-1, it may activate other phagocytosis-enhancing systems. Ficolins and mannan-binding lectin, typical serum lectins, function as weak opsonins but are strong enhancers of phagocytosis. They activate the host complement system through associated MASPs and opsonize ligands with iC3b (Endo et al. 2006). Human intelectin-1 had no association with MASPs (data not shown). However, it is possible that serum intelectin-1 binding to bacteria activates complement using a serum protease.
In conclusion, we demonstrate that serum intelectin-1 binds to mycobacteria and that intelectin-1 assists in capturing mycobacteria on the cell surface. This is the first report of the physiological and immunological functions of mammalian intelectins.
Material and methods
Materials
Anti-intelectin-1 antibodies, plasmids, cDNAs, intelectin-1-transfected cells, or prion protein-transfected RK-13 cells were prepared as described previously (Tsuji et al. 2007; Sakudo et al. 2008). In some experiments, recombinant intelectin-1 was used without purification from culture supernatant of intelectin-1-transfected cells because purified and concentrated intelectin-1 becomes insoluble and inactive in the presence of Ca2+. Galactose–Sepharose was produced by incubating epoxy-activated Sepharose 6B (GE Healthcare UK Ltd, Buckinghamshire) with galactose according to the manufacturer's instructions. 2-Acetamido-2-deoxy-4-O-beta-d-galactofuranosyl-d-glucopyranose (GalfG) was obtained from Toronto Research Chemicals Inc. (North York, ON, Canada). BCG Tokyo 172 strain was obtained from Nihon BCG Inc. (Tokyo, Japan).
Purification of human intelectin-1 from serum
Human intelectin-1 was precipitated at 4°C with galactose–Sepharose from fresh human serum or culture supernatant of human intelectin-1-transfected RK-13 cells. After washing three times with 20 mM Tris-buffered saline (pH 7.6) (TBS) containing 0.1% Triton-X100 and 2 mM CaCl2, human intelectin-1 was eluted with TBS containing 100 mM ribose, 0.1% Triton-X100, and 2 mM CaCl2. Serum intelectin-1 was also precipitated at 4°C with 1 mg of ultraviolet-killed BCG from fresh human serum with or without 10 mM EDTA. After centrifuging and removing supernatants, intelectin-1 was eluted with TBS containing 100 mM ribose and 2 mM CaCl2. The eluted intelectin-1 was resolved by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) under nonreducing or reducing conditions and was transferred to polyvinylidene difluoride membranes (Immobilon-P, Millipore, Billerica, MA). The membranes were blocked with 5% nonfat milk, and then treated for 3 h with 1 μg/mL affinity-purified rabbit anti-intelectin antibodies in 5% nonfat milk. After washing, the membranes were treated with horseradish peroxidase-conjugated donkey anti-rabbit IgG (GE Healthcare UK Ltd) and developed with the ECL Advance substrate (GE Healthcare UK Ltd).
ELISA
The concentration of intelectin-1 in human plasma was measured by sandwich ELISA. Heparinized human plasma was obtained from 17 healthy volunteers (7 males and 10 females) from 32 to 59 years old. The intelectin-1 in plasma was captured by affinity-purified rabbit anti-intelectin antibodies immobilized on 96-well plates and washed three times with TBS containing 0.05% Tween 20. The plates were incubated with TBS containing biotinylated rat anti-intelectin antibodies, 0.05% Tween 20, and 0.2% bovine serum albumin (BSA). After washing, the plates were incubated with TBS containing horseradish peroxidase-conjugated streptavidin (Thermo Fisher Scientific Inc., Rockford, IL), 0.05% Tween 20, and 0.2% BSA, washed, developed with 3,3′,5,5′-tetramethylbenzidine (Thermo Fisher Scientific Inc.), and the absorbance at 450 nm was determined. Recombinant human intelectin-1 for standards was purified from human intelectin-1-transfected RK-13 cells by using galactose–Sepharose (Tsuji et al. 2001). The concentration of intelectin-1 standard was estimated on the basis of the absorbance at 280 nm and the predicted extinction coefficient (Mach et al. 1992) of human intelectin-1. Experimental protocols were approved by the Ethical Committee of Kanagawa Cancer Center.
Flow cytometric analysis for intelectin-1 bound to the cell surface
HK-BCG was incubated for 30 min at 4°C with culture supernatant of human intelectin-1-transfected RK-13 cells. The bacteria were washed twice with 10 mM 2-[4-(2-hydroxyethyl)-1- piperazinyl]ethanesulfonic acid (HEPES)-buffered saline (pH 7.4) containing 0.1% BSA, 0.1% NaN3, and 2 mM CaCl2 (HBSC). Then, the intelectin-1-bound bacteria were incubated with HBSC containing 1 μg/mL affinity-purified rabbit anti-intelectin antibodies preadsorbed with HK-BCG, washed twice, and counterstained with fluorescein isothiocyanate-conjugated donkey anti-rabbit IgG F(ab′)2. After washing with HBSC, the mean fluorescence intensity of binding antibodies was analyzed on a FACSCalibur (BD Biosciences, San Jose, CA).
Human intelectin-1 or prion protein on the cells was measured on a flow cytometer. The prion protein was used as a control of GPI-anchored protein (Stahl and Prusiner 1991). After culturing confluent intelectin-1- or prion-protein-transfected cell or untransfected cell monolayers for 2 days in Eagle's minimum essential medium containing 5% fetal bovine serum, the cells were harvested with HEPES-buffered saline containing 10 mM EDTA, 0.1% BSA, and 0.1% NaN3. The harvested cells (1 × 106 cells) were incubated at 37°C for 30 min with or without 0.1 units/mL PI-PLC (Sigma) in phosphate-buffered saline containing 0.1% BSA and 0.1% NaN3, washed twice with HBSC, and then incubated with HBSC containing 1 μg/mL affinity-purified rabbit anti-intelectin antibodies preadsorbed with RK-13 cells or 1 μg/mL anti-prion protein, SAF32 (SPI-bio, Montigny-le-Bretonneux, France). After washing, the cells were counterstained with fluorescein isothiocyanate-conjugated goat anti-rabbit IgG F(ab′)2 or fluorescein isothiocyanate-conjugated goat anti-mouse IgG F(ab′)2, respectively. The binding antibodies were analyzed on an EPICS Altra (Beckman Coulter, Inc., Fullerton, CA).
Precipitation of recombinant intelectin-1 with galactose–Sepharose or HK-BCG
Recombinant intelectin-1 was precipitated at 4°C for 18 h with galactose–Sepharose or HK-BCG from culture supernatant of intelectin-1-transfected RK-13 cells. After washing three times with HEPES-buffered saline containing 2 mM CaCl2, intelectin-1 was eluted with TBS containing 10 mM EDTA. The eluted intelectin-1 was separated on a 10% SDS–PAGE gel under nonreducing conditions and stained with Coomassie blue.
As described previously (Tsuji et al. 2007), monomeric or dimeric intelectin-1 was prepared as the recombinant human intelectin-1 with Ser substituted for both Cys-31 and Cys-48 residues or Cys-48 residue, respectively. Both recombinant proteins showed a 40-kDa band on SDS–PAGE under reducing conditions (data not shown). Western blotting using anti-intelectin-1 antibodies showed that the 32-kDa, 60-kDa, or 120-kDa band shown in Figure 3 was intelectin-1 peptide (Tsuji et al. 2007). The 55-kDa band present in all lanes has not been identified.
MALDI-TOF
MALDI-TOF mass spectrometry was performed on an Applied Biosystems Voyager DE pro biospectrometry workstation (Applied Biosystems, Foster City, CA). Proteins were reduced with tris(2-carboxyethyl)phosphine hydrochloride (Thermo Fisher Scientific Inc.) and alkylated with iodoacetamide, and then digested for 24 h with modified trypsin (Promega, Madison, WI). The matrix used for MALDI-TOF was alpha-cyano-4-hydroxy-cinnamic acid (Sigma) in 0.05% trifluoroacetic acid. Mass spectrometry conditions were optimized for intact IgG.
Binding of HK-BCG on the intelectin-1-expressed RK-13 cells
RK-13 cells untransfected or transfected with human or mouse intelectin-1 were cultured on 24-well plates with Eagle's minimum essential medium containing 5% fetal bovine serum. The confluent cell monolayers were changed into 500 μL of fresh medium and cultured for 2 days. The cells were incubated at 37°C for 3 h with 500 μL of medium containing 50 μg of FITC-BCG and washed four times with HBSC. After fixation by 4% paraformaldehyde and staining with propidium iodide, FITC-BCG captured on the cells was observed on a laser scanning confocal microscope (Radiance 2100, Bio-Rad Laboratories, Inc., Hercules, CA). For flow cytometric analysis, the FITC-BCG-bound cells were harvested from the unfixed cell monolayers with HBSC by pipetting. The harvested cells were washed twice by centrifugation and analyzed on a flow cytometer (FACSCalibur, BD Biosciences, San Jose, CA).
BCG phagocytosis by mouse macrophages in the medium containing mouse intelectin-1
Mouse alveolar macrophages were prepared from 8-week-old C57BL/6 mice (Harlan Laboratory, Indianapolis, IN). The cells (1 × 105 cells) in broncho-alveolar lavage were incubated for 3 h in culture plates with RPMI1640 containing 5% fetal bovine serum. After removing nonadherent cells, adherent macrophages were cultured at 37°C for 3 h with 500 μL of medium containing 50 μg of HK-BCG, and then washed four times with the medium. After fixation by 4% paraformaldehyde and staining with propidium iodide, phagocytosed HK-BCG was observed by a laser scanning confocal microscope (Radiance 2100, Bio-Rad Laboratories, Inc., Hercules, CA). The images were processed by Adobe Photoshop software.
Supplementary Data
Supplementary data for this article is available online at http://glycob.oxfordjournals.org/.
Funding
The National Institutes of Health ((RO1 HL71711, DOD DAMD 17-03-1-0004) to Y.S.); Bankhead-Coley Cancer Research Program ((06BS-04-9615) to Y.S.); the Charles E. Schmidt Biomedical Foundation (to Y.S.); the Ministry of Health, Labour and Welfare ((the Grant-in-Aid for Cancer Research 19-15) to S.T.); and Kanagawa Health Foundation (to S.T.).
Supplementary Material
Acknowledgments
We are grateful to Dr S. Maeda (Research Institute of Tuberculosis, Japan Anti-Tuberculosis Association) for valuable discussions.
Conflict of interest statement
None declared.
Abbreviations
- BCG
Mycobacterium bovis bacillus Calmette-Guérin
- BSA
bovine serum albumin
- EDTA
ethylenediamine tetra-acetic acid
- ELISA
enzyme-linked immunosorbent assay
- FITC-BCG
fluorescein isothiocyanate-labeled HK-BCG
- GalfG
2-acetamido-2-deoxy-4-O-beta-d-galactofuranosyl-d-glucopyranose
- GPI
glycosylphosphatidylinositol
- HBSC
HEPES-buffered saline (pH 7.4) containing 0.1% BSA, 0.1% NaN3, and 2 mM CaCl2
- HEPES
2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid
- HK-BCG
heat-killed BCG
- MALDI-TOF
matrix-assisted laser desorption/ionization time-of-flight
- MASPs
mannan binding lectin-associated serine proteases
- PI-PLC
phosphatidylinositol-specific phospholipase C
- SDS–PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- TBS
20 mM Tris-buffered saline (pH 7.6)
References
- Abe Y, Tokuda M, Ishimoto R, Azumi K, Yokosawa H. A unique primary structure, cDNA cloning and function of a galactose-specific lectin from ascidian plasma. Eur J Biochem. 1999;261:33–39. doi: 10.1046/j.1432-1327.1999.00238.x. [DOI] [PubMed] [Google Scholar]
- Abeygunawardana C, Bush CA, Cisar JO. Complete structure of the cell surface polysaccharide of Streptococcus oralis C104: A 600-MHz NMR study. Biochemistry. 1991;30:8568–8577. doi: 10.1021/bi00099a012. [DOI] [PubMed] [Google Scholar]
- Bermudez LE, Goodman J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect Immun. 1996;64:1400–1406. doi: 10.1128/iai.64.4.1400-1406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez LE, Petrofsky M, Goodman J. Exposure to low oxygen tension and increased osmolarity enhance the ability of Mycobacterium avium to enter intestinal epithelial (HT-29) cells. Infect Immun. 1997;65:3768–3773. doi: 10.1128/iai.65.9.3768-3773.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MX, Nie P. Intelectin gene from the grass carp Ctenopharyngodon idella: cDNA cloning, tissue expression, and immunohistochemical localization. Fish Shellfish Immunol. 2007;23:128–140. doi: 10.1016/j.fsi.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Daffe M, McNeil M, Brennan PJ. Major structural features of the cell wall arabinogalactans of Mycobacterium, Rhodococcus, and Nocardia spp. Carbohydr Res. 1993;249:383–398. doi: 10.1016/0008-6215(93)84102-c. [DOI] [PubMed] [Google Scholar]
- Datta R, deSchoolmeester ML, Hedeler C, Paton NW, Brass AM, Else KJ. Identification of novel genes in intestinal tissue that are regulated after infection with an intestinal nematode parasite. Infect Immun. 2005;73:4025–4033. doi: 10.1128/IAI.73.7.4025-4033.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacour D, Gouyer V, Zanetta JP, Drobecq H, Leteurtre E, Grard G, Moreau-Hannedouche O, Maes E, Pons A, André S, et al. Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. J Cell Biol. 2005;169:491–501. doi: 10.1083/jcb.200407073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y, Takahashi M, Fujita T. Lectin complement system and pattern recognition. Immunobiol. 2006;211:283–293. doi: 10.1016/j.imbio.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Flynn JL. Lessons from experimental Mycobacterium tuberculosis infections. Microbes Infect. 2006;8:1179–1188. doi: 10.1016/j.micinf.2005.10.033. [DOI] [PubMed] [Google Scholar]
- French AT, Knight PA, Smith WD, Brown JK, Craig NM, Pate JA, Miller HR, Pemberton AD. Up-regulation of intelectin in sheep after infection with Teladorsagia circumcincta. Int J Parasitol. 2008;38:467–475. doi: 10.1016/j.ijpara.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Gerton GL, Hedrick JL. The vitelline envelope to fertilization envelope conversion in eggs of Xenopus laevis. Dev Biol. 1986;116:1–7. doi: 10.1016/0012-1606(86)90036-9. [DOI] [PubMed] [Google Scholar]
- Kuperman DA, Lewis CC, Woodruff PG, Rodriguez MW, Yang YH, Dolganov GM, Fahy JV, Erle DJ. Dissecting asthma using focused transgenic modeling and functional genomics. J Allergy Clin Immunol. 2005;116:305–311. doi: 10.1016/j.jaci.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Kuroki Y, Takahashi M, Nishitani C. Pulmonary collectins in innate immunity of the lung. Cell Microbiol. 2007;9:1871–1879. doi: 10.1111/j.1462-5822.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- Leitao EA, Bittencourt VC, Haido RM, Valente AP, Peter-Katalinic J, Letzel M, de Souza LM, Barreto-Bergter E. Beta-galactofuranose-containing O-linked oligosaccharides present in the cell wall peptidogalactomannan of Aspergillus fumigatus contain immunodominant epitopes. Glycobiology. 2003;13:681–692. doi: 10.1093/glycob/cwg089. [DOI] [PubMed] [Google Scholar]
- Mach H, Middaugh CR, Lewis RV. Statistical determination of the average values of the extinction coefficients of tryptophan and tyrosine in native proteins. Anal. Biochem. 1992;200:74–80. doi: 10.1016/0003-2697(92)90279-g. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Endo Y, Taira S, Sato Y, Fujita T, Ichikawa N, Nakata M, Mizuochi T. A novel human serum lectin with collagen- and fibrinogen-like domains that functions as an opsonin. J Biol Chem. 1996;271:2448–2454. doi: 10.1074/jbc.271.5.2448. [DOI] [PubMed] [Google Scholar]
- Nomura KH, Kobayashi R, Hirabayashi Y, Fujisue-Sakai M, Mizuguchi S, Nomura K. Involvement of blood-group-B-active trisaccharides in Ca2+-dependent cell–cell adhesion in the Xenopus blastula. Dev Genes Evol. 1998;208:9–18. doi: 10.1007/s004270050148. [DOI] [PubMed] [Google Scholar]
- Orme IM. The mouse as a useful model of tuberculosis. Tuberculosis. 2003;83:112–115. doi: 10.1016/s1472-9792(02)00069-0. [DOI] [PubMed] [Google Scholar]
- Orme IM, McMurray DN, Belisle JT. Tuberculosis vaccine development: Recent progress. Trends Microbiol. 2001;9:115–118. doi: 10.1016/s0966-842x(00)01949-1. [DOI] [PubMed] [Google Scholar]
- Pedersen LL, Turco SJ. Galactofuranose metabolism: A potential target for antimicrobial chemotherapy. Cell Mol Life Sci. 2003;60:259–266. doi: 10.1007/s000180300021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton AD, Knight PA, Gamble J, Colledge WH, Lee JK, Pierce M, Miller HR. Innate BALB/c enteric epithelial responses to Trichinella spiralis: Inducible expression of a novel goblet cell lectin, intelectin-2, and its natural deletion in C57BL/10 mice. J Immunol. 2004;173:1894–1901. doi: 10.4049/jimmunol.173.3.1894. [DOI] [PubMed] [Google Scholar]
- Quill TA, Hedrick JL. The fertilization layer mediated block to polyspermy in Xenopus laevis: Isolation of the cortical granule lectin ligand. Arch Biochem Biophys. 1996;333:326–332. doi: 10.1006/abbi.1996.0398. [DOI] [PubMed] [Google Scholar]
- Roberson MM, Wolffe AP, Tata JR, Barondes SH. Galactoside-binding serum lectin of Xenopus laevis. Estrogen-dependent hepatocyte synthesis and relationship to oocyte lectin. J Biol Chem. 1985;260:11027–11032. [PubMed] [Google Scholar]
- Russell S, Young KM, Smith M, Hayes MA, Lumsden JS. Identification, cloning and tissue localization of a rainbow trout (Oncorhynchus mykiss) intelectin-like protein that binds bacteria and chitin. Fish Shellfish Immunol. 2008;25:91–105. doi: 10.1016/j.fsi.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Sakudo A, Nakamura I, Tsuji S, Ikuta K. GPI-anchorless human prion protein is secreted and glycosylated but lacks superoxide dismutase activity. Int J Mol Med. 2008;21:217–222. [PubMed] [Google Scholar]
- Sangari FJ, Goodman J, Petrofsky M, Kolonoski P, Bermudez LE. Mycobacterium avium invades the intestinal mucosa primarily by interacting with enterocytes. Infect Immun. 2001;69:1515–1520. doi: 10.1128/IAI.69.3.1515-1520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Tomioka H, Shimizu T, Gonda T, Ota F, Sano C. Type II alveolar cells play roles in macrophage-mediated host innate resistance to pulmonary mycobacterial infections by producing proinflammatory cytokines. J Infect Dis. 2002;185:1139–1147. doi: 10.1086/340040. [DOI] [PubMed] [Google Scholar]
- Sköld M, Behar SM. Role of CD1d-restricted NKT cells in microbial immunity. Infect Immun. 2003;71:5447–5455. doi: 10.1128/IAI.71.10.5447-5455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl N, Prusiner SB. Prions and prion proteins. FASEB J. 1991;5:2799–2807. doi: 10.1096/fasebj.5.13.1916104. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Toledo MS, Takahashi HK, Straus AH. A monoclonal antibody directed to terminal residue of beta-galactofuranose of a glycolipid antigen isolated from Paracoccidioides brasiliensis: Cross-reactivity with Leishmania major and Trypanosoma cruzi. Glycobiology. 1997;7:463–468. doi: 10.1093/glycob/7.4.463. [DOI] [PubMed] [Google Scholar]
- Suzuki YA, Shin K, Lonnerdal B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry. 2001;40:15771–15779. doi: 10.1021/bi0155899. [DOI] [PubMed] [Google Scholar]
- Takano T, Sha Z, Peatman E, Terhune J, Liu H, Kucuktas H, Li P, Edholm ES, Wilson M, Liu Z. The two channel catfish intelectin genes exhibit highly differential patterns of tissue expression and regulation after infection with Edwardsiella ictaluri. Dev Comp Immunol. 2008;32:693–705. doi: 10.1016/j.dci.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Uehori J, Matsumoto M, Suzuki Y, Matsuhisa A, Toyoshima K, Seya T. Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall. J Biol Chem. 2001;276:23456–23463. doi: 10.1074/jbc.M103162200. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Yamashita M, Nishiyama A, Shinohara T, Li Z, Myrvik QN, Hoffman DR, Henriksen RA, Shibata Y. Differential structure and activity between human and mouse intelectin-1: Human intelectin-1 is a disulfide-linked trimer, whereas mouse homologue is a monomer. Glycobiology. 2007;17:1045–1051. doi: 10.1093/glycob/cwm075. [DOI] [PubMed] [Google Scholar]
- Voehringer D, Stanley SA, Cox JS, Completo GC, Lowary TL, Locksley RM. Nippostrongylus brasiliensis: Identification of intelectin-1 and -2 as Stat6-dependent genes expressed in lung and intestine during infection. Exp Parasitol. 2007;116:458–466. doi: 10.1016/j.exppara.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrackmeyer U, Hansen GH, Seya T, Danielsen EM. Intelectin: A novel lipid raft-associated protein in the enterocyte brush border. Biochemistry. 2006;45:9188–9197. doi: 10.1021/bi060570x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.