Abstract
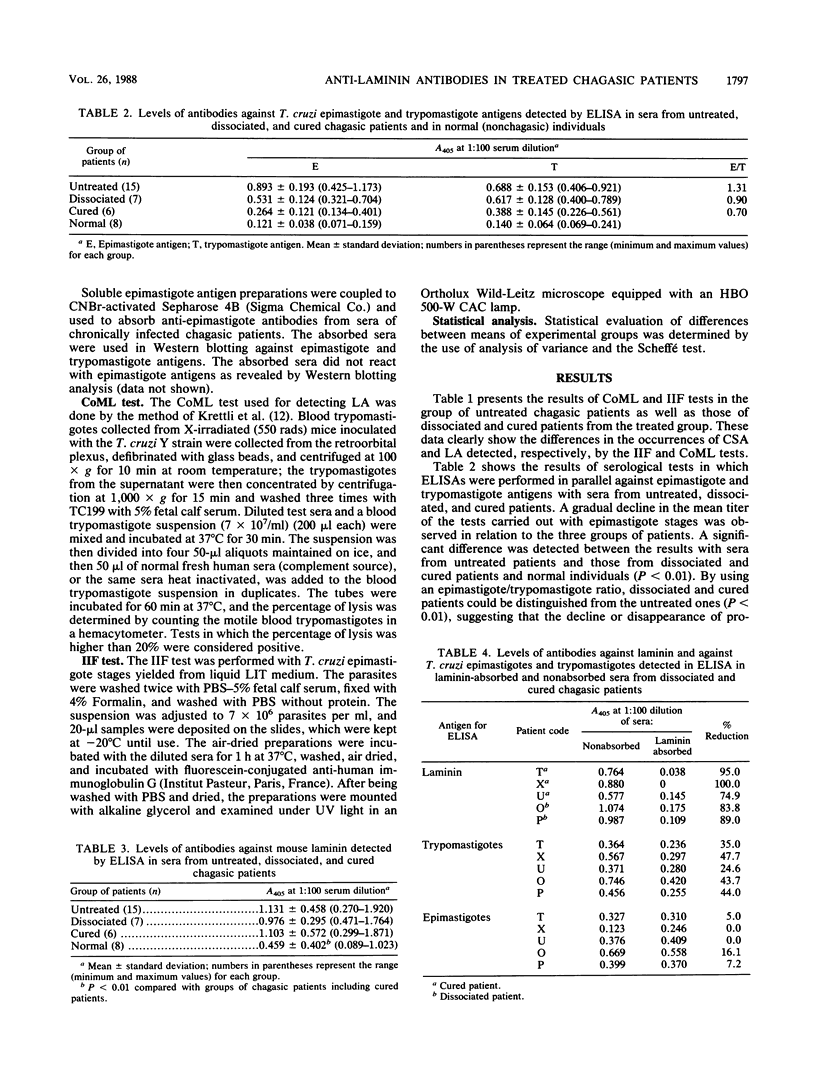
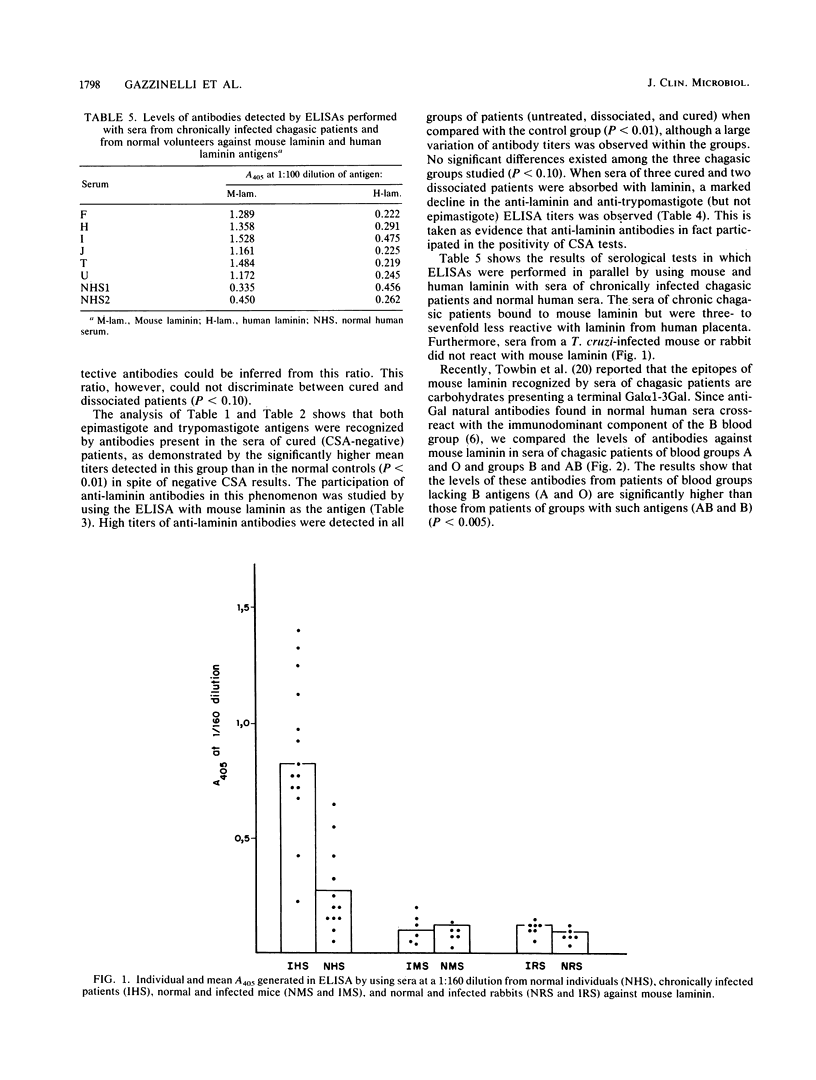
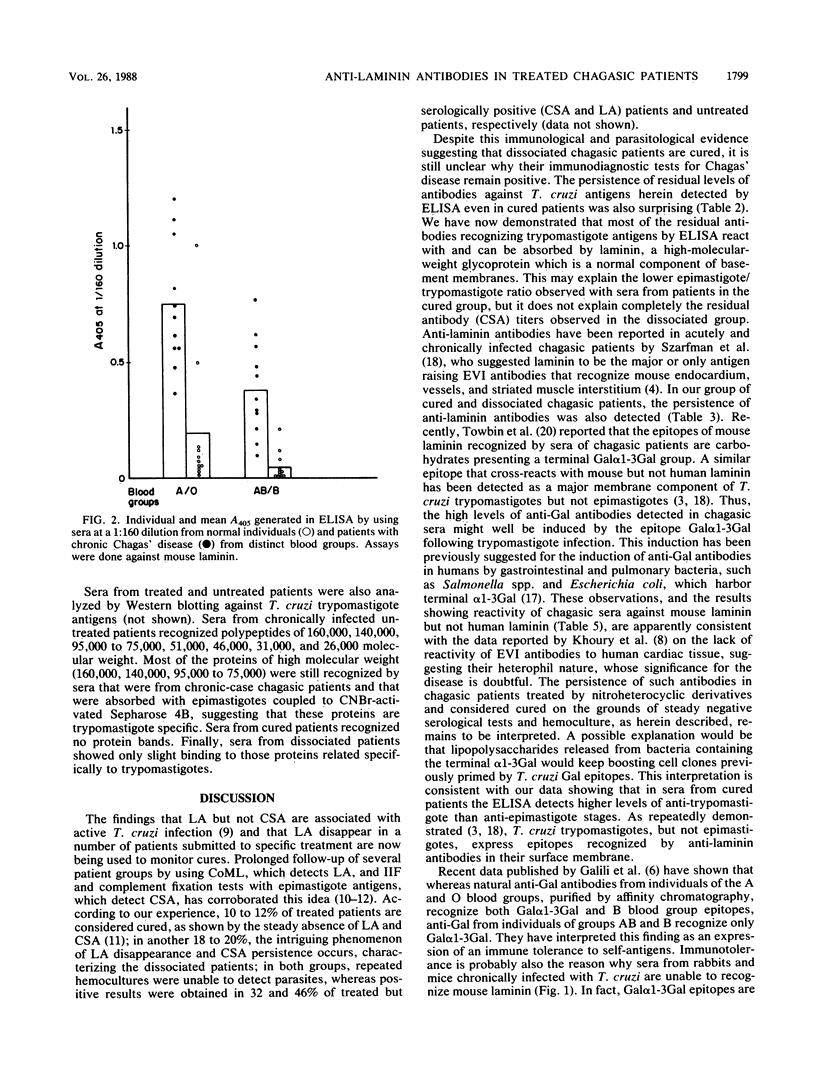
The antibody response to Trypanosoma cruzi epimastigote and trypomastigote stages, as well as to laminin, was studied in several groups of chagasic patients. In six patients who were cured of the parasite, the serum antibody titers as revealed by indirect immunofluorescence and hemagglutination tests against epimastigotes (conventional serology) and a complement-mediated lysis test with living trypomastigotes did not differ from those of normal individuals. In seven presumably cured patients, although the complement-mediated lysis test turned negative, conventional serology remained positive. Sera from this group of so-called "dissociated" patients presented significant lower mean antibody titers against epimastigote but not trypomastigote stages than did sera from 14 untreated patients (P less than 0.01). Most of the antibodies against trypomastigotes, including the residual levels found in cured patients, were absorbed by mouse laminin. In fact, significantly higher titers of anti-laminin antibodies were observed in sera from untreated chagasic patients (1.131 +/- 0.458) and cured patients (1.103 +/- 0.572) than in sera from eight normal individuals (0.459 +/- 0.402) (P less than 0.01). The anti-laminin titers were higher in sera of patients of blood group A or O than in those of patients of group B or AB. In Western blotting (immunoblotting) analysis against trypomastigotes, sera from chronic untreated patients recognized many polypeptide bands ranging from 26 to 160 kilodaltons, whereas no protein bands were observed with sera from cured patients. Only faint bands of parasite proteins were observed with sera of dissociated patients. In conjunction, the above data suggest that the anti-trypomastigote antibodies which persist after parasitological cure of patients with Chagas' disease are due mainly to cross-reactive epitopes from mouse laminin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avila J. L., Rojas M., Rieber M. Antibodies to laminin in American cutaneous leishmaniasis. Infect Immun. 1984 Jan;43(1):402–406. doi: 10.1128/iai.43.1.402-406.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener Z., Ramirez L. E., Krettli A. U., Cançado J. R. EVI antibodies in patients with Chagas' disease: relationship with anti-Trypanosoma cruzi immunoglobulins and effects of specific treatment. Mem Inst Oswaldo Cruz. 1983 Oct-Dec;78(4):437–442. doi: 10.1590/s0074-02761983000400007. [DOI] [PubMed] [Google Scholar]
- Bretaña A., Avila J. L., Arias-Flores M., Contreras M., Tapia F. J. Trypanosoma cruzi and American Leishmania spp: immunocytochemical localization of a laminin-like protein in the plasma membrane. Exp Parasitol. 1986 Apr;61(2):168–175. doi: 10.1016/0014-4894(86)90149-9. [DOI] [PubMed] [Google Scholar]
- Cossio P. M., Diez C., Szarfman A., Kreutzer E., Candiolo B., Arana R. M. Chagasic cardiopathy. Demonstration of a serum gamma globulin factor which reacts with endocardium and vascular structures. Circulation. 1974 Jan;49(1):13–21. doi: 10.1161/01.cir.49.1.13. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Galili U., Buehler J., Shohet S. B., Macher B. A. The human natural anti-Gal IgG. III. The subtlety of immune tolerance in man as demonstrated by crossreactivity between natural anti-Gal and anti-B antibodies. J Exp Med. 1987 Mar 1;165(3):693–704. doi: 10.1084/jem.165.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U., Rachmilewitz E. A., Peleg A., Flechner I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med. 1984 Nov 1;160(5):1519–1531. doi: 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury E. L., Diez C., Cossio P. M., Arana R. M. Heterophil nature of EVI antibody in Trypanosoma cruzi infection. Clin Immunol Immunopathol. 1983 May;27(2):283–288. doi: 10.1016/0090-1229(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Krettli A. U., Brener Z. Resistance against Trypanosoma cruzi associated to anti-living trypomastigote antibodies. J Immunol. 1982 May;128(5):2009–2012. [PubMed] [Google Scholar]
- Krettli A. U., Cançado J. R., Brener Z. Effect of specific chemotherapy on the levels of lytic antibodies in Chagas's disease. Trans R Soc Trop Med Hyg. 1982;76(3):334–340. doi: 10.1016/0035-9203(82)90184-5. [DOI] [PubMed] [Google Scholar]
- Krettli A. U., Weisz-Carrington P., Nussenzweig R. S. Membrane-bound antibodies to bloodstream Trypanosoma cruzi in mice: strain differences in susceptibility to complement-mediated lysis. Clin Exp Immunol. 1979 Sep;37(3):416–423. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Peters B. P., Goldstein I. J. The use of fluorescein-conjugated Bandeiraea simplicifolia B4-isolectin as a histochemical reagent for the detection of alpha-D-galactopyranosyl groups. Their occurrence in basement membranes. Exp Cell Res. 1979 May;120(2):321–334. doi: 10.1016/0014-4827(79)90392-6. [DOI] [PubMed] [Google Scholar]
- Schmuñis G. A., Cossio P. M., Szarfman A., Coarasa L., Arana R. M. Tissue-reacting antibodies (EVI antibodies) in nifurtimox-treated patients with Chagas's disease. J Infect Dis. 1978 Sep;138(3):401–404. doi: 10.1093/infdis/138.3.401. [DOI] [PubMed] [Google Scholar]
- Springer G. F. Blood-group and Forssman antigenic determinants shared between microbes and mammalian cells. Prog Allergy. 1971;15:9–77. [PubMed] [Google Scholar]
- Szarfman A., Terranova V. P., Rennard S. I., Foidart J. M., de Fatima Lima M., Scheinman J. I., Martin G. R. Antibodies to laminin in Chagas' disease. J Exp Med. 1982 Apr 1;155(4):1161–1171. doi: 10.1084/jem.155.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira V. de P., Martins E., Almeida H. de O., Soares S., de Souza H. M., de Morais C. A. Sistema ABO e formas anatomoclínicas da doença de Chagas crônica. Rev Soc Bras Med Trop. 1987 Jul-Sep;20(3):163–167. doi: 10.1590/s0037-86821987000300007. [DOI] [PubMed] [Google Scholar]
- Towbin H., Rosenfelder G., Wieslander J., Avila J. L., Rojas M., Szarfman A., Esser K., Nowack H., Timpl R. Circulating antibodies to mouse laminin in Chagas disease, American cutaneous leishmaniasis, and normal individuals recognize terminal galactosyl(alpha 1-3)-galactose epitopes. J Exp Med. 1987 Aug 1;166(2):419–432. doi: 10.1084/jem.166.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]


