Animal inoculation remains essential for many aspects of leptospirosis research. Although pathogenic leptospiral strains may be able to infect a wide variety of animals, the Golden Syrian hamster is the preferred model because its susceptibility to infection and the reproducibility of the results. Common applications include determination of strain infectivity, restoring virulence to culture-attenuated strains, assessing usefulness of potential vaccines or diagnostic antigens, and examining pathology of mammalian infection. In many respects, acute leptospirosis in the hamster reproduces the severe form of human leptospirosis (see Commentary).
This unit provides a detailed description of the procedures involved in the hamster model of leptospirosis, including housing and handling of hamsters and intraperitoneal challenge (Basic Protocol 1), and monitoring response to challenge (Basic Protocols 2 and 3, and Alternate Protocol). Methods are provided for demonstrating infection, including culture isolation of leptospires from blood and tissues (Basic Protocol 2) and serologic studies and histopathology (Basic Protocol 3). Quantitative PCR is provided as an alternative detection method (Alternate Protocol). Although leptospires disseminate to many organs, the tissues of the liver and kidney are the primary targets of infection. The liver is heavily infected during the initial stage of hematogenous dissemination. Persistent infection occurs primarily in the kidneys of animals that survive acute disease. Infection of the renal tubules leads to shedding of infectious organisms in the urine, which is the primary mode of transmission in nature. A safety policy memorandum is provided (see Strategic Planning) that should be read and signed by all staff.
In the Commentary section, a detailed rationale is presented for use of hamsters as an animal model of leptospirosis. The effects of challenge dose on the hamster model, LD50, in vitro passage of leptospiral strains, and immunological maturity are discussed. Alternative animal models are evaluated with reference to the concept of accidental versus reservoir hosts. The advantages and disadvantages of the hamster model are considered, with reference to use of alternative host animals to address specific research or vaccine-development issues.
CAUTION: Pathogenic Leptospira species are Biosafety Level 2 (BSL-2) pathogens. L. interrogans, L. kirschneri, L. noguchii, L. borgpetersenii, L. santarosai, and L. weilii are known to be pathogenic for humans. Certain strains of L. inadai, L. meyeri, L. fainei, and L. alexanderi may also be pathogenic. L. biflexa, L. wolbachii, and L. parva are thought to be nonpathogens. Follow all appropriate guidelines and regulations for the use and handling of pathogenic microorganisms. See UNIT 1A.1 and other pertinent resources (APPENDIX 1B) for more information.
CAUTION: Protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) or must conform to governmental regulations regarding the care and use of laboratory animals. This experiment requires Animal Biosafety Level 2 (ABSL-2) conditions. Follow all appropriate guidelines for the use and handling of infected animals. See UNIT 1A.1 and other pertinent resources (APPENDIX 1B) for more information. Fluids and tissues of infected animals are highly infectious. Numerous laboratory-associated infections, including deaths, have been reported (Miller et al., 1987; Pike, 1976). A safety policy memorandum is provided that should be signed by all staff (see Strategic Planning).
STRATEGIC PLANNING
Successful challenge studies require the simultaneous availability of Golden Syrian hamsters and virulent leptospiral cultures. Hamsters typically require a 1-week quarantine after shipment and should be no more than 4 weeks old on the day of challenge. Virulent leptospires typically require at least 1 week of growth after inoculation of culture medium from frozen stocks. However, leptospiral growth varies depending on the strain and the quality of the medium (UNIT 12E.1).
Prior to initiating animal challenge studies, the principal investigator should ask all personnel to read and sign a safety policy memorandum stating that they understand and agree to the following:
Leptospirosis is a well-documented laboratory hazard. Virulent Leptospira may be present in urine, blood, and tissues of infected animals and humans. Ingestion, accidental parenteral inoculation, and direct and indirect contact of skin or mucous membranes with cultures or infected tissues or body fluids—especially urine—are the primary laboratory hazards.
All personnel must submit a baseline serum sample to employee health to test for leptospiral antibodies.
All personnel must wear a gown, eyeware, gloves, mask, hat, and shoe coverings while in any room containing infected animals.
In addition to standard microbiological safety practices, cultures and infected materials will be handled in a laminar flow biological safety cabinet that has been designated for use exclusively by the principal investigator and personnel in his or her laboratory. Infected materials will be disinfected with bleach or autoclaved before disposal.
In the event of an accidental percutaneous or mucous membrane exposure to an infected animal or its tissues, the principal investigator and the employee health department should be contacted immediately for consideration of antibiotic prophylaxis with amoxicillin (500 mg, orally, three times daily) or doxycycline (100 mg, orally, twice daily) for 7 days.
INTRAPERITONEAL CHALLENGE OF HAMSTERS WITH LEPTOSPIRA
Intraperitoneal inoculation is the standard route for challenging animals with pathogenic leptospires. The primary advantage of intraperitoneal inoculation is the ease of delivering a reproducible volume of inoculum. A number of other, more biologically relevant inoculation routes have been reported, including subcutaneous (Truccolo et al., 2002) and conjunctival (Bolin and Alt, 2001) challenge models. These alternative routes more accurately reproduce aspects of natural transmission, which may be important in vaccine studies, but are less well studied than the intraperitoneal challenge route and may be more difficult to reproduce.
CAUTION: Wear appropriate personal protective equipment (see Strategic Planning) during handling of animals. Personnel should be trained in proper handling of hamsters. Golden Syrian hamsters are generally docile but may scratch or bite if startled. To avoid injury, some researchers prefer to handle hamsters with tongs and/or chain mail gloves (optional). These safety precautions are usually not necessary if researchers reach into the cage slowly and exercise patience. See Donovan and Brown (2006a) for additional instruction in handling and restraining hamsters.
Materials
3- to 4-week-old Golden Syrian hamsters (Harlan Bioproducts for Science)
Log-phase Leptospira culture for challenge (UNIT 12E.1): low-passage, virulent strain (e.g., L. interrogans serovar Copenhageni strain L1-130 or L. kirschneri serovar Grippotyphosa strain RM52)
EMJH liquid medium (see recipe)
Hamster cages, solid-bottom, with filter top to prevent escape of contaminated bedding (e.g., Ancare; http://www.ancare.com)
Hamster bedding (Sani-Chips; P.J. Murphy Forest Products, http://www.pjmurphy.net/)
Dark-field microscope (UNIT 2A.1)
Petroff-Hausser counting chamber or equivalent (also see APPENDIX 4A)
1-ml syringes
22-G needles
Additional reagents and equipment for intraperitoneal injection (Donovan and Brown, 2006b)
-
House hamsters in a room separated from the corridor by an anteroom for donning and discarding of personal protective equipment (see Strategic Planning).
CAUTION: Because infected animals shed virulent leptospires in their urine, the bedding and entire contents of the cage should be considered contaminated after challenge. Cages should be fitted with a filter top or other protective covering to prevent bedding from escaping as animals move about the cage.To prevent cross-contamination of cages during bedding changes, animals should be moved directly to new cages; a transfer cage should not be used. Double gloves should be worn during handling of infected animals to allow replacement of the outer glove between cages. If forceps are used to transfer animals, the instrument must be disinfected between cages. The author disinfects by dipping the forceps in Clidox-S (Indulab, http://www.indulab.ch), prepared by combining 1 part activator with 18 parts water and 1 part Base).Consider ordering litters of 2- to 3-week-old weanling hamsters to allow time for a ≥1-week quarantine before use, so that animals are less than 4 weeks of age on the day of challenge. -
Determine the density of the leptospiral challenge strain by dark-field microscopy using a Petroff-Hausser or similar counting chamber (also see APPENDIX 4A).
The leptospiral challenge strain should be in the log phase of growth (density >108 cells/ml) and should have been passaged less than ten times in vitro after isolation from a mammalian host. See UNIT 12E.1 for Leptospira storage and maintenance. Prepare serial 10-fold dilutions in liquid EMJH medium to yield concentrations ranging from 109 to 100 cells/ml.
-
In a biological safety cabinet, inject each designated hamster intraperitoneally (Donovan and Brown, 2006b) with 1.0 ml of the appropriate dilution using a 1-ml syringe and 22-G needle. Use three hamsters per dilution.
CAUTION: After inoculation, syringes should be discarded in a sharps disposal container without recapping the needle, since most needle-puncture injuries occur during recapping.Intraperitoneal injection of hamsters with liquid EMJH containing BSA or rabbit serum does not appear to interfere with these experiments. -
Examine animals at least twice per day after challenge.
There may be a relatively short interval (several hours) between onset of symptoms and death. Typically, animals demonstrate decreased activity, assume a hunched posture, and have ruffled fur. Appropriate endpoint criteria include loss of interest in food or water and weight loss of >10% of expected weight. Animals that survive acute infection should be euthanized 21 to 28 days after challenge to collect tissues (Basic Protocol 2) to determine whether sublethal infection is present.
HARVESTING BLOOD AND TISSUES FOR ASSESSMENT OF LEPTOSPIRA INFECTION
At the desired time following challenge, the hamsters are anesthetized with isoflurane and blood and tissues are harvested; this is a terminal surgical procedure. Inhaled anesthesia is required to ensure that animals are unconscious during the surgical procedure.
CAUTION: Use of volatile anesthetics, such as isoflurane, requires a well ventilated area and/or an appropriate scavenging system.
Materials
Hamsters infected with Leptospira (see Basic Protocol 1)
Isoflurane
Small, sealable container (e.g., Tupperware) to accommodate hamster for anesthesia
Gauze or cotton sponges
50-ml conical polypropylene centrifuge tubes
Dissecting equipment: two sets of dissecting scissors, tweezers, and scalpel
Additional reagents and equipment for blood collection by cardiac puncture (Donovan and Brown, 2006c)
NOTE: All reagents and equipment coming into contact with living cells must be sterile, and aseptic technique should be used accordingly.
Place animal in a small container (Tupperware works well) containing gauze or cotton sponges soaked with isoflurane to induce anesthesia.
-
When the animal’s breathing rate has slowed to less than one breath per 3 sec, remove the animal from the container and place the open end of a 50-ml centrifuge tube containing gauze or cotton sponges soaked with isoflurane over the nose of the animal to maintain anesthesia throughout the procedure. Ensure a snug fit by wrapping gauze around the animal’s nose.
CAUTION: Surgery on infected animals should be performed inside a biological safety cabinet to prevent exposure to infectious material.The anesthesia should be deep enough that the animal does not respond to toe pinch but not so deep that the heart stops. Optimal blood collection is performed when the heart is still beating. With animal placed on its back, open the skin via a vertical midline incision with one set of dissection scissors and tweezers.
-
To minimize risk of contaminating specimens with the animal’s skin flora, retract the skin and use a second set of dissection scissors, tweezers, and scalpel to open the peritoneal and chest cavities.
Opening of the chest cavity exposes the heart and lungs, preventing the animal from breathing and regaining consciousness. Obtain blood for culture and serology by cardiac puncture (Donovan and Brown, 2006c).
-
Obtain liver and kidney tissue for culture and histopathology.
These tissues contain the highest densities of organisms.The liver is the dark red multilobed organ in the right upper quadrant of the peritoneal cavity. Note any areas of hemorrhage. Using scissors or scalpels, obtain 1-cm × 1-cm segments of liver for culture and histopathology. To avoid crush artifacts, grasp only the margin of the organ segment with tweezers rather than the body of the organ.Hamster kidneys are normally the size, color, and shape of kidney beans. The kidneys are located on the left and right posterior walls of the peritoneal cavity and are visualized by moving the loops of intestine to one side or the other. Note any areas of hemorrhage or heterogeneity. Cut the vascular structures with scissors. To avoid crush artifacts, remove the organ by grasping only the hilum with tweezers, rather than the organ itself. One kidney should be cultured and the other submitted for histopathology. -
Consider collecting other tissues (lung or spleen) depending on the goals of the study.
The lungs are found in the chest cavities and appear as collapsed white structures after the chest cavity has been opened. Note any areas of hemorrhage. Remove the lungs by cutting the hilum with scissors. Examination of the lung is of interest because pulmonary hemorrhage is an important cause of morbidity and mortality in human disease.The spleen is a dark, red, ribbon-like structure in the left, upper peritoneal cavity and is visualized by moving the loops of intestine to the hamster’s right.
DETECTION OF LEPTOSPIRAL INFECTION
Three following three methods should be carried out in parallel to assess leptospiral infection.
Materials
Blood and organs from Leptospira-infected hamster (and blood from uninfected hamster as control in serological procedure)
Semisolid Leptospira medium with 5-fluorouracil (see recipe)
Phosphate-buffered saline (PBS; APPENDIX 2A) Culture of late logarithmic growth–phase Leptospira (UNIT 12E.1)
Neutral buffered formalin (e.g., Fisher)
70% (v/v) ethanol
Steiner-Steiner silver staining kit (optional; e.g., Sigma)
Stomacher bags (e.g., Thomas Scientific)
Microscope with dark-field optics (UNIT 2A.1)
Dedicated histopathology facility (optional)
Additional reagents and equipment for paraffin-embedding and sectioning of tissues (Zeller, 1989), hematoxylin/eosin staining (Zeller and Rogers, 1993), and Steiner-Steiner silver staining (Steiner and Steiner, 1944; reagents available as kit from Sigma and other histology suppliers)
NOTE: All reagents and equipment coming into contact with living cells must be sterile, and aseptic technique should be used accordingly.
To assess infection by standard bacteriological methods
-
1a. Inoculate blood into semisolid Leptospira medium with 5-fluorouracil at dilutions of 1/100 (by adding 0.1 ml blood to 10 ml semisolid medium) and 1/1000 (by adding 1.0 ml of the 1/100 dilution to 10 ml semisolid medium).
5-fluorouracil is added to this medium to prevent overgrowth of contaminants (Faine et al., 1999). -
2a. Inoculate tissue sample as follows. Place a 1 × 1–cm segment of liver tissue or one kidney in 1 ml EMJH liquid medium in a sterile Stomacher bag. Homogenize, then inoculate into semisolid medium with s-fluorouracil at dilutions of 1/100 (by adding 0.1 ml homogenized tissue to 10 ml semisolid medium) and 1/1000 (by adding 1.0 ml of the 1/100 dilution to 10 ml semisolid medium).
A simple way to homogenize tissue is to place the tissue and medium in a Stomacher bag, fold over the open end of the bag, and role a 100-ml cylindrical flask over the bag, forcing the contents between the flask and the hard surface of the counter top inside the biological safety cabinet. 3a. Examine a sample from each culture biweekly by dark-field microscopy for growth of leptospires.
To assess infection by serological examination
The microscopic agglutination test (MAT) described in steps 1b to 4b is a sensitive serological method of detecting exposure to Leptospira species (Faine et al., 1999).
1b. Prepare serum from the blood of the infected hamster by placing the blood in a sterile microcentrifuge tube, incubating 1 hr at 37°C, microcentrifuging 5 min at maximum speed, and transferring the medium to a separate tube. Similarly prepare serum from the blood of an uninfected hamster as control. Prepare serial two-fold dilutions of the serum in PBS.
2b. Mix each dilution with an equal volume of late log-phase Leptospira culture, then incubate 2 to 4 hr at room temperature.
3b. Examine each culture by dark-field microscopy for agglutination.
-
4b. Determine the titer endpoint, which is defined as the highest dilution resulting in 50% agglutination, measured by comparison with the density of leptospires incubated with negative control sera from uninfected hamsters (no agglutination).
Formation of agglutinating antibody indicates infection with leptospires. 50% agglutination is determined by comparing the density of organisms in the control sample (serum from uninfected hamster) versus the density of the organism in the test sample. Density of the leptospires is determined as described in UNIT 12E.1.
To assess infection by histopathology
1c. Fix a 1 × 1–cm square segment of liver tissue or one kidney in neutral buffered formalin for 8 hr, then transfer to 70% ethanol.
-
2c. Embed in paraffin and cut 4-μm sections according to standard procedures (e.g., Zeller, 1989).
The authors submit fixed tissues to a commercial pathology laboratory for embedding, sectioning, and staining. -
3c. Stain sections with hematoxylin and eosin (Zeller and Rogers, 1993) to characterize the histopathology of the organs. Perform silver staining using the technique of Steiner and Steiner (1944) to detect organisms.
Steiner-Steiner silver staining kits are available from Sigma and other suppliers. -
4c. Have the sections analyzed by a person trained in histopathology to identify the organisms and characterize the extent and severity of lesions, including assessment and grading of the location, number, and types of inflammatory cells present.
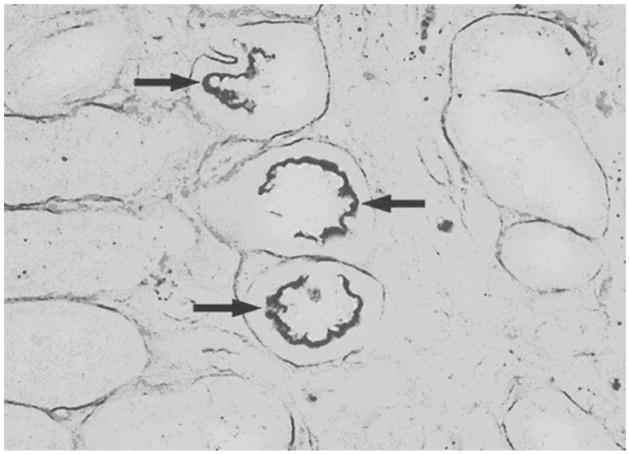
Figure 12E.2.1 shows a silver stain of hamster kidney tissue obtained 28 days after challenge showing leptospires (arrows) lining the epithelial surface of several adjacent renal tubules.
Figure 12 E.2.1.
Silver stain of hamster kidney tissue obtained 28 days after challenge. Arrows show positively-staining aggregates of leptospires lining the epithelial surface of several adjacent renal tubules. For the color version of this figure go to http://www.currentprotocols.com.
QUANTIFICATION OF INFECTION LEVELS USING REAL-TIME PCR
Culture, serology, and histopathology are sensitive methods of detecting sublethal leptospiral infection and are appropriate for most applications, including vaccine protection studies. However, these methods are not quantitative. For some types of research, such as antibiotic prophylaxis and treatment studies, a quantitative measure of leptospiral tissue burden is desirable. Amplification of a 87-bp region of the leptospiral 16S gene by quantitative (TaqMan) PCR has been shown to have a mean detection limit of two cells (Smythe et al., 2002).
Amplification and fluorescence detection is performed in an ABI Prism 7700 sequence detector. Use of other equipment may require use of different amplification conditions which should be determined empirically.
Additional Materials (also see Basic Protocols 1 and 2)
QIAamp Blood Kit (Qiagen)
DNeasy Tissue Kit (Qiagen)
TaqMan Universal PCR Master Mix (Applied Biosystems)
-
PCR primers:
Lepto F (5′-CCCGCGTCCGATTAG-3′)
Lepto R (5′-TCCATTGTGGCCGRACAC-3′)
-
TaqMan probe (Applied Biosystems):
[5′ (FAM)-CTCACCAAGGCGACGATCGGTAGC-(TAMRA) 3′]
Negative control: genomic DNA (Wilson, 1997) extracted from the nonpathogen L. biflexa (ATCC # 23582)
Positive control dilution series: genomic DNA (Wilson, 1997) extracted from serial dilutions containing 108 to 100 cells/ml of the Leptospira challenge strain
TE buffer
96-well PCR plate
ABI Prism 7700 Sequence Detection System (Applied Biosystems) with dedicated real-time PCR software
Challenge mice with Leptospira (Basic Protocol 1) and harvest blood and tissues (see Basic Protocol 2).
Extract total DNA from the blood and tissues of challenged hamsters using the QIAamp Blood Kit (Qiagen) and the DNeasy Tissue Kit (Qiagen), respectively.
-
For each sample DNA, negative control DNA, and positive control DNA dilution, prepare a PCR reaction mix (total volume, 50 μl; prepare all samples and controls in duplicate) in a PCR plate well as follows:
-
First prepare 45 μl TaqMan Universal PCR Master Mix containing:
3 pmol/μl Lepto F PCR primer
3 pmol/μl Lepto R PCR primer
2 pmol/μl TaqMan probe
Add 5 μl template DNA—i.e., sample DNA, negative control (L. biflexa) DNA, or positive control DNA dilution—to the corresponding wells.
Add 5 μl TE buffer to the designated wells in place of the DNA for a “no-template” negative control.
-
-
Conduct amplification and fluorescence detection in the ABI Prism 7700 dedicated thermal cycler using the following program, collecting the data throughout:
40 cycles: 15 sec 95°C 1 min 60°C. -
Using the dedicated real-time PCR software supplied with the ABI Prism 7700 Sequence Detection System, quantify the leptospiral DNA in the samples using the positive control dilution series of DNA from the Leptospira challenge strain as a standard curve.
UNIT 1D.3 provides more detail on the theory and practice of real-time PCR.
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps. For common stock solutions, see APPENDIX 2A; for suppliers, see SUPPLIERS APPENDIX.
BSA stock solution
Weigh out 10 g bovine serum albumin, fraction V, and add to 50 ml sterile, distilled water with constant stirring. Stir slowly to prevent foaming. If necessary, gently heat (<50°C). Once the BSA is dissolved, use immediately to prepare the complete BSA supplement (see recipe).
It is often convenient to start dissolving the BSA in the afternoon, then allow the solution to set overnight at 4°C.
The quality of BSA for use in media is important and must be determined empirically (see UNIT 12E.1).
Several vendors supply satisfactory BSA, fraction V, for use in media preparation. Before purchasing a large amount of BSA from an individual vendor, it is critical to test it by preparing the complete BSA supplement and using it to prepare the medium for a pilot experiment.
Complete BSA supplement
To 50 ml BSA stock solution (see recipe), add the following stock solutions with constant stirring in the following order:
1 ml calcium chloride stock solution (1 g CaCl2·2H2O/100 ml)
1 ml magnesium chloride stock solution (1 g MgCl2·2H2O/100 ml)
1 ml zinc sulfate stock solution (0.4 g ZnSO4·7H2O7/100 ml)
0.1 ml copper sulfate stock solution (0.3 g CuSO4·5H2O/100 ml)
10 ml ferrous sulfate stock solution (0.5 g FeSO4·7H2O/100 ml)
1 ml vitamin B12 stock solution (0.02 g/100 ml)
12.5 ml Tween 80 stock solution (10 g/100 ml)
Adjust pH to 7.4 with 2 N NaOH (~0.4 ml)
Bring the final volume to 100 ml with H2O
Sterilize by filtration through a 0.22-μm filter
-
Store indefinitely at −20°C
Although the final solution is filter sterilized, it is critically important that water for all stock solutions be sterilized by autoclaving prior to their preparation to eliminate potential contamination by saprophytic Leptospira.Leptospira require long-chain fatty acids or alcohols (provided by the Tween 20 in the above mixture) for growth, which can be toxic for growth in prepared media. Addition of albumin or animal serum can bind excess fatty acids, to enable growth of these bacteria in vitro.
EMJH basal salt solution
1 g Na2HPO4
0.3 g KH2PO4
1 g NaCl
1 ml ammonium chloride stock solution (25 g NH4Cl/100 ml)
1 ml thiamine stock solution (0.5 g thiamine/100 ml) 1 ml glycerol stock solution (10 g glycerol/100 ml)
Add components to ~800 ml glass-distilled water with stirring
Adjust pH to 7.4 with dilute NaOH or diluted HCl Bring volume to 1 liter with glass-distilled water
Sterilize by autoclaving
-
Store up to 1 month at 4°C
Volumes given are for completing a 1 liter solution.
EMJH liquid medium
-
Aseptically add 100 ml complete BSA supplement (see recipe) and 10 ml of 0.01 g/ml 5- fluorouracil (5-FU) to 890 ml EMJH basal salt solution (see recipe). Divide into aliquots as needed for experiments. Store up to 1 month at room temperature.
This medium is quite stable for routine propagation of cultures. However, for isolation from clinical samples, fresh medium should be prepared monthly.
Semisolid Leptospira medium with 5-fluorouracil
-
Add 1.5 g agar to 890 ml basal salt solution (see recipe) and autoclave. Cool to ~50°C, then add 100 ml sterile complete BSA supplement (see recipe) and 10 ml of 0.01 g/ml 5-fluorouracil (5-FU), both sterilized by filtration through a 0.22-μm filter. Store in 1-liter bottles up to 1 year at room temperature.
Primary isolation, propagation, and freezing of Leptospira cultures are often done in semisolid medium. The semisolid medium has properties similar to a suspended slurry, and can be stored in 1-liter bottles and dispensed as needed. The semisolid medium prepared in this recipe is a modified EMJH medium; by addition of the appropriate supplements, a modified, semisolid T80/40/LH medium (UNIT 12E.1) may also be prepared.
COMMENTARY
Background Information
The Golden Syrian hamster model of leptospirosis is well characterized and has a number of important advantages, including excellent reproducibility and susceptibility to a broad range of pathogenic strains (Stavitsky, 1945; Ferguson and Hamdy, 1957; Miller and Wilson, 1966; van den Ingh and Hartman, 1986). The susceptibility of hamsters to leptospiral infection may be related to the fact that these animals evolved in a dry desert habitat where they would rarely have been exposed to the moist climatic conditions conducive to transmission of leptospirosis. The fulminant, disseminated infection seen in hamsters is typical of the accidental host pattern of disease. As such, the hamster model is an appropriate model of the severe form of human leptospirosis.
Guinea pigs (Stavitsky, 1945; Faine, 1957a,b; Nally et al., 2004) and gerbils (Lewis and Gray, 1961) have also been found to be suitable animal models of fulminant-type accidental infection. The guidelines presented here are generally applicable to these alternative models. By contrast, rats and mice are reservoir or maintenance hosts in nature. Once rats and mice become immunologically mature, they are typically resistant to experimental leptospiral challenge, but may develop a sublethal infection of the renal tubules (Faine, 1962). Very young, C3H/HeJ mice can develop disseminated infection (Pereira et al., 1998). However, mice have an extremely short window of susceptibility, which can be a technical or logistical hurdle to overcome. Development of a mouse-lethal leptospiral strain by repeatedly challenging and reisolating the organism from the blood stream of mice has been reported (Koizumi and Watanabe, 2004). Leptospiral vaccine studies have been described using a mouse-adapted challenge strain obtained after more than ten passages in C3H/HeJ mice (Koizumi and Watanabe, 2004). Leptospiral vaccine studies have been described using a mouse-adapted challenge strain obtained after more than ten passages in C3H/HeJ mice (Koizumi and Watanabe, 2004).
The hamster model is not without some disadvantages. Hamsters are not allowed in certain parts of the world, such as Australia. Consequently, much of the early work by Solly Faine and colleagues was done in guinea pigs (Faine, 1957a,b) and mice (Faine, 1962). Larger animals such as dogs may be more appropriate for studies examining renal physiology of interstitial nephritis due to leptospirosis. Vaccines developed in hamsters must be validated in the target animal. Relatively few hamster-specific reagents are commercially available, making it difficult to examine the immunologic and cytokine response to infection. Likewise, hamster microarrays are not available to examine the transcriptional response of host tissues to leptospiral infection. Ultimately, gene knockouts that render mice predictably susceptible to leptospiral infection or, better yet, mice with critical genes replaced by human counterparts may become better models of human infection.
Critical Parameters
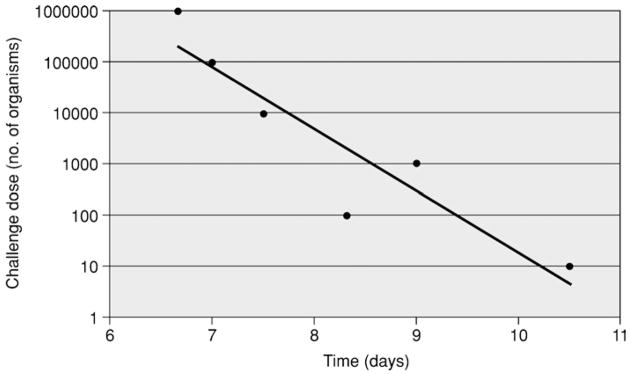
Based on animal husbandry issues and the reproducibility of the model, hamsters are the animal model of choice for leptospiral vaccine and antibiotic treatment studies. As shown in Figure 12E.2.2, the correlation between the time from challenge to 50% survival and the challenge dose is remarkably linear. When establishing the hamster model using a new challenge strain, it is essential to determine its LD50 by serial dilution. In organisms that have been passaged in vitro less than ten times after isolation from an animal host, the LD50 for intraperitoneal inoculation of 3- to 4-week-old hamsters may approximate one organism.
Figure 12E.2.2.
Time from challenge to 50% mortality. 3-week-old Golden Syrian hamsters in groups of four were challenged by intraperitoneal inoculation with serial 10-fold dilutions of Leptospira kirschneri serovar Grippotyphosa, strain RM52. Each dose was administered to hamsters in groups of four. Each point represents the time to death of the second of four hamsters. The LD50 of this strain is <10 organisms.
Troubleshooting
Leptospires rapidly lose virulence during in vitro cultivation. Loss of the ability to produce lethal infection in hamsters can occur in as few as ten passages in culture medium. Stocks of primary cultures isolated from animals should be stored in liquid nitrogen, as described in UNIT 12E.1, and thawed out when needed for animal-challenge studies. Culture attenuation can also be minimized by passaging cultures frequently enough to avoid onset of stationary phase. More highly passaged strains and strains of unknown passage number may be reisolated from hamsters to obtain a challenge strain with improved virulence.
Anticipated Results
Lethal infection may occur in <100% of hamsters that are older than 4 weeks (immunologically more mature) or that have been immunized as part of a vaccine trial. Paradoxically, increased survival can be observed when older hamsters (>4 weeks) are challenged with higher doses of certain strains (Barnett et al., 1999). Animals that survive challenge may have sublethal infection, particularly involving the kidneys (Barnett et al., 1999; Haake et al., 1999). When carried out correctly, there is generally an excellent correlation between culture isolation, serology, and histopathology for detection of sublethal infection.
Another benefit of hamsters is that an alternative, and possibly more biologically relevant, subcutaneous challenge route has been demonstrated (Trucollo et al., 2002).
Time Considerations
Successful challenge studies require coordinating the simultaneous availability of Golden Syrian hamsters and virulent leptospiral cultures. If hamsters are being shipped from another institution, contact the provider weeks to months in advance to ensure that sufficient numbers of animals of the proper age and sex will be available for shipping on the desired date. After arrival, hamsters typically require a 1-week quarantine period for acclimatization and screening for infection. Virulent leptospires typically require at least 1 week of growth after inoculation of culture medium from frozen stocks. However, leptospiral growth rates vary depending on the strain and the quality of the medium (see UNIT 12E.1 for details).
In experienced hands, intraperitoneal inoculation of hamsters (see Basic Protocol 1) takes 5 min per animal. Putting on personal protective equipment (gown, gloves, eyeware, mask, cap, and shoe covering) and preparing the work area requires 20 to 30 min. Another 20 to 30 min are required after the inoculations are completed to decontaminate the work area, discard syringes, and remove personal protective equipment. Animals should be checked 1 hr after intraperitoneal inoculation to ensure that no complications have ensued. Checking the health of animals on the day of inoculation and on subsequent days typically requires less than 1 min per cage.
Harvesting blood and tissues from hamsters (see Basic Protocol 2) takes 15 min per animal. Depending on strain virulence and challenge dose, hamsters may reach endpoint criteria as early as 3 days post challenge or as late as 21 days post challenge. Blood and tissue are immediately inoculated into semisolid culture medium (see Basic Protocol 3), which takes 5 min per specimen. Blood not used for culture takes 1 hr to clot, after which 5 min are required for centrifugation, separation of serum from cells, and storage. Cultures of primary isolates should be examined weekly and may take weeks to months to become positive. Dark-field microscopy of cultures takes 5 min per sample. The microscopic agglutination test of serum from hamsters requires 20 to 30 min to set up, followed by 2 to 4 hr of incubation time and 20 to 30 min to read the results by dark-field microscopy, depending on the number of samples. Examination of the histopathology slides takes 30 to 45 min, depending on the number of slides and the experience of the observer.
Key References
Faine et al., 1999. See above.
This monograph contains a wealth of detailed information and useful commentary and is an invaluable resource that should be on hand in any laboratory that works with animal models of leptospirosis.
Richmond, J.Y. and McKinney, W. 1999. Biosafety in Microbiological and Biomedical Laboratories, 4th ed. US Government Printing Office, Washington, D.C.
Printed version of important safety document, also available online (see Internet Resources).
Footnotes
Internet Resources
http://www.cdc.gov/od/ohs/biosfty/bmbl4/bmbl4toc.htm
Web site for the guide to Biosafety in Microbiological and Biomedical Laboratories, 4th Edition (Richmond and McKinney, 1999; see Key References).
Literature Cited
- Barnett JK, Barnett D, Bolin CA, Summers TA, Wagar EA, Cheville NF, Hartskeerl RA, Haake DA. Expression and distribution of leptospiral outer membrane components during renal infection of hamsters. Infect Immun. 1999;67:853–861. doi: 10.1128/iai.67.2.853-861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin CA, Alt DP. Use of a monovalent leptospiral vaccine to prevent renal colonization and urinary shedding in cattle exposed to Leptospira borgpetersenii serovar hardjo. Am J Vet Res. 2001;62:995–1000. doi: 10.2460/ajvr.2001.62.995. [DOI] [PubMed] [Google Scholar]
- Donovan J, Brown P. Handling and restraint. In: Coligan JE, Bierer B, Margulies DH, Strober W, editors. Current Protocols in Immunology. John Wiley & Sons; Hoboken, N.J: 2006a. pp. 1.3.1–1.3.6. [DOI] [PubMed] [Google Scholar]
- Donovan J, Brown P. Parenteral injections. In: Coligan JE, Bierer B, Margulies DH, Strober W, editors. Current Protocols in Immunology. John Wiley & Sons; Hoboken, N.J: 2006b. pp. 1.6.1–1.6.9. [DOI] [PubMed] [Google Scholar]
- Donovan J, Brown P. Blood collection. In: Coligan JE, Bierer B, Margulies DH, Strober W, editors. Current Protocols in Immunology. John Wiley & Sons; Hoboken, N.J: 2006c. pp. 1.7.1–1.7.9. [DOI] [PubMed] [Google Scholar]
- Faine S. Virulence in Leptospira. I: Reactions of guinea pigs to experimental infection with Leptospira icterohaemorrhagiae. Brit J Exp Pathol. 1957a;38:1–7. [PMC free article] [PubMed] [Google Scholar]
- Faine S. Virulence in Leptospira. II: The growth in vivo of virulent Leptospira icterohaemorrhagiae. Brit J Exp Pathol. 1957b;38:8–14. [PMC free article] [PubMed] [Google Scholar]
- Faine S. The growth of Leptospira australis B in the kidneys of mice in the incipient experimental carrier state. J Hyg. 1962;60:435–442. doi: 10.1017/s0022172400020568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faine S, Adler B, Bolin C, Perolat P. Leptospira and leptospirosis. 2. MediSci; Melbourne, Australia: 1999. [Google Scholar]
- Ferguson LC, Hamdy AH. Virulence of Leptospira pomona in hamsters and cattle. Am J Vet Res. 1957;18:35–42. [PubMed] [Google Scholar]
- Haake DA, Mazel MK, McCoy AM, Milward F, Chao G, Matsunaga J, Wagar EA. Leptospiral outer membrane proteins OmpL1 and LipL41 exhibit synergistic immunoprotection. Infect Immun. 1999;67:6572–6582. doi: 10.1128/iai.67.12.6572-6582.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi N, Watanabe H. Leptospiral immunoglobulin-like proteins elicit protective immunity. Vaccine. 2004;22:1545–1552. doi: 10.1016/j.vaccine.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Lewis C, Gray JE. Experimental Leptospira pomona infection in the Mongolian gerbil (Meriones unguiculatus) J Infect Dis. 1961;109:194–204. doi: 10.1093/infdis/109.2.194. [DOI] [PubMed] [Google Scholar]
- Miller CD, Songer JR, Sullivan JF. A twenty-five year review of laboratory-acquired human infections at the National Animal Disease Center. Am Ind Hyg Assoc J. 1987;48:271–275. doi: 10.1080/15298668791384733. [DOI] [PubMed] [Google Scholar]
- Miller NG, Wilson RB. Electron microscopy of the liver of the hamster during acute and chronic leptospirosis. Am J Vet Res. 1966;27:1071–1081. [PubMed] [Google Scholar]
- Nally JE, Chantranuwat C, Wu XY, Fishbein MC, Pereira MM, Da Silva JJ, Blanco DR, Lovett MA. Alveolar septal deposition of immunoglobulin and complement parallels pulmonary hemorrhage in a guinea pig model of severe pulmonary leptospirosis. Am J Pathol. 2004;164:1115–1127. doi: 10.1016/S0002-9440(10)63198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira MM, Andrade J, Marchevsky RS, Ribeiro dos Santos R. Morphological characterization of lung and kidney lesions in C3H/HeJ mice infected with Leptospira interrogans serovar icterohaemorrhagiae: Defect of CD4+ and CD8+ T-cells are prognosticators of the disease progression. Exp Toxicol Pathol. 1998;50:191–198. doi: 10.1016/S0940-2993(98)80083-3. [DOI] [PubMed] [Google Scholar]
- Pike RM. Laboratory-associated infections: Summary and analysis of 3921 cases. Health Lab Sci. 1976;13:105–114. [PubMed] [Google Scholar]
- Stavitsky AB. Studies on the pathogenesis of leptospirosis. J Infect Dis. 1945;76:179–192. [Google Scholar]
- Smythe LD, Smith IL, Smith GA, Dohnt MF, Symonds ML, Barnett LJ, McKay DB. A quantitative PCR (TaqMan) assay for pathogenic Leptospira spp. BMC Infect Dis. 2002;2:13. doi: 10.1186/1471-2334-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner G, Steiner G. New simple silver stain for demonstration of bacteria, spirochetes, and fungi in sections of paraffin embedded tissue blocks. J Lab Clin Med. 1944;29:868–871. [Google Scholar]
- Truccolo J, Charavay F, Merien F, Perolat P. Quantitative PCR assay to evaluate ampicillin, ofloxacin, and doxycycline for treatment of experimental leptospirosis. Antimicrob Agents Chemother. 2002;46:848–853. doi: 10.1128/AAC.46.3.848-853.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Ingh TS, Hartman EG. Pathology of acute Leptospira interrogans serotype icterohaemorrhagiae infection in the Syrian hamster. Vet Microbiol. 1986;12:367–376. doi: 10.1016/0378-1135(86)90086-6. [DOI] [PubMed] [Google Scholar]
- Wilson K. Preparation of genomic DNA from bacteria. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. John Wiley & Sons; Hoboken, N.J: 1997. pp. 2.4.1–2.4.5. [DOI] [PubMed] [Google Scholar]
- Zeller R. Fixation, embedding, and sectioning of tissues, embryos, and single cells. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. John Wiley & Sons; Hoboken, N.J: 1989. pp. 14.1.1–14.1.8. [DOI] [PubMed] [Google Scholar]
- Zeller R, Rogers M. Counterstaining and mounting of autoradiographed in situ hybridization slides. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. John Wiley & Sons; Hoboken, N.J: 1993. pp. 14.5.1–14.5.5. [DOI] [PubMed] [Google Scholar]