Abstract
The use of fertility drugs has continued to grow since their introduction in the 1960s. Accompanying this increase has been the speculation that repetitive use of these drugs can cause ovarian tumors or cancer. We recently reported that transgenic mice with chronically elevated luteinizing hormone (LH), an analog of which is commonly used in fertility regimens, develop granulosa cell (GC) tumors. In this report we show that LH induction of these tumors is highly dependent on genetic background. In CF-1 mice, chronically elevated LH invariably causes GC tumors by 5 months of age. However, in hybrid mice generated by crossing CF-1 males with C57BL/6, SJL, or CD-1 females, elevated levels of this same hormone cause a completely different phenotype resembling a luteoma of pregnancy. We also show that three genes likely control these alternative hormonal responses. This clinical correlate of elevated LH reveals remarkably distinct, strain-dependent, ovarian phenotypes. In addition, these results support the rare incidence of GC tumors in the human population, and suggest that the ability of certain fertility drugs to cause ovarian tumors may depend on an individual's genetic predisposition.
The impact of fertility drugs on ovarian cancer has been highly debated, partly because of the absence of a comprehensive epidemiological study that compares the risk of ovarian cancer associated with the use of these drugs to the risk associated with nulliparity resulting from infertility (1, 2). Although some limited studies suggest that there is no correlation between fertility drug treatments and ovarian cancer (3, 4), several case reports suggest that there may be a link (5–7). With the use of these drugs increasing, it is important to assess their long-term impact on the ovary.
Most ovulation-induction regimens elevate serum levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Both LH and FSH are members of the glycoprotein hormone family and are heterodimers that contain an α subunit common to each hormone and a unique β subunit that dictates biological specificity (8). LH and FSH are produced in pituitary gonadotropes in all mammals, and they are essential for normal female reproduction. Both promote steroidogenesis and gametogenesis by the ovary. Absence of these hormones results in infertile individuals who maintain a prepubescent state into adulthood with infantile gonads (9, 10).
To address the role of LH in ovarian tumor development, we recently developed a transgenic mouse model of chronic LH hypersecretion. This was accomplished by using a transgene composed of a bovine LH β-subunit expression cassette under the control of the α-subunit promoter of the glycoprotein hormones (11). The LH β subunit expressed from the transgene combines with the excessive α subunit present within the gonadotrope (11). The serum half-life of the LH heterodimer was also increased by the addition of the C-terminal peptide of human chorionic gonadotropin to the C terminus of the bovine LH β subunit (11). Because expression of the transgene occurs only in gonadotropes in the pituitary, serum levels of LH reflect those that could occur in a mouse if appropriate regulation of the LH β subunit were disrupted. Female transgenic mice display a variety of phenotypes, including precocious puberty (12), polycystic ovaries (11, 12), elevated androgens (12), elevated estrogens (11), elevated progesterone (11), anovulation and infertility (11–13), accelerated depletion of primordial follicles (14), and uterine receptivity defects (13), and they eventually develop granulosa cell (GC) tumors (11). Whereas these mice display chronically elevated LH, women usually have cyclic peaks and valleys of serum LH, which is also true of ovulation-induction protocols that use the LH-containing drug, human menopausal gonadotropin (hMG). Independent of cyclical pattern, the mice display many of the side effects of excessive gonadotropin stimulation that are seen in women with ovarian hyperstimulation syndrome. Herein we describe the genetic dependency of LH-induced GC tumors. The genetic heterogeneity observed in the mice and the strict requirements for the appropriate favorable genetic milieu may parallel ovarian hyperstimulation in humans, and explain, in part, the inability to assess GC tumor risk in women undergoing pharmacological treatment of infertility.
Materials and Methods
Transgenic Mice.
Mice harboring the LH-overexpression transgene (LHβCTP) have previously been described (9). Male transgenic mice have been repetitively outbred (at least 10 generations) with CF-1 females purchased from Charles River Laboratories. All mice were housed in microisolator plus units, with a 12-hr light/dark cycle, and with food and water given ad libitum. Transgene genotyping was performed by using PCR as previously described (9). Adult CF-1 males harboring the LHβCTP transgene were bred with adult females from one of the following strains: CF-1, CD-1, C57BL/6, and SJL. Male progeny were removed from each cage when they were 2 weeks old. Age of vaginal opening was used as an assessment of puberty of the female progeny. To determine whether vaginal opening had occurred, the vagina was probed daily with a Pasteur pipette probe from postpartum day 20 until opening was observed. Mice were housed for a total of 5 or 10 months prior to the collection of ovaries and blood through cardiac puncture.
Backcrosses involved breeding F1 hybrid (C57BL/6 ♀ × CF-1,Tg ♂) transgenic males with CF-1 females. At weaning, male progeny were removed from all cages. Female mice were genotyped at 4 to 6 weeks and then housed for a total of 5 months prior to the collection of ovaries and cardiac blood.
All animal protocols were approved by the Institutional Animal Use and Care Committee at Case Western Reserve University.
Hormone Assays.
Cardiac blood was collected and allowed to clot, and the serum was retained and stored at −20°C for future analyses. Serum LH and testosterone levels were measured by using previously described radioimmunoassays validated for use with mouse serum (15, 16).
Tissue Analyses.
Mice were killed by asphyxiation in a CO2 chamber. Tissues were immersed in Kahle's fixative immediately after removal from the animal and stored until embedded in paraffin. Five-micrometer sections of ovaries were stained with hematoxylin and eosin before the analysis of tumor type.
Results and Discussion
Initial studies analyzing the impact of the LH-encoding transgene involved the use of mice in a combined genetic background with contributions from the CF-1, C57BL/6, and SJL strains. In these studies, some older mice developed GC tumors (11). However, upon repetitive multigenerational breeding into the outbred CF-1 strain, the frequency of the GC tumor phenotype progressively increased to 100% when the animals were 5 months old (data not shown). To assess the possible genetic influences on the ovarian response to elevated LH, we bred CF-1 males with the transgene to females representing four different strains of mice: CF-1, CD-1, C57BL/6, and SJL. Several parameters associated with GC tumors were assessed in the F1 hybrid females that resulted from these crosses. We were unable to perform the potentially informative converse experiment of breeding CF-1 transgenic females with males from the CF-1, CD-1, C57BL/6, or SJL strains, because all of the females harboring the transgene are infertile. Thus, potential imprinting issues could not be addressed.
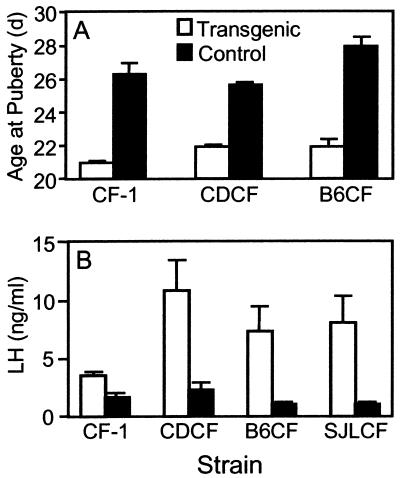
An early indicator of elevated LH in transgenic mice from the CF-1 strain is precocious vaginal opening (12), a hallmark of puberty. To determine whether the F1 hybrid female transgenic mice also underwent precocious puberty as a result of elevated LH, age at vaginal opening was determined in two F1 hybrids, (C57BL/6 ♀ × CF-1,Tg ♂) and (CD-1 ♀ × CF-1,Tg ♂); and in the parental CF-1 strain. Transgenic female mice from both F1 hybrids and the CF-1 strain displayed early vaginal opening that preceded their nontransgenic counterparts by at least 5 days (Fig. 1A), suggesting that the transgene was functional in all transgenic mice and that elevated LH early in life was independent of genetic background. To confirm that serum levels of LH were elevated in all of the F1 transgenic mice, serum was collected from 5-month-old transgenic and control mice. LH was elevated at least 4-fold in the transgenic mice compared with nontransgenic controls in all of the F1 hybrid strains at 5 months (Fig. 1B). Interestingly, 5-month-old transgenic females corresponding to the parental CF-1 strain did not display the degree of LH induction that had previously been observed in younger mice of the same strain (12). Typically, we have observed a 5- to 20-fold induction of serum LH in younger transgenic females compared with nontransgenic controls (11, 13). However, in the current study with much older females, serum LH was elevated only 2-fold (Fig. 1B). All of the mice from this strain bear GC tumors at this age, suggesting that the tumors may produce an inhibitory factor that has a negative impact on transgene expression. One possible candidate is inhibin, a common product of this type of tumor (17).
Figure 1.
Chronically elevated LH accelerates vaginal opening and increases in serum levels of LH are dependent on genetic background. (A) Age of vaginal opening for transgenic females (open bars) and nontransgenic controls (closed bars) was determined for two F1 hybrid strains and the CF-1 strain. (B) Serum levels of LH were measured in 5-month-old female transgenic (open bars) and nontransgenic (closed bars) mice representing the CF-1 parental strain and the three F1 hybrid strains. Strain designations are B6CF (C57BL/6 ♀ × CF-1,Tg ♂); CDCF (CD-1 ♀ × CF-1,Tg ♂); and SJLCF (SJL ♀ × CF-1,Tg ♂). The numbers of samples used for data sets are: CF-1 transgenics = 8, nontransgenics = 3; (C57BL/6 ♀ × CF-1,Tg ♂, transgenics = 6, nontransgenics = 7; (CD-1 ♀ × CF-1,Tg ♂, transgenics = 13, nontransgenics = 5; and (SJL ♀ × CF-1,Tg ♂), transgenics = 4, nontransgenics = 3. Values are means ± SEM.
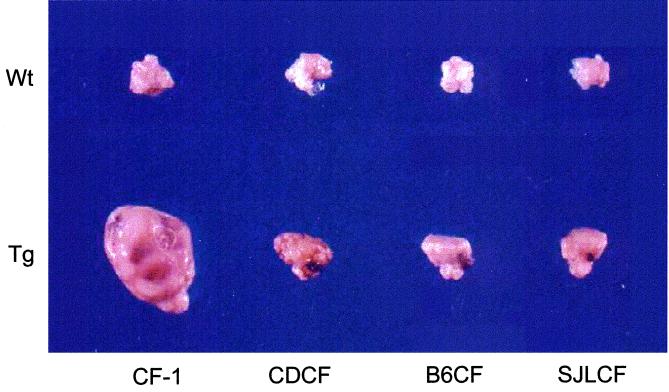
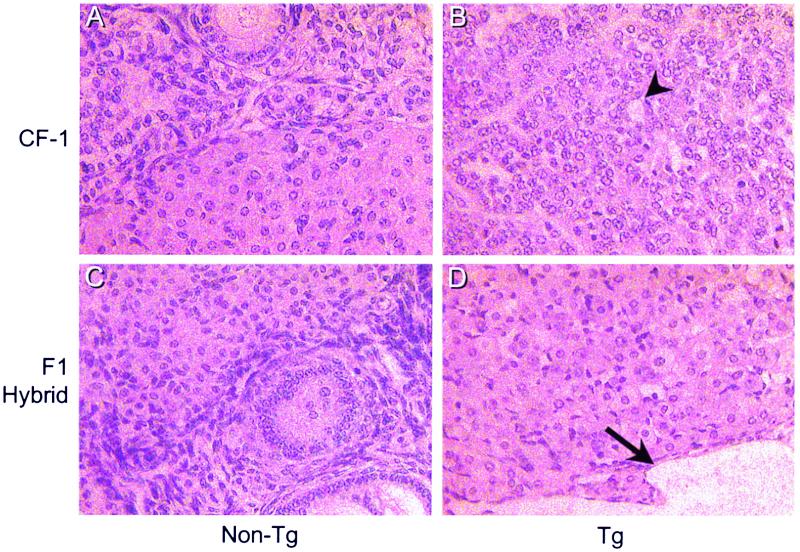
An examination of ovaries from the F1 hybrid mice indicated a profound difference between the individual strains in their response to elevated LH (Fig. 2). As expected, all mice from the CF-1 strain developed large GC tumors. In contrast, all F1 hybrid mice did not develop GC tumors (Table 1). Although the F1 hybrids failed to develop GC tumors, their ovaries were enlarged with numerous blood- and fluid-filled cysts, compared with nontransgenic control ovaries, as a pathological consequence of the altered endocrine milieu. In addition, histological analyses of the ovaries from the F1 transgenic mice generated from these crosses revealed that whereas mice from the CF-1 strain develop GC tumors, all F1 hybrid mice displayed cystic ovaries with an extensively luteinized phenotype reminiscent of a luteoma of pregnancy (Fig. 3). One F1 hybrid transgenic mouse from the (C57BL/6 ♀ × CF-1,Tg ♂) strain had a small focal proliferation of GC cells (tumorlette), similar to those reported to be associated with pregnancy, which is probably not a small neoplasm (18). This was not considered a GC tumor, because it occurred within a defined boundary, did not consume more than 50% of the ovary, and was accompanied by normal ovarian structures. To determine whether the influence of genetic background was simply a change in the latency of tumor formation, 10-month-old transgenic female mice representing the (C57BL/6 ♀ × CF-1,Tg ♂) hybrid were also examined. No GC tumors were observed in these mice (data not shown). Thus, genetic contributions from the C57BL/6 strain fully prevent the outgrowth of large GC tumors. The fact that the GC tumor phenotype was lost after a single round of breeding into strains other than CF-1 suggests that at least one gene with recessive effects must be involved in the differential ovarian response to elevated LH. In addition, these results indicate that precocious puberty and development of cysts do not necessarily predicate eventual ovarian tumor formation.
Figure 2.
GC tumor induction in response to chronically elevated LH depends on genetic background. Representative ovaries from control (Upper, Wt) or transgenic (Lower, Tg) mice from either the CF-1 parental strain or F1 hybrid strains. Hybrid strain designations are described in the legend of Fig. 1.
Table 1.
Development of GC tumors is dependent upon the CF-1 genetic background
| Mice | No. with
tumors/total no. examined
|
|
|---|---|---|
| GC tumors | Luteomas | |
| CF-1 | 8/8 | 0/8 |
| (CD-1♀ × CF-1, Tg ♂)F1 | 0/13 | 13/13 |
| (C57BL/6♀ × CF-1, Tg ♂)F1 | 0/6 | 6/6 |
| (SJL♀ × CF-1, Tg ♂)F1 | 0/4 | 4/4 |
Figure 3.
Histological examination of ovaries at 5 months from the CF-1 strain (A and B) and from the (C57BL/6 ♀ × CF-1,Tg ♂)F1 hybrids (C and D). (A) Nontransgenic CF-1. (B) Transgenic CF-1. (C) Nontransgenic F1 hybrid. (D) Transgenic F1 hybrid. The arrowhead indicates a Call-Exner body, which is a characteristic rosette-like structure common to GC tumors. The arrow indicates a fluid-filled cyst.
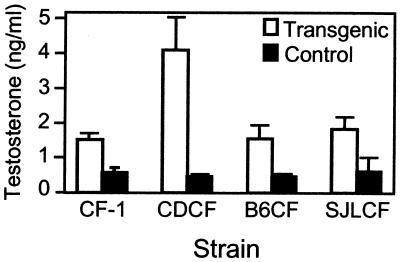
Previous studies that have examined the hormonal basis of spontaneous GC tumors in mice have implicated androgens in the tumorigenic pathway (19). An analysis of the serum levels of testosterone in the F1 hybrid mice revealed that all of the transgenic mice have elevated testosterone compared with age-matched controls. Increased serum levels of testosterone were independent of genetic composition (Fig. 4) and tumor development. Thus, we conclude that although testosterone may be necessary for GC tumor formation in these animals, it is not sufficient to initiate a tumorigenic cascade of events.
Figure 4.
Chronically elevated LH increases serum testosterone levels independently of genetic background. Serum levels of testosterone were measured in 5-month-old female transgenic (open bars) and nontransgenic (closed bars) mice representing the parental CF-1 strain and the three F1 hybrid strains. Strain designations and sample numbers are as described in Fig. 1. Values are means ± SEM.
To estimate the number of genetic modifiers that may be involved in the differential ovarian response to chronically elevated LH, we performed backcross experiments. Transgenic, F1 hybrid males corresponding to the (C57BL/6 ♀ × CF-1,Tg ♂) hybrids were crossed to CF-1 females. We selected only a single hybrid strain, (C57BL/6 ♀ × CF-1,Tg ♂), to perform the backcross experiments because of the large numbers of animals required for completion of these studies. The (C57BL/6 ♀ × CF-1,Tg ♂) strain was chosen because of the relatively high reproductive efficiency of C57BL/6, and the extensive microsatellite marker map that is available for this strain. Five-month-old female transgenic progeny from this cross were scored for appearance of the GC tumor phenotype. Of the 138 transgenic female mice generated from these crosses, 19 harbored at least one GC tumor. Seven additional mice also displayed similar tumorlettes, as observed in the single animal from the original hybrid crosses. These focal proliferations were not considered GC tumors in the statistical analysis of trait inheritance. Because serum levels of LH and testosterone and the age of vaginal opening do not correlate with GC tumor development, these assays were not performed on backcross progeny.
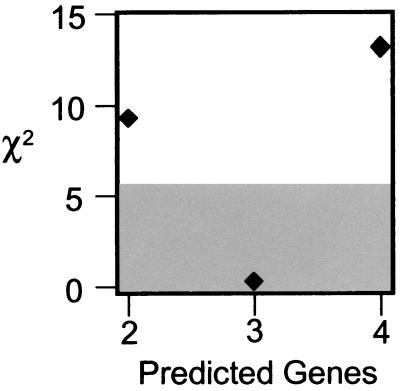
For assessment of the number of genes underlying tumor development in response to elevated LH, we tested the fit between the predicted and observed (13.8% = 19 affected mice among 138 transgenic female mice surveyed) frequency of tumor appearance. The segregation results fit a three-gene model better than all other models (Fig. 5). We assumed that each gene acted in an equal, additive, and independent manner; we also assumed that tumorigenesis showed 100% penetrance among the backcross progeny as it did among the transgenic parental line. The χ2 goodness-of-fit test had one degree of freedom, and the critical χ2 value was increased from 3.8 to 5.7, to account for multiple testing. Goodness-of-fit tests were performed for two-, three-, and four-gene models, i.e., three tests. The expected number of affected mice for the two-, three-, and four-gene models were 34.5, 17.3, and 8.6 (25%, 12.5%, and 6.25% of 138 individuals examined), respectively. Only the three-gene model displayed a good fit with the empirical data (χ2 = 0.20, P > 0.66), with both the two-gene and the four-gene models being rejected (two-gene: χ2 = 9.28, P < 0.003; four-gene: χ2 = 13.31, P < 0.0003). Indeed, the probability that three genes underlie the GC tumor trait is approximately 220- to 2,200-fold (0.66/0.003; or 0.66/0.0003) greater than either the two- or four-gene models. These results suggest that susceptibility to tumors appears to be an oligogenic trait controlled by three unlinked genes. Mapping studies are needed to validate this model and establish genetic linkage.
Figure 5.
Only the three-gene model is supported by χ2 analyses. χ2 values for the two-, three-, and four-gene models are plotted against the predicted number of genes within that model. Points outside of the shaded area reflect models that are rejected because of a χ2 value > 5.7 (P < 0.05).
Another mouse model of GC tumors has been described that also displays genetic background dependency (19, 20). In contrast to the LH overexpression model, pubertal mice from the SWR strain develop spontaneous GC tumors, and the incidence is increased with dihydroepiandrosterone (DHEA) treatment (19). At least four loci are responsible for the development of tumors in these mice (20). The model presented herein is distinct from that established by Beamer, et al. (20), in that the loci implicated impact LH induction of GC tumors in adult mice, rather than affecting their relatively infrequent spontaneous development in pubertal animals. Whether some genes contribute to both of these etiologies remains to be determined.
In conclusion, we have demonstrated that chronically elevated LH can cause GC tumors. However, the ability of LH to initiate the tumorigenic pathway is closely associated with genetic predisposition. In CF-1 mice, tumor development in response to elevated LH likely involves a small number of genes that appear to act in concert to mediate activation of this pathway. The necessity for the appropriate combinatorial genetic influence in the development of tumors in response to elevated LH may underlie the apparent inability to link infertility treatments involving ovarian stimulation with development of ovarian tumors in humans. Further work is necessary to determine the complement of predisposition genes in CF-1 mice. Such work will likely involve the use of microsatellite marker linkage analysis to identify contributing loci. Once the necessary genes are identified, dissecting their presence and function in the human ovary will be an important next step in assessing the true pathological impact of high LH on ovarian carcinogenesis. These results also suggest that epidemiological risk analyses of fertility drug association with ovarian tumor development will be incomplete until it is known whether similar oligogenic differences among individuals occurs in humans.
Acknowledgments
We thank Mr. David Peck for his excellent technical support. This work was supported by National Institutes of Health Grants HD34032 to J. H. Nilson and CA75056 to J. H. Nadeau; and F.W.A.-K. was supported by the University Hospitals of Cleveland Pathology Associates.
Abbreviations
- LH
luteinizing hormone
- GC tumor
granulosa cell tumor
References
- 1.Artini P G, Fasciani A, Cela V, Battaglia C, de Micheroux A A, D'Ambrogio G, Genazzani A R. Gynecol Endocrinol. 1997;11:59–68. doi: 10.3109/09513599709152318. [DOI] [PubMed] [Google Scholar]
- 2.Venn A, Watson L, Lumley J, Giles G, King C, Healy D. Lancet. 1995;346:995–1000. doi: 10.1016/s0140-6736(95)91687-3. [DOI] [PubMed] [Google Scholar]
- 3.Mosgaard B J, Lidegaard O, Kjaer S K, Schou G, Andersen A N. Fertil Steril. 1997;67:1005–1012. doi: 10.1016/s0015-0282(97)81431-8. [DOI] [PubMed] [Google Scholar]
- 4.Bristow R E, Karlan B Y. Fertil Steril. 1996;66:499–507. doi: 10.1016/s0015-0282(16)58557-4. [DOI] [PubMed] [Google Scholar]
- 5.Willemsen W, Kruitwagen R, Bastiaans B, Hanselaar T, Rolland R. Lancet. 1993;341:986–988. doi: 10.1016/0140-6736(93)91071-s. [DOI] [PubMed] [Google Scholar]
- 6.Rossing M A, Daling J R, Weiss N S, Moore D E, Self S G. N Engl J Med. 1994;331:771–776. doi: 10.1056/NEJM199409223311204. [DOI] [PubMed] [Google Scholar]
- 7.Parazzini F, Negri E, La Vecchia C, Moroni S, Franceschi S, Crosignani P G. Hum Reprod. 1997;12:2159–2161. doi: 10.1093/humrep/12.10.2159. [DOI] [PubMed] [Google Scholar]
- 8.Pierce J G, Parsons T F. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- 9.Kendall S K, Samuelson L C, Saunders T L, Wood C I, Camper S A. Genes Dev. 1995;9:2007–2019. doi: 10.1101/gad.9.16.2007. [DOI] [PubMed] [Google Scholar]
- 10.Friedman A J. In: Encyclopedia of Reproduction. Knobil E, Neill J D, editors. San Diego: Academic; 1998. pp. 735–744. [Google Scholar]
- 11.Risma K A, Clay C M, Nett T M, Wagner T, Yun J, Nilson J H. Proc Natl Acad Sci USA. 1995;92:1322–1326. doi: 10.1073/pnas.92.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Risma K A, Hirshfield A N, Nilson J H. Endocrinology. 1997;138:3540–3547. doi: 10.1210/endo.138.8.5313. [DOI] [PubMed] [Google Scholar]
- 13.Mann R J, Keri R A, Nilson J H. Endocrinology. 1999;140:2592–2601. doi: 10.1210/endo.140.6.6927. [DOI] [PubMed] [Google Scholar]
- 14.Flaws J A, Abbud R, Mann R J, Nilson J H, Hirshfield A N. Biol Reprod. 1997;57:1233–1237. doi: 10.1095/biolreprod57.5.1233. [DOI] [PubMed] [Google Scholar]
- 15.Keri R A, Andersen B A, Kennedy G C, Hamernik D L, Clay C M, Brace A D, Nett T M, Notides A C, Nilson J H. Mol Endocrinol. 1991;5:725–733. doi: 10.1210/mend-5-5-725. [DOI] [PubMed] [Google Scholar]
- 16.Clay C M, Keri R A, Finicle A B, Heckert L L, Hamernik D L, Marschke K M, Wilson E M, French F S, Nilson J H. J Biol Chem. 1993;268:13556–13564. [PubMed] [Google Scholar]
- 17.Gocze P M, Beamer W G, de Jong F H, Freeman D A. Gynecol Oncol. 1997;65:143–148. doi: 10.1006/gyno.1997.4635. [DOI] [PubMed] [Google Scholar]
- 18.Clement P B, Young R H, Scully R E. Semin Diagn Pathol. 1989;6:372–406. [PubMed] [Google Scholar]
- 19.Tennent B J, Shultz K L, Beamer W G. Cancer Res. 1993;53:1059–1063. [PubMed] [Google Scholar]
- 20.Beamer W G, Shultz K L, Tennent B J, Nadeau J H, Churchill G A, Eicher E M. Cancer Res. 1998;58:3694–3699. [PubMed] [Google Scholar]