Abstract
The aim of this study was to examine the utility of immunohistochemistry (IHC) in the diagnosis of leptospiral equine abortion and to compare IHC to silver staining and serology of the aborted mares. Ninety-six fetuses from 57 farms were examined using all 3 diagnostic techniques, revealing evidence of leptospiral infection in 3 fetuses (3.1%) from 3 (5.3%) different farms. A new finding in 1 of these confirmed cases of leptospiral abortion was the presence of macroscopic pinpoint grayish-white nodules that had a histologic correlate of hepatic necrosis; other histologic findings were consistent with those previously reported. IHC performed using 2 different leptospiral antisera (multivalent whole-cell rabbit antiserum and rabbit antiserum against the major outer membrane protein LipL32) yielded similar results. IHC was more sensitive (19/21 [90.5%] tissue samples) than silver staining (8/21 [38.1%] tissue samples), and more specific than serology performed using the microscopic agglutination test. The primary advantage of IHC over silver staining was the ability of IHC to identify leptospiral antigen not only as morphologically intact spiral forms.
Keywords: Abortion, equine, immunohistochemistry, Leptospira, pathology
Leptospirosis is a zoonotic bacterial disease with global distribution. Pathogenic Leptospira species infect virtually all species of mammals, including humans, and have also been isolated from birds, reptiles, amphibians, and arthropods.3 Horses are incidental hosts of several leptospiral serovars; only serovar Bratislava is suspected to be maintained in horses.6 Serologic surveys demonstrate that leptospiral infection is common in equine populations. However, most leptospiral infections in horse are subclinical.3 Clinical symptoms caused by leptospires in horses include recurrent uveitis, fever, hemoglobinuria, jaundice, stillbirth, and abortion, mainly in the last trimester. The most common serovar involved in equine abortion is Pomona, but rarely other serovars (Australis, Grippotyphosa, Icterohaemorrhagiae, Sejroe) have also been isolated from aborted equine fetuses.1,3,6 Most equine cases of Leptospira-induced abortion are sporadic. However, extreme weather conditions, especially flooding, can be responsible for accumulation of equine abortion cases in some areas.10
Several equine abortion studies found immunofluorescence (IF) to be very useful in the demonstration of leptospires in tissue samples.6,10,11,13 Drawbacks of IF are the lack of information about tissue morphology and the difficulty of preserving the results. Immunohistochemistry (IHC) provides an opportunity to examine the distribution and localization of antigen in tissue sections and enables retrospective studies using formalin-fixed and paraffin-embedded tissue samples. IHC has been described for the detection of Leptospira in pig, hamster, cattle, dog, and humans,5,9,18–20 but, to our knowledge, has not yet been reported in aborted equine fetuses.
Four newborn foals, 77 aborted equine fetuses with fetal membrane, and 15 fetuses without fetal membrane were collected during a 3-year period from 57 different farms in Hungary. Additionally, blood samples from 63 mares, which aborted or delivered these foals, were submitted for laboratory examination. After gross pathologic examination, tissue samples from various fetal organs (lung, heart, liver, spleen, kidney, brain, adrenal gland, lymph node, small intestine, thymus) and from allantochorion were fixed in 10% formalin and embedded in paraffin. Sections of 4-μm thickness were stained with hematoxylin and eosin. Liver, kidney, and fetal membranes were stained with Warthin-Starry. All tissue samples were stained with Warthin-Starry in Leptospira-positive cases.
Tissue sections of liver, kidney, and fetal membranes were examined by IHC. When Leptospira was detected, all tissue samples were examined by IHC. After antigen retrieval (in a microwave oven at 750 W, for 20 minutes in citrate buffer, pH 6.0) the samples were incubated in 3% H2O2 solution for 10 minutes and then in a 2% solution of skimmed milk powder for 20 minutes. Two antisera reagents were used to detect leptospiral antigen in each case: 1) a multivalent whole-cell rabbit antiserum conjugated to fluorescein isothiocyanate (National Veterinary Services Laboratories, Ames, IA)12 and 2) a rabbit antiserum specific for the leptospiral major outer membrane protein LipL32.9 The sections were incubated overnight with a 1: 10,000 dilution with the 2 antisera on serial sections at room temperature. Antibody binding was detected by a horseradish peroxidase–labeled polymer (EnVision+ Kit; Dako, Glostrup, Denmark). A serial section incubated with an irrelevant rabbit serum was used as a negative control. Kidney from an aborted swine fetus infected with leptospires served as the positive control. Identification of Leptospira serovars involved in the infection of the 3 equine fetuses was attempted using 8 rabbit polyclonal reference sera (Pomona, Hardjo, Canicola, Grippotyphosa, Icterohaemorrhagiae, Tarassovi, Hebdomadis, Bratislava) produced for the microscopic agglutination test (MAT) (World Health Organization/United Nations Food and Agricultural Organization Collaborating Centre for Reference and Research on Leptospirosis, N.H. Swellengrebel Laboratory, Amsterdam, The Netherlands). Serial dilutions of these sera from 1: 100 to 1: 300,000 were added to serial sections of Leptospira-positive kidney in the immunohistochemical reaction described above. Additionally, all tissue samples were examined for the presence of Chlamydiaceae-specific lipopolysaccharide (LPS), equine arteritis virus (EAV), and equine herpes virus type 1 (EHV-1). Briefly, after digestion with 0.1% pronase (Chlamydiaceae) or heat antigen retrieval (EAV, EHV-1; see above) serial sections were incubated overnight with chlamydial LPS-specific monoclonal antibody (dilution: 1: 100; Progen GmbH, Heilderberg, Germany), nucleocapsid protein-specific monoclonal antibody for EAV (dilution: 1: 1,000; produced at the Central Veterinary Institute, Budapest, Hungary) and EHV-1–specific goat serum (dilution: 1: 12,000; Veterinary Medical Research and Development, Inc., Pullman, WA) at room temperature. Antibody binding was detected by a horseradish peroxidase–labeled streptavidin-biotin kit (Universal LSAB2 Kit-HRP; Dako) for chlamydia and EAV or by peroxidase-antiperoxidase method (Dako) for EHV-1.
Samples from stomach contents and fetal membranes, and occasionally from lung, spleen, liver, and kidney, were cultured aerobically for 2 days on 10% blood agar and on nutrient agar. Sera collected immediately after abortion from 63 mares were tested by MAT using live antigens from 6 leptospiral serovars: Grippotyphosa, Hardjo, Sejroe, Icterohaemorrhagiae, Pomona, and Tarassovi. Sera with a titer of 1: 100 or greater were considered positive and further investigated using 12 additional serovars: Australis, Autumnalis, Ballum, Bataviae, Bratislava, Canicola, Hebdomadis, Javanica, Mini, Pyrogenes, Saxkoebing, and Swajizak.
Three (3.1%) cases of Leptospira-induced equine abortion from 3 (5.3%) large breeding farms were identified (Table 1). Abortifacient bacteria, EAV, or EHV-1 could not be demonstrated in any of these cases. Chlamydial LPS was observed in the normal chorionepithelial cells in Cases 1 and 2. In Case 1, a 3-year-old mare, which was pregnant for the first time, aborted in the ninth month of pregnancy. In Case 2 and 3, the mares aborted in the 10th month of pregnancy. No mares had any clinical symptoms around the time of abortion. Other cases of abortion in the herd were reported only in farm 2, Case 2.
Table 1.
Results of various laboratory tests in 3 cases of equine abortion caused by leptospiral infection.
| Case | Fetal Organs | Specific Histologic Lesions* | Warthin-Starry* | Immunohistochemistry* | MAT† |
|---|---|---|---|---|---|
| 1 | Lung | + | + | + | |
| Liver | + | + | + | Hardjo, Swajizak, Mini: 1: 200 | |
| Kidney | + | − | + | ||
| Spleen | − | − | + | Sejroe: 1: 1,600 | |
| Heart | − | + | + | ||
| Brain | + | − | − | ||
| Allantochorion | − | + | + | Pomona: 1: 800‡ | |
| 2 | Lung | − | − | + | |
| Liver | + | − | + | ||
| Kidney | + | − | + | ||
| Spleen | + | − | + | Hardjo, Sejroe: 1: 800 | |
| Heart | + | − | + | Swajizak: 1: 100 | |
| Thymus | − | − | + | ||
| Brain | − | − | − | ||
| Allantochorion | + | + | + | ||
| 3 | Lung | − | − | + | |
| Liver | + | − | + | Sejroe: 1: 3,200 | |
| Kidney | + | + | + | Grippotyphosa: 1: 800 | |
| Adrenal gland | − | + | + | Hardjo, Swajizak: 1: 400 | |
| Heart | − | + | + | Mini: 1: 200 | |
| Brain | + | − | + | ||
| Leptospira positive organs | 8/21 (38.1%) | 19/21 (90.5%) |
+ = lesions/leptospires are present;
− = lesions/leptospires are not present.
Highest titer against Leptospira serovars in the sera of aborted mares with microscopic agglutinations test at the time of abortion. MAT = microscopic agglutination test.
Four weeks after the time of abortion.
In all 3 fetuses, mild (Nos. 1 and 3) or moderate icterus (No. 2) and mild liver enlargement (Nos. 1–3) were observed. Pinpoint grayish-white nodules occurred in the liver parenchyma in fetus No. 2. In fetus No. 3 the liver was yellow and friable. In fetus No. 1 moderate splenic enlargement and in fetus No. 2 moderate mediastinal and mesenteric lymph nodes enlargement were observed. In fetus No. 2 the allantochorion was edematous on the body in a round area with a 20-cm diameter.
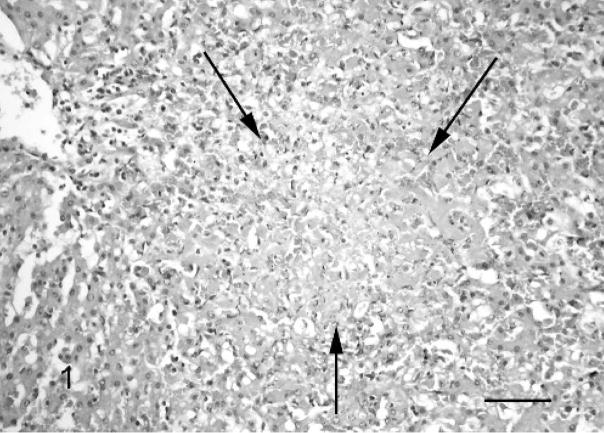
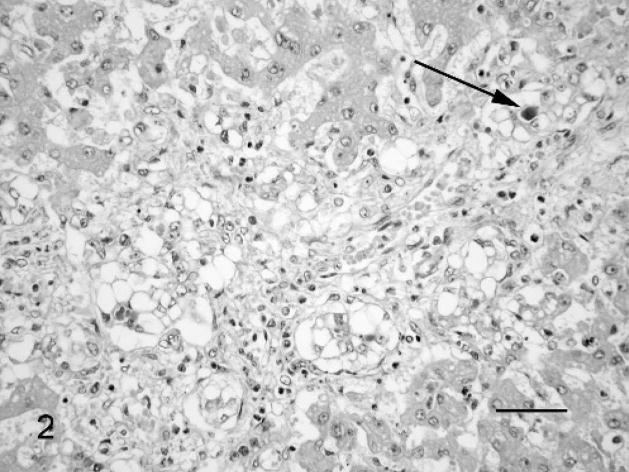
A number of histopathologic changes were found only in tissues from fetuses with leptospiral infection. Some tubuli were distended with mild to severe tubulonephrosis (Nos. 1 and 2). Additionally, mild vasculitis; perivasculitis (Nos. 1 and 2); multiple, focal, mild lympho-histiocytic interstitial nephritis (No. 3); and acute hemorrhages (No. 1) were observed in the kidney. Multifocal necrosis (Nos. 1 and 2; Fig. 1), mild to severe lympho-histiocytic, and neutrophil granulocytic infiltration in the parenchyma and in the portal triad (Nos. 1 and 2); mild vacuolar degeneration of hepatocytes; and the occurrence of some multinucleated giant hepatocytes (No. 3) were present in the liver. In fetus No. 3 a pronounced proliferation of bile ducts with severe vacuolar degeneration of the ductal epithelial cells, canalicular cholestasis, and mild lympho-histiocytic and neutrophil granulocytic infiltration in the portal triad were also evident (Fig. 2). Alterations in the lung were observed only in fetus No. 1, showing mild lympho-histiocytic vasculitis and several fresh thrombi in the blood vessels. Mild vasculitis was observed in the heart (No. 2), spleen (No. 2), and brain (No. 3). Mild lympho-histiocytic meningitis was observed in fetus No. 1. Lesions in the fetal membranes were evident only in fetus No. 2, where pronounced edema, multiple acute hemorrhages, and mild lympho-histiocytic villitis were observed. Severe lymphoid depletion in the lymph node and an increased number of megakaryocytes were seen in the spleen in fetus No. 2, but this was not felt to be specific for leptospiral infection.
Fig. 1.
Liver; Leptospira-infected equine fetus No. 1. Acute focal necrosis (arrows) in the parenchyma accompanied by mild lympho-histiocytic and neutrophil granulocytic infiltration. HE. Bar = 100 μm.
Fig. 2.
Liver; Leptospira-infected equine fetus No. 3. Proliferation of the bile ducts and vacuolar degeneration of the ductal epithelial cells, with canalicular cholestasis (arrow) and mild lympho-histiocytic and neutrophil granulocytic infiltration in the portal triad. HE. Bar = 60 μm.
Moderate to large numbers of leptospires were detected in all 3 cases with Warthin-Starry staining. The typical wavy forms of leptospires were present in the cytoplasm of different cell types (chorionepithelial cells, endothelial cells, tubular epithelial cells of the kidney) and extracellularly in the connective tissues, in the lumen of renal tubules, and in the lumen of blood vessels in various fetal organs and in the placenta. However, many tissue samples were not positive with the silver staining method. For example, in fetus No. 2 only the allantochorion was positive by Warthin-Starry staining (Table 1).
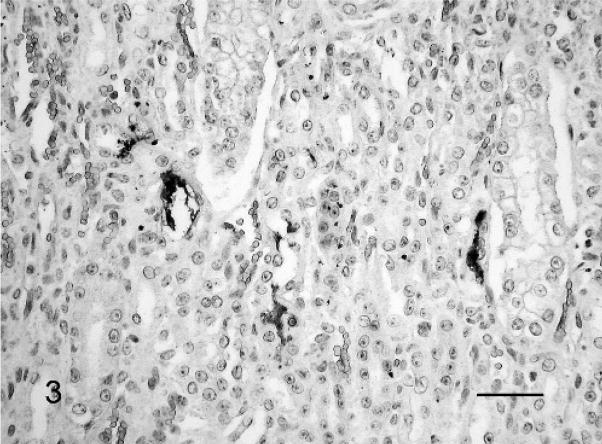
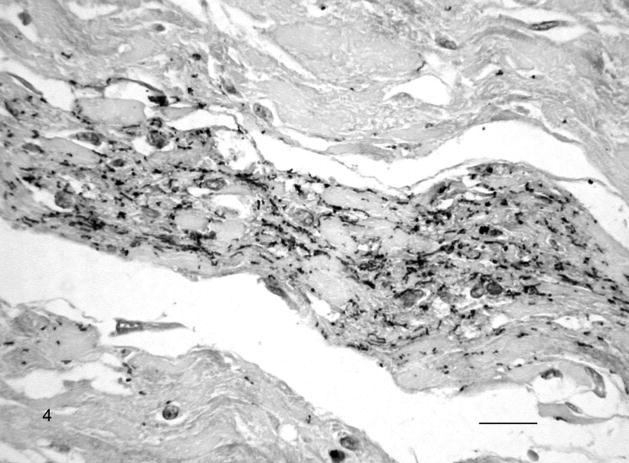
In contrast, IHC revealed a moderate to large number of leptospiral antigens in almost all of the tissue samples (Table 1). The multivalent whole-cell leptospiral antiserum and the LipL32 antiserum gave similar results. Leptospiral antigens were most abundant in the liver followed by heart, lung, and kidney (Fig. 3). Typical leptospiral wavy forms, small cocci, and large granules were observed in the cytoplasm of macrophages, chorionepithelial cells, endothelial cells, tubular epithelial cells, hepatocytes, heart muscle cells, epithelial cells in the cortex of the adrenal gland, and neurons (fetus No. 3). Typical leptospiral wavy forms and aggregates were found extracellularly in the connective tissues (Fig. 4), in the lumen of renal tubules, in the alveoli of the lung, in the sinusoids of the liver and spleen, and in the lumen of blood vessels in various fetal organs and in the placenta.
Fig. 3.
Kidney; Leptospira-infected equine fetus No. 3. Typical leptospiral wavy forms and aggregates in the lumen of several tubuli. Leptospires are detected with LipL32-specific antiserum and horseradish peroxidase–labeled polymer (EnVision+Kit). The section is counterstained with Mayer’s hematoxylin. Bar = 60 μm.
Fig. 4.
Chorioallantoic membrane; Leptospira-infected equine fetus No. 1. Large amount of extracellular leptospiral wavy forms, small cocci, and aggregates in the allantoic connective tissue. Leptospires are detected with multivalent Leptospira antiserum, and horseradish peroxidase–labeled polymer (EnVision+ Kit). The section is counterstained with Mayer’s hematoxylin. Bar = 60 μm.
Tissues were examined by IHC using serial dilutions of 8 different MAT diagnostic sera to determine whether the causative serovar could be identified. Leptospiral antigens were first detected by IHC at dilutions between 1: 100 to 1: 5,000. The amount of specific staining gradually increased and later decreased as the dilution of the sera was increased. At the end dilution leptospires were stained only scantily. Unfortunately it was not possible to identify leptospiral serovar(s) with this technique, because several reference sera reacted even at high dilutions in the same case (Table 2). However, it is possible to conclude that Pomona and Tarassovi were not involved in the infections in fetuses No. 1 and No. 2, respectively.
Table 2.
End dilution of the reference sera used in the immunohistochemistry in the cases of Leptospira-induced abortions.
| Serogroup | Fetus No. 1 | Fetus No. 2 | Fetus No. 3 |
|---|---|---|---|
| Pomona | –* | 1: 5,000 | 1: 40,000 |
| Hardjoe | 1: 40,000 | 1: 40,000 | 1: 200,000 |
| Gryppotyphosa | 1: 20,000 | 1: 200,000 | 1: 100,000 |
| Bratislava | 1: 20,000 | 1: 40,000 | 1: 20,000 |
| Tarassovi | 1: 1,000 | – | 1: 200,000 |
| Hebdomadis | 1: 40,000 | 1: 100,000 | 1: 200,000 |
| Canicola | 1: 20,000 | 1: 100,000 | 1: 200,000 |
| Icterohaemorrhagiae | 1: 10,000 | 1: 40,000 | 1: 20,000 |
– = no leptospiral antigen was recognized.
Noninfectious causes of abortion/death of newborn foals were found in 16/96 (17%) cases (twins, 2; umbilical cord torsion, 2; congenital malformation of the head, 1; trauma of the mare, 2; severe calcium deposition in the fetal organs and placenta, 2; hyperlipemia of the mare, 1; dystocia, 1; placental insufficiency, 5). Infectious causes were identified in 38/96 (40%) cases. Viruses were detected in 23 cases (EHV-1, 15; EAV, 8), bacteria in 13 cases (Chlamydophila psittaci, 1; Escherichia coli, 2; Streptococcus equinus, 1; Streptococcus equi subsp. zooepidemicus, 2; Staphylococcus equorum, 1; Pseudomonas aeruginosa, 1; leptospires, 3; Gram-negative bacteria, 2), protozoon (Encephalitozoon cuniculi) in 1 case, and fungi in 1 case (Zygomycetes spp.). No cause could be determined in 42/96 (43%) cases (data not shown). Leptospiral infection as a cause of equine abortion was diagnosed in 3/96 (3.1%) cases. The result is comparable to the 2.2–3.3% prevalence found in the USA,2,8,13 but is in contrast to the 35% prevalence reported in North Ireland.7 The mares showed no clinical signs around the time of abortion, and only single cases of abortion occurred in the herd in 2 of the 3 cases, which is characteristic for equine leptospiral abortion.3 Seropositivity for several serovars was found in all 3 mares, but the highest MAT titers were to serovar Sejroe. Interestingly, the titer was much lower in all cases compared to the findings of Donahue et al.2 Serology was not found to be specific in this study (data not shown). Two additional mares had antibodies against leptospires (for Hardjo 1: 100 or for Hardjo and Serjoe 1: 400). Noninfectious causes of abortion were evident in both cases (twins and physical trauma of the mare).
Several macroscopic lesions in fetus and fetal membranes reported to be suggestive for leptospiral infection, including swelling, yellowish discoloration and mottling of the liver, as well as swelling, perirenal edema and/or white radiating streaks in the kidney, and cystic allantoic masses, edema, areas of necrosis of the chorion, and necrotic mucoid exudates coating the chorion in the placenta.13 Of these, only icterus, manifested by yellowish discoloration of the liver and placental edema, was observed in the cases reported here. Additionally we found pinpoint grayish-white nodules in the liver of 1 fetus corresponding to acute necrosis; these have not previously been reported in aborted foals with leptospirosis, but are well known to occur in leptospiral abortion of swine.4 Several microscopic lesions have been reported in conjunction with equine leptospiral abortion, including thrombosis, vasculitis, mixed inflammatory cell infiltration, cystic adenomatosus hyperplasia, necrosis of the villi and calcification in the placenta, hepatocellular dissociation, mixed leukocytic infiltration of the portal triads, giant cell hepatopathy, multiple necrotic foci in the liver, suppurative and nonsuppurative nephritis, dilated tubules, pulmonary hemorrhages, pneumonia, myocarditis, and meningitis.13 Most of these lesions were also present in the tissues we examined, except for the proliferation of the bile ducts. This is a rather unusual finding in Leptospira-induced equine abortion, because this alteration is usually found in association with equine mycotic placentitis.16 A causative role for leptospiral infection is suggested by the fact that these changes were not found in fetuses without leptospiral infection (data not shown). However, other causes, such as toxins, cannot be excluded.
Except chlamydiae, no other pathogens were detected in these cases. As we demonstrated in a recent study, Chlamydophila psittaci infection is very common in the chorionepithelial cells of aborted equine placentas. However, the occurrence of this obligate intracellular bacteria caused no light microscopic alterations in most of the cases, and its role as an arbotifacear is not completely clear in the horse.17 According to our studies, leptospirosis seems to be the most probable cause of abortion in these cases.
This is the first report demonstrating leptospires in aborted equine fetuses and fetal membranes by IHC. Probably all important leptospiral serovars can be detected using this technique, because the multivalent Leptospira antiserum is reactive with at least 14 different Leptospira serovars,12 while the anti-LipL32 serum reacts with all pathogenic Leptospira serovars.9 Leptospires are inactivated after formalin fixation, so the infected tissue samples pose no health hazard for laboratory workers during the procedure. The method was found to be more sensitive than silver staining, as reported previously.14,18 Diagnosis of leptospirosis can be made with silver staining only by the presence of long wavy spirochetes in the tissue sections. Other leptospiral forms such as short rods, aggregates, or cocci cannot be detected by silver staining because they are not distinguishable from artifacts. Leptospires were detected by silver staining in all 3 cases, but in far fewer tissues than by IHC. The most dramatic example of the superiority of IHC was Case 2, where only the placenta was positive with silver staining, whereas the lung, liver, kidney, spleen, heart, thymus, and allantochorion tissues were positive by IHC. Compared to culture, the gold standard method in the diagnosis of leptospiral infections, the sensitivity of IHC was 78% and the specificity was 100%.15 Additionally the technique had several advantages over silver staining: 1) it enabled the specific demonstration of leptospires together with light microscopic changes in tissue sections, 2) leptospires could be demonstrated not only as whole bacteria but also as intra- and extracellular granules, and 3) the samples could be easily evaluated on low magnification (100–200×) because of the good contrast of the red-stained leptospiral antigens over the blue background staining. IHC can be used as a rapid staining procedure for diagnosis as well as retrospective studies.
Serovar identification is important for epidemiologic studies and for potential development of a vaccine for equine leptospirosis.3 Some authors have suggested the use of serovar specific polyclonal antibodies on tissue sections for serovar identification. These sera, when highly diluted, should react only with the homolog serovars.10,11 Unfortunately, we were unable to confirm these earlier findings, because several serovar-specific polyclonal reference sera produced for MAT were strongly cross-reactive. Serovar-specific monoclonal antibodies may be appropriate for serovar determination by IHC.
In conclusion, the two IHC methods we describe here are rapid and relatively inexpensive and provide an opportunity for the safe and specific detection of leptospires in tissue samples of animal origin. IHC can be used for the histopathologic diagnosis of leptospirosis either as a primary approach or when the etiology of equine abortion is uncertain because of inconclusive silver staining results or when the placenta is unavailable for examination.
Acknowledgments
The excellent technical assistance of Mrs. Ágnes Mészáros is gratefully acknowledged. The discovery of LipL32 and production of LipL32 antiserum was supported by Public Health Service grant AI-34431 (to D. A. H.) from the National Institute of Allergy and Infectious Diseases.
References
- 1.Bernard WV, Bolin C, Riddle T, Durando M, Smith BJ, Tramontin RR. Leptospiral abortion and leptospiruria in horses from the same farm. J Am Vet Med Assoc. 1993;202:1285–1286. [PubMed] [Google Scholar]
- 2.Donahue JM, Smith BJ, Poonacha KB, Donahue JK, Rigsby CL. Prevalence and serovars of leptospira involved in equine abortions in central Kentucky during the 1991–1993 foaling seasons. J Vet Diagn Invest. 1995;7:87–91. doi: 10.1177/104063879500700114. [DOI] [PubMed] [Google Scholar]
- 3.Donahue JM, Williams NM. Emergent causes of placentitis and abortion. Vet Clin N Am Equine. 2000;16:443–456. doi: 10.1016/s0749-0739(17)30088-3. [DOI] [PubMed] [Google Scholar]
- 4.Ellis WA. Leptospirosis. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, editors. Diseases of Swine. Blackwell Science; Oxford, UK: 1999. pp. 483–493. [Google Scholar]
- 5.Ellis WA, Robertson GM, Hustas L, Kirby M. Detection of leptospires in tissue using an immunoperoxidase staining procedure. Aust Vet J. 1983a;60:364–367. doi: 10.1111/j.1751-0813.1983.tb02849.x. [DOI] [PubMed] [Google Scholar]
- 6.Ellis WA, Bryson DG, O’Brien JJ, Neil SD. Leptospiral infection in aborted equine foetuses. Equine Vet J. 1983b;15:321–324. doi: 10.1111/j.2042-3306.1983.tb01811.x. [DOI] [PubMed] [Google Scholar]
- 7.Ellis WA. Equine leptospirosis. In: Wernery U, Wade JF, Mumford JA, Kaaden OR, editors. Proc 8th Int Conf Equine Inf Dis. R&W Publications; Newmarket, UK: 1999. pp. 155–158. [Google Scholar]
- 8.Giles RC, Donahue JM, Hong CB, Tuttle PA, Petrites-Murphy MB, Poonacha KB, Roberts AW, Tramontini RR, Smith B, Swerzek TW. Causes of abortion, stillbirth, and perinatal death in horses: 3,527 cases (1986–1991) J Am Vet Med Assoc. 1993;203:1170–1175. [PubMed] [Google Scholar]
- 9.Haake DA, Chao G, Zuerner RL, Barnett JK, Barnett D, Mazel M, Matsunaga J, Levett PN, Bolin CA. The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect Immun. 2000;68:2276–2285. doi: 10.1128/iai.68.4.2276-2285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinde H, Hietala SK, Bolin CA, Dowe JT. Leptospiral abortion in horses following a flooding incident. Equine Vet J. 1996;28:327–330. doi: 10.1111/j.2042-3306.1996.tb03097.x. [DOI] [PubMed] [Google Scholar]
- 11.Kitson-Piggot AW, Prescott JF. Leptospirosis in horses in Ontario. Can J Vet Res. 1987;51:448–451. [PMC free article] [PubMed] [Google Scholar]
- 12.Miller DA, Wilson MA, Kirkbride CA. Evaluation of multivalent leptospira fluorescent antibody conjugates for general diagnostic use. J Vet Diagn Invest. 1989;1:146–149. doi: 10.1177/104063878900100210. [DOI] [PubMed] [Google Scholar]
- 13.Poonacha KB, Donahue JM, Giles RC, Hong CB, Petrites-Murphy MB, Smith BJ, Swerzek TW, Tramontin RR, Tuttle PA. Leptospirosis in equine fetuses, stillborn foals, and placentas. Vet Pathol. 1993;30:362–369. doi: 10.1177/030098589303000405. [DOI] [PubMed] [Google Scholar]
- 14.Scanziani E, Sironi G, Mandelli G. Immunoperoxidase studies on leptospiral nephritis of swine. Vet Pathol. 1989;26:442–444. doi: 10.1177/030098588902600510. [DOI] [PubMed] [Google Scholar]
- 15.Scanziani E, Luini M, Fabbi M, Pizzocaro P. Comparison between specific immunoperoxidase staining and bacteriological culture in the diagnosis of renal leptospirosis of pigs. Res Vet Sci. 1991;50:229–232. doi: 10.1016/0034-5288(91)90112-2. [DOI] [PubMed] [Google Scholar]
- 16.Swerzek TW, Donahue JM. Equine mycotic placentitis. In: Kirkbride CA, editor. Laboratory Diagnosis of Livestock Abortion. Iowa State University Press; Ames, IA: 1990. pp. 229–236. [Google Scholar]
- 17.Szeredi L, Hotzel H, Sachse K. High prevalence of chlamydial (Chlamydophila psittaci) infection in fetal membranes of aborted equine fetuses. Vet Res Commun. 2005;29(Suppl 1):37–49. doi: 10.1007/s11259-005-0835-1. [DOI] [PubMed] [Google Scholar]
- 18.Yener Z, Keles H. Immunoperoxidase and histological examinations of leptospiral nephritis in cattle. J Vet Med A. 2001;48:441–447. doi: 10.1046/j.1439-0442.2001.00355.x. [DOI] [PubMed] [Google Scholar]
- 19.Wild CJ, Greenlee JJ, Bolin CA, Barnett JK, Haake DA, Cheville NF. An improved immunohistochemical diagnostic technique for canine leptospirosis using antileptospiral antibodies on renal tissue. J Vet Diagn Invest. 2002;14:20–24. doi: 10.1177/104063870201400105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaki SR, Shieh WJ. Leptospirosis associated with outbreak of acute febrile illness and pulmonary haemorrhage, Nicaragua, 1995. Lancet. 1996;347:535–536. doi: 10.1016/s0140-6736(96)91167-8. [DOI] [PubMed] [Google Scholar]