Abstract
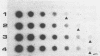
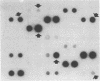
The sensitivity and specificity of an immune dot blot test (IDBT), which relies on a 125I-labeled genus-specific monoclonal antibody to detect the Chlamydia lipopolysaccharide (LPS) antigen, were improved by pretreatment of specimens with proteinase K. This enzyme destroys protein A and therefore eliminates false-positive reactions caused by the presence of Staphylococcus aureus. Proteinase K treatment also improved the ability of the assay to detect the Chlamydia LPS antigen. When the improved IDBT was compared with culture for detection of C. trachomatis in 1,394 urogenital specimens obtained from a genitourinary medicine clinic, the overall sensitivity was 96%, and LPS antigen was detected in 76 of 83 (92%) specimens that yielded less than 10 inclusions in culture. The specificity and positive and negative predictive values of the test were 97, 81.5, and 99%, respectively. Of 123 conjunctival swabs, 7 were positive by both tests and 4 swabs were positive only by IDBT. This improved IDBT provides a simple, reliable alternative to culture for the detection of C. trachomatis in urogenital and conjunctival specimens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Kuo C. C., Wang S. P., Altman J. A new Chlamydia psittaci strain, TWAR, isolated in acute respiratory tract infections. N Engl J Med. 1986 Jul 17;315(3):161–168. doi: 10.1056/NEJM198607173150305. [DOI] [PubMed] [Google Scholar]
- Krech T., Gerhard-Fsadni D., Hofmann N., Miller S. M. Interference of Staphylococcus aureus in the detection of Chlamydia trachomatis by monoclonal antibodies. Lancet. 1985 May 18;1(8438):1161–1162. doi: 10.1016/s0140-6736(85)92467-5. [DOI] [PubMed] [Google Scholar]
- Mallinson H., Roberts C., Bruce White G. B. Staphylococcal protein A; its preparation and an application to rubella serology. J Clin Pathol. 1976 Nov;29(11):999–1002. doi: 10.1136/jcp.29.11.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J. Rapid diagnosis of sexually transmitted diseases--speed has a price. Diagn Microbiol Infect Dis. 1986 Mar;4(3):185–189. doi: 10.1016/0732-8893(86)90097-0. [DOI] [PubMed] [Google Scholar]
- Storey C. C., Mearns G., Richmond S. J. Immune dot blot technique for diagnosing infection with Chlamydia trachomatis. Genitourin Med. 1987 Dec;63(6):375–379. doi: 10.1136/sti.63.6.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Thomas B. J., Osborn M. F. Evaluation of enzyme immunoassay (Chlamydiazyme) for detecting Chlamydia trachomatis in genital tract specimens. J Clin Pathol. 1987 Feb;40(2):194–199. doi: 10.1136/jcp.40.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornley M. J., Zamze S. E., Byrne M. D., Lusher M., Evans R. T. Properties of monoclonal antibodies to the genus-specific antigen of Chlamydia and their use for antigen detection by reverse passive haemagglutination. J Gen Microbiol. 1985 Jan;131(1):7–15. doi: 10.1099/00221287-131-1-7. [DOI] [PubMed] [Google Scholar]
- Tjiam K. H., van Heijst B. Y., van Zuuren A., Wagenvoort J. H., van Joost T., Stolz E., Michel M. F. Evaluation of an enzyme immunoassay for the diagnosis of chlamydial infections in urogenital specimens. J Clin Microbiol. 1986 Apr;23(4):752–754. doi: 10.1128/jcm.23.4.752-754.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]