Abstract
Chondrocytes isolated from a variety of sources, including auricular (AU) and articular (AR) cartilage, can differ in cell behavior, growth, and extracellular matrix (ECM) production, which can impact neocartilage properties in tissue engineering approaches. This behavior is also affected by the surrounding microenvironment, including soluble factors, biomaterials, and mechanical loading. The objective of this study was to investigate differences in juvenile AU and AR chondrocyte behavior when encapsulated in radically polymerized hyaluronic acid hydrogels. When implanted in vivo, differences in macroscopic appearance, mechanical properties, glycosaminoglycan content, and collagen content were observed depending on the chondrocyte type encapsulated. Specifically, AU constructs exhibited construct growth and neo-cartilage formation with increases in aggregate modulus and ECM accumulation with culture, whereas AR constructs retained their construct size and remained translucent with only a minimal increase in the compressive modulus. When cultured in vitro, both cell types remained viable and differences in gene expression were observed for type I and II collagens. Likewise, differences in gene expression were noted after dynamic mechanical loading, where stimulated AR constructs exhibited 2.3- and 1.5-fold increases in type II collagen and aggrecan over free-swelling controls, while AU samples exhibited smaller fold increases of 1.4- and 1.3-fold, respectively. Thus, these data indicate that the specific cell source, cell/material interactions, and loading environment are important in the final properties of tissue-engineered products.
Introduction
One of the major challenges for cartilage tissue engineering is determining an appropriate cell source for delivery. The most obvious choice is chondrocytes since they are natively found in cartilage and serve to maintain and remodel their surrounding matrix. Chondrocytes can be isolated from a variety of sources, including articular (AR), auricular (AU), nasoseptal, and costal cartilage, which have discrete chemical, physical, and mechanical properties. Chondrocyte behavior is dependent on where it is isolated from, and thus it is important to determine how chondrocytes from each source function and behave in an engineered environment (e.g., biomaterial, soluble factors, and mechanical loading).
To date, researchers have shown differences among chondrocyte sources with respect to cell yields, proliferation rates, and phenotype retention.1–4 In a study by van Osch et al., higher cell yields and faster proliferation rates were obtained for AU chondrocytes when compared to AR chondrocytes.1 Further, these AU chondrocytes were able to retain their chondrocytic phenotype when cultured in alginate beads. Likewise, nasal chondrocytes can proliferate four times faster than AR chondrocytes in monolayer,3 and can be seeded at very low seeding densities with an 838-fold expansion in one passage without extensive dedifferentiation.2 With the addition of growth factors, AU, nasal, and costal chondrocytes all exhibit increased proliferation, GAG/DNA content, and upregulation of type II collagen expression; however, redifferentiation was achieved only in AU and nasal chondrocyte cell pellets.4 These studies provide motivation for exploring multiple chondrocyte sources toward tissue engineering approaches.
Source-dependent differences can also be found in the resulting engineered cartilage tissues, including differences in construct size, gene expression, biochemical content, and mechanical properties. Panossian et al. showed that AU chondrocyte–seeded samples produced neocartilage with greater biochemical and histological similarity to that of native cartilage than AR counterparts when implanted in vivo.5 Xu et al. have also shown that AU chondrocytes encapsulated in fibrin exhibited the highest equilibrium modulus compared to those encapsulated with AR and costal chondrocytes.6 In a comparison study of bovine nasal, AR, costal, and AU chondrocytes grown on poly(L-lactide-ɛ-caprolactone) scaffolds,7 construct size and gene expression varied with chondrocyte type. AU chondrocyte–seeded constructs had the largest diameter, while costal chondrocyte–seeded constructs had the greatest thickness. In addition, costal chondrocytes, followed by nasoseptal, AR, and AU chondrocytes, had the highest expression of type II collagen and aggrecan. However, despite cell differences, Johnson et al. demonstrated that AR, AU, and costal chondrocytes were all able to form new cartilaginous matrix when cultured in fibrin glue–cartilage composites in vivo.8 This information is important toward isolating a specific cell source, yet the behavior of cells is dependent on the biomaterial used as a carrier. Specifically, variables in biomaterial design, such as mechanics, permeability, and incorporation of biological motifs, can dictate cellular behavior. Thus, data on the source-dependent differential responses of chondrocytes can assist in biomaterial choice, scaffold type, and cartilage repair application, e.g., in plastic, reconstructive, or orthopaedic surgery.
For our work, we utilized a hydrogel scaffold based on hyaluronic acid (HA), a linear polysaccharide found natively in cartilage that is degraded by hyaluronidases (Hyal) and binds to a variety of cell surface receptors.9 Due to its potential biological activity (e.g., cell-mediated degradation and cell binding), it is particularly interesting as a scaffolding material for tissue engineering applications. HA was modified via its hydroxyl group with methacrylic anhydride for photopolymerization, which allows for the formation of a three-dimensional hydrogel with both temporal and spatial control. Previously, we illustrated the diversity of hydrogel properties through alterations in macromer concentration and molecular weight and showed compositions that support the viability of encapsulated chondrocytes.10–12 The objective of this study was to investigate and characterize the differences in cell behavior of AU and AR chondrocytes when encapsulated and cultured in these HA hydrogels.
Materials and Methods
Macromer synthesis
Methacrylated HA was synthesized as previously reported.13 Briefly, methacrylic anhydride (Sigma Aldrich, St. Louis, MO) was added to a solution of 1 wt% HA (Lifecore, Chaska, MN, MW = 64 kDa) in deionized water, adjusted to a pH of 8 with 5 N sodium hydroxide, and reacted on ice for 24 h. The macromer solution was purified via dialysis (MW cutoff 6–8 kDa) against deionized water for a minimum of 48 h with repeated changes of water. The final product was obtained by lyophilization and stored at −20°C in powder form prior to use. The macromer was sterilized using a germicidal lamp in a laminar flow hood for 30 min and dissolved in a sterile solution of phosphate-buffered saline (PBS) containing 0.05 wt% 2-methyl-1-[4-(hydroxyethoxy)phenyl]-2-methyl-1-propanone (Irgacure 2959, I2959, Ciba, Tarrytown, NY) for cell encapsulation.
Chondrocyte isolation and photoencapsulation
Cartilage tissue was harvested in a sterile fashion from the ears (AU) and the knees (AR) of 3- to 6-month-old swine. The harvested cartilage was cut into ∼1 mm3 pieces, washed in PBS, and digested overnight at 37°C in Dulbecco's Modified Eagle's Medium (DMEM) containing 0.1% and 0.05% collagenase (Worthington, Lakewood, NJ) for AU and AR cartilage, respectively. Digested tissue was passed through a 100 μm filter and centrifuged to obtain a chondrocyte pellet. Chondrocytes were washed with PBS, counted using a hemacytometer, and determined viable using the trypan blue exclusion dye test prior to encapsulation. Chondrocytes (40 million cells/mL) were photoencapsulated in hydrogels by suspension in a 2 wt% macromer (methacrylated HA) solution, injection into sterile molds (50 μL volume), and polymerization with ultraviolet light for 10 min using a long-wave ultraviolet lamp (Topbulb, East Chicago, IN, F8T5BLB).
In vivo and in vitro culture methods
For in vivo culture, constructs (n = 5/group per time point) were implanted subcutaneously in nude mice within 2 h of gelation. Nude mice were anesthetized with isoflurane, and five subcutaneous pockets were made using an incision and blunt dissection. One chondrocyte/hydrogel construct was placed in each of these pockets, and the wound was closed with sterile stainless steel skin clips. After 6 and 12 weeks, mice were euthanized and constructs were harvested for analysis. NIH guidelines for the care and use of laboratory animals (NIH Publication #85-23 Rev. 1985) were observed.
For in vitro culture, AU- and AR-seeded constructs, fabricated in the same manner as described above, were cultured for 14 days in DMEM supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, 1% sodium bicarbonate, 1% nonessential amino acids, and 50 μg/mL ascorbic acid 2-phosphate (growth media). Media were changed every 2 days. Relative gene expression and histology were analyzed after 6 and 14 days of culture.
Mechanical stimulation
Loading parameters for in vitro mechanical stimulation in the bioreactor system were validated using an Instron 5848 Microtester equipped with a 50 N load cell and the Wave-Maker Software package. Acellular HA macromer solution was polymerized between glass plates (2.25 mm thickness), and cylindrical hydrogels were produced with a 5 mm diameter biopsy punch. Samples (n = 3) were immersed in a PBS custom bath at room temperature and positioned between two impermeable platens. A 5% static compression was applied; after equilibrium was achieved, dynamic compression was applied with a sinusoidal waveform at a 10% amplitude and a frequency of 1 Hz. Loading was carried out for a minimum of 10 min, and each test was repeated three times. Larger amplitudes (15%) and higher frequencies (3 Hz) were also tested. For mechanical stimulation of cell-seeded constructs within the bioreactor system, hydrogels (n = 4/group per time point) were formed in the same fashion with a seeding density of 40 million cells/mL. After 5 days of subculture, the hydrogels were immersed in growth media and subjected to uniaxial unconfined cyclic compression (5% tare, 10% strain, 1 Hz) in a custom-made bioreactor.14 Dynamic loading was carried out for 3 h per day for 1 or 5 consecutive days at 37°C and 5% CO2 in a humidified environment. Samples were removed from the bioreactor after each mechanical loading session. At the end of loading on day 1 or 5, samples were removed from culture and analyzed for gene expression and compared to free-swelling controls.
Mechanical testing
Explanted samples (n = 5) were cored with a 3/16 inch (4.76 mm) diameter punch and weighed (wet weight). Cored samples were mechanically tested in confined compression in a PBS bath. To ensure complete confinement, samples were initially loaded in creep to a tare load of 5 g until reaching equilibrium (defined as less than 10 μm of change in 10 min) before undergoing stress relaxation. Stress relaxation was carried out by applying a ramped displacement (1 μm/s) to 10% strain, after which the sample was allowed to relax to equilibrium (defined as less than 0.5 g of change in 10 min). The equilibrium-confined compression aggregate modulus (HA) for each sample was calculated by dividing the equilibrium load by the sample cross-sectional area and the applied strain. Native AU and AR cartilage samples were similarly tested (n = 5).
Biochemical analysis
For biochemical analysis (n = 5), mechanically tested samples were lyophilized, weighed (dry weight), and digested in a proteinase K solution (200 μg/mL proteinase K (Roche, Mannheim, Germany), 100 mM ammonium acetate, pH 7.0) overnight at 60°C. Proteinase K was then inactivated at 100°C for 5 min. Total DNA, GAG, and collagen contents were determined using the PicoGreen dsDNA assay,15 the dimethylmethylene blue dye method16 with chondroitin sulfate (CS) as a standard, and the hydroxyproline assay17 using a collagen to hydroxyproline ratio of 7.25,18,19 respectively. Values reported for DNA, GAG, and collagen content were normalized to the sample wet weight. The proteinase K digestion solution was used as a negative control.
Viability
The viability of AU and AR chondrocytes in the HA hydrogel was determined using an MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltertrazolium) cell proliferation assay. Mitochondrial activity (n = 3) was assessed on days 0 (2 h after encapsulation), 7, and 14 of in vitro culture. Briefly, 100 μL of MTT reagent was added to 1 mL of media and incubated for 4 h. Samples were then removed from the media, homogenized in the detergent solution with a tissue grinder, and incubated for 4 h before reading in a spectrophotometer. Absorbance readings at 570 nm were normalized to day 0 values to account for differences in mitochondrial activity between cell sources.
Gene expression analysis
Samples (n = 4) were homogenized in Trizol reagent (Invitrogen, Carlsbad, CA) with a tissue grinder, and RNA was extracted according to the manufacturer's instructions. RNA concentration was determined using an ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE). One microgram of RNA from each sample was reverse transcribed into cDNA using reverse transcriptase (Superscript II, Invitrogen) and oligoDT (Invitrogen). Polymerase chain reaction (PCR) was performed on an Applied Bio-systems (Foster City, CA) real-time PCR system using a 25 μL reaction volume for SybrGreen and TaqMan (5′-nuclease) reactions. Primers and probes specific for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), type I and type II collagen, aggrecan, and hyaluronidases (Hyal) 1, 2, and 3 are listed in Table 1. SybrGreen reaction was used for all genes except type II collagen, and GAPDH was used as the housekeeping gene. The relative gene expression was calculated using the ΔΔCt method, where fold difference was calculated using the expression 2−ΔΔCt.
Table 1.
Porcine Primer and Probe Sequences for Real-Time PCR
| Gene | Forward primer | Reverse primer | Probe |
|---|---|---|---|
| GAPDH | CGTCCCTGAGACACGATGGT | CCCGATGCGGCCAAAT | |
| Type I collagen30 | GGCTCCTGCTCCTCTTAGCG | CATGGTACCTGAGGCCGTTC | |
| Type II collagen | CTCCTGGCACGGATGGT | CTGGAGGGCCCTGAGC | CCCAAAGGCGCATCTG |
| Aggrecan31 | GCAGACCAGAAGCTGTGTGAGA | TGACGATGCTGCTCAGGTGT | |
| HYAL132 | TGCCCTATGCCCAGATCTTC | CAGCTCCTCCCGAGACAGAA | |
| HYAL232 | AGGGCTTAGCGAGATGGATCT | TGTCAGGTAATCCTTGAGGTATTGG | |
| HYAL332 | TGAACTTGTGCAGACCATTGGT | GCCAACACTCCTCCTCAGAACT |
Histological analysis
For histological analysis, constructs were fixed in 10% formalin for 24 h, embedded in paraffin, and processed using standard histological procedures. The histological sections (7 μm thick) were stained for chondroitin sulfate and collagen distributions using the Vectastain ABC kit (Vector Labs, Burlingame, CA) and the DAB substrate kit for peroxidase (Vector Labs). Sections were predigested in 0.5 mg/ mL Hyal for 30 min at 37°C and incubated in 0.5 N acetic acid for 4 h at 4°C to swell the samples prior to overnight incubation with primary antibodies at dilutions of 1:100, 1:200, and 1:3 for CS (mouse monoclonal anti-CS, Sigma, St. Louis, MO), and type I (mouse monoclonal anticollagen type 1, Sigma) and type II (mouse monoclonal anticollagen type II, Developmental Studies Hybridoma Bank, Iowa City, IA) collagen antibodies, respectively. Nonimmune controls underwent the same procedure without primary antibody incubation.
Statistical analysis
All values are reported as the mean ± the standard error of the mean. ANOVA was used to determine significant differences among groups, with p ≤ 0.05. Sensitivity of the data was analyzed using a nonparametric Wilcoxon Rank-Sum test, given small sample sizes.
Results
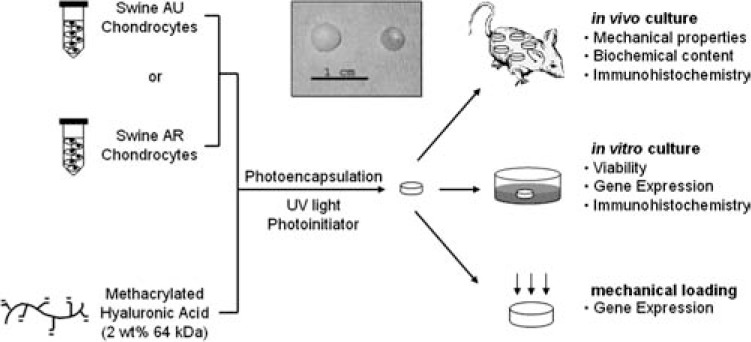
For this study, differences in cell behavior and neo-cartilage production between AU and AR chondrocyte–seeded HA hydrogels were investigated subcutaneously in nude mice and under various culture conditions. Additionally, the influence of dynamic mechanical loading on chondrocyte gene expression was investigated. A general schematic of the experimental layout for this study is illustrated in Figure 1.
FIG. 1.
General schematic of chondrocyte isolation, encapsulation, and analysis. Inset: macroscopic image of explanted AU (left) and AR (right) chondrocyte–seeded constructs after 12 weeks of in vivo subcutaneous culture.
In vivo culture
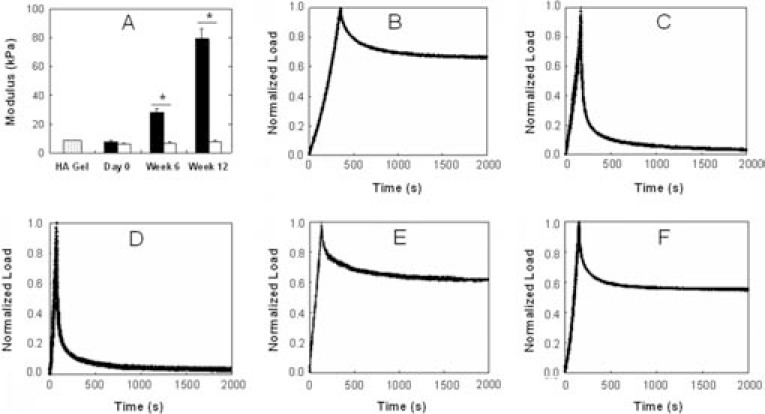
The macroscopic appearance (Fig. 1 inset) of AU and AR chondrocyte–seeded samples after 12 weeks of culture in vivo was dramatically different. AU constructs increased in size in all dimensions and were shiny and white in appearance after subcutaneous culture, while AR samples more closely resembled implanted constructs and retained their size and translucency. The neocartilage mechanical properties reflected these macroscopic observations, where the confined compression aggregate modulus of AU explants (28.2 ± 2.2 kPa, 79.3 ± 6.7 kPa) was significantly greater than that of AR explants (7.0 ± 0.3 kPa, 8.0 ± 0.7 kPa) after 6 and 12 weeks, respectively (Fig. 2), and increased with culture. When normalized to their peak load, stress relaxation curves for AU explants more closely resembled those of native AU and AR cartilages, while those of AR explants more closely resembled profiles of HA hydrogel alone with a high peak load followed by rapid relaxation. The aggregate modulus of AU constructs represented ∼226% and ∼31% of native AU and AR cartilages, respectively, while the modulus of AR constructs only represented ∼23% and ∼3% of the native tissue values, respectively.
FIG. 2.
Mechanical properties of explanted constructs. (A) Modulus of AU (black) and AR (white) chondrocyte–seeded constructs compared to HA hydrogel alone (shaded). Representative stress relaxation curves for AU chondrocyte–seeded (B) and AR chondrocyte–seeded (C) explants after 12 weeks of in vivo culture compared to HA hydrogel alone (D) and to native AU (E) and AR (F) cartilages. Stress relaxation curves are normalized to peak stress. Significant differences (p ≤ 0.05) between AU and AR groups are denoted by asterisks (*).
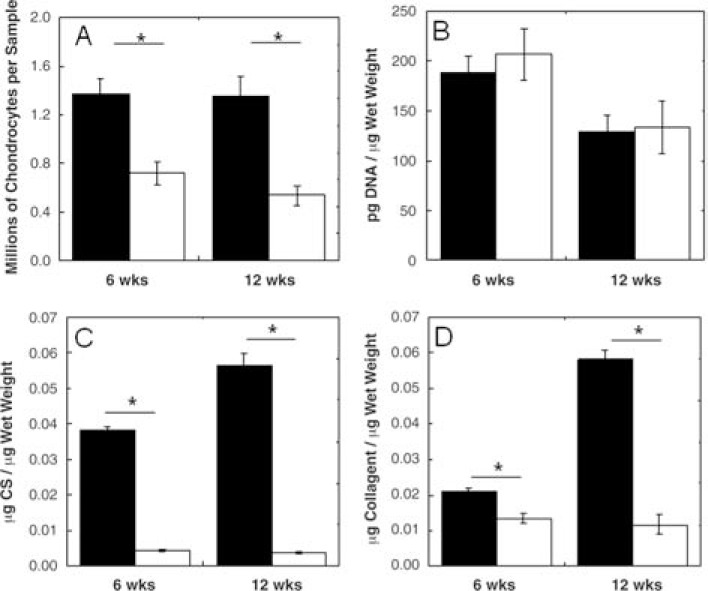
A decrease in water content was observed in both AU (87.8% ± 2.7% water) and AR (95.0% ± 1.4% water) constructs after 12 weeks when compared to HA hydrogel alone (97.0% ± 0.3% water), with a greater decrease observed for AU constructs. After 12 weeks of subcutaneous culture, the DNA content was significantly greater in AU constructs (1.35 ± 0.17 million chondrocytes) versus AR constructs (0.54 ± 0.08 million chondrocytes. However, the DNA content was similar between these groups when normalized to the wet weight (Fig. 3). It is important to note that cell loss was observed during photoencapsulation. Thus, the number of cells encapsulated may be less than the seeding density would suggest, yet still provides information on comparisons between cell types. Sulfated-GAG and collagen contents also reflect the macroscopic differences between the two cell sources. After 12 weeks of in vivo culture, AU constructs were comprised of 0.056 ± 0.003 μg CS/μg wet weight and 0.58 ± 0.003 μg collagen/μg wet weight, while AR constructs contained 0.004 ± 0.0002 μg CS/μg wet weight and 0.012 ± 0.003 μg collagen/μg wet weight (Fig. 3). The biochemical content of AU constructs more closely resembled that of native cartilage tissues. Specifically, the GAG content was ∼92% of AU and ∼65% of AR cartilages, and the collagen content was ∼64% of AU and ∼57% of AR cartilages after 12 weeks of in vivo culture. The orientation of these extracellular matrix (ECM) components was not assessed in this work.
FIG. 3.
Biochemical content of AU (black) and AR (white) explants reported as millions of chondrocytes per sample (A) and DNA content (B), CS (C), and collagen content (D) per wet weight. Significant differences (p ≤ 0.05) between AU and AR groups are denoted by asterisks (*).
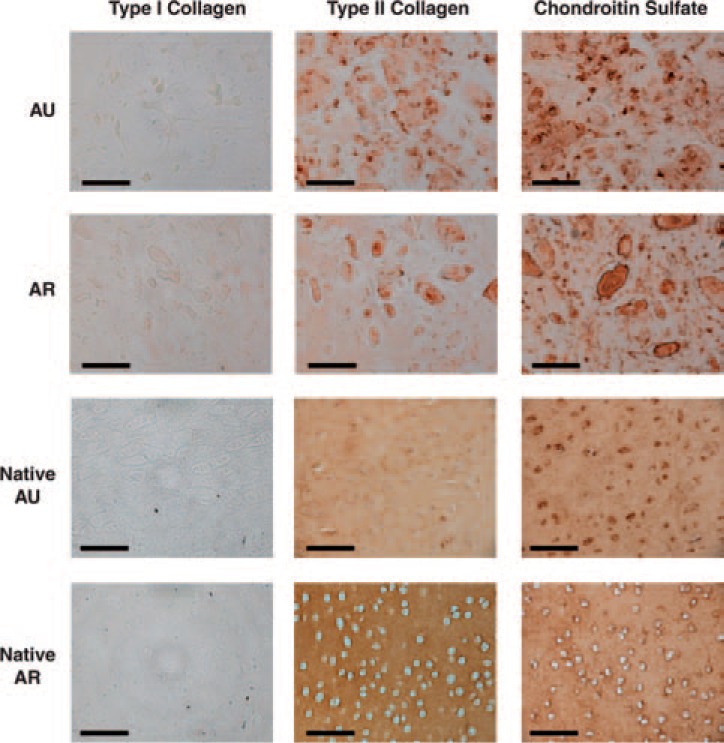
Immunohistochemistry of the constructs (Fig. 4) was used to assess the distribution of cells and ECM components in the neocartilage. Greater type II collagen staining versus type I collagen staining was observed for both AU and AR constructs. An even distribution of type II collagen and CS was observed in AU constructs, while more clustering of cells and ECM proteins was observed in AR constructs.
FIG. 4.
Representative sections of AU and AR constructs stained for type I and II collagens and chondroitin sulfate after 12 weeks of subcutaneous in vivo culture compared to native cartilage tissue sections. Scale bars = 100 μm. Color images available online at www.liebertpub.com/ten.
In vitro culture
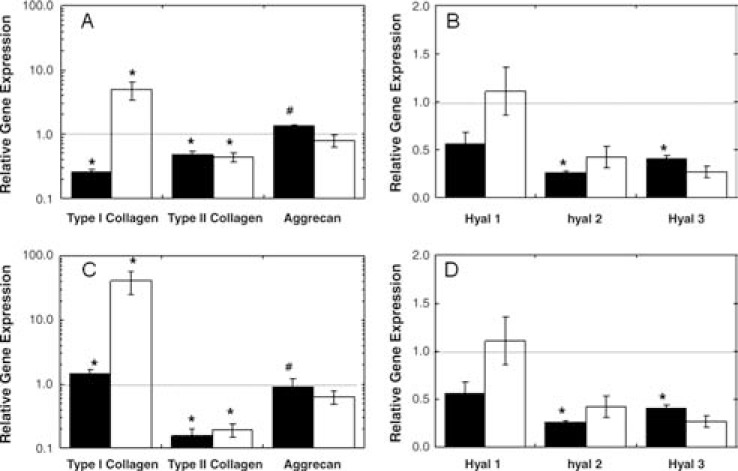
With dramatic differences observed in neocartilage formation in vivo, short-term in vitro cultures were used to investigate the potential causes of these differences, including comparisons of viability, gene expression, and ECM deposition. AU and AR chondrocytes showed comparable viability in HA hydrogels, and both exhibited increases in mitochondrial activity from day 7 to 14 (Fig. 5). Gene expression data (Fig. 6) were collected at 6 and 14 days of culture and normalized to the gene expression of the respective chondrocytes at the time of encapsulation. Although similar trends in gene expression were observed for AU and AR chondrocytes in HA hydrogels, the type I and type II collagen expression was significantly different between the two cell sources. Specifically, the normalized type I collagen expression was greater in AR samples and the normalized type II collagen expression was greater in AU samples. Both groups exhibited a trend of increased type I collagen expression with culture time and fairly constant type II collagen expression, though downregulated when compared to respective cells at encapsulation. Compared to AU samples, AR constructs exhibited equal or higher levels of three isoforms of hyaluronoglucosaminidase (an enzyme that degrades HA) expression.
FIG. 5.
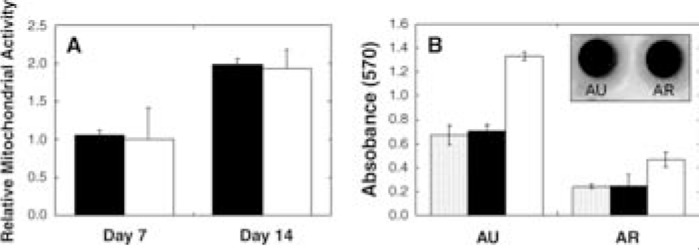
(A) Relative mitochondrial activity of AU (black) and AR (white) hydrogels cultured in vitro for 7 and 14 days. Values were normalized to day 0 absorbance readings. (B) Absorbance readings at 570 nm for AU and AR constructs at days 0 (shaded), 7
FIG. 6.
Relative gene expression for AU (A, B) and AR (C, D) constructs after 6 (black) and 14 (white) days of in vitro culture. GAPDH was used as the housekeeping gene, and expression was normalized to cells encapsulated at day 0. Significant differences (p ≤ 0.05) between AU and AR groups are denoted by asterisks (*) using parametric and nonparametric statistics, or hashes (#) using parametric only.
Histological analysis indicated pericellular staining of type II collagen and evenly distributed staining for CS throughout the gel in both AU and AR samples after 14 days of in vitro culture (data not shown). Although staining for both type I and II collagens was observed in the perimeter of the hydrogel, no type I collagen staining was observed within the core of the hydrogel. Positive staining for type II collagen and aggrecan was observed for both in vivo and in vitro cultures; however, type II collagen was more distributed within the constructs after 12 weeks of in vivo culture.
Mechanical stimulation
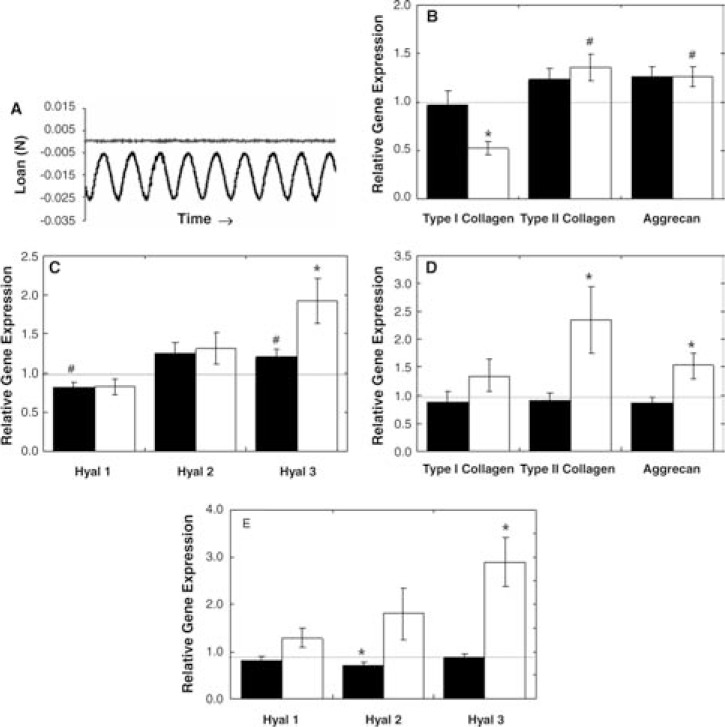
To examine whether AU and AR chondrocytes respond to mechanical loading in HA hydrogels, the constructs were subjected to dynamic loading in vitro. Validation of mechanical loading parameters was performed on acellular HA hydrogels (Fig. 7A). Hydrogels were subjected to a 5% tare strain with a superimposed dynamic axial strain of 10% at 1 Hz, where load and displacement during cyclic compression were acquired. Load response showed no capping or other deviation from the sinusoidal path under these conditions, indicating that the HA hydrogel remained in contact with the indenter without any evidence of lift-off. Further analyses at higher frequencies (3 Hz) and larger magnitudes (15% dynamic strain) showed similar results (data not shown).
FIG. 7.
Validation of mechanical loading of HA hydrogels at 5% tare, 10% strain, and frequency of 1 Hz (A). Relative gene expression of dynamically loaded AU (B, C) and AR (D, E) hydrogels for 1 (black) and 5 (white) days normalized to free-swelling controls. Significant differences (p ≤ 0.05) between free-swelling and mechanically loaded samples are denoted by asterisks (*) using parametric and nonparametric statistics, and hashes (#) using parametric only.
Using these validated loading parameters, dynamic mechanical stimulation was applied to cell-seeded HA constructs, and the effects of stimulation were monitored with relative gene expression (Fig. 7). Upregulation of type II collagen and aggrecan was evident for both AU and AR samples after 5 days of consecutive loading, where significant differences for loaded constructs over free-swelling constructs were noted for AU samples with only parametric statistics, while significant differences (p ≤ 0.05) for AR samples were determined with both parametric and non-parametric tests. For loaded AR constructs, 2.3- and 1.5-fold increases in type II collagen and aggrecan, respectively, over free-swelling controls were observed, while only 1.4- and 1.3-fold increases were observed for loaded AU samples. Additionally, type I collagen gene expression was significantly downregulated for AU constructs after 5 days of loading. Cyclic compressive loading also increased gene expression of Hyal3 for both cell sources, with greater increases observed for AR samples (2.9-fold) over AU samples (1.9-fold).
Discussion
Chondrocytes can be isolated from a variety of sources, and they behave and respond differently to their local environment (e.g., exposure to mechanical forces, soluble factors, varying compositions of ECM molecules, or scaffold material) depending on the chondrocyte source.20 Thus, it is important to investigate the response of specific chondrocyte sources to this microenvironment for assessment of tissue engineering approaches. This study focused specifically on differences in neocartilage production by AU and AR chondrocytes in HA hydrogels. AU cartilage is a non-load-bearing and elastic tissue found primarily in the ear and epiglottis, whose primary role is to maintain shape and structure. Alternatively, AR cartilage is a hyaline cartilage that is capable of distributing mechanical loads and providing low friction articulation in diarthrodial joints. Therefore, chondrocytes isolated from these two types of cartilage differ in the ECM they produce in order to carry out their respective physiological roles.21
Our laboratory and others have been developing HA hydrogels for a range of biological applications. HA is a linear polysaccharide found natively in all forms of cartilage, interacts with cells via CD44, ICAM-1, and RHAMM cell surface receptors9, and plays a role in cell proliferation, migration, morphogenesis, inflammation, and wound repair.22 Thus, HA-based hydrogels are being developed for their potential to simulate a native ECM environment,12,23 since HA can potentially interact with chondrocytes and direct cell behavior. In these hydrogels, dramatic differences in neocartilage formation were observed in vivo depending on whether AU or AR chondrocytes were used as a cell source, suggesting differential cell interactions with the HA scaffold. These interactions could be due to factors such as changes in cellular-specific binding to the hydrogels, differences in matrix degradation through enzyme production, and the reproduction of the environment seen natively by these cells.
After 12 weeks of subcutaneous in vivo culture, AU chondrocyte–seeded hydrogels exhibited construct growth and ECM production. The decrease in water content and the increase in GAG content correlated to an increase in compressive equilibrium aggregate modulus from 6 to 12 weeks, in accordance with previously established data.24,25 The change in water content indirectly influences mechanical properties since it represents an increase in ECM in the constructs. Since the number of chondrocytes per sample remained relatively constant, a decrease in DNA content per wet weight from 6 to 12 weeks was observed and may be attributed to the accumulation of newly synthesized ECM (i.e., increased GAG and collagen content). In addition, immunohistological results reflect positive staining for type II collagen and CS and an absence of type I collagen within the construct, suggesting phenotype retention. Although not measured in this study, we previously showed that neo-cartilage formed by AU chondrocytes in HA gels also produced elastin, but the elastin production decreased with in vitro cell expansion.11
In contrast, AR chondrocyte–seeded hydrogels exhibited little neocartilage formation, and the constructs more closely resembled the HA hydrogel alone in macroscopic appearance and mechanical properties. Stress relaxation curves suggest a higher permeability of AR constructs compared to AU constructs and native cartilage. Also, the lower aggregate modulus can be correlated to the high water content, an indication of less ECM production. However, immuno-histological stains for AR samples also showed evidence of type II collagen and CS deposition, indicating cartilage ECM production in HA hydrogels in vivo. Although both AU and AR chondrocyte–seeded samples showed type II collagen and CS deposition, the distribution of ECM molecules differ, with more clustering in the AR constructs. Within the HA hydrogel, AR chondrocytes appeared to undergo interstitial growth, forming lacunae and isogenous groups surrounded by pericellular and territorial matrix. Since the orientation of the collagen and CS in the constructs was not assessed, no conclusions can be made regarding this potential difference with respect to mechanics.
With in vitro culture, AU and AR chondrocyte mitochondrial activity (when normalized to day 0 absorbance readings) was similar in HA hydrogels, and cell proliferation was observed from day 7 to 14 for both cell sources. The hydrated hydrogel environment allowed cells to maintain a rounded morphology, which can assist in cell proliferation and phenotype retention. However, it is important to note that a lower mitochondrial activity level was observed for AR chondrocytes in HA hydrogels on day 0 compared to AU chondrocytes, which may have played a role in the differences in neocartilage formation. Free radical polymerization effects on cell death were deemed negligible, as the photoinitiating system implemented has been used extensively and others have shown it to be cytocompatible,26 and AR chondrocytes exposed to the photoinitiator and radical polymerization conditions have shown comparable cell survival to unexposed controls.27 Thus, free radicals present during polymerization should not significantly affect the survival or mitochondrial activity of either type of chondrocyte.
Further, differences in gene expression were observed in short-term in vitro cultures. Natively, less type I collagen and more type II collagen are found in AR versus AU cartilage.21 Thus, differences in gene expression may reflect differences in the primary cells rather than differential response in vitro. However, the data suggest that AU chondrocytes behave more like primary cells than AR chondrocytes when cultured in vitro in HA hydrogels, since AR chondrocytes exhibited a greater increase in type I collagen and a greater decrease in type II collagen expression during culture compared to isolated cells. Hyaluronidase expression within the hydrogels was also examined to gain insight on the potential catabolism of the HA scaffold by encapsulated cells. Hyals are enzymes that cleave the β-1,4-glycosidic bonds between glucuronic acid and N-acetylglucosamine28 and are responsible for HA turnover, which can affect cell migration, differentiation, and matrix catabolism. Hyal 1 cleaves HA into small molecular masses of less than 20 kDa,29 while Hyal 2, the most prominent Hyal in cartilage, hydrolyzes high molecular mass HA into intermediate-sized fragments of approximately 20 kDa.30 Hyal 3 has also been detected in cartilage, but little is known about its enzymatic activity.31 With in vitro culture, both AU and AR constructs exhibited decreased gene expression of Hyal 2 and 3, suggesting downregulation of HA turnover and matrix catabolism.
With in vivo and in vitro cultures, AU chondrocyte–seeded constructs exhibited greater neocartilage formation and better phenotype retention. However, in these cultures, the encapsulated chondrocytes are exposed to a non-load-bearing environment that more closely resembles that of native AU cartilage. Thus, it was hypothesized that exposure to external mechanical stimulation would have a positive effect on AR chondrocytes, since they have responded to dynamic compression loading regimes in other scaffolds.32 Hydrogels are capable of transducing mechanical loads, which may stimulate ECM production via cell signaling resulting from cell deformation or mechanotransduction by cell surface receptors. However, it is important to note that scaffold chemistry and properties, as well as loading regime can affect chondrocyte response.33 When dynamic unconfined compression loading was applied to the chondrocyte-seeded HA hydrogels, a greater increase in type II collagen and aggrecan expression over unloaded hydrogels was observed for AR versus AU constructs after 5 days of consecutive loading. Thus, the data indicate that dynamic compression alone, without the addition of growth factors, is capable of stimulating type II collagen and aggrecan expression. Further, studies have shown catabolic and anabolic effects of compressive loading, hinting at a structural remodeling effect of the newly synthesized matrix through loading.34 In our study, Hyal gene expression also increased with mechanical stimulation for Hyal 2 and 3, which suggests that mechanical loading may stimulate local HA turnover and matrix remodeling.
In conclusion, we have shown that AU and AR chondrocytes photoencapsulated in HA hydrogels exhibit differences in cell behavior in both in vivo and in vitro cultures, and differ in their response to mechanical stimulation. AU chondrocytes excelled in producing neocartilage in vivo, in a subcutaneous environment that more closely resembled native AU cartilage, while AR chondrocytes exhibited enhanced gene expression in a mechanically loaded environment, more akin to that of a loaded joint environment. Thus, the differences in cell behavior of various chondrocyte sources in combination with specific biomaterials must be taken into account when designing scaffolds for cartilage tissue engineering. The next steps in the development of this hydrogel system for clinical implementation include assessment in large animal models that mimic specific clinical instances in human. Specifically, the use of autologous cells in these HA gels from various sources in a variety of defects should be investigated and the mechanics and ECM productions should be analyzed.
Acknowledgments
Support for this research was provided through NIH grants (K22DE015761 and RO3AR053668) and NSF Graduate Research Fellowships (to C.C. and I.E.E.). The authors would like to thank Mark Randolph for his generous donation of swine tissue.
References
- 1. van Osch G.J.V.M., Mandl E.W., Jahr H., Koevoet W., Nolst-Trenite G., and Verhaar J.A. Considerations on the use of ear chondrocytes as donor chondrocytes for cartilage tissue engineering. Biorheology 41, 411, 2004 [PubMed] [Google Scholar]
- 2. Hicks D.L., Sage A.B., Schumacher B.L., Sah R.L., and Watson D. Growth and phenotype of low-density nasal septal chondrocyte monolayers. Otolaryngol Head Neck Surg 133, 417, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Kafienah W., Jakob M., Demarteau O., Frazer A., Barker M.D., Martin I., and Hollander A.P. Three-dimensional tissue engineering of hyaline cartilage: comparison of adult nasal and articular chondrocytes. Tissue Eng 8, 817, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Tay A.G., Farhadi J., Suetterlin R., Pierer G., Heberer M., and Martin I. Cell yield, proliferation, and postexpansion differentiation capacity of human ear, nasal, and rib chondrocytes. Tissue Eng 10, 762, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Panossian A., Ashiku S., Kirchhoff C.H., Randolph M.A., and Yaremchuk M.J. Effects of cell concentration and growth period on articular and ear chondrocyte transplants for tissue engineering. Plast Reconstr Surg 108, 392, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Xu J.W., Zaporojan V., Peretti G.M., Roses R.E., Morse K.B., Roy A.K., Mesa J.M., Randolph M.A., Bonassar L.J., and Yaremchuk M.J. Injectable tissue-engineered cartilage with different chondrocyte sources. Plast Reconstr Surg 113, 1361, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Isogai N., Kusuhara H., Ikada Y., Ohtani H., Jacquet R., Hillyer J., Lowder E., and Landis W.J. Comparison of different chondrocytes for use in tissue engineering of cartilage model structures. Tissue Eng 12, 691, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Johnson T.S., Xu J.W., Zaporojan V.V., Mesa J.M., Weinand C., Randolph M.A., Bonassar L.J., Winograd J.M., and Yaremchuk M.J. Integrative repair of cartilage with articular and nonarticular chondrocytes. Tissue Eng 10, 1308, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Menzel E.J., and Farr C. Hyaluronidase and its substrate hyaluronan: biochemistry, biological activities and therapeutic uses. Cancer Lett 131, 3, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Burdick J.A., Chung C., Jia X., Randolph M.A., and Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules 6, 386, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chung C., Mesa J., Miller G.J., Randolph M.A., Gill T.J., and Burdick J.A. Effects of auricular chondrocyte expansion on neocartilage formation in photocrosslinked hyaluronic acid networks. Tissue Eng 12, 2665, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chung C., Mesa J., Randolph M.A., Yaremchuk M., and Burdick J.A. Influence of gel properties on neocartilage formation by auricular chondrocytes photoencapsulated in hyaluronic acid networks. J Biomed Mater Res A 77, 518, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smeds K.A., Pfister-Serres A., Miki D., Dastgheib K., Inoue M., Hatchell D.L., and Grinstaff M.W. Photocrosslinkable polysaccharides for in situ hydrogel formation. J Biomed Mater Res 54, 115, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Mauck R.L., Byers B.A., Yuan X., and Tuan R.S. Regulation of cartilaginous ECM gene transcription by chondrocytes and MSCs in 3D culture in response to dynamic loading. Biomech Model Mechanobiol 6, 113, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Singer V.L., Jones L.J., Yue S.T., and Haugland R.P. Characterization of PicoGreen reagent and development of a fluorescence-based solution assay for double-stranded DNA quantitation. Anal Biochem 249, 228, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Farndale R.W., Sayers C.A., and Barrett A.J. A direct spectrophotometric microassay for sulfated glycosaminogly-cans in cartilage cultures. Connect Tissue Res 9, 247, 1982 [DOI] [PubMed] [Google Scholar]
- 17. Stegemann H., and Stalder K. Determination of hydroxyproline. Clin Chim Acta 18, 267, 1967 [DOI] [PubMed] [Google Scholar]
- 18. Herbage D., Bouillet J., and Bernengo J.C. Biochemical and physiochemical characterization of pepsin-solubilized type-II collagen from bovine articular cartilage. Biochem J 161, 303, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Williamson A.K., Chen A.C., and Sah R.L. Compressive properties and function-composition relationships of developing bovine articular cartilage. J Orthop Res 19, 1113, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Chung C., and Burdick J.A. Engineered cartilage tissue. Adv Drug Deliv Rev, 60, 243, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Naumann A., Dennis J.E., Awadallah A., Carrino D.A., Mansour J.M., Kastenbauer E., and Caplan A.I. Immunochemical and mechanical characterization of cartilage subtypes in rabbit. J Histochem Cytochem 50, 1049, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Chen W.Y., and Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen 7, 79, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Shu X.Z., Liu Y., Luo Y., Roberts M.C., and Prestwich G.D. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules 3, 1304, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Armstrong C.G., and Mow V.C. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J Bone Joint Surg Am 64, 88, 1982 [PubMed] [Google Scholar]
- 25. Kempson G.E., Muir H., Swanson S.A., and Freeman M.A. Correlations between stiffness and the chemical constituents of cartilage on the human femoral head. Biochim Biophys Acta 215, 70, 1970 [DOI] [PubMed] [Google Scholar]
- 26. Bryant S.J., Nuttelman C.R., and Anseth K.S. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomater Sci Polym Ed 11, 439, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Williams C.G., Malik A.N., Kim T.K., Manson P.N., and Elisseeff J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 26, 1211, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Chow G., Knudson C.B., and Knudson W. Expression and cellular localization of human hyaluronidase-2 in articular chondrocytes and cultured cell lines. Osteoarthritis Cartilage 14, 849, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Frost G.I., Csoka A.B., Wong T., and Stern R. Purification, cloning, and expression of human plasma hyaluronidase. Biochem Biophys Res Commun 236, 10, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Lepperdinger G., Strobl B., and Kreil G. HYAL2, a human gene expressed in many cells, encodes a lysosomal hyaluronidase with a novel type of specificity. J Biol Chem 273, 22466, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Flannery C.R., Little C.B., Hughes C.E., and Caterson B. Expression and activity of articular cartilage hyaluronidases. Biochem Biophys Res Commun 251, 824, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Mauck R.L., Soltz M.A., Wang C.C.B., Wong D.D., Chao P.H.G., Valhmu W.B., Hung C.T., and Ateshian G.A. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng Trans ASME 122, 252, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Bryant S.J., Chowdhury T.T., Lee D.A., Bader D.L., and Anseth K.S. Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Ann Biomed Eng 32, 407, 2004 [DOI] [PubMed] [Google Scholar]
- 34. de Croos J.N.A., Dhaliwal S.S., Grynpas M.D., Pilliar R.M., and Kandel R.A. Cyclic compressive mechanical stimulation induces sequential catabolic and anabolic gene changes in chondrocytes resulting in increased extracellular matrix accumulation. Matrix Biol 25, 323, 2006 [DOI] [PubMed] [Google Scholar]