Abstract
Objective
The NHLBI Family Heart Study (FHS) genome-wide linkage scan identified a region of chromosome 7q with a logarithm of odds score of 4.9 for body mass index (BMI).
Design
We report the results of fine mapping the linkage peak using 1020 single nucleotide polymorphisms (SNPs) to test for association to obesity in families exhibiting linkage to chromosome 7. Association observed in linked families (284 obese cases/ 381 controls) was examined in an independent set of unrelated FHS participants (172 obese cases/308 controls) to validate the observed association. Two dichotomous obesity phenotypes were studied based on clinical BMI cutoffs and the sex-specific distribution of both BMI and leptin levels.
Results
Using a P-value of 0.01 as criteria for association in the linked families, a P-value of 0.05 as criteria for association in the unrelated sample, and requiring consistency in the direction of the effect of the minor allele between the two samples, we identified two coding SNPs in the NYD-SP18 gene with minor alleles increasing the risk of obesity. Adjustment for exercise, smoking and FTO genotype did not influence the result in linked families, but improved the result in the unrelated sample. Carrying a minor allele of the nonsynonymous SNP rs6971091 conferred an odds ratio of at least 2 for obesity defined by both BMI and leptin levels.
Conclusion
The effect of the NYD-SP18 SNP on obesity was larger than the effect of FTO in FHS families. Publicly available results from genome-wide association studies support the association between NYD-SP18 and BMI. The NYD-SP18 gene is described as testes development related, but little is known about the gene’s function or the mechanism by which it may influence risk for obesity.
Keywords: leptin, single nucleotide polymorphism, association
Introduction
A genome-wide linkage scan for body mass index (BMI) in the National Heart, Lung and Blood Institute (NHLBI) Family Heart Study (FHS) identified a quantitative trait locus on chromosome 7q32.3 with a logarithm of odds (LOD) score of 4.9, peaking at D7S1804 (136.95 cM; 131.9 Mb).1 The region of interest on chromosome 7 includes the leptin gene and has also been implicated by several other linkage studies for BMI, obesity and body fat phenotypes (see Feitosa et al.,1 2002 for review). In 2003, Li et al.2 reported the result of dense microsatellite mapping across a 40 cM region from 7q22.1 to 7q35 and concluded that two or more genes in the region may influence obesity. The most significant results reported were in a region upstream of the leptin gene, flanked by markers D7S692 and D7S523 at approximately 121–123 cM. In contrast, Platte et al.3 reported linkage and association with BMI in the old order Amish population for the region flanked by D7S1804 and D7S3070, at approximately 137–163 cM. Previously, we reported an association between variants 5′ of the leptin gene and BMI that was limited to men in FHS,4 but the minimal association among women suggested that other genes under the linkage peak may also influence BMI and obesity.
In the present study, we sought to locate the position of genes that may influence obesity on chromosome 7q31–7q34 by fine mapping the linkage region using single nucleotide polymorphisms (SNPs) to test for association. As a part of the linkage follow-up study, leptin levels have been ascertained for a subset of the original FHS participants. Circulating leptin levels are primarily determined by amount of body fat,5 whereas BMI cannot always distinguish between obesity and an athletic physique.6 Therefore, in addition to using traditional BMI cutoffs to define obesity, information on both BMI and leptin levels was used to define a dichotomous obesity phenotype for association testing. Leptin levels differ substantially by sex, which has been attributed to differences in percent body fat and hormone levels between sexes.7 Incorporating sex-specific leptin level cutoffs was designed to reduce the potential for misclassification of individuals into the obese group that may occur using a BMI only definition for obesity.
In addition to studying two independent samples from the FHS to validate observed effects on chromosome 7, we incorporated information from publicly released results of genome-wide association (GWA) studies performed for BMI and obesity. Recently, several reports have implicated the fat mass and obesity-associated FTO gene (fatso) to have a substantial and replicated effect increasing risk for obesity.8–10 A SNP in FTO was genotyped and accounted for in analyses of chromosome 7 polymorphisms. The gene region implicated by analyses in our FHS data was evaluated for evidence of association in GWA results from (1) the Diabetes Genetics Initiative GWA for BMI in the control sample11 and (2) the Framingham Heart Study 100K GWA for BMI.12
Methods
Two independent samples were selected from FHS for fine mapping and validation of identified SNP association. Families from the original genome-wide linkage study for BMI were evaluated to determine a family-specific LOD score using the microsatellite genotyping. The family sample selected for follow-up consisted of the 220 families that exhibited evidence for linkage to BMI on chromosome 7q with a positive family-specific LOD score. By selecting families with evidence for linkage, we sought to increase the frequency of the associated alleles in the study sample and thereby increase the power to detect association.13
To validate SNP association identified in the linked family set, an independent unrelated sample was selected from individuals whose families were recruited as part of FHS, but who were not included in the genome scan. Unrelated cases and controls were selected from the tails of the BMI distribution, choosing equal numbers of individuals from the four study centers by sex. The study was approved by the institutional review boards of the participating institutions and appropriate informed consent was obtained from all participants.
All genotyping was performed on one of two platforms. In the linked families, 426 SNPs were genotyped using TaqMan technology implemented on the ABI PRISM 7900HT Sequence Detection system (Applied Biosystems; Foster City, CA, USA) in the Family Heart Study genotyping laboratory at Boston University School of Medicine. The ABI genotyping is performed one SNP at a time, thus only the SNPs with evidence for association in the linked families were genotyped in the unrelated sample. In addition, both linked families and unrelated case/control subjects were genotyped using Illumina (San Diego, CA, USA) custom genotyping implemented with the BeadArray technology, which genotypes SNPs in a multiplex experiment. A total of 594 SNPs were genotyped in all samples. SNP selection for the ABI genotyping was prioritized based on known and hypothetical genes within the chromosome 7q22–q36 region. SNP selection for the Illumina genotyping was designed to fill gaps in ABI genotyping and used the Tagger software 14 to identify tag-SNPs after accounting for the ABI genotyping. SNPs were selected to have a minor allele frequency (MAF) greater than 5% in HapMap, except for coding SNPs, which were selected with MAF greater than 1%. The resulting coverage was most dense in a 10.8Mb region centered under the linkage peak beginning at the LEP gene, where the average maximum r2 was 0.71, with 58% coverage at an r2 of 0.8. Assessing the entire 17Mb region, we achieved 43% coverage at an r2 of 0.8 due to a more gene focused approach to genotyping at the boundaries of the linkage region.
Two dichotomous phenotypes were created to categorize participants as obese cases and non-obese controls. First, a crude BMI cutoff according to World Health Organization (WHO) guidelines was used to define obesity as BMI ≥30kg/m2 and controls as normal or under-weight with BMI ≤25 kg/m2.15 Second, a dichotomous phenotype was created using cutoffs defined by the sex-specific distributions of both BMI and leptin levels. For BMI, a total of 2882 women and 2450 men from the entire FHS were evaluated to identify the 25th and 75th percentiles. Leptin levels were measured for members of the linked families and the unrelated case and control sample. These 924 women and 872 men were used to identify the 25th and 75th percentiles. The obese BMI/leptin phenotype was defined for women as BMI ≥30.7821 and leptin ≥29.1 (N=107 linked families, N=64 unrelated) and for men as BMI ≥30.2923 and leptin ≥10.5 (N=81 linked families, N=49 unrelated). The control BMI/leptin phenotype was defined for women as BMI ≤22.926 and leptin ≤10.9 (N=92 linked families, N=80 unrelated) and for men BMI ≤24.8555 and leptin ≤3.9 (N=79 linked families, N=81 unrelated). This BMI/leptin phenotype uses more stringent criteria for defining case and control status, and thus fewer cases and controls meet the criteria for the BMI/ leptin trait than met criteria for the definition based on WHO BMI cutoffs. All participants reporting a diagnosis of diabetes were excluded to create a homogenous sample whose obesity status has not been influenced by diabetes medications or lifestyle changes consequential to a diabetes diagnosis.
Members of the linked families, excluding diabetics, were studied for all 1020 SNPs for both traits. Logistic generalized estimating equations were used to estimate the odds ratio (OR) for both obesity phenotypes associated with the minor SNP allele and adjusted for age, age2, age3, study center and sex accounting for multiple observations within a family. The unrelated nondiabetic case/control sample was studied for all SNPs with a P-value ≤0.01 for association with either obesity trait in the linked families. A P-value ≤0.05 in the unrelated case/control sample and consistency in the direction of effect of the OR between the linked families and unrelated case/control set was considered evidence of replication. The SNPs identified by the primary replication-based approach were reevaluated to incorporate additional covariate adjustment for multifactorial influences on obesity.
Results
The characteristics of the cases and controls in the linked families and the unrelated sample are shown in Table 1. The mean BMI and leptin levels are similar for cases/controls in the linked families compared to the unrelated sample. Mean ages are in the early 50 s across all samples. The proportion of subjects with hypertension or high cholesterol is higher in the case groups, but comparable between families and unrelated.
Table 1.
Descriptive characteristics of cases and controls in linked families and the unrelated sample
| Linked families |
Unrelated |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cases |
Controls |
Cases |
Controls |
|||||
| Female | Male | Female | Male | Female | Male | Female | Male | |
| Obesity (N) | 164 | 120 | 239 | 142 | 99 | 73 | 159 | 149 |
| BMI (kg/m2) | 36.3±5.3 | 34.2±4.1 | 22.1±1.9 | 22.9±1.6 | 36.4±6.0 | 34.3±4.0 | 21.5±1.8 | 22.9±1.7 |
| Leptin (ng/ml) | 41.3±22.0 | 15.5±9.9 | 11.8±5.8 | 3.7±1.8 | 39.1±19.5 | 14.5±8.5 | 10.3±4.6 | 4.2±2.3 |
| Age (years) | 53.5±13.4 | 51.1±13.0 | 50.6±14.0 | 50.2±15.2 | 50.9±12.1 | 52.4±11.2 | 51.0±12.6 | 51.5±14.7 |
| % Hypertensive | 35.98 | 40.0 | 16.74 | 22.54 | 42.42 | 38.36 | 10.06 | 20.13 |
| % High cholesterol | 29.27 | 33.33 | 26.78 | 24.65 | 47.47 | 27.40 | 27.67 | 22.82 |
Abbreviation: BMI, body mass index.
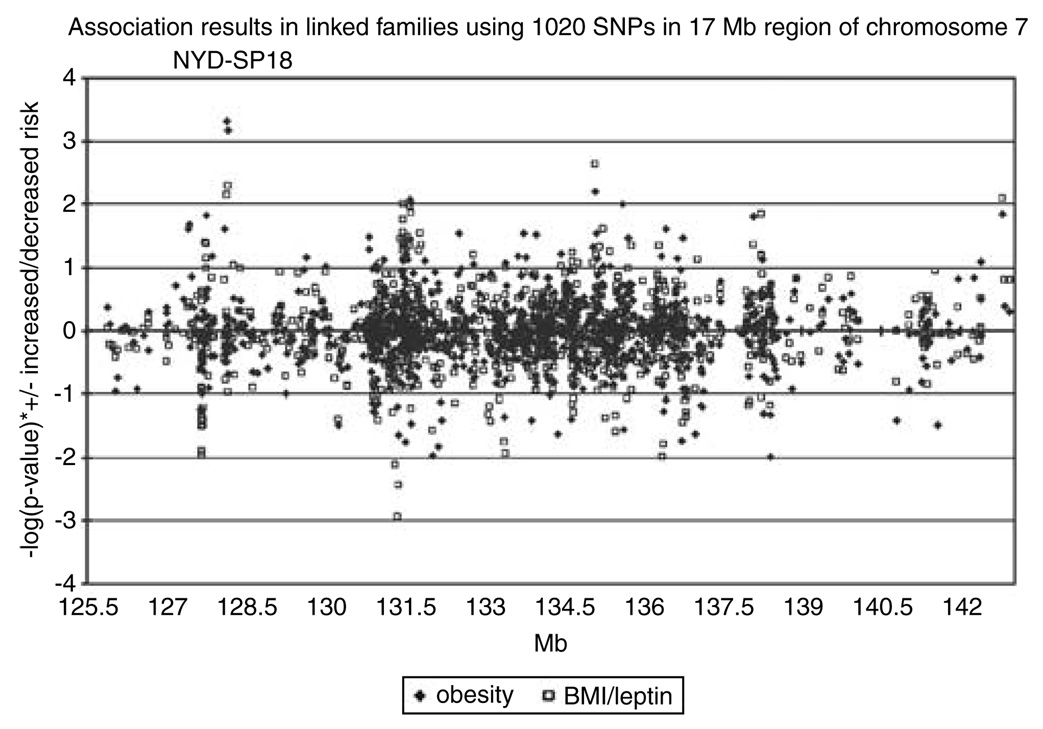
Figure 1 portrays the results of association to both obesity phenotypes across the fine-mapped region in linked families. A total of 12 SNPs had P-values≤ 0.01 for either the clinically obese phenotype or the BMI/leptin obesity phenotype in the linked families (Table 2). Of these, two SNPs had P≤0.05 in the case/control sample and consistency in the direction of the OR for the minor allele (bolded in Table 2). One of the two SNPs met the replication criteria for both phenotypes. The SNPs identified are in strong linkage disequilibrium (LD) with one another (D′ =0.99, r2=0.97), and both are located in the coding region of the gene NYD-SP18 (FAM137A). Because the SNPs are in such strong LD, a haplotype did not improve evidence for association over that seen for single SNPs.
Figure 1.
P-values plotted on the y axis with positive values for odds ratios (ORs >1; minor allele increases risk of obesity) and negative values for ORs <1 (minor allele protective for obesity). Physical position in megabase pairs is depicted by x axis.
Table 2.
Twelve SNPs with P-values ≤0.01 in linked families adjusted for age, age2, age3, study center and sex
| SNP | Position | Gene | Linked families |
Unrelated |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Obesity |
BMI/leptin |
Obesity |
BMI/leptin |
|||||||
| OR | P-value | OR | P-value | OR | P-value | OR | P-value | |||
| rs745229 | 128146126 | NYD-SP18 | 2.13 | 0.0005 | 2.20 | 0.007 | 1.52 | 0.03 | 1.62 | 0.06 |
| rs6971091 | 128150522 | 128150522 | 2.09 | 0.0007 | 2.25 | 0.005 | 1.53 | 0.03 | 1.66 | 0.05 |
| rs11771570 | 131311468 | 0.70 | 0.17 | 0.40 | 0.008 | 1.29 | 0.25 | 1.64 | 0.09 | |
| rs6943360 | 131355683 | 0.66 | 0.06 | 0.33 | 0.001 | 1.14 | 0.52 | 1.22 | 0.47 | |
| rs4731839 | 131376671 | 0.48 | 0.02 | 0.30 | 0.003 | 1.29 | 0.31 | 1.45 | 0.27 | |
| rs281877 | 131472007 | KIAA1550|PLXNA4 | 1.52 | 0.08 | 2.45 | 0.01 | 1.09 | 0.70 | 1.03 | 0.93 |
| rs717005 | 131600752 | PLXNA4 | 1.95 | 0.008 | 2.62 | 0.01 | 1.10 | 0.69 | 0.71 | 0.25 |
| rs6950129 | 131616006 | PLXNA4 | 2.07 | 0.01 | 2.95 | 0.01 | 1.06 | 0.82 | 0.66 | 0.26 |
| rs12531707 | 133378770 | EXOC4|KIAA1699 | 0.60 | 0.04 | 0.39 | 0.008 | 0.86 | 0.53 | 0.87 | 0.65 |
| rs10257539 | 135090454 | 1.76 | 0.006 | 2.34 | 0.002 | 0.75 | 0.15 | 0.88 | 0.62 | |
| rs7811360 | 135611338 | 2.06 | 0.01 | 1.52 | 0.33 | 0.73 | 0.26 | 0.85 | 0.69 | |
| rs17854363 | 142763844 | FAM131B | 1.80 | 0.01 | 2.28 | 0.008 | 1.10 | 0.64 | 0.88 | 0.64 |
Abbreviations: BMI, body mass index; OR, odds ratio; SNP, single nucleotide polymorphism. SNPs meeting replication criteria in the unrelated sample are given in bold.
To address concerns that unrecognized population stratification may generate a false positive result, we performed a family-based association test (FBAT) in the linked families for the two SNPs in NYD-SP18. Both SNPs had P-values <0.01 for the dichotomous obesity trait using the FBAT test (rs745229, P-value=0.004; rs6971091, P-value=0.008), and both had P-values <0.05 for the BMI/leptin dichotomous trait (rs745229, P-value=0.03; rs6971091, P-value=0.04). For both SNPs, the minor alleles (frequency=0.23) were associated with obesity, which was consistent with the findings from GEE.
To address the issue of other genetic and environmental factors that influence obesity, additional covariates were included in the GEE modeling for NYD-SP18 SNPs. Four additional variables were added to the GEE model: (1) a quantitative variable derived to reflect the total exercise activity metabolic index (met—minutes per week); (2) a quantitative variable reflecting the average number of hours spent watching television per day, an index of a sedentary lifestyle; (3) a dichotomous variable identifying current smokers and (4) FTO minor allele for SNP rs9939609. Adjusting for these covariates had little effect on the ORs and P-values in the linked families. Adjusting for covariates in the case/control sample increased the ORs and decreased the P-value for association to NYD-SP18 SNP rs6971091, particularly for the association to the BMI/leptin trait (Table 3). Adjusted results in the case/control sample for rs6971091 produced an OR=2 and P=0.02 for the BMI/ leptin obese phenotype. Association to the FTO SNP was not statistically significant in either linked families or case/ control. Intermediate analyses suggested that adjustment for exercise, TV and smoking attenuated a stronger crude effect from FTO minor alleles on risk. The OR estimate of 1.6 for FTO minor allele carriers in the case/control is similar in effect size to literature reports.8 A comparison of the population attributable risk (PAR) for the NYD-SP18 and FTO SNPs in linked families indicates a higher risk associated with the NYD-SP18 minor allele (PAR=0.35) than that of FTO minor allele (PAR=0.21). However, among the unrelated sample, the PAR estimates are both 0.29.
Table 3.
Results for NYD-SP18 adjusting for FTO minor allele, exercise, TV watching and smoking to estimate the OR for association to the BMI/leptin phenotype
| SNP | Position | Gene | Linked families |
Unrelated case control |
||
|---|---|---|---|---|---|---|
| OR | P-value | OR | P-value | |||
| rs6971091 | 128150522 | NYD-SP18 | 2.3 | 0.008 | 2.0 | 0.02 |
| rs9939609 | 52378028 | FTO | 1.4 | 0.22 | 1.6 | 0.12 |
Abbreviations: OR, odds ratio; SNP, single nucleotide polymorphism.
Discussion
While the 7q31–34 region is one of the most widely replicated regions for linkage to obesity and BMI, there remains uncertainty as to the number and position of the genes that may account for this linkage. Using genotyping information from 1020 SNPs typed in families showing strong linkage to BMI in this region, we found evidence for association to obesity with SNPs in the gene NYD-SP18. Using an independent sample of unrelated individuals, the increased risk for obesity associated with the minor allele was validated. The minor alleles had a frequency of 0.23 and in the linked family sample provided an OR estimate for obesity on the order of 2.1, while the unrelated sample provided a minimally adjusted OR estimate of 1.5. The OR estimate from the linked families may overestimate the effect because of the selection criteria of linkage to the region. However, the effect size in the unrelated sample is larger when additional covariates are included in the model to account for activity, smoking and FTO genotype, reflecting the multifactorial influences on BMI and obesity.
To address the contribution of the identified SNP to the linkage signal, we assessed the LOD score for BMI in this selected subset of the original families. Among families selected for linkage, the LOD score for BMI was 16.76. Adjusting for the NYD-SP18 nonsynonymous SNP as a covariate in linkage analysis, the resulting LOD score was 15.31, representing a 9% reduction in the LOD score. The result suggests that the SNP accounts for a portion of the linkage signal. The 1020 genotyped SNPs in linked families cover a large chromosomal region, but the density of typing could still be improved, and thus it is possible that other genes in the region also influence BMI. The power in the unrelated sample to validate an association observed in the linked families is limited. For an SNP with an MAF of 25%, we had 56% power to detect an OR of 1.5 and 95% power to detect an OR of 2. Thus, some of the non-replication of modest findings from linked families may represent false-negative results. Nonetheless, the nonsynonymous NYD-SP18 SNP appears to account for a portion of the linkage signal and association was validated in independent samples.
Recently, a GWA study of BMI was performed in conjunction with a diabetes GWA.11 A GWA analysis was performed for BMI separately in the diabetic cases and nondiabetic control sample. The two SNPs in NYD-SP18 reported here with association to obesity traits in FHS exhibited modest association to BMI in the nondiabetic sample. The SNP rs745229 was genotyped and generated a P-value of 0.057 for association to BMI, and the SNP rs6971091 was not directly genotyped but instead was computationally imputed and a P-value of 0.048 was observed (http://www.broad.mit.edu/diabetes/scandinavs/TEXT/DGI_full_table_bmi_cfsp_1106_controls_all_sexes_allm.txt). Both SNPs are reported with positive β estimates for an additive model, which replicates the direction of effect observed in FHS, and minor allele frequencies were similar at 0.25. In addition, the results identified rs339078 with a P-value of 0.02 associated with BMI, and this SNP is located physically between the aforementioned coding SNPs.
The Framingham Heart Study recently released the results of GWA analyses.16 Results for the mean BMI phenotype derived from averaging BMI across seven clinical examinations 12 were examined for the NYD-SP18 region. Only two of the 100K SNPs were located within the transcription start and stop of the gene. The SNP rs339093 with an MAF of 0.07 generated an FBAT P-value of 0.016 for the mean BMI trait in 62 informative families. The independent results from the control sample of the diabetes GWA and these Framingham Heart Study results further support the role of NYD-SP18 in BMI and obesity.
NYD-SP18 is thought to be related to testes development (LocusID: 84691). The SNP rs745229 is a synonymous polymorphism in exon 3 and rs6971091 is a nonsynonymous polymorphism substituting glutamic acid for lysine at amino-acid position 242 in exon 4. Information from the SNPeffect database (http://snpeffect.vib.be/snp_main.php?id=27249604) suggests that the amino-acid substitution at rs6971091 may influence HSP70 (70 kDa heat shock protein) binding affinity and could result in a misfolded native protein.17,18 A sample of 16 participants from the linked families, half of whom carried the minor allele of the identified SNPs, was sequenced for seven NYD-SP18 exons and the putative promoter region and no novel coding polymorphisms were revealed. Sequencing was designed for the shorter isoform of the gene that aligns with FAM137A. Additional sequencing in the exons and promoter of the longer NYD-SP18 isoform are planned.
The rationale for the involvement of NYD-SP18 in regulating BMI is largely speculative at this point. The gene has been labeled as testes development related and is known to be expressed in the testes,19 which fosters speculation that it may influence testosterone levels, but this has not been demonstrated. Testosterone levels have been studied extensively as they relate to obesity and leptin levels. Both BMI and leptin levels have been shown to be negatively correlated to testosterone levels in men, but the correlation in women was different depending on obesity; leptin and testosterone levels were positively correlated in non-obese women and negatively correlated among women with higher BMI.20 A second tissue in which NYD-SP18 has been shown to be expressed is the salivary gland.19 Expression in the salivary gland may suggest an influence on digestion, as saliva contains enzymes that initiate starch digestion, but it may also support the testosterone connection. Salivary glands metabolize testosterone, which is detectable in saliva, and salivary gland hormone concentrations may be influenced by testosterone metabolism.21 The testosterone–obesity association suggests that one of the first steps to dissecting the influence of NYD-SP18 on obesity may be to determine whether the polymorphism has any influence on circulating or salivary testosterone levels.
In summary, we have identified a nonsynonymous polymorphism in the NYD-SP18 gene that increases risk of obesity in two independent samples from the FHS. In the FHS families exhibiting linkage to this region, the attributable risk estimate for the NYD-SP18 minor allele is larger than that of the FTO polymorphism that was recently identified and replicated as an obesity candidate gene, and among the unrelated FHS sample, the attributable risk estimates were similar. The association to NYD-SP18 is further supported by nominally significant P-values for association to BMI in publicly available GWA results. Though the pathway through which NYD-SP18 is associated to obesity is unclear, further research into the gene’s expression and replication of this finding are warranted.
Acknowledgements
This study was supported by National Heart, Lung, and Blood Institute grants NHLBI RO-1 HL68891-06. This article is presented on behalf of the investigators of the NHLBI Family Heart Study.
References
- 1.Feitosa MF, Borecki IB, Rich SS, Arnett DK, Sholinsky P, Myers RH, et al. Quantitative-trait loci influencing body-mass index reside on chromosomes 7 and 13: the National Heart, Lung, and Blood Institute Family Heart Study. Am J Hum Genet. 2002;70:72–82. doi: 10.1086/338144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li WD, Li D, Wang S, Zhang S, Zhao H, Price RA. Linkage and linkage disequilibrium mapping of genes influencing human obesity in chromosome region 7q221–7q35. Diabetes. 2003;52:1557–1561. doi: 10.2337/diabetes.52.6.1557. [DOI] [PubMed] [Google Scholar]
- 3.Platte P, Papanicolaou GJ, Johnston J, Klein CM, Doheny KF, Pugh EW, et al. A study of linkage and association of body mass index in the Old Order Amish. Am J Med Genet. 2003;121:71–80. doi: 10.1002/ajmg.c.20005. [DOI] [PubMed] [Google Scholar]
- 4.Jiang Y, Wilk JB, Borecki I, Williamson S, DeStefano AL, Xu G, et al. Common variants in the 5′ region of the leptin gene are associated with body mass index in men from the National Heart, Lung, and Blood Institute Family Heart Study. Am J Hum Genet. 2004;75:220–230. doi: 10.1086/422699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paracchini V, Pedotti P, Taioli E. Genetics of leptin and obesity: a HuGE review. Am J Epidemiol. 2005;162:101–114. doi: 10.1093/aje/kwi174. [DOI] [PubMed] [Google Scholar]
- 6.Yang W, Kelly T, He J. Genetic epidemiology of obesity. Epidemiol Rev. 2007;29:49–61. doi: 10.1093/epirev/mxm004. [DOI] [PubMed] [Google Scholar]
- 7.Elbers JM, Asscheman H, Seidell JC, Frolich M, Meinders AE, Gooren LJ. Reversal of the sex difference in serum leptin levels upon cross-sex hormone administration in transsexuals. J Clin Endocrinol Metab. 1997;82:3267–3270. doi: 10.1210/jcem.82.10.4284. [DOI] [PubMed] [Google Scholar]
- 8.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dina C, Meyre D, Gallina S, Durand E, Korner A, Jacobson P, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39:724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 10.Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 12.Fox CS, Heard-Costa N, Cupples LA, Dupuis J, Vasan RS, Atwood LD. Genome-wide association to body mass index and waist circumference: the Framingham Heart Study 100K project. BMC Med Genet. 2007;8:S18. doi: 10.1186/1471-2350-8-S1-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fingerlin TE, Boehnke M, Abecasis GR. Increasing the power and efficiency of disease-marker case-control association studies through use of allele-sharing information. Am J Hum Genet. 2004;74:432–443. doi: 10.1086/381652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Bakker PI, Yelensky R, Pe’er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–1223. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization technical Report Series. 2000;894(i–xii):1–253. [PubMed]
- 16.Cupples LA, Arruda HT, Benjamin EJ, D’Agostino RB, Sr, Demissie S, DeStefano AL, et al. The Framingham Heart Study 100K SNP genome-wide association study resource: overview of 17 phenotype working group reports. BMC Med Genet. 2007;8:S1. doi: 10.1186/1471-2350-8-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reumers J, Conde L, Medina I, Maurer-Stroh S, Van Durme J, Dopazo J, et al. Joint annotation of coding and non-coding single nucleotide polymorphisms and mutations in the SNPeffect and PupaSuite databases. Nucleic Acid Res. 2008;36:D825–D829. doi: 10.1093/nar/gkm979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudiger S, Germeroth L, Schneider-Mergener J, Bukau B. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 1997;16:1501–1507. doi: 10.1093/emboj/16.7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson JM, Castle J, Garrett-Engele P, Kan Z, Loerch PM, Armour CD, et al. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science. 2003;302:2141–2144. doi: 10.1126/science.1090100. [DOI] [PubMed] [Google Scholar]
- 20.Soderberg S, Olsson T, Eliasson M, Johnson O, Brismar K, Carlstrom K, et al. A strong association between biologically active testosterone and leptin in non-obese men and women is lost with increasing (central) adiposity. Int J Obes Relat Metab Disord. 2001;25:98–105. doi: 10.1038/sj.ijo.0801467. [DOI] [PubMed] [Google Scholar]
- 21.Cefalu WT, Pardridge WM, Chaudhuri G, Judd HL. Serum bioavailability and tissue metabolism of testosterone and estradiol in rat salivary gland. J Clin Endocrinol Metab. 1986;63:20–28. doi: 10.1210/jcem-63-1-20. [DOI] [PubMed] [Google Scholar]