Abstract
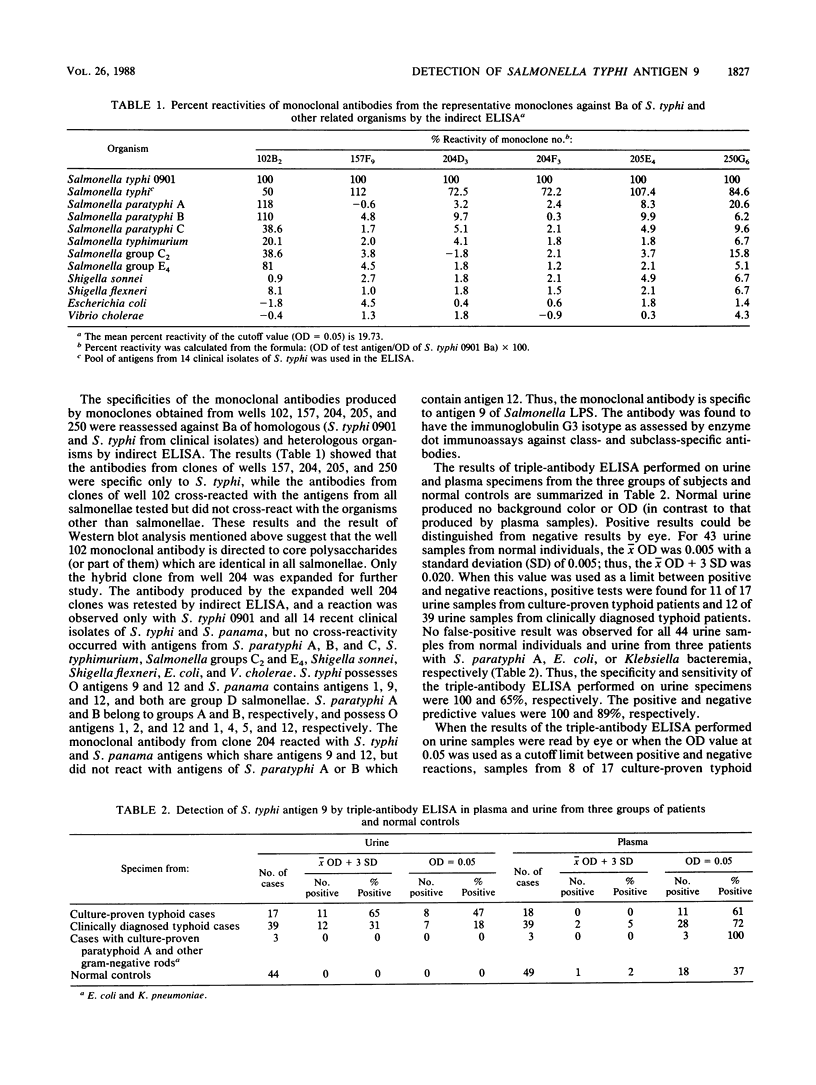
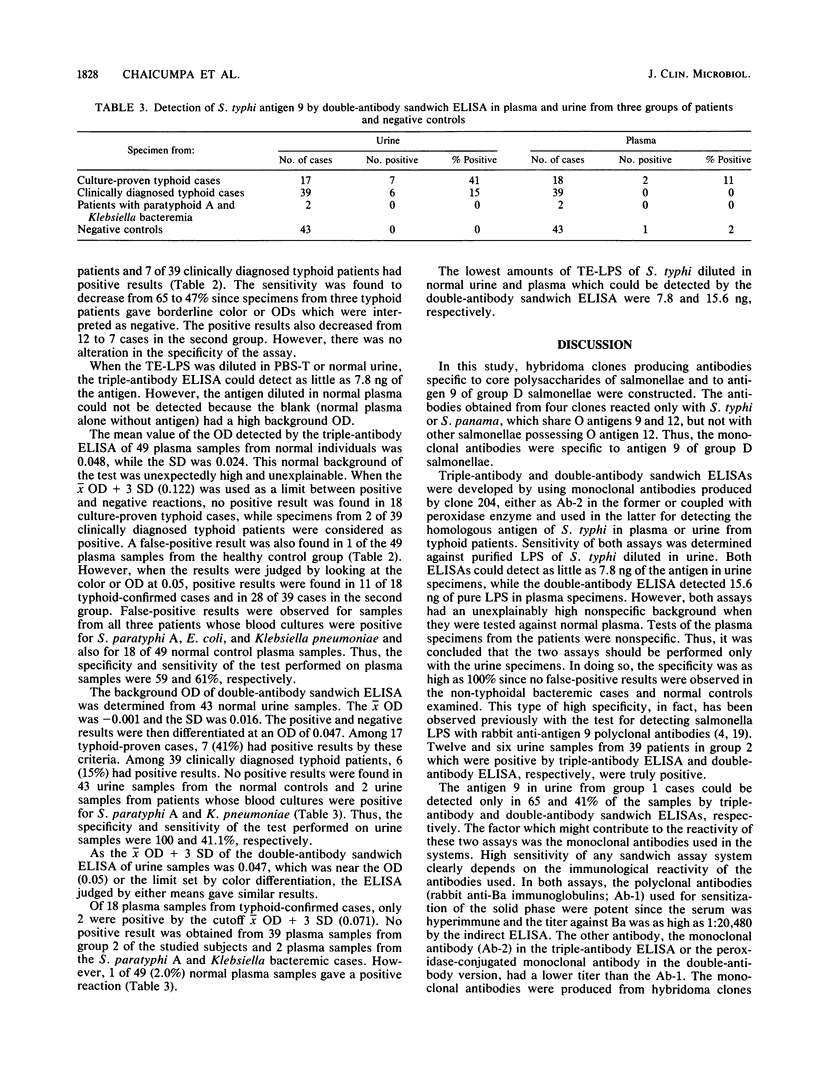
Monoclonal antibodies were raised against Barber antigen (Ba) of Salmonella typhi 0901. Antibodies produced to antigen 9 of group D salmonellae were used in double- and triple-sandwich antibody enzyme-linked immunosorbent assays (ELISAs) for detecting antigen 9 in urine and plasma specimens from three groups of patients and 49 controls. The triple-antibody ELISA detected the antigen in urine samples from 11 of 18 (65%) patients with hemoculture-proven typhoid (group 1) and 12 of 39 (31%) patients with clinical features compatible with typhoid but whose hemocultures were negative (group 2). This ELISA was negative in three patients from whom Salmonella paratyphi A, Escherichia coli, and Klebsiella pneumoniae (group 3) were isolated by hemoculture and in all healthy controls. The double-antibody sandwich ELISA was positive in 41 and 15% of urine samples from patients in groups 1 and 2, respectively, and was negative with samples from two patients from group 3 and all controls. The sensitivity and specificity compared with those for healthy controls were 65 and 100%, respectively, for the triple-antibody ELISA. Although as little as 7.8 ng of homologous lipopolysaccharide could be detected, background in clinical specimens prevented accurate interpretation of the detection of this antigen in serum. Results were best with urine specimens.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appassakij H., Bunchuin N., Sarasombath S., Rungpitarangsi B., Manatsathit S., Komolpit P., Sukosol T. Enzyme-linked immunosorbent assay for detection of Salmonella typhi protein antigen. J Clin Microbiol. 1987 Feb;25(2):273–277. doi: 10.1128/jcm.25.2.273-277.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber C., Vladoianu I. R., Dimache G. Contributions to the study of Salmonella. Immunological specificity of proteins separated from Salmonella typhi. Immunology. 1966 Oct;11(4):287–296. [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carlsson M., Hedin A., Inganäs M., Härfast B., Blomberg F. Purification of in vitro produced mouse monoclonal antibodies. A two-step procedure utilizing cation exchange chromatography and gel filtration. J Immunol Methods. 1985 May 10;79(1):89–98. doi: 10.1016/0022-1759(85)90395-3. [DOI] [PubMed] [Google Scholar]
- Edelman R., Levine M. M. Summary of an international workshop on typhoid fever. Rev Infect Dis. 1986 May-Jun;8(3):329–349. doi: 10.1093/clinids/8.3.329. [DOI] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Gupta A. K., Rao K. M. Simultaneous detection of Salmonella typhi antigen and antibody in serum by counter-immunoelectrophoresis for an early and rapid diagnosis of typhoid fever. J Immunol Methods. 1979;30(4):349–353. doi: 10.1016/0022-1759(79)90017-6. [DOI] [PubMed] [Google Scholar]
- Hewitt J., Coates A. R., Mitchison D. A., Ivanyi J. The use of murine monoclonal antibodies without purification of antigen in the serodiagnosis of tuberculosis. J Immunol Methods. 1982 Dec 17;55(2):205–211. doi: 10.1016/0022-1759(82)90032-1. [DOI] [PubMed] [Google Scholar]
- John T. J., Sivadasan K., Kurien B. Evaluation of passive bacterial agglutination for the diagnosis of typhoid fever. J Clin Microbiol. 1984 Oct;20(4):751–753. doi: 10.1128/jcm.20.4.751-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khusmith S., Tharavanij S., Patarapotikul J., Koonrangsesomboon D. Characterisation of monoclonal antibodies against blood forms of Plasmodium falciparum by the immunofluorescent test. Asian Pac J Allergy Immunol. 1984 Jun;2(1):91–95. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindmark R., Thorén-Tolling K., Sjöquist J. Binding of immunoglobulins to protein A and immunoglobulin levels in mammalian sera. J Immunol Methods. 1983 Aug 12;62(1):1–13. doi: 10.1016/0022-1759(83)90104-7. [DOI] [PubMed] [Google Scholar]
- Rockhill R. C., Rumans L. W., Lesmana M., Dennis D. T. Detection of Salmonella typhi D, Vi, and d antigens, by slide coagglutination, in urine from patients with typhoid fever. J Clin Microbiol. 1980 Mar;11(3):213–216. doi: 10.1128/jcm.11.3.213-216.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockhill R. C., Rumans L. W., Lesmana M. Slide coagglutination for Salmonella typhi antigens in broths inoculated with feces from typhoid fever patients. Southeast Asian J Trop Med Public Health. 1981 Dec;12(4):528–532. [PubMed] [Google Scholar]
- Shetty N. P., Srinivasa H., Bhat P. Coagglutination and counter immunoelectrophoresis in the rapid diagnosis of typhoid fever. Am J Clin Pathol. 1985 Jul;84(1):80–84. doi: 10.1093/ajcp/84.1.80. [DOI] [PubMed] [Google Scholar]
- Sundararaj T., Ilango B., Subramanian S. A study on the usefulness of counter immuno-electrophoresis for the detection of Salmonella typhi antigen in the sera of suspected cases of enteric fever. Trans R Soc Trop Med Hyg. 1983;77(2):194–197. doi: 10.1016/0035-9203(83)90067-6. [DOI] [PubMed] [Google Scholar]
- Svenungsson B., Jörbeck H., Lindberg A. A. Diagnosis of Salmonella infections: specificity of indirect immunofluorescence for rapid identification of Salmonella enteritidis and usefulness of enzyme-linked immunosorbent assay. J Infect Dis. 1979 Dec;140(6):927–936. doi: 10.1093/infdis/140.6.927. [DOI] [PubMed] [Google Scholar]
- Taylor D. N., Harris J. R., Barrett T. J., Hargrett N. T., Prentzel I., Valdivieso C., Palomino C., Levine M. M., Blake P. A. Detection of urinary Vi antigen as a diagnostic test for typhoid fever. J Clin Microbiol. 1983 Oct;18(4):872–876. doi: 10.1128/jcm.18.4.872-876.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Tsang R. S., Chau P. Y., Lam S. K., La Brooy J. T., Rowley D. Antibody response to the lipopolysaccharide and protein antigens of Salmonella typhi during typhoid infection. I. Measurement of serum antibodies by radioimmunoassay. Clin Exp Immunol. 1981 Dec;46(3):508–514. [PMC free article] [PubMed] [Google Scholar]