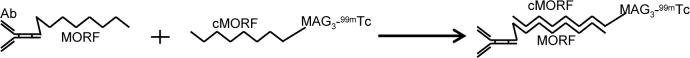Abstract
In treating tumors by pretargeting, the antitumor antibody and the cytotoxic effector (e.g. toxins, radioactivity) are separately administered. Therefore pretargeting is more complicated with many variables. We are conducting studies to understand the influence of each variable using a novel recognition pair of mutually complementary phosphorodiamidate morpholino oligomers (i.e. MORF/cMORF). Earlier we developed a semiempirical model capable of accurately predicting the behavior of a radiolabeled cMORF effector with variations in dosages and timing. We have now extended the model to predict the effector behavior, in particular, its maximum percent tumor accumulation (MPTA) in mice pretargeted with three different MORF-conjugated antibodies (MN14, B72.3 and CC49). The MN14 and the CC49 target different antigens in the same tumor, while the CC49 and the B72.3 target the same antigen but with very different tumor accumulation. By comparing the pretargeting results of these three antibodies with our prediction, we confirmed that the MPTA of the radiolabeled cMORF effector in the LS174T tumor is independent of the antibodies. In conclusion, the MPTA cannot be improved through the use of different pretargeting antibodies, although different antibodies may improve the maximum absolute tumor accumulation, the heterogeneity, and/or the tumor-to-normal tissue ratios of the effector. This conclusion will apply equally well to effectors carrying a fluorescent probe, an anticancer agent, or a radioactive imaging agent.
Keywords: Pretargeting, Anticancer, Antibodies, Tumor, Radioimmunotargeting
INTRODUCTION
Pretargeting involves at least two injections, the first to target tumor with an antitumor antibody and the second to deliver the effector carrying a fluorescent probe, an anticancer agent, or an imaging radionuclide as in this study. Because this approach combines the superior tumor targeting property of antibodies with the rapid pharmacokinetics of a small effector, it is proving to be increasingly useful for both tumor imaging and radiotherapy (1-5). It has been shown that pretargeting can greatly improve the tumor delivery of radionuclides over the conventional direct-targeting by radiolabeled antibodies (6-11). Clinical radiotherapeutic trials are also showing promise in the treatment of solid tumors (12-13). A novel pretargeting approach is under development in this laboratory using the recognition pair of an 18 mer phosphorodiamidate morpholino oligomer and its complement (i.e. MORF/cMORF) (14, 15), as illustrated in Fig 1.
Fig 1.
Principle of pretargeting using morpholino oligomers as the recognition system
For convenience, the pretargeting variables may be divided into two categories. For a two-step pretargeting approach, the variables of category 1 include tumor model, pretargeting antibody, and effector. The variables of category 2 include those relating to dosage and timing, specifically, the dosages of pretargeting antibody and effector, the pretargeting interval, and the detection time. We have developed a semiempirical model to predict the biodistribution of the cMORF effector in pretargeted tumored mice with variations in the category 2 variables (16, 17). This prediction model is based on the pharmacokinetics of the pretargeting antibody, the pharmacokinetics of the radiolabeled effector administered alone, the accessibility of the antibody for the radiolabeled effector in the pretargeted tumor and normal tissues, the quantitative relationships between pretargeting antibody and effector, and finally the delivery property of the radiolabeled effector to the tumor and normal tissues. In this report, we are extending this model to investigate one category 1 variable, namely the pretargeting antibody.
One important concept associated with the semiempirical model and the category 1 variables is the Maximum Percent Tumor Accumulation (MPTA) of the cMORF effector in %ID or %ID/g. Our previous investigations indicated that, for a given solid tumor model (tumor host, type, size, and location) and a fixed dosage of pretargeting antibody, the absolute tumor accumulation of effector increases linearly with increasing effector dosage until saturation and then levels off (16, 17). When plotted as the percent accumulation (i.e. %ID/g), the curve is horizontal until saturation and then declines. In the horizontal range, the percent accumulation corresponds to the maximum percent accumulation (i.e. MPTA). The MPTA and the constant slope of the absolute accumulation curve in the linear range are related by MPTA= slope × 100 % / tumor weight. Similar dosage studies of effectors have been reported elsewhere although in less detail (18, 19).
It can be easily shown that the MPTA must be independent of the nature of pretargeting antibody. Recently (20), we have provided an expression of effector accumulation Q as a function of several factors:
where F is the cardiac output in grams of blood per second (g/s), f is the fraction of the cardiac output reaching the tumor, W is the tumor weight (g), C is the blood level of the effector in %ID/g and E is the trapping efficiency (the retained fraction of the effector reaching the tumor). If the dosage of cMORF effector is below that required to saturate the MORF-antibody in tumor, E will be a constant. However, if the dosage of cMORF effector is above that required dosage, when the MORF in tumor becomes saturated, E will become zero such that the additional cMORF effector delivered to tumor cannot be retained. Thus, the percent tumor accumulation of cMORF effector just before the saturation of the pretargeting antibody in tumor will be the maximum percent tumor accumulation (i.e. MPTA):
| (1) |
This equation shows that the MPTA is independent of the pretargeting antibody and dependent only on the tumor and the effector. Thus, provided that the dosage does not exceed that dosage required to saturate the effector-binding sites presented by the pretargeting antibody in tumor, the percent tumor accumulation of the effector will be maximum at the value of MPTA and constant with increasing effector dosage. Of course, if the tumor model is changed, f may change and E may also be different due to the variance of diffusion barriers.
The prediction model
1. Tumor
In this study, the experimental conditions were arranged to ensure that the tumor accumulation of the effector would be at MPTA. The effector dosage was selected based on the experimentally determined accessibility of the MORF-antibody for the labeled cMORF effector, the average number of MORFs per antibody (gpm), and the MORF-antibody accumulation in tumor (%ID/g). We have shown that indium-111 (111In) may be used to radiolabel the antibody MN14 for use in measuring its concentration in tumor and normal tissues and that 99mTc may be used to radiolabel the cMORF effector for use in measuring the accessibility of antibody in these tissues (16).
For an accessibility of 100% in tumor, the dosage of MORF-antibody (Dantibody μg) that required just to be saturated in tumor by any fixed effector dosage (DCmorf , μg) can be calculated by equation 2:
| (2) |
Where %ID/g antibody is the tumor accumulation of MORF-antibody and Mantibody and McMORF are the molecular weights of the antibody and cMORF effector respectively.
2. Blood
By the nature of pretargeting, the radiolabeled effector is always designed to be excreted rapidly such that at the time of detection its blood level is usually minimal. In addition, at this time, the blood level of the pretargeting antibody will also be low and can be assumed to be a constant because of its slow pharmacokinetics. Thus the total blood concentration of effector (Cblood) will be the sum of the concentrations of antibody-bound (bound Cblood) and free (free Cblood) effector. The bound Cblood can be calculated from the saturation of the circulating antibody by equation 3 and, as a first approximation, the free Cblood may be estimated from the experimental clearance curve of the labeled effector in tumored mice not receiving the pretargeting antibody (17). The accessibility of MORF-antibody in blood has been shown to be about 100% (16).
| (3) |
3. Normal Organs
In principle, the level in normal organs may be estimated in the same way as in blood. However, the following observation simplifies the estimation and eliminates the need to measure the accessibility. In our previous studies with the MN14 antibody, over the wide range tested we found the organ-to-blood ratios of antibody-bound effector are constant in all organs other than kidneys (see immediately below), independent of the dosages of antibody and effector as well as the pretargeting interval. Our interpretation is that the accessible MORF-MN14 in these normal organs is in equilibrium with MORF-MN14 in blood (17). As described below, we show that this equilibrium of MORF-antibodies between normal organs and blood still holds for MORF-CC49 and MORF-B72.3 as well.
4. Kidney
The kidney is an unique organ with respect to pretargeting since the cMORF effector clears exclusively via this organ and its accumulation is independent of the presence or absence of MORF-antibody therein (17). Thus the kidney accumulation is a property of the radiolabeled cMORF effector and independent of the pretargeting antibody provided that any pharmacokinetic effects can be ignored. For example, if the blood level of a particular antibody is unusually high at the time of effector administration, more of the effector will be bound in circulation and less will be free to clear and accumulate in the kidneys. However, this high level of antibody is seldom encountered in pretargeting.
Based on the above semiempirical model and the MPTA expression, we now report on the agreement of the experimental results with predictions in pretargeting with three different antibodies. The antiCEA antibody MN14 and the antiTAG-72 antibody CC49 target different antigens in the same tumor, while the CC49 and B72.3 target the same TAG-72 antigens but with a large difference in tumor accumulation.
MATERIALS AND METHODS
As before, the base sequences of MORF and its complement (cMORF) were respectively 5’-TCTTCTACTTCACAACTA-linker-amine and 5’-TAGTTGTGAAGTAGAAGA-linker-amine (GeneTools, Philomath, OR). The murine anti-CEA antibody MN14 was a gift from Immunomedics (Morris Plains, NJ). The antiTAG-72 antibody CC49, also murine, was produced by Strategic Biosolutions (Ramona, CA) from the CC49 murine hybridoma cell line, a gift from Dr Schlom (Laboratory of Tumor Immunology and Biology, Center for Cancer Research, NCI, NIH, Bethesda, MD). The first generation antiTAG-72 antibody B72.3 was a gift from NCI BRB Preclinical Repository (Rockville, MD). The S-acetyl NHS-MAG3 was synthesized in house (21) and the structure confirmed by elemental analysis, proton NMR, and mass spectroscopy. The Hydralink linker was from Solulink Biosciences (San Diego, CA). The DTPA cyclic anhydride was from Sigma (St Louis, MO). The p-SCN-Benzyl-DTPA was from Macrocyclics (Dallas, TX). The P-4 resin (Bio-Gel P-4 Gel, medium) was purchased from Bio-Rad Laboratories (Hercules, CA) and the Sephadex G-100 resin was from Pharmacia Biotech (Uppsala, Sweden). The 99Mo-99mTc generator and the 111InCl3 were both from Perkin Elmer Life Science Inc (Boston, MA). All other chemicals were reagent grade and used without purification.
99m Tc-labeled cMORF, 111 In-labeled antibodies, and MORF-conjugated antibodies
The MAG3-cMORF was synthesized as previously described (22). To radiolabel, 50 μL of 99mTc-pertechnetate generator eluate was introduced into a combined solution consisting of 30 μL (> 0.5 µg) of MAG3-MORF in pH 5.2 NH4OAc buffer, 10 μL of 50 μg/μL Na2tartrate·2H2O in a pH 9.2 buffer, and 3 μL of 4 μg/μL SnCl2·2H2O in ascorbate-HCl solution (1 μg/μL Na·ascorbate in 10 mM HCl). The solution was heated at 100 °C for 20 min. The labeling efficiency was routinely greater than 95% as determined by size exclusion HPLC.
While the MN14 and B72.3 were successfully conjugated with the DTPA cyclic anhydride as previously described (15), difficulties were experienced in conjugating the CC49 and labeling with 111In in this manner. Therefore p-SCN-Benzyl-DTPA was used for this antibody. Briefly, a CC49 solution (1.7 μg/μL) in 20 mM PBS, pH 7.2, was mixed with a 0.80 μg/μL p-SCN-Benzyl-DTPA solution in 0.5 M NaCO3-NaHCO3 buffer (molar ratio = 2/8, pH 9.3) at a DTPA-to-antibody molar ratio of 55 and the mixture allowed to react overnight (15−24 h). The average number of DTPAs per antibody (gpm) was determined by analyzing the 111In-labeled conjugation mixture by size exclusion HPLC before purification and calculated by multiplying the DTPA-to-antibody molar ratio by the fraction of radioactivity on the antibody. Purification was achieved on a 0.7×20 cm Sephadex G-100 glass Econo-column. The labeling with 111In of the three antibodies was as previously described (15).
The MORF-antibodies were synthesized using a commercial Hydralink linker (23). The antibodies were first conjugated with succinimidyl 4-hydrazinonicotinate acetone hydrazone while the MORF was conjugated with succinimidyl 4-formylbenzoate. After purification, combining the hydrazine-modified antibody and the benzaldehyde-modified MORF resulted in hydrazone formation. Purification of the MORF-antibodies from the free MORF was also achieved on a 0.7×20 cm Sephadex G-100 glass Econo-column and the gpm was determined following routine procedure described previously (15).
Animal study
All animal studies were performed with the approval of the UMMS Institutional Animal Care and Use Committee. For tumor induction, 106 LS174T colon cancer cells were injected into the left thigh of each Swiss NIH nude mouse (Taconic Farms, Germantown, NY). The animals were used at 12−13 days when the tumors were about 0.5 g. To measure the biodistribution of each antibody, three groups (N = 4) of tumored mice received via a tail vein 30 μg (20−30 μCi ) of 111In-labeled B72.3, MN14, or CC49. After 48 h, the mice were sacrificed for biodistribution by exsanguination via heart puncture under halothane anesthesia. In the pretargeting studies, three groups (N=4) of tumored mice received via a tail vein 48, 23 and 11 μg MORF-conjugated B72.3, MN14, and CC49 respectively. After 48 h, each mouse received 1 μg (100 μCi) of labeled cMORF also via a tail vein. Animals were sacrificed for biodistribution 3 h later.
At sacrifice, samples of blood and other organs were removed, weighted, and counted in a NaI(Tl) well counter (Cobra II automatic gamma counter, Packard Instrument Company, CT) along with a standard of the injectate. Blood and muscle were assumed to constitute 7 % and 40 % of body weight respectively. The tumored thigh was excised and the skin and as much of the muscle and bone as possible were removed before counting. The radioactivity therein was attributed to the tumor since the radioactivity levels in bone and muscle were negligible. After the tumor thigh was counted, the tumor mass was dissected to isolate the residual bone and muscle so that their weights could be subtracted to provide the net tumor weight.
RESULTS
Biodistribution of 111 In labeled antibodies
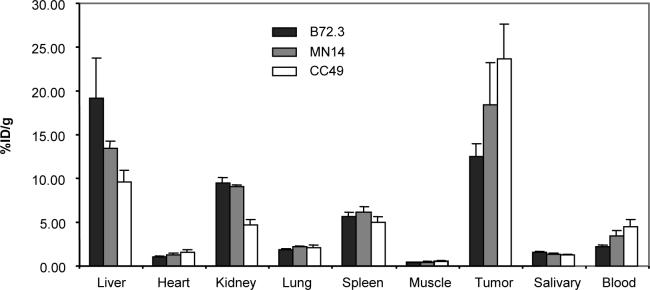
The 48 h biodistributions of the three antibodies radiolabeled with 111In in LS174T tumored mice are presented in Fig 2. As shown, while the accumulations in heart, lung, spleen, muscle, and salivary gland were generally antibody independent, the liver accumulations were highly dependent. Significant differences in kidney accumulations occurred only for the labeled CC49 antibody (Student's t test, p < 0.05). The tumor accumulations varied in proportion to the blood levels such that the tumor-to-blood ratios for these antibodies are essentially identical (5.6, 5.4, and 5.3 for B72.3, MN14, and CC49 respectively). The 48 h tumor accumulations of the 111In labeled antibodies in %ID/g and corresponding average tumor weights in gram in parentheses were 12.5 ± 1.4 (0.65 ± 0.12), 16.7 ± 3.9 (0.58 ± 0.13), and 23.6 ± 4.0 (0.57 ± 0.13) for B72.3, MN14 and CC49 respectively.
Fig 2.
Biodistributions (%ID/g) of the three 111In labeled antibodies at 48 h. Error bars indicate one SD (N = 4)
Biodistribution of labeled cMORF in pretargeted mice
The conjugation of B72.3, MN14, and CC49 with MORF provided an average of 0.53, 0.83, 1.22 gpm respectively. The tumor accumulation of the cMORF effector earlier determined in the mice pretargeted with MORF-MN14, 6.24 ± 0.41 %ID/g for a 0.52 ± 0.05 g LS174T tumor, was predicted to be the MPTA of cMORF effector in this study with each of the three antibodies (17). Using the molecular weights of cMORF (6331 Da) and antibody (160 kDa) and the 48 h 111In-antibody tumor accumulations (Fig. 2), the dosage of MORF-antibody that must be administered such that the accessible MORFs in tumor at 48 h are just saturated with the cMORF effector can be estimated by equation 2 for any given dosage of cMORF effector. In this manner and assuming the MPTA is independent of antibody, the calculation for an effector dosage of 2 μg provides 48, 23 and 11 μg as the dosages of MORF conjugated B72.3, MN14, and CC49 respectively. While these antibody dosages were used in the animal studies, the dosage of effector was reduced from 2 to 1 μg to ensure the MORF binding sites in tumor would not be saturated (i.e. MPTA conditions).
Table 1 lists the 3 h biodistributions of labeled cMORF effector in tumored mice pretargeted 48 h earlier by each of the three MORF-antibodies at the dosages selected. Since the labeled cMORF effector was administered at a dosage lower than that required for saturation, the tumor accumulations should be the MPTAs. The experimental values in each organ are now compared with the theoretical expectations below.
Table 1.
The 3 h biodistributions (%ID/g) of labeled cMORF effector in tumored mice pretargeted 48 h earlier by each of the MORF-MN14, -CC49, and B72.3 antibodies. Tumor sizes (g) are also included.
| Organ | MN14 | CC49 | B72.3 |
|---|---|---|---|
| Liver | 0.84±0.15 | 0.63±0.08 | 1.35±0.24 |
| Heart | 0.55±0.09 | 0.30±0.05 | 0.85±0.24 |
| Kidney | 4.10±1.33 | 3.57±0.71 | 3.41±0.48 |
| Lung | 0.85±0.16 | 0.68±0.06 | 1.38±0.41 |
| Spleen | 0.44±0.06 | 0.41±0.05 | 0.77±0.15 |
| Muscle | 0.33±0.19 | 0.18±0.03 | 0.45±0.13 |
| Tumor | 6.28±1.47 | 6.16±0.47 | 8.89±1.28 |
| Sal. gland | 0.59±0.11 | 0.44±0.07 | 1.02±0.24 |
| Blood |
2.26±0.38 |
1.60±0.09 |
3.75±0.99 |
| Tumor size (g) | 0.50±0.09 | 0.54±0.11 | 0.43±0.23 |
1. Tumor
Because both the pharmacokinetics and the average number of MORF per antibody differed among the antibodies, the dosage of each MORF-antibody was adjusted to provide the same accessible MORF concentration in tumor. Otherwise, a different dosage of effector would be required to meet the MPTA conditions for each antibody.
Fig 3 shows the experimental MPTAs from Table 1 along with the corresponding average tumor size (g). The tumor accumulations (6.28 ± 1.47 and 6.16 ± 0.47 %ID/g) at 3 h in mice pretargeted 48 h earlier with the MN14 and CC49 antibodies are statistically identical to each other and to that predicted (6.24 ± 0.41 %ID/g), while that for B72.3 (8.89 ± 1.28 %ID/g) is significantly higher than predicted (Student's t test, p < 0.05). We have observed previously an inverse relationship between tumor accumulation and tumor size (17) to suggest that the higher tumor accumulation in this case is most likely due to the smaller tumor size of 0.43 g.
Fig 3.

Tumor accumulations of effector at 3 h by pretargeting with three different MORF-antibodies 48 h earlier. Error bars signify one SD (N = 4). The horizontal line at 6.24 %ID/g is the predicted value.
2. Blood
At the detection time of 3 h, the blood level of free effector was earlier determined to be 0.04 %ID/g (15). The total blood levels of effector can then be predicted by adding this free effector level to the antibody-bound effector levels calculated from equation 3. In animals pretargeted 48 h earlier with the MORF-MN14, the experimental blood level of cMORF effector agrees well within two SDs with the predicted as shown in Table 2a. One standard deviation was fixed at 25% of the predicted as before (17). The difference in the case of animals receiving MORF-B72.3 is just outside this limit while the difference in the case of MORF-CC49 is larger. These discrepancies may be related to different pharmacokinetic behavior of the 111In-DTPA-B72.3 used to trace the pharmacokinetics of the MORF-conjugated antibody.
Table 2.
Comparisons between experimental and predicted values for (a) blood levels (%ID/g) and organ-to blood ratios of antibody-bound effector at 3 h post injection of cMORF effector in LS174T tumored mice having received one of three MORF conjugated antibodies 48 h before injection of cMORF effector. Values presented as the average with one SD (N = 4)
| (a) Blood level | ||||
|---|---|---|---|---|
| B72.3 | MN14 | CC49 | ||
| Experimental | 3.75±0.99 | 2.26±0.38 | 1.60±0.09 | |
| Predicted | 2.27±0.57 | 2.51±0.63 | 2.41±0.60 | |
| (b) Organ-to blood ratios | ||||
|---|---|---|---|---|
| Predicted* | B72.3 | MN14 | CC49 | |
| Liver/blood | 0.31 | 0.30±0.08 | 0.26±0.03 | 0.23±0.05 |
| Spleen/blood | 0.15 | 0.16±0.02 | 0.12±0.02 | 0.15±0.04 |
| Lung/blood | 0.40 | 0.34±0.06 | 0.33±0.05 | 0.36±0.04 |
| Heart/blood | 0.20 | 0.21±0.01 | 0.22±0.00 | 0.15±0.03 |
| Muscle/blood | 0.11 | 0.11±0.02 | 0.14±0.09 | 0.10±0.02 |
Taken from (17)
3. Liver, spleen, lung, heart, and muscle
As mentioned above, the organ-to-blood ratios of antibody-bound effector were earlier found experimentally to be constants with respect to the dosages of antibody and effector as well as the pretargeting interval (17). The experimental and predicted ratios from this study are listed in Table 2b. To derive these experimental ratios, it was necessary to obtain the value for the bound effector by subtracting the free effector level in each normal organ from the experimental organ accumulations in the same manner as that described above for blood. The free effector levels at 3 h were earlier determined to be 0.27, 0.17, 0.12, 0.06, and 0.03 %ID/g for liver, spleen, lung, heart, and muscle respectively (17). As shown, the experimental ratios for all three antibodies are within two SDs of that predicted. The predicted ratios in the table are taken from previous pretargeting studies with MN14 (17) and reproduced in this study. As is evident in Table 2 b, the ratios for the pretargetings using B72.3 and CC49 antibodies are also in agreement with the predicted.
4. Kidney
The kidney accumulations of the effector were 3.41±0.48, 4.10±1.33 and 3.57±0.71 %ID/g in the mice pretargeted with MORF conjugated B72.3, MN14, and CC49 respectively and agree within 2 SD with the predicted 5.44±1.51 %ID/g obtained from the kidney accumulations measured during several previous studies (17), again reflecting that the kidney accumulation is a determinant of the effector only. In our case, the influence of the different pharmacokinetics of each antibody on the kidney levels of MORF may be neglected since, independent of the antibody, most of the effector clears through the kidneys at 3 h (15).
DISCUSSION
We showed previously that the biodistribution of the cMORF effector in pretargeted mice can be predicted by our semiempirical model with variations in three category-2 variables, namely the dosage of pretargeting antibody, the dosage of effector, and the pretargeting interval (17). Therefore this approach may help avoid trial-and-error studies of pretargeting optimization. Several rules govern the selection of the category-2 variables for optimal pretargeting. The pretargeting interval should be selected to provide the required tumor-to-blood ratio. The detection time should be selected to allow the free effector to clear to the background radioactivity level required. The dosage of the pretargeting antibody is not a critical variable since any convenient dosage may be selected although it should be below that required to saturate the tumor antigenic sites. With a too high antibody dosage, higher than necessary levels of antibody will be present in circulation and normal organs at the time of effector administration. To achieve an optimal pretargeting outcome, the effector dosage should be such that the accessible antibody localized in tumor is just saturated by the effector. A lower effector dosage will still provide the MPTA (%ID or %ID/g) but a lower absolute accumulation in nmol or ng, thus providing a less favorable tumor-to-blood ratio. Conversely, an effector dosage in excess of that necessary to saturate the antibody in tumor will still provide the maximum absolute accumulation but a lower percent tumor accumulation, since a less percent of the effector will be retained in tumor.
In this study, we have extended the semiempirical model to predict the influence of the category-1 variables although the utility of our model regarding these variables is still qualitative. At this stage, all the parameters in the MPTA expression of equation 1 are not available yet. However, some predictions from this expression may improve our understanding of tumor delivery. In particular, we have predicted that the MPTA is independent of antibody based on equation 1, either targeting different antigens or having different pharmacokinetics. This experimental study has confirmed this prediction. Recently, we had also shown that the gpm of an antibody does not change the MPTA (23).
In the course of our investigations, several misconceptions residing in this and possibly other laboratories become clarified. Firstly, for solid tumors, the MPTA of the effector cannot be increased by increasing the number of effector-binding sites in tumor, for example, by administering a larger dosage of antibody (17), by increasing the gpm (23), or by other means (however, it is possible to increase the absolute tumor accumulation by, for example, amplification pretargeting, in which a multivalent polymer is administered between the pretargeting antibody and the effector (24)). Secondly, the benefit of pretargeting is usually not in achieving a higher absolute or percent tumor accumulation compared to conventional targeting, since the rapid clearance of the effector limits its delivery and therefore the absolute or percent tumor accumulations by pretargeting is often lower than that achievable by conventional targeting. Thirdly, pretargeting cannot provide tumor-to-blood ratios superior to that achieved by directly labeled antibody since the effector cannot target more pretargeted antibody than exists in tumor and normal tissues. However, as mentioned earlier, the advantage of pretargeting rests in combining the tumor affinity of the antitumor antibody with the rapid clearance of the effector (6-11). In this way, pretargeting can greatly improve the tumor delivery of cytotoxic agents, such as radioactive effectors, by providing a comparable tumor-to-blood ratio in approximately 1 h post administration of the effector to that achieved by radiolabeled antibodies in days and therefore greatly reduce the cytotoxicity to normal tissues.
However, it should be mentioned that certain tumor-to-normal-organ ratios may increase if the antibody in those organs, but not in tumor, become inaccessible to the effector due to metabolism or some other mechanism (25-27). In our experience, this phenomenon applies especially to liver in the case of MORF pretargeting. For example, in this investigation, the liver accumulations of CC49, MN14, and B72.3 measured by the 111In labeled antibodies were 9.6, 13.4, and 19.2 %ID/g respectively at 48 h (Fig 2). If the accessibility of the MORF-antibodies for the cMORF effector were 100%, the liver accumulations would have been about 5.1, 10.1, 19.3 %ID/g based on equation 3, rather than the much lower observed accumulations of 0.63, 0.84, 1.35 %ID/g.
To our knowledge, this report describes the first comparison of different antibodies for pretargeting under MPTA conditions. Several previous studies have compared different antibodies for pretargeting and reported antibody-dependent differences in the tumor accumulation of effector (8, 28-31). However, these earlier studies compared antibodies at the same dosage of antibodies and the same dosage of effector without considering the saturation of pretargeting antibody by effector in tumor. In this investigation, if each of the three antibodies had been administered at the same dosage and the effector were at a fixed dosage sufficient to saturate any of the three MORF-antibodies in tumor, the CC49 would have similarly provided a higher tumor accumulation of the effector than the other two. However, even in the case of CC49, the effector accumulation would have been lower than that achieved herein under the MPTA conditions.
Although the MPTA can not be improved by the use of different antibodies, there is often a need to consider different antibodies for pretargeting (32). Advanced recombinant and antibody-engineering technologies are now increasingly in use to develop antitumor antibodies with improved specificity, diminished cross reactivity with normal tissues, increased binding affinity, alleviated immunogenicity, reduced heterogeneity of antibody deposition in tumor, etc. These include intact antibodies targeting different antigens, antibodies with different pharmacokinetics, antibody fragments, and genetically engineered small antibody constructs (diabodies, minibodies, affibodies, etc). Reports on a large number of these pretargeting studies have appeared in recent years (33-36). Furthermore, if the dosage of effector is above that required to saturate the MORF-antibody in tumor, both the absolute and the percent tumor accumulation of the effector will depend upon the antibody, since antibodies differ in tumor accumulation (28), affinity for their antigen or the effector (37), the disappearance rate by either internalization or shedding of their antigen complexes, and the accessibility in tumor for the effector (38, 39).
In conclusion, from the results in mice pretargeted with three different antibodies, we have confirmed experimentally the prediction that the MPTA of the effector is independent of the pretargeting antibody in the LS174T tumor model. Although it is possible to improve tumor-to-normal-tissue ratios, the use of different antibodies can not improve the MPTA of labeled cMORF effector by pretargeting. While this investigation is concerned with an effector radiolabeled with an imaging radionuclide, the conclusions arrived at herein will apply equally well to effectors carrying other imaging labels such as fluorophores or cytotoxic labels such as therapeutic radionuclides.
ACKNOWLEDGEMENTS
The authors are grateful to Dr Griffiths (Immunomedics, Morris Plains, NJ) for providing the antiCEA antibody MN14, to Dr Schlom (Laboratory of Tumor Immunology and Biology, Center for Cancer Research, NCI, NIH, Bethesda, MD) for providing the CC49 hybridoma, and to Dr Raurgemma (NCI Preclinical Repository, Rockville, MD) for providing the B72.3 antibody. Financial support was from the National Institute of Health (CA94994 and CA107360).
Financial support: Financial supports are from the National Institute of Health (CA94994 and CA107360).
REFERENCES
- 1.Sharkey RM, Goldenberg DM. Advances in radioimmunotherapy in the age of molecular engineering and pretargeting. Cancer Invest. 2006;24:82–97. doi: 10.1080/07357900500449553. [DOI] [PubMed] [Google Scholar]
- 2.Reilly RM. Radioimmunotherapy of solid tumors: the promise of pretargeting strategies using bispecific antibodies and radiolabeled haptens. J Nucl Med. 2006;47:196–199. [PubMed] [Google Scholar]
- 3.Goldenberg DM, Sharkey RM, Paganelli G, Barbet J, Chatal JF. Antibody pretargeting advances cancer radioimmunodetection and radioimmunotherapy. J Clin Oncol. 2006;24:823–834. doi: 10.1200/JCO.2005.03.8471. [DOI] [PubMed] [Google Scholar]
- 4.Corneillie TM, Whetstone PA, Meares CF. Irreversibly binding anti-metal chelate antibodies: Artificial receptors for pretargeting. J Inorg Biochem. 2006;100:882–90. doi: 10.1016/j.jinorgbio.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Sharkey RM, Karacay H, Cardillo TM, et al. Improving the delivery of radionuclides for imaging and therapy of cancer using pretargeting methods. Clin Cancer Res. 2005;11:7109s–7021s. doi: 10.1158/1078-0432.CCR-1004-0009. [DOI] [PubMed] [Google Scholar]
- 6.Karacay H, Brard PY, Sharkey RM, et al. Therapeutic advantage of pretargeted radioimmunotherapy using a recombinant bispecific antibody in a human colon cancer xenograft. Clin Cancer Res. 2005;11:7879–7885. doi: 10.1158/1078-0432.CCR-05-1246. [DOI] [PubMed] [Google Scholar]
- 7.Sharkey RM, Cardillo TM, Rossi EA, et al. Signal amplification in molecular imaging by pretargeting a multivalent, bispecific antibody. Nat Med. 2005;11:1250–1255. doi: 10.1038/nm1322. [DOI] [PubMed] [Google Scholar]
- 8.Pagel JM, Hedin N, Subbiah K, et al. Comparison of anti-CD20 and anti-CD45 antibodies for conventional and pretargeted radioimmunotherapy of B-cell lymphomas. Blood. 2003;101:2340–2348. doi: 10.1182/blood-2002-03-0874. [DOI] [PubMed] [Google Scholar]
- 9.Subbiah K, Hamlin DK, Pagel JM, et al. Comparison of immunoscintigraphy, efficacy, and toxicity of conventional and pretargeted radioimmunotherapy in CD20-expressing human lymphoma xenografts. J Nucl Med. 2003;44:437–445. [PubMed] [Google Scholar]
- 10.Magnani P, Paganelli G, Modorati G, et al. Quantitative comparison of direct antibody labeling and tumor pretargeting in uveal melanoma. J Nucl Med. 1996;37:967–971. [PubMed] [Google Scholar]
- 11.Sung C, van Osdol WW. Pharmacokinetic comparison of direct antibody targeting with pretargeting protocols based on streptavidin-biotin binding. J Nucl Med. 1995;36:867–876. [PubMed] [Google Scholar]
- 12.Kraeber-Bodéré F, Rousseau C, Bodet-Milin C, et al. Targeting, toxicity, and efficacy of 2-step, pretargeted radioimmunotherapy using a chimeric bispecific antibody and 131I-labeled bivalent hapten in a phase I optimization clinical trial. J Nucl Med. 2006;47:247–255. [PubMed] [Google Scholar]
- 13.Forero-Torres A, Shen S, Breitz H, et al. Pretargeted radioimmunotherapy (RIT) with a novel anti-TAG-72 fusion protein. Cancer Biother Radiopharm. 2005;20:379–390. doi: 10.1089/cbr.2005.20.379. [DOI] [PubMed] [Google Scholar]
- 14.Liu G, Mang'era K, Liu N, Gupta S, Rusckowski M, Hnatowich DJ. Tumor pretargeting in mice using 99mTc-labeled morpholino, a DNA analog. J Nucl Med. 2002;43:384–391. [PubMed] [Google Scholar]
- 15.Liu G, He J, Dou S, et al. Eur J Nucl Med Mol Imaging. Pretargeting in tumored mice with radiolabeled morpholino oligomer showing low kidney uptake. 2004;31:417–424. doi: 10.1007/s00259-003-1393-9. [DOI] [PubMed] [Google Scholar]
- 16.Liu G, He J, Dou S, Gupta S, Rusckowski M, Hnatowich DJ. Further investigations of morpholino pretargeting in mice--establishing quantitative relations in tumor. Eur J Nucl Med Mol Imaging. 2005;32:1115–1123. doi: 10.1007/s00259-005-1853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu G, Dou S, He J, Liu X, Rusckowski M, Hnatowich Predicting the biodistribution of radiolabeled cMORF effector in MORF-pretargeted mice. Eur J Nucl Med Mol Imaging. 2007;34:237–246. doi: 10.1007/s00259-006-0222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharkey RM, Karacay H, Richel H, McBride WJ, Rossi EA, Chang K, Yeldell D, Griffiths GL, Hansen HJ, Goldenberg DM. Optimizing bispecific antibody pretargeting for use in radioimmunotherapy. Clin Cancer Res. 2003;9(10 Pt 2):3897S–3913S. [PubMed] [Google Scholar]
- 19.Gautherot E, Le Doussal JM, Bouhou J, Manetti C, Martin M, Rouvier E, Barbet J. Delivery of therapeutic doses of radioiodine using bispecific antibody-targeted bivalent haptens. J Nucl Med. 1998;39:1937–1943. [PubMed] [Google Scholar]
- 20.Liu G, Dou S, Pretorius PH, Liu X, Rusckowski M, Hnatowich DJ. Pretargeting CWR22 prostate tumor in mice with MORF-B72.3 antibody and radiolabeled cMORF. Eur J Nucl Med Mol Imaging. 2007 doi: 10.1007/s00259-007-0606-z. [Epub ahead of print] PMID: 17909792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winnard P, Jr, Chang F, Rusckowski M, Mardirossian G, Hnatowich DJ. Preparation and use of NHS-MAG3 for technetium-99m labeling of DNA. Nucl Med Biol. 1997;24:425–32. doi: 10.1016/s0969-8051(97)00027-9. [DOI] [PubMed] [Google Scholar]
- 22.Liu G, Dou S, He J, et al. Radiolabeling of MAG3-morpholino oligomers with 188Re at high labeling efficiency and specific radioactivity for tumor pretargeting. Appl Radiat Isot. 2006;64:971–978. doi: 10.1016/j.apradiso.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He J, Liu G, Dou S, Gupta S, Rusckowski M, Hnatowich DJ. An improved method for covalently conjugating morpholino oligomers to antitumor antibodies. Bioconjug Chem. 2007;18:983–988. doi: 10.1021/bc060208v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He J, Liu G, Gupta S, Zhang Y, Rusckowski M, Hnatowich DJ. Amplification targeting: a modified pretargeting approach with potential for signal amplification-proof of a concept. J Nucl Med. 2004;45:1087–1095. [PubMed] [Google Scholar]
- 25.Liu G, Liu C, Zhang S, et al. Investigations of 99mTc morpholino pretargeting in mice. Nucl Med Commun. 2003;24:697–705. doi: 10.1097/00006231-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Goodwin DA, Meares CF, Watanabe N, et al. Pharmacokinetics of pretargeted monoclonal antibody 2D12.5 and 88Y-Janus-2-(p-nitrobenzyl)-1,4,7,10-tetraazacyclododecanetetraacetic acid (DOTA) in BALB/c mice with KHJJ mouse adenocarcinoma: a model for 90Y radioimmunotherapy. Cancer Res. 1994;54:5937–5946. [PubMed] [Google Scholar]
- 27.Liu G, Zhang S, He J, et al. The influence of chain length and base sequence on the pharmacokinetic behavior of 99mTc-morpholinos in mice. Q J Nucl Med. 2002;46:233–243. [PubMed] [Google Scholar]
- 28.Karacay H, Sharkey RM, McBride WJ, et al. Pretargeting for cancer radioimmunotherapy with bispecific antibodies: role of the bispecific antibody's valency for the tumor target antigen. Bioconjug Chem. 2002;13:1054–1070. doi: 10.1021/bc0200172. [DOI] [PubMed] [Google Scholar]
- 29.Cardillo TM, Karacay H, Goldenberg DM, et al. Improved targeting of pancreatic cancer: experimental studies of a new bispecific antibody, pretargeting enhancement system for immunoscintigraphy. Clin Cancer Res. 2004;10:3552–3561. doi: 10.1158/1078-0432.CCR-03-0340. [DOI] [PubMed] [Google Scholar]
- 30.Rossi EA, Sharkey RM, McBride W, et al. Development of new multivalent-bispecific agents for pretargeting tumor localization and therapy. Clin Cancer Res. 2003;9:3886S–3896S. [PubMed] [Google Scholar]
- 31.van Schaijk FG, Boerman OC, Soede AC, et al. Comparison of IgG and F(ab')2 fragments of bispecific anti-RCCxanti-DTIn-1 antibody for pretargeting purposes. Eur J Nucl Med Mol Imaging. 2005;32:1089–1095. doi: 10.1007/s00259-005-1796-x. [DOI] [PubMed] [Google Scholar]
- 32.Goldenberg DM, Sharkey RM, Barbet J, Chatal J-F. Radioactive antibodies: Selective targeting and treatment of cancer and other diseases. Applied Radiology. 2007;36:10–29. [Google Scholar]
- 33.Rossi EA, Goldenberg DM, Cardillo TM, McBride WJ, Sharkey RM, Chang CH. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc Natl Acad Sci U S A. 2006;103:6841–6846. doi: 10.1073/pnas.0600982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albrecht H, Denardo GL, Denardo SJ. Monospecific bivalent scFv-SH: effects of linker length and location of an engineered cysteine on production, antigen binding activity and free SH accessibility. J Immunol Methods. 2006;310:100–116. doi: 10.1016/j.jim.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Nobs L, Buchegger F, Gurny R, Allemann E. Biodegradable nanoparticles for direct or two-step tumor immunotargeting. Bioconjug Chem. 2006;17:139–145. doi: 10.1021/bc050137k. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Xing J, Zhang Q, et al. Optimal design of Ig 5' primers for construction of diverse phage antibody library established to select anti-HAb18GEF and anti-DOTA-Y Fabs for hepatoma pretargeting RIT. Front Biosci. 2006;11:1733–1749. doi: 10.2741/1919. [DOI] [PubMed] [Google Scholar]
- 37.Barbet J, Kraeber-Bodere F, Vuillez JP, Gautherot E, Rouvier E, Chatal JF. Pretargeting with the affinity enhancement system for radioimmunotherapy. Cancer Biother Radiopharm. 1999;14:153–166. doi: 10.1089/cbr.1999.14.153. [DOI] [PubMed] [Google Scholar]
- 38.Casalini P, Luison E, Menard S, Colnaghi MI, Paganelli G, Canevari S. Tumor pretargeting: role of avidin/streptavidin on monoclonal antibody internalization. J Nucl Med. 1997;38:1378–1381. [PubMed] [Google Scholar]
- 39.Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, et al. Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): a strategy for vascular immunotargeting of drugs. Proc Natl Acad Sci U S A. 1999;96:2379–2384. doi: 10.1073/pnas.96.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]