Summary
Although the factors contributing to the progression of prostate cancer (PCa) remain incompletely understood, androgens have long been recognized to play a central role in this process. Upon entering PCa cells, androgens bind to a cognate nuclear receptor, the androgen receptor (AR). The activated AR translocates to the nucleus, binds as a dimer to androgen response elements (AREs) in the promoter of target genes, where it recruits the coactivator proteins necessary for the formation of a productive transcriptional complex, an event crucial for PCa cell viability. For many decades, the androgen dependency of PCas has been exploited therapeutically by androgen ablation strategies. While initially successful, these forms of therapy almost inevitably fail eventually, and an androgen depletion independent (ADI) disease emerges, for which currently no cure is available. Studies from our laboratory and others demonstrate that despite low circulating levels of functional androgens, the AR is critical for the proliferation and survival of ADI PCa cells. Recent data indicate that alterations in the expression and/or activity of AR coactivator proteins occur during PCa progression that can foster ADI activation of the AR. Here, we have investigated the role of the coactivator p300 in AR transcriptional activity and progression of PCa.
Introduction
Activation of the AR in ADI PCa cells has been attributed to mechanisms of AR hypersensitivity (AR amplification and/or mutations), promiscuous activation of the AR (by adrenal androgens, non-androgenic steroids and even anti-androgens), and outlaw AR pathways (AR activated by growth factors and cytokines, thereby bypassing the need for androgens) (1,2). Recently, the importance of the involvement of AR co-activator proteins in ADI AR activation is increasingly being recognized. Under physiological conditions coactivators are necessary for the formation of a productive transcriptional AR complex by facilitating DNA occupancy, chromatin remodelling, recruitment of general transcription factors associated with the RNA polymerase II holocomplex, as well as ensuring appropriate folding of the AR, AR protein stability, and/or proper AR subcellular distribution (3). In the progression of PCa a subset of these coactivators has been shown to be overexpressed, and this overexpression has been demonstrated to substantially contribute to the ADI mechanisms of AR activation described above. Therefore, AR coactivators have been suggested as valuable targets for therapeutic intervention (4).
The transcriptional coactivator p300 has been shown to regulate gene transcription through several distinct mechanisms. In addition to its potential to act as a direct bridge between DNA-bound transcription factors and the basal transcriptional machinery and to serve as a scaffold interacting with and assembling a number of other transcriptional regulators, p300 possesses histone acetyl transferase (HAT) activity by which it renders the chromatin environment more easily accessible for the transcriptional machinery. Apart from histones, p300 has also been shown to acetylate other proteins such as transcription factors and coregulators, resulting in modulation of transcription through altered protein-protein interactions, protein-DNA interactions, nuclear retention or protein half life of certain proteins (5). In terms of its involvement in AR signaling in PCa cells, a role for p300 has previously been described in androgen-dependent activation of the AR. Full ligand-induced transcriptional activity of the AR, recruitment of coactivators to the AR transcriptional complex and prostate cancer cell growth was shown to depend on its direct acetylation by p300 HAT activity (6,7).
Here, we describe the importance of p300 in ligand-independent ADI AR activity and prostate cancer progression.
Results
p300 expression is increased in PCa cells and correlates with PCa cell proliferation and aggressive tumor features
To investigate whether p300 might be involved in the development and/or progression of PCa, we assessed the expression of p300 in tissue samples of 95 patients with biopsy-proven PCa by performing immunohistochemistry. Expression of p300 was evaluated by digital image analysis as well as visual grade. These studies demonstrated overall p300 expression to be elevated in neoplastic tissues when compared to benign adjacent tissues (Fig. 1). Moreover, our analysis revealed a positive correlation of p300 expression on PCa biopsy with expression of MIB-I, an in situ marker of cell proliferation (P=0.009). In line with these findings, siRNA-mediated silencing of p300 expression in the PCa adenocarcinoma cell line LNCaP led to a decrease in cell proliferation in vitro. Review of the clinical information of the patient population studied, as well as biopsy and prostatectomy findings allowed for a correlative analysis of p300 expression and several clinicopathological parameters. This analysis demonstrated that high levels of p300 on biopsy predict larger tumor volumes (P<0.001), extraprostatic extension of disease (P=0.003), seminal vesicle involvement at prostatectomy (P=0.002) as well as PCa progression after surgery (P=0.01). In terms of genotypic and cellular changes, higher levels of p300 correlated positively with non-diploid DNA content and p300 expression tended to be positively correlated with high Gleason scores, which correspond to more undifferentiated tumors (8). Taken together, these data clearly point towards an association of p300 expression in PCa with cell proliferation and more aggressive disease.
Fig. 1. High p300 expression in PCa tissues.
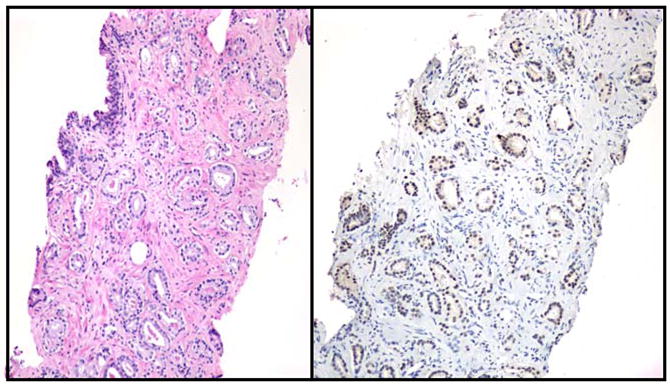
Formalin-fixed, paraffin-embedded PCa tissues from needle biopsies were stained with hematoxylin/eosin (left panel) or p300 immunohistochemistry was performed as described (right panel) (8).
p300 is required for ADI activation of the AR
Having established that p300 expression is elevated in PCa and correlates with aggressive tumor features, we were interested in exploring the impact of increased p300 expression on some key features of PCa progression. To this end, we first investigated whether p300 is important for ADI activation of the AR by non-androgenic ligands such as interleukin-6 (IL-6), a cytokine of particular interest to prostate cancer disease. Both IL-6 and its receptor have been shown to be expressed in PCa cells. Several independent studies have revealed that plasma levels of IL-6 are increased in patients with ADI PCa disease. Moreover, high IL-6 serum levels correlate with poor prognosis. Importantly, IL-6 has been shown to regulate AR transcriptional activity in the absence of androgens. Mechanistically, IL-6 exerts its effect on PCa cells and AR activity through activation of the JAK/STAT, MAPK and PI3K signaling pathways (9).
To determine whether p300 is involved in ADI transactivation of the AR by IL-6, LNCaP cells were transfected with an AR-dependent prostate specific antigen (PSA) promoter reporter construct and stimulated with 50ng/ml IL-6. In agreement with previous reports, we found increased reporter activity following IL-6 treatment. Blocking the MAPK pathway with the inhibitor PD98059 reduced the IL-6-mediated activation of the AR. Interestingly, transfection of p300 into cells reversed this inhibition, suggesting that p300 is a final target for the MAPK pathway during IL-6 stimulation. To further assess the role of p300 in this model, we used adenoviral E1A, which sequesters p300, thereby inhibiting its HAT activity. Transfection of E1A into LNCaP cells inhibited IL-6-mediated activation of the AR. To assess if the E1A-mediated repression is due to its specific interaction with p300, cells were cotransfected with increasing amounts of p300. Overexpression of p300 reversed the E1A-mediated inhibition. A mutant p300 that lacks HAT activity (p300-HAT) did not reverse the E1A-mediated inhibition, indicating that the HAT activity of p300 is necessary in order for p300 to functionally interact with the AR. AR protein expression levels remained constant during these experiments, suggesting that these events take place at the transcriptional level. To assess a direct role for p300 in transcriptional activation of endogenously expressed AR target genes, small interfering RNAs were used to downregulate p300 expression. LNCaP cells were transfected with either siRNA oligonucleotides specifically designed to target p300 or non-specific oligonuleotides as control. After transfection, cells were treated with 50ng/ml IL-6. Cells were subjected to immunocytochemistry using antibodies specific to p300, AR and PSA. Treatment of cells with IL-6 enhanced PSA expression. However, IL-6 had no effect on PSA expression in cells that were previously transfected with siRNA against p300. These results were confirmed using Western blot to detect PSA protein expression. Again, AR protein levels remained constant during these experiments, including during disruption of p300. Our data indicate that p300 is directly involved in transactivation of the AR by IL-6 under ADI conditions. It should be noted, however, that transfection of p300 did not further increase activation of the AR by IL-6 and did not induce AR activation when transfected in the absence of IL-6, indicating that p300 is necessary but not sufficient to induce AR activation (10).
p300 modulates nuclear morphology in PCa
Morphologic changes in the structure of cells, primarily the nuclei, are characteristic features of most cancers. Significantly, quantifiable nuclear features of PCa cells have been shown to correlate with disease progression. Because we had demonstrated that p300 expression correlates with PCa progression following prostatectomy, we addressed whether p300 plays a role in nuclear morphologic changes in PCa cells. To this end, we examined tissue samples from our patient population with biopsy-proven PCa, and tested whether p300 expression correlated with nuclear alterations measured and quantified by digital image analysis (DIA). Interestingly, we found significant associations between p300 expression and several nuclear alterations in PCa tissues, such as nuclear area, minimum diameter, DNA mass, and DNA index. To test if these alterations were a direct effect of p300 on PCa cell structure, we transfected a p300 expression construct or empty vector into the LNCaP-C4-2 PCa cell line, a well-characterized model for ADI PCa. Following transfection, cells were stained with Feulgen dye and subjected to DIA to assess nuclear alterations. Remarkably, we observed increases in many of these same features, such as nuclear area, perimeter, and minimum/maximum diameter, in cells transfected with p300. Transfection of LNCaP-C4-2 cells with an expression construct encoding the p300 homologue CBP or a vector harboring a non-coding sequence of similar size did not induce changes in nuclear features, indicating these effects to be specific for p300. Upon further analysis, we determined that the nuclear alterations associated with and induced by p300, including nuclear perimeter, area, and minimum/maximum diameter, each independently correlated with a more aggressive phenotype as judged by Ki-67 expression and extraprostatic extension of the tumor at the time of surgery (11). These results therefore provide compelling evidence that the changes in nuclear morphology induced by p300 in PCa cells are of clinical importance and correlate with aggressive prostate cancer. These observations further support our hypothesis that p300 plays an important role in the ADI progression of PCa.
Alterations in nuclear structure frequently involve deregulation of nuclear matrix proteins, such as lamin, PC-1 and NMP149, which show differential expression in cancer tissues. We therefore examined whether an increase in p300 expression could affect expression of factors involved in nuclear structure formation. To this end, LNCaP-C4-2 cells were transfected with an expression construct for p300 or empty vector. 48 hours after transfection, expression of several nuclear structure related factors, including lamin A/C, lamina-associated peptide 2, laminins and tubulins, was evaluated. Real time PCR and semiquantitative PCR measurements revealed increases in the expression of lamin A/C. Elevated lamin A/C expression was confirmed by western blotting on nuclear extracts from LNCaP-C4-2 (11). In summary, these data are the first to show direct regulation of quantifiable nuclear features by a transcriptional coactivator, through a mechanism involving regulation of nuclear lamin A/C levels.
Discussion
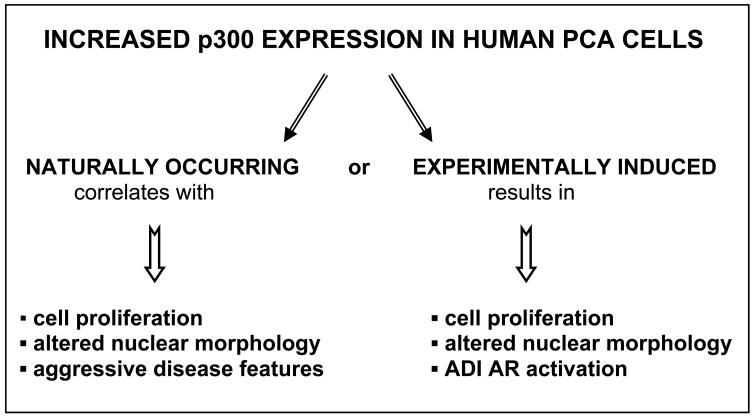
Taken together, our data point towards a central role for p300 expression as a determinant for the development of aggressive PCa features. Noteworthy, comparison of the findings obtained from the clinical material available from 95 patients with biopsy-proven PCa that were treated with radical retropubic prostatectomy without neoadjuvant therapy and studies on p300 overexpression in PCa cell lines in culture reveals remarkable similarities (Fig. 2). Indeed, both naturally occurring and experimentally induced increases in p300 expression in PCa cells correlates with enhanced cell proliferation and modulation of nuclear morphology. Moreover, consistent with a role for p300 in AR activation in the ADI state of the disease, p300 was shown to be necessary for IL-6 induced activation of AR activity under androgen deprived conditions.
Fig. 2.
Comparison of the effects of increased p300 expression in PCa tissues and cultured PCa cells.
Recently, the neuropeptide bombesin was shown to be able to enhance the HAT activity of p300 protein while leaving p300 expression levels unaltered (12). In view of these findings and the observation that p300 HAT activity is critical for ADI AR activity (10), it will be important the explore whether growth factors and cytokines such as IL-6, that are frequently overexpressed in ADI can have an additional affect on p300 activity. Finally, as p300 as well as other co-activators are emerging as potential therapeutic targets, efforts should be directed towards identifiying the mechanisms underlying and the factors driving p300 overexpression.
References
- 1.Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst. 2001;93:1687–97. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- 2.Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. 2004;351:1488–90. doi: 10.1056/NEJMp048178. [DOI] [PubMed] [Google Scholar]
- 3.Heinlein CA, Chang C. Androgen receptor (AR) coregulators: an overview. Endocr Rev. 2002;23:175–200. doi: 10.1210/edrv.23.2.0460. [DOI] [PubMed] [Google Scholar]
- 4.Heemers HV, Tindall DJ. Androgen receptor coregulatory proteins as potential therapeutic targets in the treatment of prostate cancer. Curr Cancer Ther Rev. 2005;1:175–86. [Google Scholar]
- 5.Kalkhoven E. CBP and p300 : HATs for different occasions. Biochem Pharmacol. 2004;68:1145–55. doi: 10.1016/j.bcp.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 6.Fu M, Wang C, Reutens AT, et al. p300 and p300/cAMP-response element-binding protein-associated factor acetylate the androgen receptor at sites governing hormone-dependent transactivation. J Biol Chem. 2000;275:20853–60. doi: 10.1074/jbc.M000660200. [DOI] [PubMed] [Google Scholar]
- 7.Fu M, Rao M, Wang C, et al. Acetylation of androgen receptor enhances coactivator binding and promotes prostate cancer cell growth. Mol Cell Biol. 2003;23:8563–75. doi: 10.1128/MCB.23.23.8563-8575.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debes JD, Sebo TJ, Lohse CM, et al. p300 in prostate cancer proliferation and progression. Cancer Res. 2003;63:7638–40. [PubMed] [Google Scholar]
- 9.Culig Z, Steiner H, Bartsch G, et al. Interleukin-6 regulation of prostate cancer cell growth. J Cell Biochem. 2005;95:497–505. doi: 10.1002/jcb.20477. [DOI] [PubMed] [Google Scholar]
- 10.Debes JD, Schmidt LJ, Huang H, et al. p300 mediates androgen-independent transactivation of the androgen receptor by interleukin 6. Cancer Res. 2002;62:5632–6. [PubMed] [Google Scholar]
- 11.Debes JD, Sebo TJ, Heemers HV, et al. p300 modulates nuclear morphology in prostate cancer. Cancer Res. 2005;65:708–12. [PubMed] [Google Scholar]
- 12.Gong J, Zhu J, Goodman OB, Jr, et al. Activation of p300 histone acetyltransferase activity and acetylation of the androgen receptor by bombesin in prostate cancer cells. Oncogene. 2006;25:2011–21. doi: 10.1038/sj.onc.1209231. [DOI] [PubMed] [Google Scholar]