Abstract
Purpose
Androgens are essential for prostatic growth and development but they also have a significant role in prostate disease pathogenesis. Dihydrotestosterone, the primary prostatic androgen, is transformed from testosterone by types 1 and 2 5α-reductase and, thus, a potential therapeutic benefit could be achieved through the inhibition of 5α-reductase.
Materials and Methods
A literature review was performed using PubMed®/MEDLINE® and congress abstracts to examine evidence supporting the potential of 5α-reductase inhibitors in the primary prevention of prostate cancer and in limiting the progression of diagnosed disease.
Results
Prostate disease development is associated with increased expression of each 5α-reductase isoenzyme with over expression of type 1 of particular importance in prostate cancer development and progression. The 2 5α-reductase inhibitors currently clinically available are finasteride, a type 2 5α-reductase inhibitor, and dutasteride, a dual 5α-reductase inhibitor. Dual inhibition by dutasteride has been shown to translate into a greater degree and consistency of dihydrotestosterone suppression compared with finasteride. The Prostate Cancer Prevention Trial showed that finasteride significantly decreased the 7-year risk of prostate cancer in men with prostate specific antigen 3.0 ng/ml or less, while the ongoing Reduction by Dutasteride of Prostate Cancer Events study is assessing whether dutasteride decreases the risk of biopsy detectable prostate cancer in men with prostate specific antigen 2.5 to 10 ng/ml and a previous negative biopsy. Small-scale studies have demonstrated potential effects of 5α-reductase inhibition in prostate cancer treatment that warrant further investigation, while dutasteride use in men undergoing expectant treatment is also being examined.
Conclusions
The inhibition of 5α-reductase represents a valid target for prostate cancer risk reduction and treatment strategies. The greater suppression of dihydrotestosterone observed with agents that inhibit each 5α-reductase isoenzyme may translate into enhanced outcomes and studies are under way to test this hypothesis.
Keywords: prostate, prostatic neoplasms, cholestenone 5 alpha-reductase, androgens, dihydrotestosterone
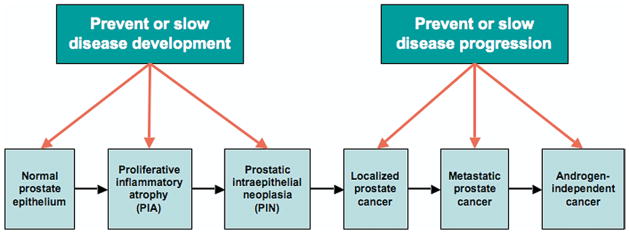
Prostate cancer is the most common cancer and a leading cause of cancer death in men. Decreasing the risk of cancer (primary prevention) could offer substantive benefits, while slowing or preventing disease progression (secondary prevention) or delaying progression in those in whom curative therapy (tertiary prevention) fails could decrease prostate cancer related deaths, decrease the need for intervention and improve quality of life in patients whose mortality will not ultimately be prostate cancer related (fig. 1).
Fig. 1.
Prostate cancer natural history
The androgen axis is a target for approaches aimed at decreasing the prostate cancer risk or slowing tumor progression. Androgens are essential for prostatic development, growth and function. However, later in life they have a significant role in the pathogenesis of BPH and prostate cancer. Since the mid 20th century, it has been clear that DHT and not testosterone is the primary androgen responsible for the AR mediated growth and survival of normal, hyperplastic and malignant prostate tissue. Testosterone is converted to the more potent DHT by 5αR isoenzymes. Therefore, tissues with a high concentration of these enzymes, such as the prostate, act to concentrate the effects of circulating testosterone. Thus, a potential therapeutic benefit could be achieved through inhibiting DHT conversion. This offers the potential to prevent, delay or possibly treat prostate cancer, providing a different approach to individual and population management of this prevalent disease. We examined the basic science and clinical evidence supporting the potential of 5αR inhibitors in the primary prevention of prostate cancer and in limiting the progression of diagnosed disease.
THE AR AXIS
Development and maintenance of the adult prostate requires a functional AR axis. Because testosterone is lipophilic, it can diffuse freely into target cells and bind to and activate AR. However, in tissues such as the prostate it is first converted to DHT by types 1 and 2 5αR. DHT dissociates more slowly from AR and induces a receptor conformation that is more resistant to degradation than testosterone.1 DHT is also intrinsically about twice as potent as testosterone at stimulating prostate growth2 and this combination of effects augment the androgenic signal in tissues where 5αR is highly expressed.
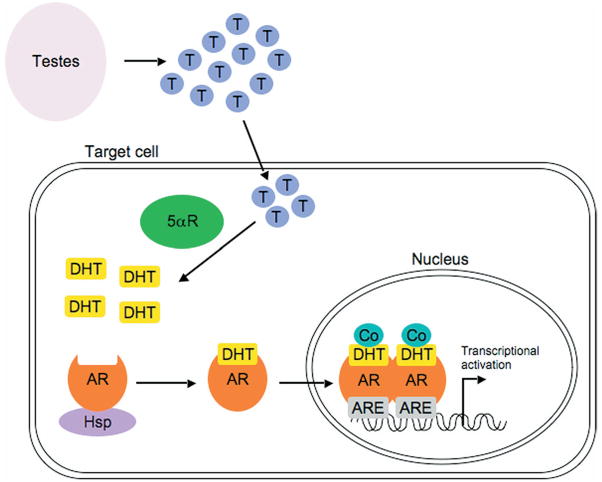
DHT mediates its effects via high affinity binding to AR. In the basal unliganded state AR is bound in the cytoplasm to heat shock protein molecular chaperones and co-chaperones (fig. 2). DHT binding to the AR causes heat shock protein dissociation, allowing AR translocation into the nucleus. Here AR binds AREs as a dimer in the promoter and enhancer regions of target genes. ARE bound AR requires coactivator proteins to transcriptionally activate target genes. Thus, the AR induced assembly of these multiprotein complexes results in a finely regulated level of target gene transcription.
Fig. 2.
AR signaling pathway in healthy prostate. In androgen responsive target cells testosterone (T) is converted to DHT by 5αR enzymes. DHT binds to AR and causes dissociation of heat shock proteins (Hsp), allowing translocation of DHT-AR complex into nucleus, where it binds to AREs. Recruitment of coactivator proteins (Co) enables transcriptional activation of target genes. Modified from Tindall and Dehm.50
ANDROGEN EFFECTS IN THE HEALTHY PROSTATE
Effects on Prostatic Gene Expression
Current knowledge of androgen regulated genes involved in normal prostatic function comes mainly from large-scale gene expression studies. To date more than 200 androgen regulated genes have been identified, of which the best characterized are PSA, kallikrein 2 and prostatic acid phosphatase. Prostatic secretions are rich in polyamines and several enzymes involved in their synthesis have been shown to be androgen regulated, as have genes involved in lipogenesis, cell cycle regulation and cell survival.3 Therefore, through interaction with AR androgens have a central role in regulating genes involved in normal prostate function, growth and survival.
Effects on Prostate Cell Development
DHT binding to the nuclear AR in prostatic stromal cells stimulates the production and secretion of paracrine growth and survival factors. They diffuse from the stroma to the epithelium, influencing growth and differentiation.4 At the same time AR occupancy by its ligand in AR positive luminal cells in the epithelium up-regulates the expression of growth factors that diffuse across the basement membrane to affect stromal cells.5 In the healthy prostate this complex epithelial-stromal signaling relationship results in homeostasis with proliferative and apoptotic indexes in the prostate balanced with no net growth, although cells are continuously being replaced.
ANDROGEN EFFECTS IN PROSTATE CANCER DEVELOPMENT AND PROGRESSION
Prostate cancer development appears to be a multistep process with proliferative inflammatory atrophy and high grade PIN representing precursors (fig. 1). Chronic/recurrent inflammation along with dietary and inherited factors are thought to be responsible for prostate cancer initiation and promotion.6 Precursor lesions may then progress to latent low grade tumors, which can progress slowly for decades to clinically significant invasive disease.
Androgens and AR are important throughout all stages of disease progression. Whereas AR is a key regulator of prostatic maintenance and survival in the healthy prostate, in prostate cancer it functions as an inducer of uncontrolled cell growth. In the healthy prostate basal cells differentiate into AR negative TA cells that express basal and secretory cell markers. TA cells clonally expand, differentiate and migrate to the luminal layer, where they form secretory cells. Many investigators hypothesize that TA cells give rise to prostate cancer with malignant changes allowing the morphological features and gene expression of basal and secretory cells. Normally TA cells express no or only low levels of AR. However, proliferating cells in proliferative inflammatory atrophy variably express higher levels of AR with expression further enhanced in high grade PIN.7 DHT binding to AR in these transformed cells drives proliferation. Therefore, prostatic carcinogenesis depends on a fundamental change in the AR signaling pathway, involving conversion from a paracrine to an autocrine mechanism for androgen stimulated growth.
The importance of androgens in prostate cancer development has been highlighted by a study of recurrent chromosomal rearrangements in prostate tumor cells.8 Recurrent chromosomal rearrangements may lead to the juxtaposition of a promoter and/or enhancer of 1 gene with an oncogene, resulting in altered expression of an oncogenic protein or fusion of 2 genes to create an abnormal protein with different activity. Studies have demonstrated that ERG, ETV1 and ETV4 are over expressed in mutually exclusive fashion in prostate cancer but not in PIN or benign epithelium.8,9 These genes fuse with TMPRSS2, a type 2 transmembrane-bound serine protease that is highly expressed in prostate tissue and whose expression is androgen sensitive.8 Fusion allows androgens to stimulate over expression of the ERG/ETV1 and TMPRSS2 fusion gene, the implication being that androgen stimulation is directly responsible for promotion of an aberrant fusion gene involved in tumorigenesis.
AR activity appears to be important throughout all stages of disease progression. Treatment for advanced prostate cancer involves the primary modulation of AR activity through the deprivation of circulating androgens by surgical/chemical means. Although more than 80% of patients initially show a positive response to therapy, those with metastatic prostate cancer eventually experience progression with the development of ADI tumors.10 Evidence suggests that increased AR expression and androgen binding are required for the transition to androgen independence.11 It may seem paradoxical that this transition can occur during androgen deprivation but it is becoming clear that the low androgen levels achieved during therapy may be sufficient for AR activation.12
Although ADI tumors are resistant to further attempts at blocking androgen action, AR remains critical for their growth and survival.3,13 During ADI progression prostate cancer relies on various cellular pathways, some involving the AR and others bypassing it. Pathways involving the AR include receptor amplification or mutation, deregulation of growth factors or cytokines and coactivator alteration.14 AR gene amplification leads to increased expression and enhanced activation by low androgen levels, while AR gene mutations can increase the number of ligands that can activate the receptor. Deregulated growth factors can also activate AR and they usually involve an alteration in coactivator function or expression. One of the most important pathways bypassing AR involves the deregulation of apoptotic genes. The tumor suppressor gene PTEN and the anti-apoptotic gene Bcl-2 have important roles, leading eventually to cell survival. It is also thought that prostate cancer cells may develop neuroendocrine-like behavior, secreting neuropeptides that induce adjacent cell growth, enabling them to survive therapeutic interventions. However, we still have much to learn about the mechanisms by which prostate cancer survives following androgen deprivation therapy.
PHYSIOLOGICAL ROLE OF 5αR IN THE PROSTATE
Testosterone is the key nuclear androgen in many tissues, such as muscle. The 5αR isoenzymes enhance the androgen signal by converting testosterone to the more potent DHT, which also stabilizes the AR complex in its active form. However, in experimental models high testosterone concentrations mimic the effects of DHT. The greatest difference between the 2 androgens occurs at low concentrations. A testosterone threshold exists below which little or no prostate stimulation occurs. In contrast, even at low concentrations prostate growth is stimulated by DHT. Hence, it appears that a major role of 5αR is to ensure normal prostate function at low circulating testosterone levels.2
EXPRESSION OF 5αR IN HEALTH AND DISEASE
The importance of 5αR in male sexual development is best illustrated by examining 5αR deficiency. Mutations in type 2 5αR cause male pseudohermaphroditism and affected males have increased plasma testosterone with decreased DHT.15 External genitalia are ambiguous at birth and virilization occurs at puberty but the prostate remains small and facial/body hair is decreased. Neither BPH nor prostate cancer has been observed in patients with type 2 5αR gene mutations.15 This natural genetic model highlights the importance of DHT in prostate development and the potential role for 5αR in prostate disease.
Cellular localization of the 2 isoenzymes in normal prostate tissue has been examined in several studies with conflicting results, reflecting the different assay methods used and the nature of the specimens examined. An early study using Northern blotting after the physical separation of stromal and epithelial cells indicated type 1 5αR expression in epithelial and stromal cells, and type 2 5αR expression only in stromal cells.16 However, an in situ hybridization study to directly localize 5αR mRNA suggested that type 2 5αR is also expressed in epithelial cells.17 Studies of 5αR isoenzyme distribution in normal vs hyperplastic and malignant prostate tissue have shown that expression of the 2 isoenzymes is increased in hyperplastic prostate tissue and type 1 expression is increased in prostate cancer relative to BPH tissue.18 Furthermore, the expression of type 2 5αR has been shown to be lower in localized prostate cancer than in normal or hyperplastic tissue.19
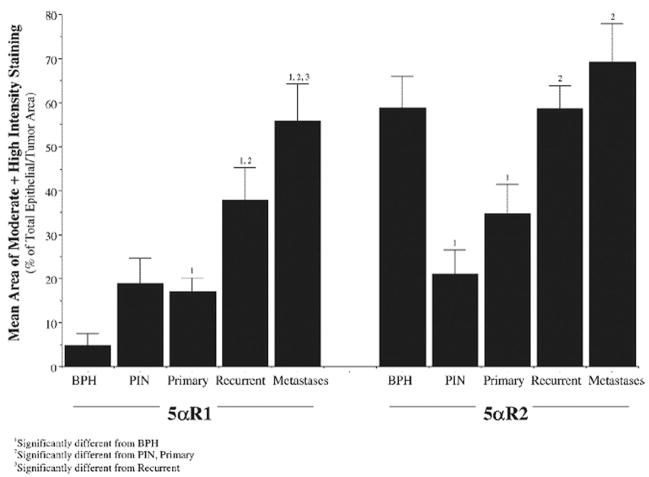
Immunostaining for type 1 5αR has been shown to be low to moderate in intensity and primarily nuclear in BPH, whereas in prostate cancer high intensity, primarily cytoplasmic staining is frequently observed.20 Further study has demonstrated that, while type 1 expression in BPH is low, it increases steadily in PIN, and in primary, recurrent and metastatic prostate cancer (fig. 3).21 In contrast, type 2 expression is lower in PIN and primary cancer compared with that in BPH and no different from that in BPH in recurrent and metastatic prostate cancer. Another study has also shown higher type 1 than type 2 expression in recurrent prostate cancer, androgen stimulated benign prostate and androgen stimulated prostate cancer,22 and a 2.1-fold increase in type 1 expression in metastatic vs primary cancer.23 Therefore, prostate disease development is associated with increased expression of the 2 isoenzymes. In particular type 1 over expression may be important in prostate cancer development and progression. The difference in isoenzyme expression profiles in healthy and malignant prostate tissue may be relevant when examining the effects of different 5αR inhibitors for prostate cancer prevention and treatment.
Fig. 3.
Type 1 and 2, 5αR immunostaining in BPH, PIN, primary prostate cancer, recurrent cancer and metastases. Reproduced with permission from Thomas et al.21
EFFECTS OF 5αR INHIBITORS ON CIRCULATING AND INTRAPROSTATIC ANDROGENS
Fundamental differences exist between androgen ablation and 5αR inhibition. Castration decreases serum testosterone and DHT, resulting in hypogonadism. It decreases intraprostatic DHT by 50% to 80% and the remaining DHT arises from the conversion of adrenal androgen precursors. The inhibition of 5αR markedly decreases serum DHT but, because testosterone is not decreased, sexual dysfunction is uncommon. Although 5αR inhibition causes a more marked intraprostatic DHT reduction than castration, intraprostatic testosterone increases. Therefore, the effect of 5αR inhibition on prostate function is not as great as castration and the combination of the 2 methods has been shown to be more effective than either alone in an animal model of prostate cancer.24
Two 5αR inhibitors are currently under investigation in prostate cancer chemoprevention, that is the type 2 5αR inhibitor finasteride, and the dual 5αR inhibitor dutasteride. Dutasteride is 45-fold more effective in inhibiting type 1 5αR and 2-fold more effective in inhibiting type 2 than finasteride.25 This dual inhibition translates into a greater degree and consistency of DHT suppression.25 In a phase II study in 399 patients with BPH the mean ± SD decrease in serum DHT at week 24 in those receiving 0.5 mg dutasteride was 94.7% ± 3.3% compared with 70.8% ± 18.3% in patients receiving 5.0 mg finasteride (p <0.001).26 At least 90% DHT suppression was noted in 85.4% of patients on dutasteride compared with only 2.2% of patients on finasteride.27
Since low DHT concentrations can stimulate prostate growth, it is important to consider the effect of 5αR inhibition on intraprostatic DHT.28 In a study of 81 men with clinically localized prostate cancer mean intraprostatic DHT was 93% and 99% lower, respectively, in men who received 0.5 or 3.5 mg dutasteride for 4 months before radical prostatectomy compared with those who received no therapy.29 This compares favorably with the 68% to 86% decreases seen with finasteride.25 Given that type 1 5αR over expression may be important in prostate cancer development and progression,21 the dual inhibition of dutasteride may be particularly relevant in risk reduction.
The DHT decrease through 5αR inhibition is accompanied by a reciprocal increase in testosterone. In 46 men who received 5 mg dutasteride or placebo for 6 to 10 weeks before radical prostatectomy mean intraprostatic DHT decreased from 6,178.8 pg/gm with placebo to 177.2 pg/gm with dutasteride, while intraprostatic testosterone increased from 124.5 pg/gm with placebo to 2,502 pg/gm with dutasteride.30 However, the total intraprostatic androgen concentration remained almost 60% lower in the dutasteride group. Given the lower potency of testosterone vs DHT, 5αR inhibitor treatment significantly decreases the total androgen effect.
EFFECTS OF 5αR INHIBITORS ON GENE EXPRESSION
The effects of dutasteride on gene regulation have been investigated in androgen responsive prostate cancer cells.31 Microarray gene expression analysis showed that dutasteride disrupted cellular pathways involved in metabolism, catalytic activity, cell growth/maintenance, protein metabolism, signal transduction and many other processes in LNCaP cells. The expression of caspase 7 and 8, which are components of the FasL/tumor necrosis factor-αapoptotic signaling pathway, also increased with dutasteride administration.31
HISTOLOGICAL EFFECTS OF 5αR INHIBITORS
There is strong evidence that 5αR inhibitors decrease prostate volume and induce atrophy and apoptosis in benign prostatic epithelium.32 There are increasing data to suggest similar effects in prostate cancer. Finasteride has decreased the LNCaP cell growth rate in a dose dependent manner,33 while a later study showed that dutasteride inhibits LNCaP cell proliferation, displaying more potent inhibition of DHT induced PSA accumulation than finasteride.34
A recent study compared the effects of finasteride and dutasteride on prostate cancer growth.24 Rats bearing the Dunning R-3327H xenograft were randomized to varying doses of daily finasteride or dutasteride for 55 days, after which the weight and DHT content of ventral prostate and H tumor were determined. In surgically castrated controls H rat prostate cancer growth essentially stopped following castration, correlating with a greater than 90% decrease in serum testosterone, and testosterone and DHT in H tumor tissue. The level of testosterone and DHT also decreased by more than 90% in ventral prostatic tissue, correlating with a 70% gland weight reduction. Finasteride decreased the tumor DHT concentration by only 40% (p not significant) and it had a minimal effect on tumor growth. However, it significantly decreased normal ventral prostate weight and the DHT concentration. This is because H tumor tissue expresses increased levels of type 1 5αR but undetectable to low levels of type 2 5αR. In contrast, dutasteride treatment decreased ventral prostate and H tumor DHT concentration and gland weight, demonstrating the importance of type 1 5αR inhibition in this prostate cancer model.
The study also examined the response of LNCaP cells to 5αR inhibition alone and in combination with androgen ablation.24 Equimolar dosing with dutasteride demonstrated greater inhibition of LNCaP growth in intact nude mice compared with finasteride. However, castration produced a greater decrease in LNCaP growth compared with either 5αR inhibitor. Only the combination of dutasteride and castration produced greater inhibition of tumor growth than castration. Therefore, this study provides support for the hypothesis that dutasteride dual 5αR inhibition vs finasteride type 2 inhibition translates into greater efficacy in situations in which type 1 5αR over expression occurs.
In a study in 46 men undergoing radical prostatectomy pretreatment for 6 to 10 weeks with 5 mg dutasteride caused increased apoptosis and a trend toward decreased microvessel density vs placebo.30 Tumor volume was decreased, the percent of atrophic epithelium increased and the stromal-to-gland ratio doubled in the dutasteride group.35 In 5 patients with prostate cancer treated with finasteride atrophic effects similar to but less prominent than those in androgen ablation controls were seen.36 Androgen deprivation changes with finasteride were noticeable in Gleason grade 2–3 tumors but they were minimal in grade 4 tumors. Collectively these results support the concept that 5αR inhibitors could slow the progression or even cause the regression of early prostate cancer.
CLINICAL EVIDENCE FOR BENEFITS OF 5αR INHIBITORS IN PROSTATE CANCER TREATMENT
Clinical evidence for 5αR inhibitors in preventing prostate cancer came first from studies of BPH that recorded prostate cancer development in the safety evaluations. Two large-scale studies, including the Proscar Long-Term Efficacy and Safety Study, and the Medical Therapy of Prostatic Symptoms study, provided preliminary evidence for an effect of finasteride on prostate cancer development. The Proscar Long-Term Efficacy and Safety Study randomized 3,040 men with moderate to severe lower urinary tract symptoms to receive 5 mg finasteride or placebo for 4 years. Biopsies were performed in 325 finasteride treated men and in 320 placebo recipients. Prostate cancer diagnosis was similar in the finasteride and placebo groups (4.7% vs 5.1%).37 Although it was not statistically significant, assessment of 53 end of study needle biopsy specimens in men with baseline PSA greater than 4 ng/ml showed that patients on placebo were more likely to have a Gleason score of at least 7 and a tumor at least 10% of the total prostate volume compared with finasteride treated patients.38 The Medical Therapy of Prostatic Symptoms study randomized 3,047 men with moderate lower urinary tract symptoms to placebo, doxazosin, finasteride or a combination of doxazosin and finasteride with a mean followup of 4.4 years. A subpopulation of 1,327 men underwent biopsy and, although the incidence was not statistically significant, prostate cancer positive biopsies were lower in the finasteride group (5.3%) than in the other groups (8.3% for placebo, 7.4% for doxazosin and 7.9% for the doxazosin/finasteride combination).39 In patients receiving finasteride either as monotherapy or in combination the overall decrease in prostate cancer diagnosis was approximately 20% compared with that of placebo, similar to that in the PCPT.40
The PCPT was a 7-year study in 18,882 men that showed that finasteride decreased the risk of prostate cancer by 24.8% vs placebo (p <0.001).40 However, this overall finding has been tempered by the increased incidence of Gleason grade 7–10 cancer in the finasteride group. This is now believed to be due in part to a PSA driven ascertainment bias, whereby PSA had statistically significantly better sensitivity and area under the ROC curve for detecting prostate cancer in the finasteride arm than in the placebo arm.41 This bias would be expected to contribute to a greater detection of overall and high grade prostate cancer with finasteride. The likely cause of the bias is that finasteride decreased PSA to a lesser extent in men with high grade cancer. Because PSA values in the finasteride arm were increased by a factor of 2.0 to 2.3 and adjusted values greater than 4.0 ng/ml resulted in a for-cause biopsy recommendation, the differential PSA decrease in high and low grade cancers would result in more for-cause biopsies in finasteride treated men with high grade cancer. These data also imply that finasteride was less effective at decreasing the volume of high grade compared with low grade cancers. A potential reason for this is that type 1, 5αR is more highly expressed in localized high grade than in low grade cancers. Support for this hypothesis comes from a recent study in which type 1 and 2, 5αR expression was increased in localized high grade vs low grade cancer.42 This suggests that dual inhibition may be more effective for the prevention and/or treatment of these cancers than type 2, 5αR inhibition alone.
Increased type 1, 5αR expression in prostate cancer provides a strong rationale for dual inhibitor use in chemoprevention, whereby the greater DHT suppression with dutasteride26 may translate into improved outcomes. Retrospective analyses of prostate cancer incidence in 3 large-scale dutasteride BPH studies provided encouraging support for this hypothesis.43 A total of 4,325 men with BPH but no history or evidence of prostate cancer were randomized to 0.5 mg dutasteride or placebo daily for 2 years. Prostate cancer detection was determined by for-cause biopsies during the 2 years or the first 3 months of an open label extension. The cumulative incidence of prostate cancer was significantly lower in the dutasteride group compared with the placebo group at 27 months (1.2% vs 2.5%, p = 0.002). Gleason grading was available for 27 patients and the proportion with a score of at least 7 was 30% in dutasteride treated subjects vs 41% in placebo recipients (not statistically different).
These findings prompted the initiation of the REDUCE study.44 This 4-year, randomized, placebo controlled trial is evaluating the efficacy and safety of daily dutasteride in approximately 8,000 men at increased risk for prostate cancer. The REDUCE study was specifically designed to enroll men at high risk, a group likely to be optimum candidates for chemoprevention in any future population based screening approach.
The PCPT and REDUCE trials were designed to investigate the effects of 5αR inhibitors on prostate cancer development. However, prostate cancer presents other opportunities for intervention. Active surveillance without treatment is a strategy that is used in some patients with early prostate cancer. Treatment with a 5αR inhibitor during expectant management has the potential to the delay progression to clinically significant disease (secondary prevention), so that surgical/radiological intervention may not become indicated. A North American study has been initiated in which men undergoing expectant management of prostate cancer are being randomized to 0.5 mg dutasteride or placebo daily for 3 years.45
There have also been studies examining 5αR inhibitor treatment at later points during the prostate cancer life cycle (tertiary prevention). One study investigated 10 mg finasteride daily in 120 men who had previously undergone radical prostatectomy and had detectable serum PSA.46 These patients were at high risk for clinical relapse and finasteride treatment delayed an increase in median PSA by 9 months during 1 year and by 14 months during 2 years vs placebo. Another study in 28 men with metastatic prostate cancer found that 10 mg finasteride daily significantly decreased serum PSA at 3 and 6 weeks compared with placebo (−22.9% vs −2.9% and −15.1% vs 11.7%, respectively, p <0.05).47 Dutasteride has been investigated in combination with bicalutamide before brachytherapy for clinically localized prostate cancer.48 After 3 months of 50 mg bicalutamide and 0.5 mg dutasteride daily in 31 patients average prostate volume decreased by 33.6% and transition zone volume decreased by 39.8%. These reductions compare favorably with those for luteinizing hormone-releasing hormone agonists with or without antiandrogen and they were associated with fewer side effects.
The positive effects of 5αR inhibition seen in these small-scale studies coupled with an absence of significant safety concerns result in a favorable risk-benefit profile and warrant further investigation. Recent interest has been directed toward combination AR targeted therapy to simultaneously target multiple AR pathways.49 The use of 5αR inhibitors in conjunction with luteinizing hormone-releasing hormone agonists, geldanamycin analogues, kinase inhibitors and/or novel bulky antiandrogens to target specific points in the AR cascade appears to be a rational approach for prostate cancer treatment that warrants phase II investigation.
CONCLUSIONS
Androgen activation of AR has a key role in the development and maintenance of normal and aberrant prostatic growth. Inhibition of 5αR isoenzymes to decrease DHT has demonstrated benefits in the primary prevention of prostate cancer and potential in limiting disease progression in men with diagnosed disease. The greater suppression of DHT observed with agents that inhibit both 5αR isoenzymes may translate into enhanced outcomes in these populations and studies are under way to test this. The use of 5αR inhibitors as part of combination treatment to slow progression during later stages of prostate cancer also warrants further investigation.
Abbreviations and Acronyms
- 5αR
5α-reductase
- ADI
androgen depletion independent
- AR
androgen receptor
- ARE
androgen response element
- BPH
benign prostatic hyperplasia
- DHT
dihydrotestosterone
- PCPT
Prostate Cancer Prevention Trial
- PIN
prostatic intraepithelial neoplasia
- PSA
prostate specific antigen
- REDUCE
Reduction by Dutasteride of Prostate Cancer Events
- TA
transit amplifying
References
- 1.Zhou ZX, Lane MV, Kemppainen JA, French FS, Wilson EM. Specificity of ligand-dependent androgen receptor stabilization: receptor domain interactions influence ligand dissociation and receptor stability. Mol Endocrinol. 1995;9:208. doi: 10.1210/mend.9.2.7776971. [DOI] [PubMed] [Google Scholar]
- 2.Wright AS, Thomas LN, Douglas RC, Lazier CB, Rittmaster RS. Relative potency of testosterone and dihydrotestosterone in preventing atrophy and apoptosis in the prostate of the castrated rat. J Clin Invest. 1996;98:2558. doi: 10.1172/JCI119074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dehm SM, Tindall DJ. Molecular regulation of androgen action in prostate cancer. J Cell Biochem. 2006;99:333. doi: 10.1002/jcb.20794. [DOI] [PubMed] [Google Scholar]
- 4.Kurita T, Wang YZ, Donjacour AA, Zhao C, Lydon JP, O’Malley BW, et al. Paracrine regulation of apoptosis by steroid hormones in the male and female reproductive system. Cell Death Differ. 2001;8:192. doi: 10.1038/sj.cdd.4400797. [DOI] [PubMed] [Google Scholar]
- 5.Joseph IB, Nelson JB, Denmeade SR, Isaacs JT. Androgens regulate vascular endothelial growth factor content in normal and malignant prostatic tissue. Clin Cancer Res. 1997;3:2507. [PubMed] [Google Scholar]
- 6.Nelson WG, De Marzo AM, DeWeese TL, Isaacs WB. The role of inflammation in the pathogenesis of prostate cancer. J Urol, part 2. 2004;172:S6. doi: 10.1097/01.ju.0000142058.99614.ff. [DOI] [PubMed] [Google Scholar]
- 7.De Marzo AM, Putzi MJ, Nelson WG. New concepts in the pathology of prostatic epithelial carcinogenesis. Urology. 2001;57:103. doi: 10.1016/s0090-4295(00)00952-3. [DOI] [PubMed] [Google Scholar]
- 8.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 9.Tomlins SA, Mehra R, Rhodes DR, Smith LR, Roulston D, Helgeson BE, et al. TMPRSS2: ETV4 gene fusions define a third molecular subtype of prostate cancer. Cancer Res. 2006;66:3396. doi: 10.1158/0008-5472.CAN-06-0168. [DOI] [PubMed] [Google Scholar]
- 10.Roy-Burman P, Tindall DJ, Robins DM, Greenberg NM, Hendrix MJ, Mohla S, et al. Androgens and prostate cancer: are the descriptors valid? Cancer Biol Ther. 2005;4:4. doi: 10.4161/cbt.4.1.1563. [DOI] [PubMed] [Google Scholar]
- 11.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 12.Mohler JL, Gregory CW, Ford OH, 3rd, Kim D, Weaver CM, Petrusz P, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 13.Li TH, Zhao H, Peng Y, Beliakoff J, Brooks JD, Sun Z. A promoting role of androgen receptor in androgen-sensitive and -insensitive prostate cancer cells. Nucleic Acids Res. 2007;13:2767. doi: 10.1093/nar/gkm198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. 2004;351:1488. doi: 10.1056/NEJMp048178. [DOI] [PubMed] [Google Scholar]
- 15.Imperato-McGinley J, Zhu YS. Androgens and male physiology the syndrome of 5alpha-reductase-2 deficiency. Mol Cell Endocrinol. 2002;198:51. doi: 10.1016/s0303-7207(02)00368-4. [DOI] [PubMed] [Google Scholar]
- 16.Bruchovsky N, Sadar MD, Akakura K, Goldenberg SL, Matsuoka K, Rennie PS. Characterization of 5alpha-reductase gene expression in stroma and epithelium of human prostate. J Steroid Biochem Mol Biol. 1996;59:397. doi: 10.1016/s0960-0760(96)00125-2. [DOI] [PubMed] [Google Scholar]
- 17.Pelletier G, Luu-The V, Huang XF, Lapointe H, Labrie F. Localization by in situ hybridization of steroid 5alpha-reductase isozyme gene expression in the human prostate and preputial skin. J Urol. 1998;160:577. [PubMed] [Google Scholar]
- 18.Iehle C, Radvanyi F, Gil Diez de Medina S, Ouafik LH, Gerard H, Chopin D, et al. Differences in steroid 5alpha-reductase iso-enzymes expression between normal and pathological human prostate tissue. J Steroid Biochem Mol Biol. 1999;68:189. doi: 10.1016/s0960-0760(99)00030-8. [DOI] [PubMed] [Google Scholar]
- 19.Luo J, Dunn TA, Ewing CM, Walsh PC, Isaacs WB. Decreased gene expression of steroid 5 alpha-reductase 2 in human prostate cancer: implications for finasteride therapy of prostate carcinoma. Prostate. 2003;57:134. doi: 10.1002/pros.10284. [DOI] [PubMed] [Google Scholar]
- 20.Thomas LN, Douglas RC, Vessey JP, Gupta R, Fontaine D, Norman RW, et al. 5alpha-reductase type 1 immunostaining is enhanced in some prostate cancers compared with benign prostatic hyperplasia epithelium. J Urol. 2003;170:2019. doi: 10.1097/01.ju.0000091804.20183.81. [DOI] [PubMed] [Google Scholar]
- 21.Thomas LN, Lazier CB, Gupta R, Norman RW, Troyer DA, O’Brien SP, et al. Differential alterations in 5alpha-reductase type 1 and type 2 levels during development and progression of prostate cancer. Prostate. 2005;63:231. doi: 10.1002/pros.20188. [DOI] [PubMed] [Google Scholar]
- 22.Titus MA, Gregory CW, Ford OH, 3rd, Schell MJ, Maygarden SJ, Mohler JL. Steroid 5alpha-reductase isozymes I and II in recurrent prostate cancer. Clin Cancer Res. 2005;11:4365. doi: 10.1158/1078-0432.CCR-04-0738. [DOI] [PubMed] [Google Scholar]
- 23.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 24.Xu Y, Dalrymple SL, Becker RE, Denmeade SR, Isaacs JT. Pharmacologic basis for the enhanced efficacy of dutasteride against prostatic cancers. Clin Cancer Res. 2006;12:4072. doi: 10.1158/1078-0432.CCR-06-0184. [DOI] [PubMed] [Google Scholar]
- 25.Frye SV. Discovery and clinical development of dutasteride, a potent dual 5alpha-reductase inhibitor. Curr Top Med Chem. 2006;6:405. doi: 10.2174/156802606776743101. [DOI] [PubMed] [Google Scholar]
- 26.Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5 alpha-reductase inhibitor. J Clin Endocrinol Metab. 2004;89:2179. doi: 10.1210/jc.2003-030330. [DOI] [PubMed] [Google Scholar]
- 27.Roehrborn CG, Andriole G, Schalken JA, Wilson T, Clark RV. Dutasteride, a novel dual 5alpha-reductase inhibitor, reduces serum DHT to a greater extent than finasteride and achieves finasteride maximal reduction in a larger proportion of patients. Presented at XVIIIth Congress of European Association of Urology; Madrid, Spain. March 12–15, 2003. [Google Scholar]
- 28.Wright AS, Douglas RC, Thomas LN, Lazier CB, Rittmaster RS. Androgen-induced regrowth in the castrated rat ventral prostate: role of 5alpha-reductase. Endocrinology. 1999;140:4509. doi: 10.1210/endo.140.10.7039. [DOI] [PubMed] [Google Scholar]
- 29.Gleave M, Qian J, Andreou C, Pommerville P, Chin J, Casey R, et al. The effects of the dual 5alpha-reductase inhibitor dutasteride on localized prostate cancer—results from a 4-month pre-radical prostatectomy study. Prostate. 2006;66:1674. doi: 10.1002/pros.20499. [DOI] [PubMed] [Google Scholar]
- 30.Andriole GL, Humphrey P, Ray P, Gleave ME, Trachtenberg J, Thomas LN, et al. Effect of the dual 5alpha-reductase inhibitor dutasteride on markers of tumor regression in prostate cancer. J Urol. 2004;172:915. doi: 10.1097/01.ju.0000136430.37245.b9. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt LJ, Murillo H, Tindall DJ. Gene expression in prostate cancer cells treated with the dual 5 alpha-reductase inhibitor dutasteride. J Androl. 2004;25:944. doi: 10.1002/j.1939-4640.2004.tb03166.x. [DOI] [PubMed] [Google Scholar]
- 32.Rittmaster RS, Norman RW, Thomas LN, Rowden G. Evidence for atrophy and apoptosis in the prostates of men given finasteride. J Clin Endocrinol Metab. 1996;81:814. doi: 10.1210/jcem.81.2.8636309. [DOI] [PubMed] [Google Scholar]
- 33.Bologna M, Muzi P, Biordi L, Festuccia C, Vicentini C. Finasteride dose-dependently reduces the proliferation rate of the LNCaP human prostatic cancer cell line in vitro. Urology. 1995;45:282. doi: 10.1016/0090-4295(95)80019-0. [DOI] [PubMed] [Google Scholar]
- 34.Lazier CB, Thomas LN, Douglas RC, Vessey JP, Rittmaster RS. Dutasteride, the dual 5alpha-reductase inhibitor, inhibits androgen action and promotes cell death in the LNCaP prostate cancer cell line. Prostate. 2004;58:130. doi: 10.1002/pros.10340. [DOI] [PubMed] [Google Scholar]
- 35.Iczkowski KA, Qiu J, Qian J, Somerville MC, Rittmaster RS, Andriole GL, et al. The dual 5-alpha-reductase inhibitor dutasteride induces atrophic changes and decreases relative cancer volume in human prostate. Urology. 2005;65:76. doi: 10.1016/j.urology.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 36.Civantos F, Watson RB, Pinto JE, Korman RB, Soloway MS. Finasteride effect on prostatic hyperplasia and prostate cancer: a comparative clinicopathologic study of radical prostatectomies. J Urol Pathol. 1997;6:1. [Google Scholar]
- 37.Andriole GL, Guess HA, Epstein JI, Wise H, Kadmon D, Crawford ED, et al. Treatment with finasteride preserves usefulness of prostate-specific antigen in the detection of prostate cancer: results of a randomized, double-blind, placebo-controlled clinical trial. PLESS Study Group. Proscar Long-term Efficacy and Safety Study. Urology. 1998;52:195. doi: 10.1016/s0090-4295(98)00184-8. [DOI] [PubMed] [Google Scholar]
- 38.Yang XJ, Lecksell K, Short K, Gottesman J, Peterson L, Bannow J, et al. Does long-term finasteride therapy affect the histologic features of benign prostatic tissue and prostate cancer on needle biopsy? PLESS Study Group. Proscar Long-Term Efficacy and Safety Study. Urology. 1999;53:696. doi: 10.1016/s0090-4295(98)00579-2. [DOI] [PubMed] [Google Scholar]
- 39.Andriole G, Bautista M, Crawford D, Kusec J, McConnell JD, Lucia S, et al. Prostate cancer (CAP) detection in the Medical Therapy of Prostatic Symptoms (MTOPS) trial. J Urol. 2003;169(suppl):120. abstract 466. [Google Scholar]
- 40.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 41.Thompson IM, Chi C, Ankerst DP, Goodman PJ, Tangen CM, Lippman SM, et al. Effect of finasteride on the sensitivity of PSA for detecting prostate cancer. J Natl Cancer Inst. 2006;98:1128. doi: 10.1093/jnci/djj307. [DOI] [PubMed] [Google Scholar]
- 42.Thomas LN, Douglas RC, Lazier CB, Gupta R, Norman RW, Murphy PR, et al. Levels of 5alpha-reductase type 1 and type 2 are increased in high grade compared to low grade prostate cancer. J Urol. 2008;179:147. doi: 10.1016/j.juro.2007.08.155. [DOI] [PubMed] [Google Scholar]
- 43.Andriole GL, Roehrborn C, Schulman C, Slawin KM, Somerville M, Rittmaster RS. Effect of dutasteride on the detection of prostate cancer in men with benign prostatic hyperplasia. Urology. 2004;64:537. doi: 10.1016/j.urology.2004.04.084. [DOI] [PubMed] [Google Scholar]
- 44.Andriole G, Bostwick D, Brawley O, Gomella L, Marberger M, Tindall D, et al. Chemoprevention of prostate cancer in men at high risk: rationale and design of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial. J Urol. 2004;172:1314. doi: 10.1097/01.ju.0000139320.78673.2a. [DOI] [PubMed] [Google Scholar]
- 45.Fleshner N, Gomella LG, Cookson MS, Finelli A, Evans A, Taneja S, et al. Delay in the progression of low-risk prostate cancer: rationale and design of the Reduction by Dutasteride of Clinical Progression Events in Expectant Management (REDEEM) trial. Contemp Clin Trials. 2007;28:763. doi: 10.1016/j.cct.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Andriole G, Lieber M, Smith J, Soloway M, Schroeder F, Kadmon D, et al. Treatment with finasteride following radical prostatectomy for prostate cancer. Urology. 1995;45:491. doi: 10.1016/s0090-4295(99)80021-1. [DOI] [PubMed] [Google Scholar]
- 47.Presti JC, Jr, Fair WR, Andriole G, Sogani PC, Seidmon EJ, Ferguson D, et al. Multicenter, randomized, double-blind, placebo controlled study to investigate the effect of finasteride (MK-906) on stage D prostate cancer. J Urol. 1992;148:1201. doi: 10.1016/s0022-5347(17)36860-x. [DOI] [PubMed] [Google Scholar]
- 48.Merrick GS, Butler WM, Wallner KE, Galbreath RW, Allen ZA, Kurko B. Efficacy of neoadjuvant bicalutamide and dutasteride as a cytoreductive regimen before prostate brachytherapy. Urology. 2006;68:116. doi: 10.1016/j.urology.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 49.Singh P, Uzgare A, Litvinov I, Denmeade SR, Isaacs JT. Combinatorial androgen receptor targeted therapy for prostate cancer. Endocr Relat Cancer. 2006;13:653. doi: 10.1677/erc.1.00797. [DOI] [PubMed] [Google Scholar]
- 50.Tindall DJ, Dehm SM. Regulation of androgen receptor signaling in prostate cancer. Expert Rev Anticancer Ther. 2005;5:63. doi: 10.1586/14737140.5.1.63. [DOI] [PubMed] [Google Scholar]