Abstract
Purpose
The sperm-derived SPANX family proteins can be found expressed in human tumors. Here, we aimed to perform a comprehensive study to evaluate immunotherapeutic relevance of one of its members, SPANX-B. We wanted to test its expression pattern in human tumors; and to evaluate CD4+ and CD8+ T cell responses in healthy humans after in vitro immunizations.
The Experimental Design
Expression of SPANX-B in human malignancies, including a multi-tumor tissue array of 145 primary tumors, was assessed utilizing RT/PCR, western blotting and immunohistochemical analysis. T cell immunogenicity and immunodominant epitopes of SPANX-B were studied using in vitro immunizations of healthy human donor-derived leukocytes.
Results
SPANX-B was abundantly expressed in melanoma and carcinomas of lung, ovary, colon and breast. In melanoma, tissue array data indicated that it was expressed in advanced and metastatic disease. Unlike most tumor-associated antigens, SPANX-B was an immunogenic antigen that was recognized by circulating T cell precursors in healthy humans. Importantly, these T cells were readily expanded to generate SPANX-B –specific helper CD4+ and cytolytic CD8+ T cells that recognized unique immunodominant epitopes: at least one HLA-DR-restricted Pep-9 epitope (SPANX-B12–23) and two HLA-A2-restricted Pep-2 and Pep-4 epitopes (respectively, SPANX-B23–31 and SPANX-B57–65). The CD8+ T cells were fully functional to recognize and lyse HLA-A2-expressing tumors, including primary human melanomas.
Conclusions
SPANX-B is an immunogenic sperm-derived antigen that is expressed in a number of human tumors. SPANX-B is also efficiently recognized by the human T cell immune arm, indicating its significant value for the development of protective and therapeutic cancer vaccines.
Keywords: SPANX, immunogenicity of TAA, antitumor CTL, MHC epitope
Introduction
Hominid SPANX family (a sperm protein associated with the nucleus on the chromosome X) consists of highly homologous proteins that share about 40–50% similarity with each other. SPANX is encoded by an ancestral SPANX-N and its descendant SPANX-A/D genes (1) that contain two exons separated by a 650 bp intron with an inserted retroviral LTR sequence (2). SPANX-A/D has five members (SPANX-A1, -A2, -B, -C, and –D) classified on the basis of diagnostic amino acid substitutions. The SPANX-A1, -C, and –D genes encode 97 a.a. proteins; while the SPANX-B gene, which can have up to a dozen copies, express 108 a.a. protein (3). SPANX is not expressed in nongametogenic adult tissues and its biological function is not well understood. However, based on their unique expression and localization in subpopulations of spermatids and spermatozoa (4), SPANX proteins were proposed to participate in mammalian spermatogenesis. SPANX-A and SPANX-B are localized in nuclear craters and cytoplasmic droplets of ejaculated spermatozoa, respectively (5).
SPANX is a typical Cancer-testis (CT) antigen, since SPANX genes are also specifically expressed in variety of human tumors and in normal testis (2;4;6). To date, the initial observation that SPANX-C (originally designated CTp11) was expressed in human melanoma (6) was expanded demonstrating that the other members (SPANX-A1, -A2, and -B) were also present in melanoma, bladder carcinomas and hematological malignancies and myeloma (5–8). The SPANX-positivity may also indicate the presence of more aggressive skin tumors, particularly in distant, nonlymphatic metastatic melanomas (8). SPANX appears to be an immunogenic antigen in humans, as sera of cancer patients contained high titers of SPANX antibody presumably generated during necrosis of malignant cells (7;8). However, the therapeutic relevance of the presence of SPANX antibody remains unknown, as successful tumor eradication would primarily depend on the activation of antigen/tumor-specific CD4+ helper and CD8+ cytolytic T cells. It is also unclear whether humans contain the SPANX-specific immune effector cells, since tumor- associated antigen (TAA) –reactive cells could be eliminated due to thymic selection. Furthermore, there is no comprehensive study which examines the clinical and therapeutic relevance of these antigens, specifically SPANX-B.
Here, we demonstrate that SPANX-B is widely expressed in human malignancies, particularly in melanoma and lung, ovarian and breast carcinomas. In melanoma specifically, its expression was associated with advanced and metastatic disease. Moreover, we show for the first time that SPANX-B is also recognized by the human T cell immune arm. Human peripheral blood contains T cell precursors that recognize SPANX-B, which can be readily expanded to generate the SPANX-B –specific CD4+ and cytolytic CD8+ T cells. We have also mapped their recognition epitopes demonstrating that SPANX-B contains at least one immunodominant HLA-DR- and two HLA-A2 –restricted epitopes. These data, taken together, clearly indicate that SPANX-B is a potent and clinically relevant therapeutic antigen that may be successfully exploited in cancer immunotherapy.
Materials and Methods
Human Peripheral Blood Cell Isolation
Human peripheral blood samples (PBMCs) were collected from healthy donors in accordance with Human Subject Protocol #2003054 by the Health Apheresis Unit and the Clinical Core Laboratory of the National Institute on Aging (NIA). CD4+ T cells were isolated from PBMCs by negative selection using a human CD4 subset column kit (R&D Systems Inc., Minneapolis, MN) after Ficoll-Paque (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) density gradient separation according to the manufacturers’ instructions. CD8+ T cells were isolated using CD8 beads (Invitrogen, in a ratio 1 μl beads per 1 × 108 T cells in PBS with 0.1% BSA and 2mM EDTA). Beads were removed from cells using DETACHaBEAD CD4/CD8 reagent (Dynal Biotech/Invitrogen Corp.). Cell purity was determined by FACS which resulted in 94% CD4+ T cells and 98% CD8+ T cells. Cells were cultured in RPMI with 10% FBS and 5% Human serum (clone medium). Monocyte/macrophage-enriched PBMCs by plastic adherence were used for isolation of DCs. Briefly, immature dendritic cells ( DCs) were generated by five day culturing adherent cells in cRPMI with 10% FBS and 5% human serum containing 20 ng/mL rhIL-4 and 30 ng/mL rhGM-CSF. DC were matured with overnight treatment with 10 ug/mL LPS (Sigma).
Reagents and cells
Recombinant SPANX-B protein fused with MBP was produced and purified from E.coli using the pMAL protein expression and purification system according to manufacturer’s instructions (New England Biolabs, Inc., Beverly, MA). Peptide sequences used in this work: Pep-1 (SPANX-C80–89), LQMEEEEFM; Pep-2 (SPANX-B23–31), EANEANKTM; Pep-3 (SPANX-B2–10), GQQSSVRRL; Pep-4 (SPANX-B57–65), LVVRYRRNV; Pep-5 (SPANX-C12–35), RSVPCESNEVNETMPETPTGDSDP; Pep-6 (SPANX-B42–65), QPAPKKMKTSESSTILVVRYRRNV; Pep-7 (SPANX-B66–89), KRTSPEELVNDHARENRINPDQME; Pep-8 (SPANX-B80–103), ENRINPDQMEEEEFIEITTERPKK; Pep-9 (SPANX-B12–23), RSVPCESNEANE; Pep-9-Mod (SPANX-B12–23 -scrambled), RSAPCASAEVNE; Pep-10 (SPANX-C8–16), GGVKRSVPC; Pep-11 (SPANX-C86–94), EFMEIMVEI; Pep-12 (SPANX-BC43–51), KTSESSTIL; Pep-G1 (SPANX-B11–31), KRSVPCESNEANEANEANKTM; Pep-G2 (SPANX-B21–41), ANEANEANKTMPETPTGDSDP; MOPC (9) (MOPC Igλ91–101), ALWFRNHFVFGGGTK; and influenza HLA-A2 peptide M (Flu M158–61), GILGFVFTL. All peptides were custom designed and purchased from AnaSpec (AnaSpec, Inc., San Jose, CA). The SPANX-B peptide-specific antibody ANEA-I0117 was generated by immunizing rabbits with SPANX-B-specific synthetic peptides. Antibodies specific to human IFNγ and to HLA-A,B,C were purchased from BD pharmingen (San Jose, CA); antibodies to HLA-DR, HLA-DP and HLA-DQ were from Leinco (St. Louis, MO).
The majority of cell lines, such as 938 Mel, Jurkat, CCRF-CEM (CEM, CCL-119), and CRL 7002, were purchased from ATCC. UACC1273 cell line was provided by Dr. Ashani T. Weeraratna (NIA). The cells were grown in cRPMI supplemented with 10% FBS. Human embryonic kidney cells (HEK293, ATCC) were grown in cDMEM with 10% FBS; ovarian cancer cell lines 2008, BG-1, and OVCAR3 (a generous gift of Dr. Patrice Morin, NIA, Baltimore, MD) were grown in cMcCoy’s 5A medium supplemented with 10% FBS. Human primary melanoma cells Tc526, Tc624, Tc2492, Tc2547 and Tc938 (a generous gift of Dr. Nicholas Restifo, NCI, Bethesda, MD) were cultured in cRPMI supplemented with 10% FBS. All cells were maintained in a 37°C humidified 5% CO2. Collection of paraffin non small cells lung cancer (NSCLC) blocks from seven different patients was supplied by the Department of Pathology, Civile Maggiore Hospital, Verona, Italy.
T cell stimulation
To generate CD4+ T cell lines, CD4+ T cells (2 × 106) were stimulated with autologous DC pulsed with either SPANX-B protein (2 ug/mL), or with SPANX-B peptides (10 μg/ml) in the presence of IL-2 (20 U/mL) for seven days. T cells were re-stimulated once every week two times. To generate CD8+ CTL lines, 2 × 106 CD8+ T cells were stimulated with autologous DC pulsed with either SPANX-B protein (2 ug/mL) and SPANX-B peptides (10 ug/mL) in the presence of IL-2 (20U/mL) and IL-15 (10 ng/mL) for seven days. T cells were weekly restimulated with peptide-loaded DCs for several times. To test antigen-specific activity of CD4+ T cell lines, all experiments were performed in triplicates in 96-well plates with 1 × 105 CD4+ T cells/well. The cells were stimulated with autologuos DCs incubated overnight with SPANX-B protein, or SPANX-B or control peptides (0.1 and 0.01 μg/mL) in clone medium with (20 U/mL). T cell activity is judged by IFNγ secretion in culture supernatants by ELISA after 48 hours incubation (see below and (10)). For most experiments, unless specified, 1:5 Target:Effector ratio was used.
Detection of IFNγ by ELISA, as described elsewhere (10). Briefly, 96-well flat-bottom plates were coated with 2 μg/mL of anti-human IFNγ antibody (BD pharmingen, San Jose, CA) to capture secreted IFNγ. The captured IFNγ was detected with 0.5 μg/mL the biotin-conjugated mouse anti-human IFNγ antibody and streptavidin-HRP (BD pharmingen, San Jose, CA). The assay was visualized with TMB peroxidase solution B (KPL, Gaithersburg, MD) and read at OD450.
CTL assay and HLA blocking experiments
Tumor cell lines (target cells, 2 × 106) were incubated in 200 μL of Fetal bovine serum (FBS) with 200 μCi of Na251Cr04 (PerkinElmer, Billerica, MA) for 2 hours at 37°C. Cells were washed with RPMI three times and resuspended in cRPMI with 10% FBS at 1 × 105 cells/mL. CTL assay was performed in triplicates in 96-well round bottom plates with 1 × 104/well Cr51-labeled target cells. The target cells were co-cultured at indicated ratios with effector cells (peptide- specific CD8+ T cells) for 6 hours. The specific 51Cr-release is calculated using formula: ((test sample release – spontaneous release)/(maximum release – spontaneous release)) × 100). Maximum release is for the target cells alone lysed with 2% of Triton X.
The MHC class I and class I inhibition assays
HLA specific mAbs or control isotype matched IgG were preincubated with peptide-pulsed DCs at concentration of 10 μg/mL for one hour at 4°C. Cells were washed with PBS, irradiated at 4500 rad and mixed with T cells at indicated ratios. To block HLA class I expression, tumor cells lines were pretreated with 10 ug/mL of anti-HLA-A,B,C antibodies (BD Pharmingen, San Jose, CA) for one hour at 4°C. Cells were washed with PBS and labeled with 51Cr as indicated above. IFNγ production was determined by ELISA after 48h incubation as described (10).
Detection of SPANX-B expression human tumors
SPANX-B mRNA expression was tested and confirmed using RT/PCR utilizing combinations of two different sets of primers that amplifies spliced messages, such as forward and reverse primers designed in house (PRSPANXB-Lar-1: 5′-ATGGGCCAACAATCCAGTGT-3′, and PRSPANXB-Lar-R1: 5′-CTTTTTAGGTCTTTCAGTCGT-3′, respectively); and forward and reverse SPANX-B primers reported by others (11) (5′-ACTGTAGACATCGAAGAACC-3′, and 5′-TTG1ATTCTGTTCTCTCGGGC-3′). Total RNA, extracted from frozen cell pellets using RNeasy Mini Kit (Qiagen, MD), was reverse transcribed using M-MLV RT (Invitrogen) and amplified using 2U Taq DNA polymerase (New England Biolabs Inc., Beverly, MA): 35 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min. Control amplification was for expression of GAPDH using PRuGAPDH-1 and PRuGAPDH-R1 (5′-TGTGGAAGGGCTCATGACCACAGTCCAT-3′, and 5′-GCCTGCTTCACCACCTTCTTGATG-3′, respectively).
SPANX-B protein expression was detected using ANEA-I0117 anti-SPANX-B Ab (1:5000) in Western blotting of tumor lysates with 30 μg total protein separated in reducing 14% PAAG gels (Invitrogen, Carlsbad, CA). Bands were detected with ECL plus western blotting detection system (GE healthcare, Little Chalfont Buckinghamshire, UK).
Immunohistochemistry stainings for SPANX-B were performed on Multi-Tumor Tissue Arrays (T-MTA-5, Tissue Array Research Program, NCI, www.cancer.gov/tarp) or on paraffin embedded biopsies of human with melanoma and NSCLC lung cancer (pathology collection of Ospedale Borgo Trento Piazzale Stefani, Verona, Italy). Briefly, the tissue slides were treated with series of xylene (three times for 5 min), ethanol 100% (two times for 2min), ethanol 90%, ethanol 80%, ethanol 75% (one time for 2 min), PBS and water. Antigen retrieval was performed with citrate buffer in a steamer for 25 min. Slides were rinsed with PBS and blocked for 5 min with V block solution (LabVision Corp, Fremont, CA) and one hour with blocking buffer (0.2% Triton X-100, 0.2% casein, 0.2% BSA, 5% normal goat serum, 0.2% gelatin and 0.02% NaN3). To perform immunohistochemistry staining on cultured cells, 3×104 cells tumors cell lines were seeded in an 8-well chamber slide (Lab-Tek II Chamber slide system). Cells were washed twice with PBS and fixed with 300 μL of Methanol: H2O2 3% for 30 min. After several PBS washes, the cells were blocked with ultra V block solution (Lab Vision Corp,), rinsed with PBS and blocked with 200 μL of blocking buffer for one hour. The slides were treated with ANEA-I0117 anti-SPANX-B Ab (1:500) or control rabbit Ig for 2h at room temperature. Slides were washed three times in PBS, incubated for 30 min with biotinylated goat anti-rabbit antibody (Lab Vision Corp,), washed three times with PBS and incubated with streptavidin- peroxidase (Lab Vision Corp) for 20 min. Protein expression was visualized with DAB (Lab Vision corporation); and counterstained with hematoxilyn.
Data analysis of the Mannheim data set
Scaled gene expression data were generated from the Affymetrix HG-U133 microarray chips for 45 primary cultures of melanoma biopsies using accession number GSE4843 (Mannheim data set, www.ncbi.nlm.nih.gov/geo). This data was loaded into GeneSpring GX 7.3 (Agilent Technologies, Palo Alto, CA, USA) and normalized as previously described (12). Normalized data for the Motif 2 set of genes (averaged within each sample expression pattern (12)) was compared to the expression pattern for SPANXB1 (probe set 220921_at) and the correlation coefficient was calculated; and statistical significance of variation of SPANX-B between the cohorts was calculated using Student’s two-tailed t-test.
Results
SPANX-B protein is uniquely expressed in human tumors
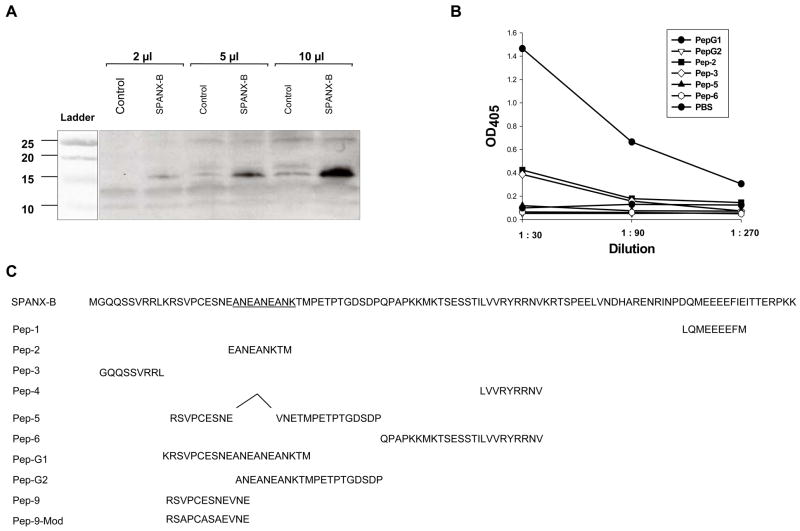
SPANX-B can be often found in the list of highly expressed genes in microarray study of human tumors, including hematological malignancies and breast cancer (11;13). To confirm this, we have tested its expression using two antibodies, a rabbit 3ANEA Ab and mouse SPANX-B Ab. The 3ENEA Ab was reported to be specific for the amino-terminal portion of SPANX-B in human spermatozoa (14), and it specifically detected an expected size (about 15 Kd) band in SPANX-B- trasduced murine RL5 cells in western blotting (Fig.1a). The second SPANX-B Ab was produced from mice DNA immunized with SPANX-B-expressing plasmid. It was also specific for SPANX-B, as it preferentially recognized PepG1 peptide (Fig.1b) encoding the amino-terminus of SPANX-B (Fig.1c), but not other region-specific peptides, such as PepG2, Pep-2, Pep-3 and Pep-6 (Fig.1b,c).
Figure 1. The specificity of antibody and expression of SPANX-B in human immortalized tumor cell lines. (A).
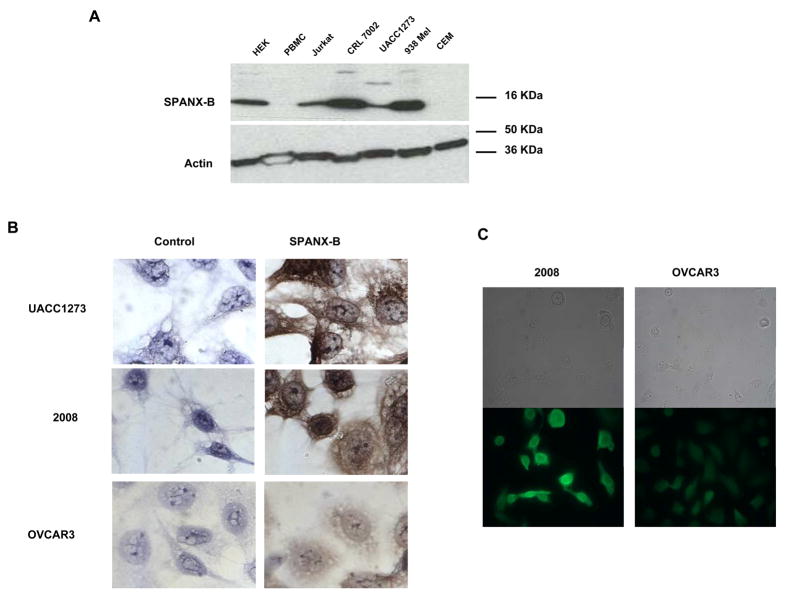
ANEA-I0117 antibody recognized SPANX-B expressed in mouse erythroleukemia RL5 cells. Shown, western blotting with ANEA-I0117 anti-SPANX-B antibody on lysates of RL5 cells (2μl, 5μl and 10 μl, respectively) transiently transduced with plasmid expressing SPANX-B (SPANX-B). Control lysates were made from untransduced parental RL5 cells (Control). (B) Murine anti-SPANX-B antibody, which was generated from mice DNA immunized with plasmid expressing SPANX-B, recognized unique SPANX-B –specific insert of SPANX-B (encoded in PepG1 and Pep-2 paptides, C). Shown, ELISA assay on plates coated with various peptides that are depicted in C. (C) Protein sequence of SPANX-B and locations of the synthetic peptides used is shown. Complete list of peptides used in the work is shown in the Materials and Methods section. Pep-5 does not contain 6 a.a. insert of SPANX-B (underlined). (D) The expression of SPANX-B protein was evaluated by Western blotting with ANEA-I0117 Ab (1:5000) in lysates of cell lines, such as HEK293 (HEK), Jurkat, CRL 7002, UACC1273, 938 Mel, CCRF-CEM (CEM), and normal human donor PBMC (upper panel). The blot was stripped and hybridized with anti-actin Ab (lower panel). Shown, (E and F) immunohistochemistry and immunofluorescent assay, respectively, of melanoma (UACC1273) and ovarian carcinoma (2008 and OVCAR3) cell lines. The cells were stained with ANEA-I0117 Ab (left and bottom panels in E and F, respectively); and control IgG (right and upper panels in E and F, respectively).
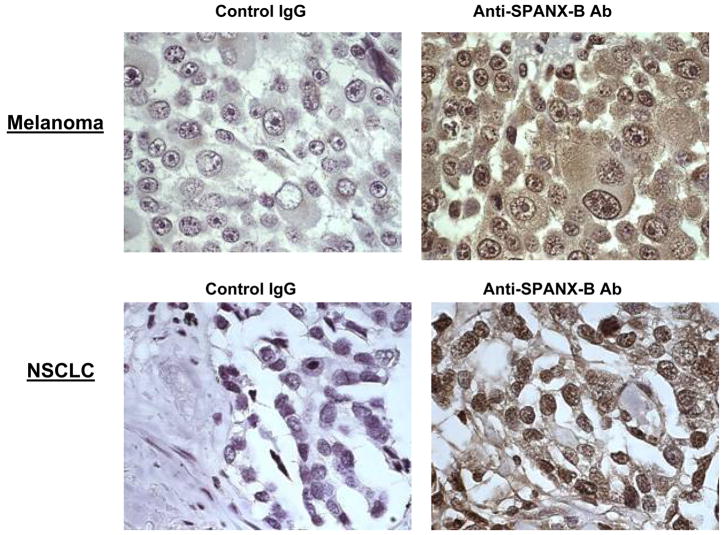
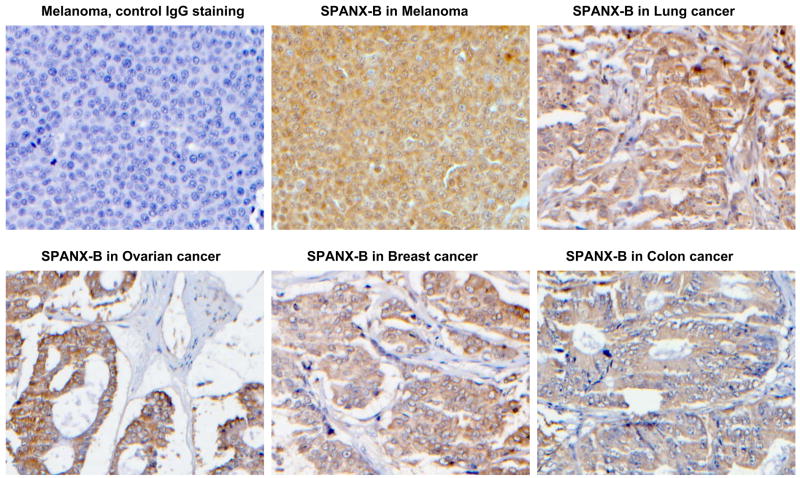
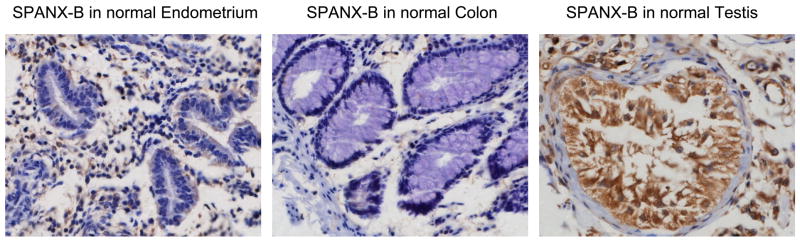
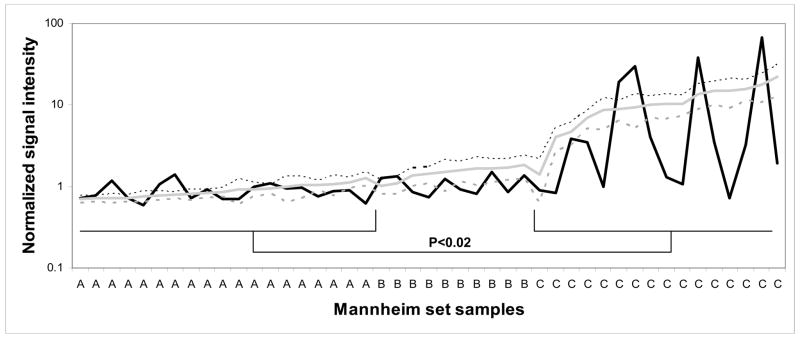
In support, it failed to recognize Pep-5 peptide that encoded the overlapping PepG1, but homologous with SPANX-C, portion (Fig.1b,c). In contrast, it reacted with a second peptide Pep-2 that only represented unique for SPANX-B insert of PepG1 (Fig.1b,c). In concordance with RT/PCR data (data not shown), SPANX-B was detected in lysates of immortalized human tumor lines, such as melanoma cells (UACC1273 and 938 Mel, Fig.1d), and carcinomas of colon (HCC2998), and ovarian (IGROV 1), renal (786-0 and UO-31) and NSCLC (NCI H226) (data not shown). In contrast, SPANX-B was not detected in lysates of normal human PBMCs (Fig.1d) and cell lines, such as HCT116 and HCT15 (colon cancer), OVCAR3 and OVCAR4 (ovarian cancer), and A498 and ACHN (renal cancer) and HOP62 and NCI H522 (NSCLC) (data not shown). These data were also supported by the immunohistochemistry staining of the cells. For example, SPANX-B –expressing cells, such as UACC1273 and 2008 cells (Fig.1d), but not SPANX-B negative OVCAR3 cells, were also positive both in immunohistochemistry (Fig.1e) and immunofluorescence (Fig.1f) stainings. The staining of primary human tumor samples revealed that it was expressed in human melanoma (2/2) and lung carcinoma (5/5, NSCLC) (Fig.2a). Furthermore, the survey of a panel of human primary 145 tumors on a multitumor tissue microarray (T-TMA) has revealed that SPANX-B was expressed in ovarian, colon, breast and lung cancers, besides melanoma (see a representative picture in Fig. 2b). Although we did not intend to study normal human tissues, SPANX-B was detected in the limited numbers of normal tissues included in T-TMA, such as normal endometrium and colon (Fig.2c). However, it was abundantly expressed in Sertoli cells of normal testis (Fig.2c), indicating that SPANX-B is a typical cancer/testis –associated (CT) antigen. The expression of SPANX-B was variable, ranging from an abundant production in melanoma and ovarian carcinomas, to low or almost undetectable levels in T cell tumors (Jurkat and CEM, respectively, Fig.1d). At least in melanoma, the presence of SPANX-B may indicate a metastatic stage of the disease, as its expression was associated with a group of genes expressed in melanoma with the highest metastatic potential (Cohort C in Mannheim data set (12), correlation co-efficient 0.503, Fig.2d). In contrast, we did not find any association in Cohort A that consisted of highly proliferative cells with low metastatic potential.
Figure 2. SPANX-B is expressed in human primary tumors.
(A) Immunohistochemistry staining of slides from paraffin embedded human primary melanoma (top panel) and non-small cell lung carcinoma (NSCLC, bottom panel); and T-TMA slides with human tumors (B) and normal tissues (C). The ANEA-I0117 Ab (SPANX-B) and control isotype-matched Ab (control IgG) were used at 1:500 dilution. (D) SPANX-B is mostly expressed in metastatic melanomas. Normalized signal intensity data for 77 genes previously identified as being associated with increased metastatic potential was averaged in each of 45 melanoma sample data sets (Mannheim data set) (12). This averaged profile (grey shaded line) was plotted against that of SPANX-B1 (black solid) across all samples and a positive correlation coefficient of 0.503 was calculated. Melanoma lines are labeled according to their cohort membership (12). Dotted lines mark the 95% confidence interval for the averaged profile. P-value is for comparisons between Cohorts A and C.
Characterization of SPANX-B-specific CD4+ T cell responses
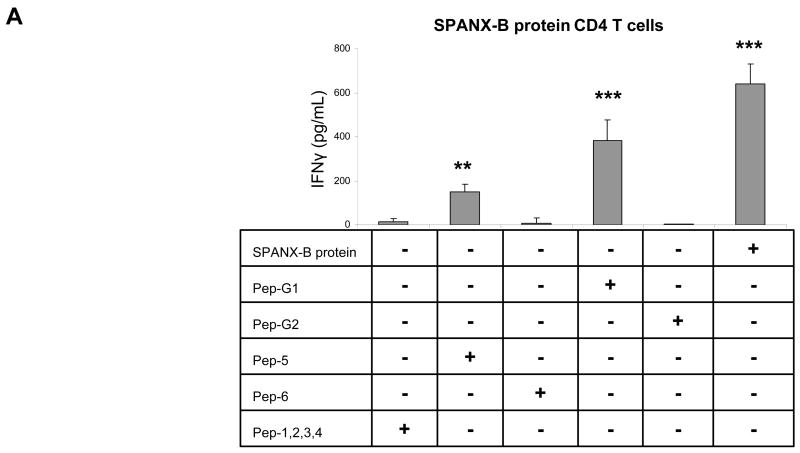
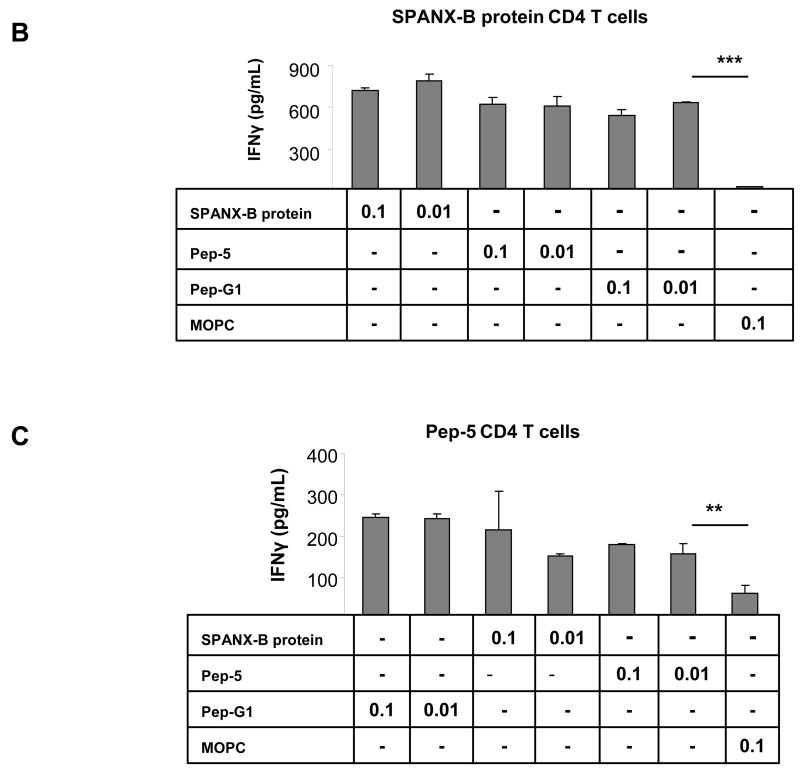
The clinical and therapeutic relevance of TAA depends on its ability to induce and expand the antigen-specific T cell precursors. To test this, human peripheral blood CD4+ T cells were stimulated with autologous DCs incubated with SPANX-B protein. As a result, this allowed to generate SPANX-B –specific CD4+ T cell lines from almost every human donor; the cells specifically recognized SPANX-B- treated DCs, but not control antigen-treated DCs, by secreting IFNγ (SPANX-B protein, Fig.3a). To determine an immunodominant epitope, the CD4+ T cells were stimulated with DCs pulsed with synthetic peptides to various parts of SPANX-B (Fig.1c). Among them, two peptides from the amino-terminal portion of SPANX-B, SPANX11–31 or SPANX12–35 (Pep-G1 and Pep-5, Fig.1c), activated the CD4+ T cell lines to secrete IFNγ (Fig.3a,b). In contrast, the CD4+ T cells failed to respond to DCs pulsed with other SPANX-B peptides, including peptides SPANX21–41 (Pep-G2) and SPANX42–65 (Pep-6), or mixture of Peptides 1–4 (Pep-1,2,3,4, Fig.3a), or control MOPC peptide Fig.3b,c). Together, the MHC class II immunodominant region (epitope) is probably located within overlapping portion of Pep-G1 and Pep-5 between residues 11 and 35 of SPANX-B. The region was recognized by almost every normal human donor CD4+ T cells tested (9/9), indicating that humans contain a pre-existing pool of precursor T cells specific for SPANX-B. In support, SPANX-B -specific CD4+ T cell lines were also independently and readily generated using peptides Pep-5 and Pep-G1 (Fig.3c,d, respectively) that also specifically and in a dose-dependent manner recognized DCs incubated with SPANX-B protein.
Figure 3. SPANX-B induces CD4+ T cell responses.
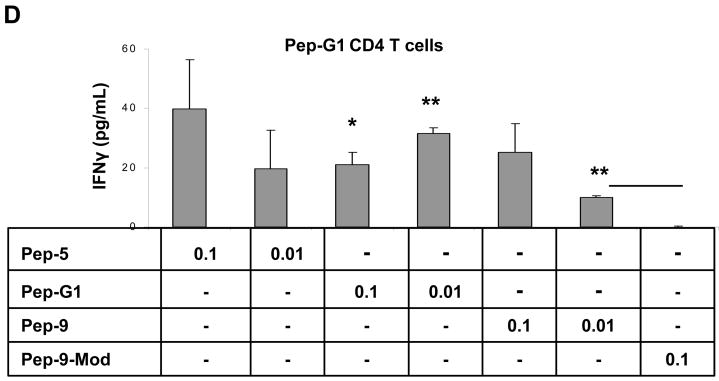
(A and B) SPANX-B –specific CD4+ T cell lines were generated by repeated stimulations of human T cells with irradiated autologous iDC treated with SPANX-B protein. The immunodominant region of SPANX-B is located in the overlapping portion of synthetic peptides Pep-G1 and Pep-5, as the T cell line can be also activated to secrete IFNγ with irradiated mDCs pulsed with 1μg/ml Pep-G1 or Pep-5, but not with individual peptides (Pep-G2, Pep-6) or mixture of peptides (Pep-1, -2, -3, -4) specific to other regions of SPANX-B. The CD4+ T cell lines generated to SPANX-B protein (B), or Pep-5 (C), or Pep-G1 (D) specifically and reciprocally recognize DCs pulsed with titrated amounts (μg/ml) of SPANX-B protein, or Pep-5, or Pep-G1, or Pep-9 and secrete IFNγ (pg/ml). The T cells were not activated with DCs pulsed with control murine class II peptide (MOPC) or with scrambled Pep-9 (Pep-9-Mod, D). **P<0.01 and ***P<0.001 value is for comparison with the group indicated by line. Shown, mean ± SEM of representative and reproducible results of at least three independent experiments performed in triplicates.
The immunodominant epitope of SPANX-B is recognized in HLA-DR-restricted fashion
To fine map the epitope, a shorter peptide Pep-9 (SPANX-B12–23, Fig.1c), which represents an overlapping portion of peptides Pep-5 and Pep-G1, was tested. Autologous DCs pulsed Pep-9 were indeed able to activate the SPANX-B-specific CD4+ T cell lines (independently generated to SPANX-B protein, or Pep-1, or Pep-G1) to secrete IFNγ (a representative result on the Pep-G1-specific CD4+ T cells is shown in Fig.3d). In contrast, the CD4+ T cells did not respond when stimulated with scrambled Pep-9 peptide (Pep-9-Mod, Fig.3d). Together, the CD4+ T cells recognized an immunodominant epitope encoded “RSVPCESNEANE” sequence of Pep-9 (Fig.1c).
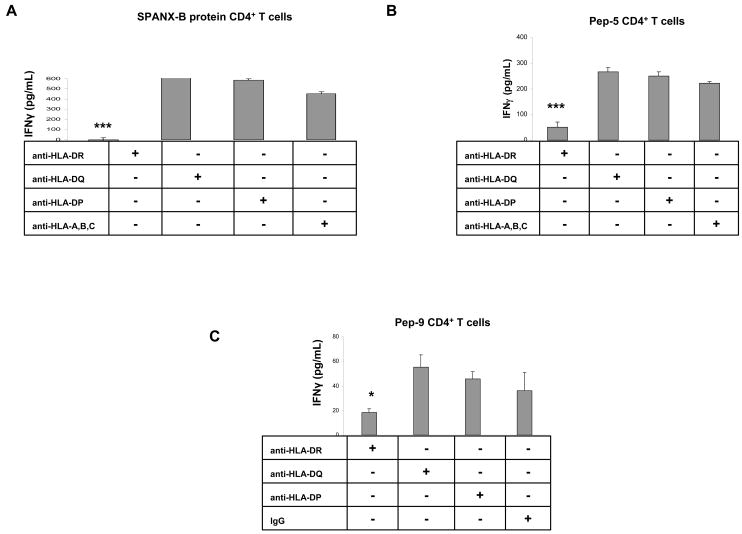
Next, to characterize HLA specificity, the CD4+ T cell lines were stimulated with autologous DCs pulsed with Pep-9 in the presence of antibody that blocked HLA-DR, or HLA-DQ, or HLA-DP molecules. The stimulation of the SPANX-B-specific CD4+ T cells was not affected by the presence of control antibody or antibodies that block HLA-DQ, or HLA-DP, or anti-MHC class I (Fig.4a–c). In contrast, anti-HLA-DR Ab completely abrogated the Pep-9 –induced IFNγ secretion from all CD4+ T cell lines specific to SPANX protein (Fig.4a), or Pep-5 (Fig.4b), or Pep-9 (Fig.4c), respectively. Thus, the peptide Pep-9 represents an immunodominant epitope recognized by human CD4+ T cells in HLA-DR- restricted fashion.
Figure 4. The MHC Class II immunodominant epitope of SPANX-B is presented in the context of HLA-DR.
The Pep-9 –induced activation of the CD4+ T cell lines, i.e. generated to SPANX-B protein (A), or Pep-5 (B), or Pep-9 (C) can be abrogated by the presence of anti-HLA-DR Ab. In contrast, the presence of antibodies to HLA-DQ, or HLA-DP or MHC class I (anti-HLA-A,B,C) did not affect activity of CD4+ T cells. ***P<0.001 value is for comparison with the group indicated by line. Shown, mean ± SEM of representative and reproducible results of two independent experiments performed in triplicates for each panel.
SPANX-B is also recognized by CD8+ T cells in HLA-A2 context
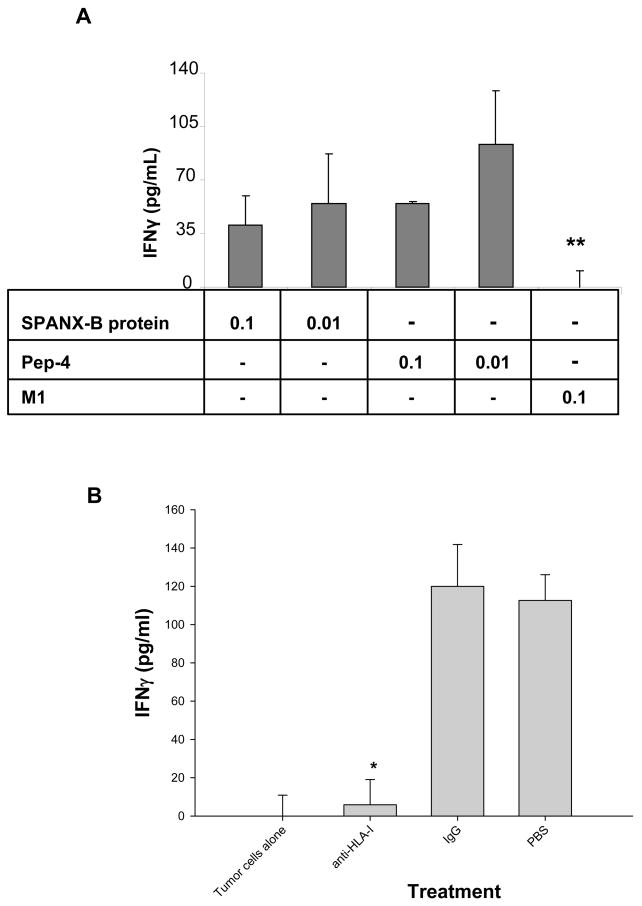
To exploit the full therapeutic value of TAAs, the induction of CD8+ T cells is required. Computer analysis of SPANX-B protein has revealed two 9-mer peptides designated Pep-2 and Pep-4 (SPANX-B23–31 and SPANX-B57–65, respectively) that contained moderate Parker binding scores for HLA-A2 (www-bimas.cit.nih.gov). To test whether they are recognized by human CD8+ T cells, HLA-A2-positive peripheral blood CD8+ T cells were stimulated with autologuos DCs incubated with SPANX-B protein. As a result, SPANX-B –specific CD8+ T cell lines were generated from every donor PBL (3/3). The CD8+ T cells secreted IFNγ upon incubation with irradiated autologous DCs that were pulsed with SPANX-B protein (Fig.5a), or Pep-4 (Fig.5a), or Pep-2 (data not shown). Reciprocally, the CD8+ T cells that were independently generated by Pep-2 or Pep-4 -pulsed DCs also recognized SPANX-B protein- pretreated DCs (data not shown). The recognition was specific, as no IFNγ was produced when the CD8+ T cells were stimulated with DCs pulsed with control HLA-A2 –positive Matrix 1 influenza peptide (M1, Fig.5a). Furthermore, this was the MHC class I restricted recognition, as SPANX-B –induced activation was specifically and significantly abrogated by the presence of anti-MHC-I, but not control, antibody (Fig.5b).
Figure 5. SPANX-B also induces CD8+ T cell responses.
The SPANX-B –specific CD8+ T cell lines were generated by stimulating human CD8+ T cells with DCs fed with SPANX-B protein (A), or Pep-2 (B) or Pep-4 (not shown). The CD8+ T cell lines specifically recognized and secreted IFNγ (pg/ml) upon stimulation with DCs incubated with titrated amounts (μg/ml) of SPANX-B protein, or Pep-4, but not control M1 Flu peptide (M1). (B) The CD8+ T cell recognition is the MHC class I restricted, as the presence of anti-HLA I Ab, but not control isotype-matched Ab (IgG), abrogated IFNγ secretion from the SPANX-B –specific CD8+ T cells stimulated with mDCs pulsed with Pep-2. The same was observed for other CD8+ T cell lines generated by Pep-2 - or Pep-4 –pulsed DCs (not shown). *P<0.05 (A) and **P<0.01 (B) value is for comparison with IgG and M1 groups, respectively. Shown, mean ± SEM of representative and reproducible results of at least two independent experiments performed in triplicates for each panel.
The SPANX-B –specific CD8+ T cells kill human tumor in HLA-A2 context
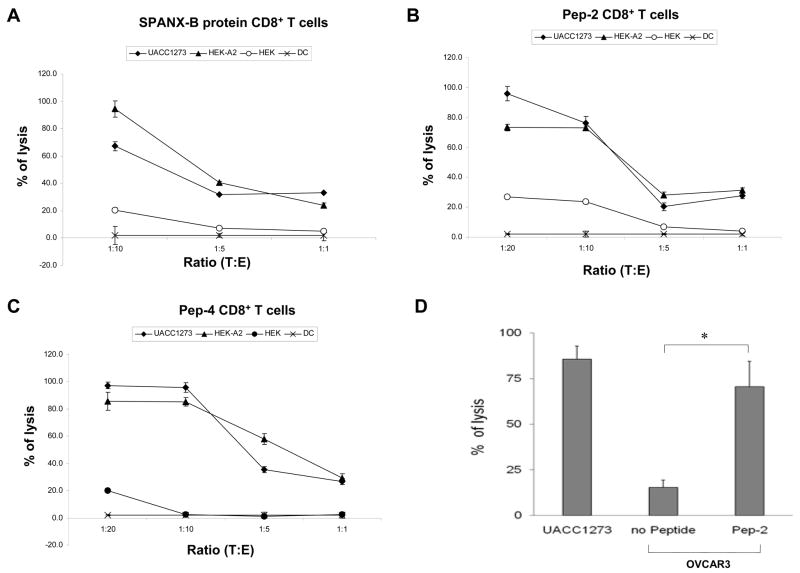
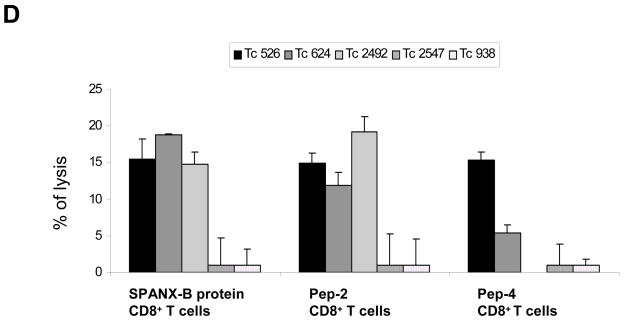
Next, we have tested whether the CD8+ T cells can recognize human tumors that express SPANX-B. As shown in Fig.6, the CD8+ T cells were able to kill melanoma cells. For example, they recognized and lysed SPANX-B-expressing and HLA-A2-positive UACC1273 melanoma cells (UACC1273, Fig.6a–c). Several lines of evidence indicate that is indeed HLA-A2-restricted response. First, the CD8+ T cells did not lyse HLA-A2-negative but SPANX-B –expressing melanoma cells 938 Mel (data not shown). Second, all three types of CD8+ T cell lines (independently generated to SPANX-B protein, or Pep-2, or Pep-4) not only killed UACC1273 melanoma cells, but also lysed HLA-A2 –expressing HEK293 cells that were pulsed with Pep-2 (HEK-A2, Fig.6a–c). In contrast, the CD8+ T cells did not kill HLA-A2-negative parental HEK293 cells (HEK, Fig.6a–c). Lastly, the CD8+ T cells could not lyse HLA-A2-positive OVCAR3 cells that did not express SPANX-B, unless pulsed with Pep-2 (Fig.6d). Furthermore and importantly, all three CD8+ lines also recognized and lysed primary tumors from melanoma patients that expressed HLA-A2 (Tc526, Tc624 and Tc2492, Fig.6d), while HLA-A2-negative tumors were not affected (Tc2547 and Tc938, Fig.6e). Thus, humans contain a readily available proportion of circulating CD8+ T cells (at least precursors) that recognize two epitopes of SPANX-B, Pep-2 and Pep-4, presented on the MHC class I molecules in the HLA-A2 context.
Figure 6. The SPANX-B –specific CD8+ T cells recognize and kill HLA-A2-expressing melanoma cells.
Effector CD8+ T cells (E) generated by stimulating with SPANX-B protein (A), or Pep-2 (B), or Pep-4 (C) were mixed with target (T) UACC1273 melanoma cells at indicated ratio (X-axis) to perform cytolytic 6-hour Cr release assay. Control targets were parental (HEK) or HLA-A2 –expressing (HEK-A2) HEK293 cells that were pulsed with 1 μg/ml Pep-2. Autologous DCs alone were used as a negative control group. (D) The CD8+ T cells did not lyse SPANX-B –negative but HLA-A2-positive OVCAR3 cells, unless pulsed with Pep-2. (A–D) Shown, percentage of cytotoxicity (Y-axis) of a representative experiment performed in triplicate and repeated at least twice. (E) Effector Effector CD8+ T cells also recognize and kill HLA-A2-expressing (Tc526, Tc624 and Tc2492), but not HLA-A2-negative (Tc2547 and Tc938) primary human melanoma cells. Shown, mean ± SEM (%, Y-axis) of Cr release of the Pep-2 –specific CD8+ T cells at T:E of 1:10. Comparable cytolysis was also detected from the SPANX-B protein- and Pep-4 –specific CD8+ effector cells (data not shown).
Discussion
Tumor- associated antigens (TAAs) are the main targets of cancer immunotherapy. TAAs, as self-antigens, are usually poorly immunogenic and their immune effector cells may have been subjected to thymic selection and elimination. In contrast, a group of TAAs that are expressed in embryonic cells (OFA-iLRP (15)) or in various immune privileged sites (cancer-testis antigens designated by their unique expression in the named tissues) are highly immunogenic in humans. Importantly, their antigen-specific precursor and effector T cells appear to be preserved in adult humans and cancer patients, which can be associated with a favorable cancer outcome. The induction of embryonic OFA antigen- recognizing CTLs was used as a positive control in renal carcinoma patients immunized with renal tumor RNA- transfected DCs (16). Hence, OFA-iLRP-specific CD4+ and CD8+ T cells are shown to be readily expanded from the peripheral blood of healthy donors or patients with B cell malignancies and renal cell carcinoma (16–18). The CD8+ cells were fully active and able to kill antigen-expressing tumors, such as acute myeloid leukemia and chronic lymphocytic leukemia cells (17;18).
SPANX family proteins, specifically SPANX-C (CTp11), are usually produced in normal human sperm cells, but was found also expressed in melanoma and cancer cells of kidney, bladder and prostate (2;4;6). However, due to the lack of specific antibodies that discriminate each member of the family, no systemic study on the expression of SPANX-B in human tumors was reported. To date, its expression in melanoma, testicular germ cell tumors, and hematopoietic malignancies was detected mostly by the use of RT/PCR analysis (2;7;19;20). Here, we re-evaluated RT/PCR results using two different SPANX-B specific antibodies that were specific to a unique 6-a.a insert only present in SPANX-B. We have found SPANX-B in a number of different tumors, besides melanoma. It is worth noting that SPANX-B –specific exon 1 fragment was also amplified by RT/PCR in all ten randomly chosen positive samples. Together, the antibodies used in this study are specific, although due to a high homology between SPANX-A/D members the issue of cross-reactivity may not be fully resolved. Overall, the survey of a panel of tumors on a multi-tumor tissue microarray indicated that SPANX-B was expressed in carcinomas of breast, lung, colon and ovary. A functional relevance of our results remains unknown, as SPANX should not be expressed in nongametogenic adult tissues. It is tempting to speculate that SPANX-B may facilitate motility and metastasis of tumors and lead to more aggressive stage of the disease. First, Westbrook et al. suggested that the presence of SPANX (family members not specified) could be an indicator for the presence of more aggressive skin tumors, particularly in distant, nonlymphatic metastatic melanomas (8). Furthermore, SPANX-B was among genes that was over expressed in human breast cancer cell lines that metastasize to lung (see ref. (13)). Lastly, we have also found that SPANX-B was also preferentially expressed in Cohort C of the Mannheim data set that represented the pattern of genes associated with highly metastatic motif 2 (12).
SPANX proteins, including SPANX-B, appear to be immunogenic in humans, as the sera of patients with hematopoietic malignancies that express SPANX-B also contained SPANX-B - reactive antibodies (7). Despite this, it remains unknown whether SPANX-B can elicit T cell responses in humans. It is hardly arguable fact that the successful cancer therapy would require induction of the antigen-specific cellular immune responses. The therapeutic value/potency of TAAs is closely linked with their ability to elicit long-lasting CD4+ T helper and CD8+ CTLs. As such, our data suggest that SPANX-B is indeed a potentially valuable therapeutic antigen. First, healthy humans contain circulating SPANX-B -specific T cell precursors. Second, these T cells can be readily expanded to generate both helper CD4+ T cells and cytolytic CD8+ T cells utilizing in vitro immunizations with SPANX-B treated DCs. Third, SPANX-B is not only expressed in human melanomas, but it is also processed and presented on their MHC class I molecules. SPANX-B has at least two immunodominant HLA-A2 –restricted Pep-2 and Pep-4 epitopes that alone can be used to elicit SPANX-B –specific CD8+ CTLs. Fourth, and importantly, the SPANX-B –specific CD8+ T cells generated from normal human PBLs can recognize and efficiently kill the HLA-matched human primary melanomas. Lastly, we have determined at least one HLA-DR-restricted and immunodominant Pep-9 epitope against which the SPANX-B –specific CD4+ T cells can be readily generated from normal human PBLs. Thus, it is tempting to speculate that this ability would provide an additional important benefit for the immunotherapy of cancers as a source of T helper cells. In fact, CD8+ T cells primed in the absence of CD4+ T cell help are shown to undergo TRAIL-dependent death upon antigen restimulation, despite their ability to mediate effector functions such as cytotoxicity (21). At present, this possibility is not testable in vivo primarily due to the fact that SPANX-B is only expressed in higher primates and no proper animal models exist so far. Our attempts to establish a murine model that expresses SPANX-B in poorly-immunogenic tumor cells failed, as SPANX-B rendered the cells immunogenic and inhibited tumor progression in mice (Biragyn, unpublished observation). Taken together, SPANX-B is not only a CT antigen expressed in a majority of human tumors, but it is also a very attractive therapeutic target antigen. Our data indicate that SPANX-B is an immunogenic antigen that can readily expand and activate both arms of T cell immunity, helper CD4+ T cells and CD8+ CTLs, to recognize and kill HLA-matched SPANX-B –expressing melanomas.
Figure 7.
Acknowledgments
We are grateful to Karen Madara (Apheresis Unit and the Clinical Core Laboratory, NIA) for providing human blood samples; Dr. Nicholas Restifo (NCI) for the gift of human primary melanoma cells; Dr. Pat Morin (NIA) for the gift of ovarian cancer cell lines; Dr. Paul Goldsmith (NCI) for the gift of ANEA-I0117 Abs and SPANX-B protein; Ana Lustig (NIA) for critical reading of the manuscript and helpful comments and suggestions. Dr. Keith S. Hoek’s research was supported by the Swiss National Foundation (3100A0-103671) and Oncosuisse (OCS-01927-08-2006). This research was supported by the Intramural Research Program of the National Institute on Aging and Center for Cancer Research of the National Cancer Institute, NIH.
Footnotes
Statement of Clinical Relevance
Despite a recent surge in the discovery of novel tumor-associated antigens (TAAs), the need for well-defined antigens with a therapeutic or even protective value remains high. SPANX family proteins are usually produced in spermatozoids, but they were shown to be also expressed in human tumors. Here, we have performed a comprehensive study evaluating clinical and immunotherapeutic relevance of one of its member, SPANX-B. We report that SPANX-B is uniquely expressed in a number of human tumors. Moreover, we demonstrate for the first time that SPANX-B is an immunogenic antigen that is recognized by human T cells inducing helper CD4+ and cytolytic CD8+ T cell responses. Taken together, we provide important and detailed information on the clinical relevance of SPANX-B, and as such it would have significant practical value for the development of potent protective and therapeutic vaccines to combat cancer.
References
- 1.Kouprina N, Mullokandov M, Rogozin IB, Collins NK, Solomon G, Otstot J, et al. The SPANX gene family of cancer/testis-specific antigens: rapid evolution and amplification in African great apes and hominids. Proc Natl Acad Sci U S A. 2004 Mar 2;101(9):3077–82. doi: 10.1073/pnas.0308532100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zendman AJ, Zschocke J, van Kraats AA, de Wit NJ, Kurpisz M, Weidle UH, et al. The human SPANX multigene family: genomic organization, alignment and expression in male germ cells and tumor cell lines. Gene. 2003 May 8;309(2):125–33. doi: 10.1016/s0378-1119(03)00497-9. [DOI] [PubMed] [Google Scholar]
- 3.Kouprina N, Pavlicek A, Noskov VN, Solomon G, Otstot J, Isaacs W, et al. Dynamic structure of the SPANX gene cluster mapped to the prostate cancer susceptibility locus HPCX at Xq27. Genome Res. 2005 Nov;15(11):1477–86. doi: 10.1101/gr.4212705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westbrook VA, Schoppee PD, Vanage GR, Klotz KL, Diekman AB, Flickinger CJ, et al. Hominoid-specific SPANXA/D genes demonstrate differential expression in individuals and protein localization to a distinct nuclear envelope domain during spermatid morphogenesis. Mol Hum Reprod. 2006 Nov;12(11):703–16. doi: 10.1093/molehr/gal079. [DOI] [PubMed] [Google Scholar]
- 5.Westbrook VA, Diekman AB, Naaby-Hansen S, Coonrod SA, Klotz KL, Thomas TS, et al. Differential nuclear localization of the cancer/testis-associated protein, SPAN-X/CTp11, in transfected cells and in 50% of human spermatozoa. Biol Reprod. 2001 Jan;64(1):345–58. doi: 10.1095/biolreprod64.1.345. [DOI] [PubMed] [Google Scholar]
- 6.Zendman AJ, Cornelissen IM, Weidle UH, Ruiter DJ, van Muijen GN. CTp11, a novel member of the family of human cancer/testis antigens. Cancer Res. 1999 Dec 15;59(24):6223–9. [PubMed] [Google Scholar]
- 7.Wang Z, Zhang Y, Liu H, Salati E, Chiriva-Internati M, Lim SH. Gene expression and immunologic consequence of SPAN-Xb in myeloma and other hematologic malignancies. Blood. 2003 Feb 1;101(3):955–60. doi: 10.1182/blood-2002-06-1930. [DOI] [PubMed] [Google Scholar]
- 8.Westbrook VA, Schoppee PD, Diekman AB, Klotz KL, Allietta M, Hogan KT, et al. Genomic organization, incidence, and localization of the SPAN-x family of cancer-testis antigens in melanoma tumors and cell lines. Clin Cancer Res. 2004 Jan 1;10(1 Pt 1):101–12. doi: 10.1158/1078-0432.ccr-0647-3. [DOI] [PubMed] [Google Scholar]
- 9.Bogen B, Lambris JD. Minimum length of an idiotypic peptide and a model for its binding to a major histocompatibility complex class II molecule. EMBO J. 1989;8:1947–52. doi: 10.1002/j.1460-2075.1989.tb03599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiavo R, Baatar D, Olkhanud P, Indig FE, Restifo N, Taub D, et al. Chemokine receptor targeting efficiently directs antigens to MHC class I pathways and elicits antigen-specific CD8+ T-cell responses. Blood. 2006 Jun 15;107(12):4597–605. doi: 10.1182/blood-2005-08-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lilljebjorn H, Heidenblad M, Nilsson B, Lassen C, Horvat A, Heldrup J, et al. Combined high-resolution array-based comparative genomic hybridization and expression profiling of ETV6/RUNX1-positive acute lymphoblastic leukemias reveal a high incidence of cryptic Xq duplications and identify several putative target genes within the commonly gained region. Leukemia. 2007 Oct;21(10):2137–44. doi: 10.1038/sj.leu.2404879. [DOI] [PubMed] [Google Scholar]
- 12.Hoek KS, Schlegel NC, Brafford P, Sucker A, Ugurel S, Kumar R, et al. Metastatic potential of melanomas defined by specific gene expression profiles with no BRAF signature. Pigment Cell Res. 2006 Aug;19(4):290–302. doi: 10.1111/j.1600-0749.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- 13.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005 Jul 28;436(7050):518–24. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kouprina N, Noskov VN, Pavlicek A, Collins NK, Schoppee Bortz PD, Ottolenghi C, et al. Evolutionary diversification of SPANX-N sperm protein gene structure and expression. PLoS ONE. 2007;2(4):e359. doi: 10.1371/journal.pone.0000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biragyn A, Schiavo R, Olkhanud P, Sumitomo K, King A, McCain M, et al. Tumor-associated embryonic antigen-expressing vaccines that target CCR6 elicit potent CD8+ T cell-mediated protective and therapeutic antitumor immunity. J Immunol. 2007 Jul 15;179(2):1381–8. doi: 10.4049/jimmunol.179.2.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su Z, Dannull J, Heiser A, Yancey D, Pruitt S, Madden J, et al. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 2003 May 1;63(9):2127–33. [PubMed] [Google Scholar]
- 17.Siegel S, Wagner A, Kabelitz D, Marget M, Coggin J, Jr, Barsoum A, et al. Induction of cytotoxic T-cell responses against the oncofetal antigen-immature laminin receptor for the treatment of hematologic malignancies. Blood. 2003 Dec 15;102(13):4416–23. doi: 10.1182/blood-2003-01-0198. [DOI] [PubMed] [Google Scholar]
- 18.Siegel S, Wagner A, Friedrichs B, Wendeler A, Wendel L, Kabelitz D, et al. Identification of HLA-A*0201-presented T cell epitopes derived from the oncofetal antigen-immature laminin receptor protein in patients with hematological malignancies. J Immunol. 2006 Jun 1;176(11):6935–44. doi: 10.4049/jimmunol.176.11.6935. [DOI] [PubMed] [Google Scholar]
- 19.Salemi M, Calogero AE, Castiglione R, Tricoli D, Asero P, Rosa R, et al. Expression of SpanX proteins in normal testes and in testicular germ cell tumours. Int J Androl. 2006 Apr;29(2):368–73. doi: 10.1111/j.1365-2605.2005.00615.x. [DOI] [PubMed] [Google Scholar]
- 20.Salemi M, Calogero AE, Bosco P, Castiglione R, La VS, Borgione E, et al. Expression of SpanX mRNA in testicular germ cell tumors. Hum Cell. 2006 Aug;19(3):87–90. doi: 10.1111/j.1749-0774.2006.00014.x. [DOI] [PubMed] [Google Scholar]
- 21.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005 Mar 3;434(7029):88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]