Abstract
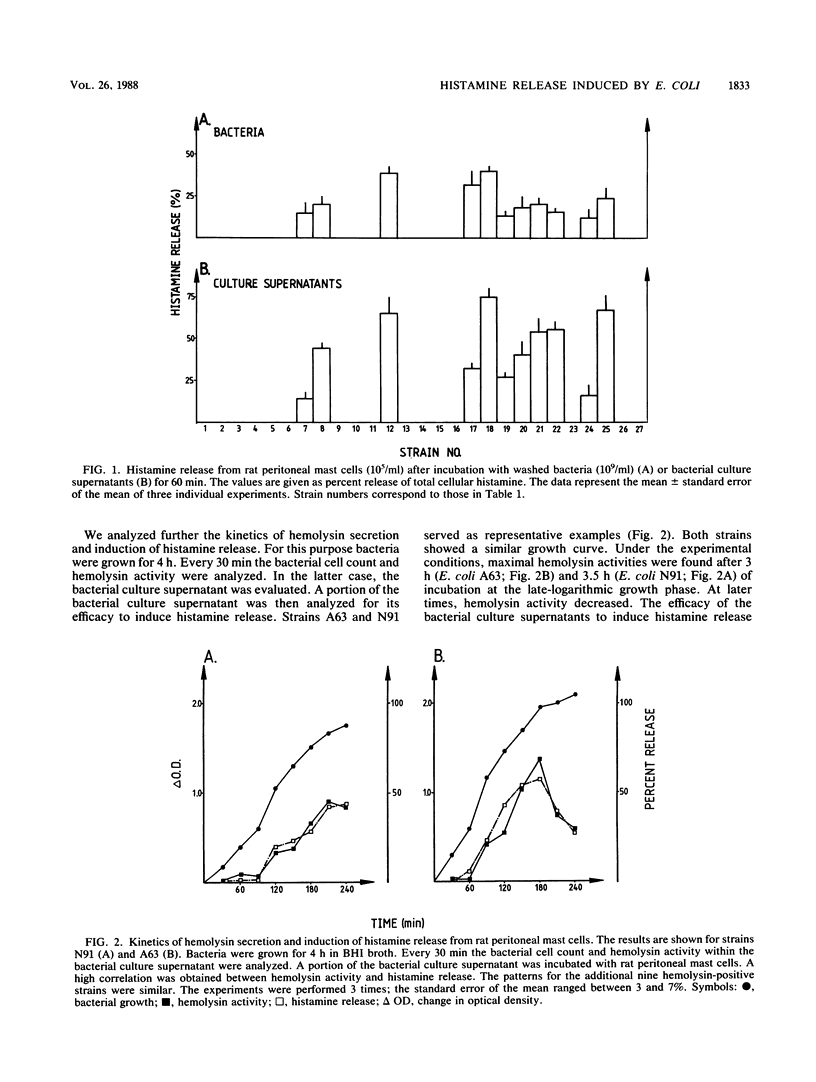
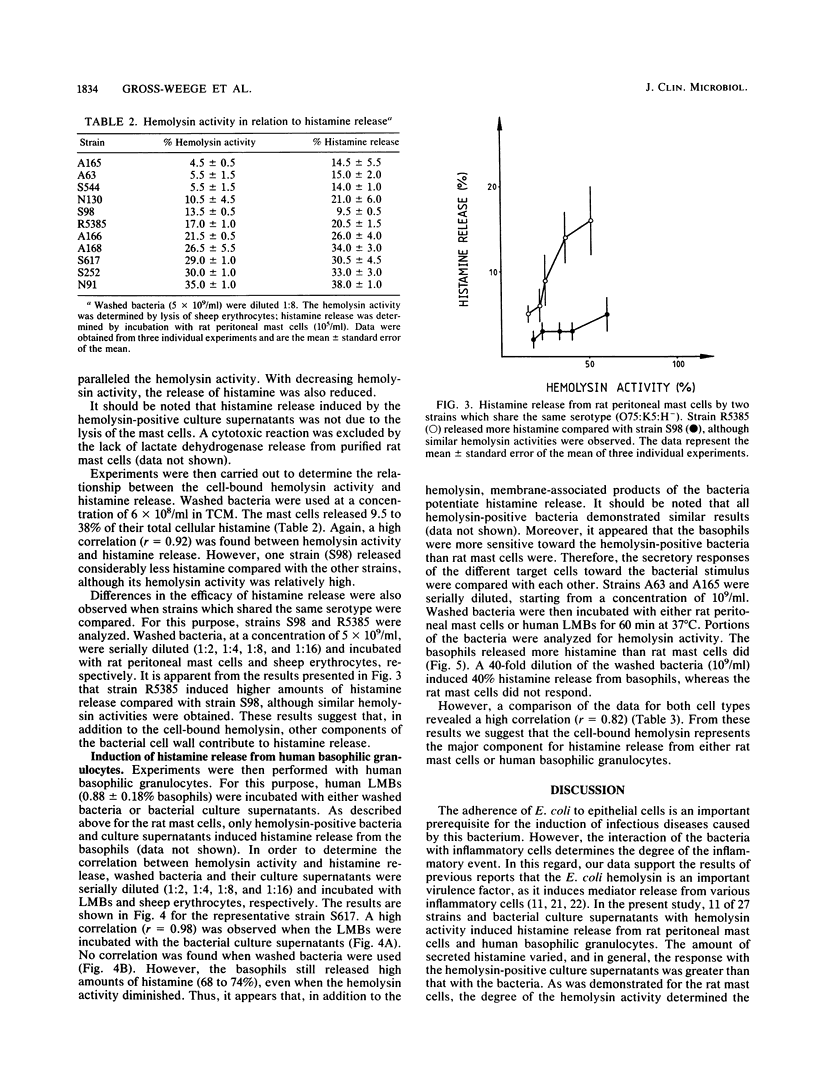
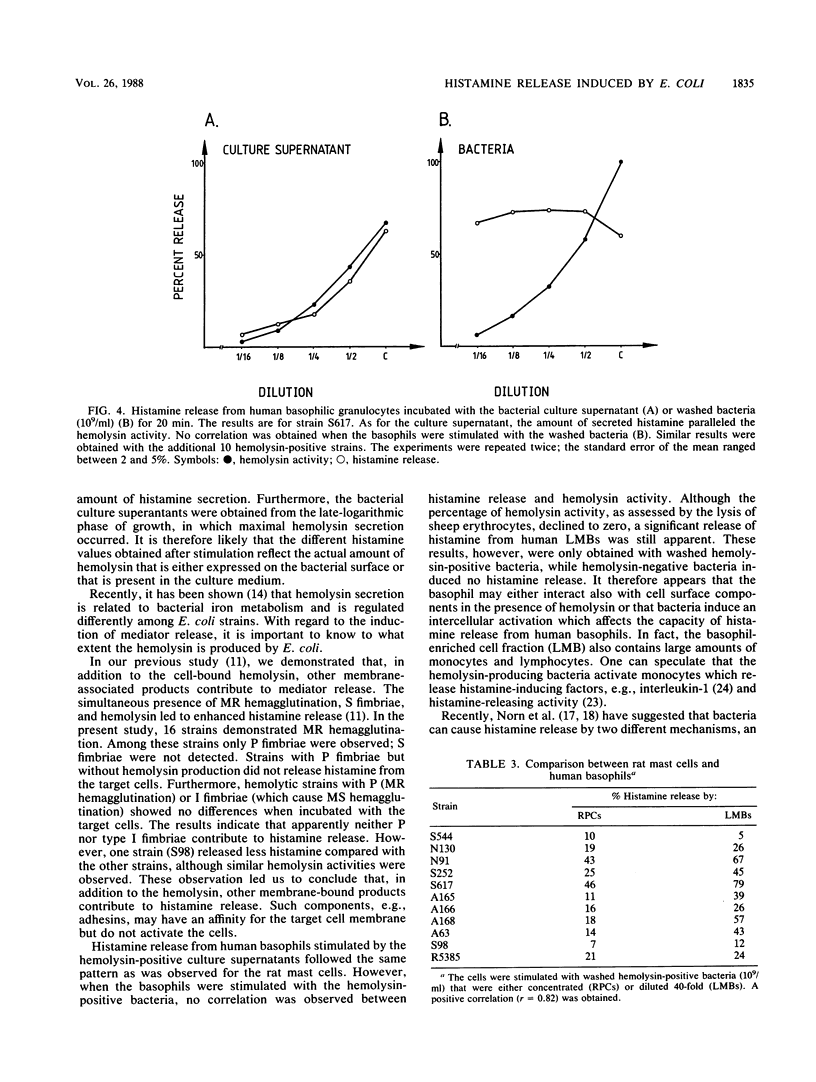
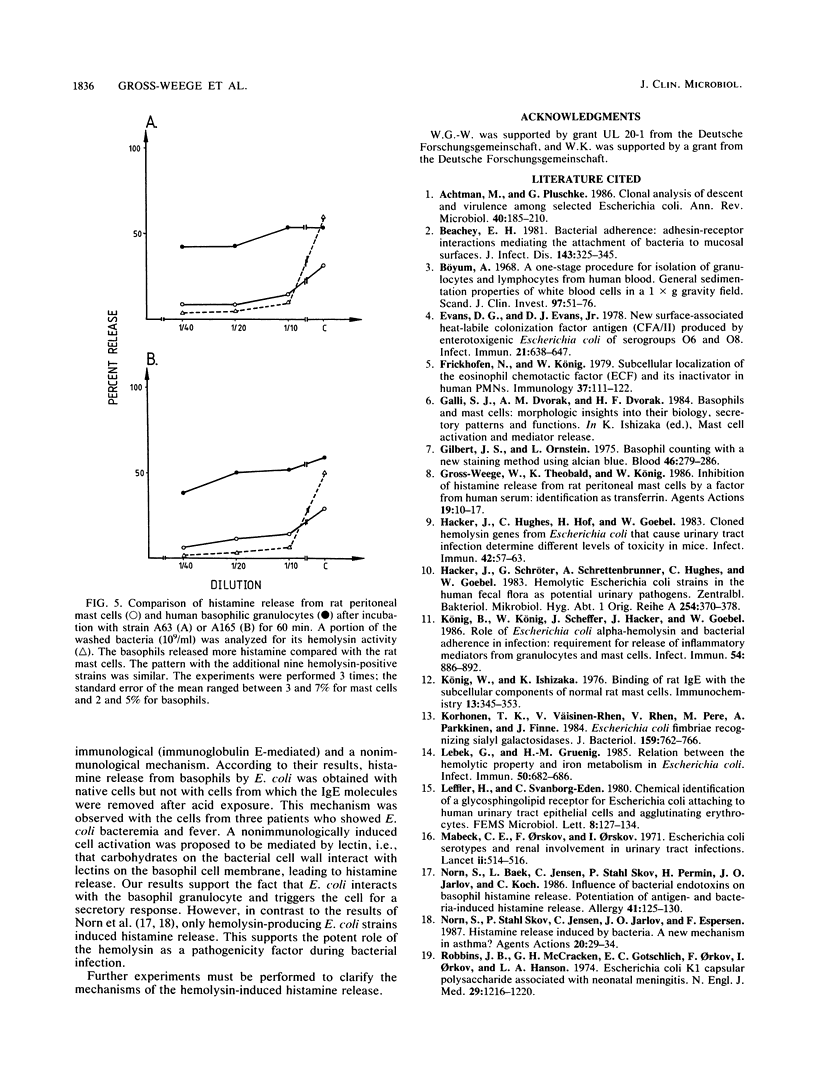
We investigated the role of 27 disease-relevant Escherichia coli strains isolated from humans in the induction of histamine release from rat peritoneal mast cells and human basophilic granulocytes. Our data indicated that only the hemolysin-positive (HLY+) bacteria and the hemolysin-positive culture supernatants induced histamine release. For the latter, the hemolysin activity determined the degree of histamine secretion. Incubation of the target cells with washed HLY+ bacteria revealed a different secretory response. For the rat mast cells, histamine release paralleled expression of hemolysin activity, with the exception of strain S98 (O75:K5:H- HLY+), which induced less histamine, although its hemolysin activity was relatively high. No correlation between histamine secretion and hemolysin activity was observed when human basophils were stimulated with the HLY+ bacteria. Large amounts of histamine were still released, even when the hemolysin activity declined to zero. Our results support the potent role of the E. coli hemolysin as a pathogenicity factor in bacterial infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Pluschke G. Clonal analysis of descent and virulence among selected Escherichia coli. Annu Rev Microbiol. 1986;40:185–210. doi: 10.1146/annurev.mi.40.100186.001153. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Böyum A. A one-stage procedure for isolation of granulocytes and lymphocytes from human blood. General sedimentation properties of white blood cells in a 1g gravity field. Scand J Clin Lab Invest Suppl. 1968;97:51–76. [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr New surface-associated heat-labile colonization factor antigen (CFA/II) produced by enterotoxigenic Escherichia coli of serogroups O6 and O8. Infect Immun. 1978 Aug;21(2):638–647. doi: 10.1128/iai.21.2.638-647.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frickhofen N., König W. Subcellular localization of the eosinophil chemotactic factor (ECF) and its inactivator in human polymorphonuclear leucocytes (PMN). Immunology. 1979 May;37(1):111–122. [PMC free article] [PubMed] [Google Scholar]
- Gilbert H. S., Ornstein L. Basophil counting with a new staining method using alcian blue. Blood. 1975 Aug;46(2):279–286. [PubMed] [Google Scholar]
- Gross-Weege W., Theobald K., König W. Inhibition of histamine release from rat peritoneal mast cells by a factor from human serum--identification as transferrin. Agents Actions. 1986 Oct;19(1-2):10–17. doi: 10.1007/BF01977250. [DOI] [PubMed] [Google Scholar]
- Hacker J., Hughes C., Hof H., Goebel W. Cloned hemolysin genes from Escherichia coli that cause urinary tract infection determine different levels of toxicity in mice. Infect Immun. 1983 Oct;42(1):57–63. doi: 10.1128/iai.42.1.57-63.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Schröter G., Schrettenbrunner A., Hughes C., Goebel W. Hemolytic Escherichia coli strains in the human fecal flora as potential urinary pathogens. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 May;254(3):370–378. [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen-Rhen V., Rhen M., Pere A., Parkkinen J., Finne J. Escherichia coli fimbriae recognizing sialyl galactosides. J Bacteriol. 1984 Aug;159(2):762–766. doi: 10.1128/jb.159.2.762-766.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König B., König W., Scheffer J., Hacker J., Goebel W. Role of Escherichia coli alpha-hemolysin and bacterial adherence in infection: requirement for release of inflammatory mediators from granulocytes and mast cells. Infect Immun. 1986 Dec;54(3):886–892. doi: 10.1128/iai.54.3.886-892.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König W., Ishizaka K. Binding of rat IgE with the subcellular components of normal rat mast cells. Immunochemistry. 1976 Apr;13(4):345–353. doi: 10.1016/0019-2791(76)90346-3. [DOI] [PubMed] [Google Scholar]
- Lebek G., Gruenig H. M. Relation between the hemolytic property and iron metabolism in Escherichia coli. Infect Immun. 1985 Dec;50(3):682–686. doi: 10.1128/iai.50.3.682-686.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norn S., Baek L., Jensen C., Skov P. S., Permin H., Jarløv J. O., Koch C. Influence of bacterial endotoxins on basophil histamine release. Potentiation of antigen- and bacteria-induced histamine release. Allergy. 1986 Feb;41(2):125–130. doi: 10.1111/j.1398-9995.1986.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Norn S., Skov P. S., Jensen C., Jarløv J. O., Espersen F. Histamine release induced by bacteria. A new mechanism in asthma? Agents Actions. 1987 Feb;20(1-2):29–34. doi: 10.1007/BF01965622. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., McCracken G. H., Jr, Gotschlich E. C., Orskov F., Orskov I., Hanson L. A. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974 May 30;290(22):1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- Sarff L. D., McCracken G. H., Schiffer M. S., Glode M. P., Robbins J. B., Orskov I., Orskov F. Epidemiology of Escherichia coli K1 in healthy and diseased newborns. Lancet. 1975 May 17;1(7916):1099–1104. doi: 10.1016/s0140-6736(75)92496-4. [DOI] [PubMed] [Google Scholar]
- Scheffer J., König W., Hacker J., Goebel W. Bacterial adherence and hemolysin production from Escherichia coli induces histamine and leukotriene release from various cells. Infect Immun. 1985 Oct;50(1):271–278. doi: 10.1128/iai.50.1.271-278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer J., Vosbeck K., König W. Induction of inflammatory mediators from human polymorphonuclear granulocytes and rat mast cells by haemolysin-positive and -negative E. coli strains with different adhesins. Immunology. 1986 Dec;59(4):541–548. [PMC free article] [PubMed] [Google Scholar]
- Schulman E. S., Liu M. C., Proud D., MacGlashan D. W., Jr, Lichtenstein L. M., Plaut M. Human lung macrophages induce histamine release from basophils and mast cells. Am Rev Respir Dis. 1985 Feb;131(2):230–235. doi: 10.1164/arrd.1985.131.2.230. [DOI] [PubMed] [Google Scholar]
- Subramanian N., Bray M. A. Interleukin 1 releases histamine from human basophils and mast cells in vitro. J Immunol. 1987 Jan 1;138(1):271–275. [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]


