Abstract
The majority of the genome in animals and plants is transcribed in a developmentally regulated manner to produce large numbers of non–protein-coding RNAs (ncRNAs), whose incidence increases with developmental complexity. There is growing evidence that these transcripts are functional, particularly in the regulation of epigenetic processes, leading to the suggestion that they compose a hitherto hidden layer of genomic programming in humans and other complex organisms. However, to date, very few have been identified in genetic screens. Here I show that this is explicable by an historic emphasis, both phenotypically and technically, on mutations in protein-coding sequences, and by presumptions about the nature of regulatory mutations. Most variations in regulatory sequences produce relatively subtle phenotypic changes, in contrast to mutations in protein-coding sequences that frequently cause catastrophic component failure. Until recently, most mapping projects have focused on protein-coding sequences, and the limited number of identified regulatory mutations have been interpreted as affecting conventional cis-acting promoter and enhancer elements, although these regions are often themselves transcribed. Moreover, ncRNA-directed regulatory circuits underpin most, if not all, complex genetic phenomena in eukaryotes, including RNA interference-related processes such as transcriptional and post-transcriptional gene silencing, position effect variegation, hybrid dysgenesis, chromosome dosage compensation, parental imprinting and allelic exclusion, paramutation, and possibly transvection and transinduction. The next frontier is the identification and functional characterization of the myriad sequence variations that influence quantitative traits, disease susceptibility, and other complex characteristics, which are being shown by genome-wide association studies to lie mostly in noncoding, presumably regulatory, regions. There is every possibility that many of these variations will alter the interactions between regulatory RNAs and their targets, a prospect that should be borne in mind in future functional analyses.
Introduction
Genome sequencing projects have shown that the numbers of protein-coding genes and the extent of protein-coding sequences do not change appreciably across the vertebrates nor indeed across the metazoa as a whole, despite large differences in developmental complexity [1]. On the other hand, the extent of non–protein-coding intronic and intergenic sequences in genomes does increase with developmental complexity, suggesting that these sequences may contain increasingly elaborate regulatory information [1].
In recent years it has also become evident that the vast majority of the mammalian genome, and that of other complex organisms, is transcribed, apparently in a developmentally regulated manner, to produce large numbers of ncRNAs that are antisense, intergenic, interleaved, or overlapping with protein-coding genes [2]–[5]. In addition, there are increasing reports (Figure 1) of the functionality of individual ncRNAs in mammals (Table 1), other animals (see, e.g., [6]–[8]), plants (e.g., [9],[10]), and fungi (e.g., [11]), particularly in relation to developmental processes [12]. These include the involvement of ncRNAs in the regulation of the expression of homeotic genes [7],[13], oncogenes [14], and metabolic genes [15], as well as in the regulation of skeletal development [16], eye development [17], epithelial-to-mesenchymal transition [18], and subcellular structures [19]–[22], among many others (for reviews and additional examples, see [4], [12], [23]–[25]).
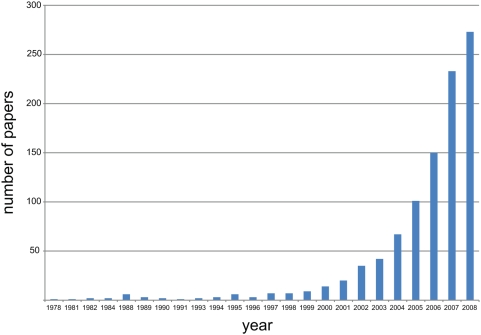
Figure 1. The recent rise in papers on ncRNAs.
The number of indexed Medline entries with the words “non-coding RNA”, “noncoding RNA”, “non-protein-coding RNA” or “ncRNA” in the title, abstract or keywords is plotted per year. Data courtesy of Ryan J. Taft.
Table 1. Examples of functional mammalian noncoding RNAs.
| Name | Characteristics | Function | Experimental Methodologya | References |
| Air | 108 kb; transcribed from an antisense promoter located in intron 2 of Igf2r | Regulates genomic imprinting of a cluster of autosomal genes on mouse chromosome 17 | Mutagenesis; FISH; ChIP; RNA-IP | [156],[189],[196] |
| BACE1AS | ∼2 kb; transcribed antisense to beta-secretase-1 (BACE1) gene, elevated in Alzheimer disease | Regulates BACE1 expression in vitro and in vivo, influences amyloid-beta 1–42 levels | shRNA and siRNA knockdown | [197] |
| BC1 | 152 nt; expressed by a specific subset of neurons in the central and peripheral nervous system; dendritic location | Affects exploratory behaviour and anxiety, represses translation by targeting initiation factor 4A helicase | In vivo knockout, biochemical analyses | [100],[198] |
| Borg | ∼2.8 kb; induced by bone morphogenic proteins (BMPs) and osteogenic proteins | Regulates BMP-induced differentiation of C2C12 cells into osteoblastic cells | Antisense knockdown | [199] |
| CCND1 associated ncRNAs | Unspecified sizes; transcribed from the promoter region of the cyclin D (CCND1) gene; induced by DNA damage signals | Allosterically modifies the RNA-binding protein TLS (“translocated in liposarcoma”), leading to inhibition of CREB-binding protein and histone acetyltransferase activities to repress cyclin D1 | ChIP; RNA-IP; siRNA knockdown | [200] |
| CUDR | ∼2.2 kb; up-regulated in a doxorubicin-resistant human squamous carcinoma | Regulates drug sensitivity, cellular transformation and apoptosis | Overexpression | [201] |
| EGO | ∼1.0 and 1.7 kb; highly expressed in human bone marrow and in eosinophil development | Regulates expression of myelin basic protein and eosinophil-derived neurotoxin mRNAs | siRNA knockdown | [202] |
| DHFR upstream | Unspecified size; transcribed upstream of the dihydrofolate reductase (DHFR) gene | Regulates DHFR expression by formation of triple helix in the DHFR promoter | siRNA knockdown,; ChIP; RNA-IP, other | [15] |
| Evf-2 | ∼3.8 kb; antisense to Dlx6; developmentally regulated, expressed in the ventral forebrain | Cooperates with Dlx-2 in vivo to increase the transcriptional activity of the Dlx-5/6 enhancer in a target and homeodomain-specific manner | Overexpression; siRNA knockdown; mutagenesis; ChIP | [154] |
| Gadd7 | 754 nt; induced by lipotoxic-stress | Regulates lipid-induced oxidative and ER stress | Mutagenesis; shRNA knockdown | [203] |
| GAS5 | ∼7 kb; growth arrest-specific transcript, multiple splice isoforms, encodes several snoRNAs in its introns, down-regulated in breast cancer | Controls apoptosis and the cell cycle in lymphocytes | siRNA knockdown; overexpression | [204] |
| H19 | 2.3 kb; imprinted (maternal allele active) at the Igf2 locus; strongly expressed during embryogenesis; up-regulated in various tumours | Complex functions, influences growth by way of a cis control on Igf2 expression implicated as both a tumour suppressor and an oncogene | siRNA knockdown; overexpression; in vivo knockout | [205]–[207] |
| HOTAIR | 2.2 kb; transcribed from the HOXC locus from a position intergenic and antisense to the flanking HOXC11 and HOXC12 genes | Epigenetically silences gene expression at the HOXD locus | siRNA knockdown | [13] |
| HOTAIRM1 | 483 nt; specific to the myeloid lineage | Involved in RA-induced expression of HOXA1 and HOXA4 during myeloid differentiation, and induction of myeloid differentiation genes CD11b and CD18 | shRNA knockdown | [49] |
| Hsr1 | ∼600 nt; ubiquitously expressed | Required for heat shock response | siRNA and antisense knockdown | [208] |
| IGS RNAs | 150–300 nt; originate from the intergenic spacers (IGS) that separates rRNA genes; bind to the chromatin remodelling complex NoRC | Required for the nucleolar localization of NoRC and epigenetic control of the rDNA locus | Mutagenesis; antisense knockdown | [209] |
| Kcnq1ot1 | 91 kb; paternally expressed from Kcnq1 imprinting control region | Mediates organization of a lineage-specific nuclear domain involved in epigenetic silencing of the Kcnq1 imprinting control region | Mutagenesis; ChIP; RNA/DNA FISH | [22],[155],[157],[210] |
| Khps1a | 1,290 nt; originates from the CpG island and overlaps a tissue-dependent differentially methylated region of Sphk1 | Regulates DNA methylation in the tissue-dependent differentially methylated region of Sphk1 | Overexpression | [211] |
| lincENC1 | Size unspecified; ∼181 kb from Enc1 | Regulates cell proliferation in embryonal stem cells | shRNA knockdown | [32] |
| MEG3 | ∼1.6 kb; maternally expressed from the Dlk1-Gtl2 imprinted locus | Regulates p53 expression, inhibits cell proliferation in the absence of p53 | Overexpression, mutagenesis | [192] |
| MEN ε/β (Neat1) | ∼3.5 and ∼23 kb; up-regulated upon muscle differentiation; transcribed from the multiple endocrine neoplasia 1 (MEN1) locus | Required for the structural integrity of nuclear paraspeckles | Antisense, siRNA knockdown; overexpression; FISH | [19]–[21] |
| ncR-uPAR | ∼350 bp; upstream of human protease-activated receptor-1 (PAR-1) gene | Up-regulates Par-1 promoter | Overexpression | [212] |
| Nkx2.2AS | 4.3 kb; antisense to Nkx2, preferentially expressed in the nervous system | Enhances oligodendrocytic differentiation | Overexpression | [213] |
| NRON | ∼2.7 kb; enriched in placenta, muscle, and lymphoid tissues | Modulates NFAT nuclear trafficking | siRNA knockdown | [214] |
| p15AS | 38.4 kb; antisense to the tumour suppressor gene p15 | Epigenetically silences p15 expression | Overexpression | [14] |
| PCGEM1 | 1,643 nt; prostate tissue-specific and prostate cancer-associated | Inhibits apoptosis induced by doxorubicin | Overexpression | [215] |
| PRINS | ∼3.6 kb; elevated expression in psoriatic epidermis; regulated by the proliferation and differentiation state of keratinocytes. | Required for cell viability after serum starvation | siRNA knockdown | [42] |
| PINC | ∼1 and 1.6 kb; developmentally regulated, expressed in mammary gland | Performs dual roles in cell survival and regulation of cell-cycle progression | siRNA knockdown; FISH | [216] |
| RepA | ∼1.6 kb; internal to Xist | Recruits the Polycomb complex, PRC2, to the inactive X chromosome, with Ezh2 serving as the RNA binding subunit | ChIP; FISH; overexpression; shRNA knockdown | [128] |
| SAF | 1.5 kb; transcribed from the opposite strand of intron 1 of the human Fas gene | Regulates Fas-mediated but not TNF-alpha-mediated apoptosis | Overexpression | [217] |
| SatIII | Various sizes up to >1.4 kb; transcribed from satellite DNA associated with, and localized in, nuclear stress bodies | Mediates recruitment of RNA processing factors to, and formation of, nuclear stress bodies | Antisense and siRNA knockdown | [218] |
| SCA8 | Predicted gene product underpinning the triplet repeat expansion-induced neurodegenerative disease Spinocerebellar Ataxia 8 | Induces late-onset, progressive neurodegeneration in the Drosophila retina; associates with the RNA binding protein staufen | Ectopic expression in Drosophila; genetic modifier screen | [39] |
| TERRA / TelRNAs | Various sizes; transcribed from and associated with telomeres; contain UUAGGG repeats | May form G-quartet structures with telomere DNA; inhibit telomerase activity | RNA FISH; oligo-nucleotide inhibition | [219],[220] |
| Tsix | ∼40 kb; antisense to Xist | Epigenetically silences Xist expression by inhibiting RepA recruitment of polycomb complexes to maintain the active X chromosome in females | Mutagenesis | [128], [221]–[223] |
| TUG1 | 6.7 kb; expressed in the developing retina and brain | Required for the proper formation of photoreceptors in the developing rodent retina | shRNA knockdown | [17] |
| UCA1 | 1,442 nt; expressed in embryonic development and bladder cancer | Enhances tumorigenic behaviour of bladder cancer cells in vitro and in vivo | Overexpression | [224] |
| Xist | ∼17 kb; mosaic expression in females | Epigenetically controls dosage compensation by silencing one of the two X chromosomes | Mutagenesis | for recent review see [99] |
| Y RNAs | 83–112 nt; up-regulated in cancer; bound by Ro autoantigen | Regulate cell DNA replication and cell proliferation | siRNA knockdown | [47],[225] |
| Zeb2NAT | >680 nt; antisense to Zeb2, a transcriptional repressor of E-cadherin | Regulates splicing of the Zeb2 5′ UTR | Overexpression | [226] |
| Zfh-5AS | ∼10 kb; expressed in particular regions of the developing brain | Regulates expression of the transcription factor Zfh-5 mRNA | Mutagenesis | [227] |
This list is not exhaustive, and there are other examples of functional ncRNAs in mammals (see e.g. [228]) as well as of regulatory and structural ncRNAs in other animals, plants, fungi (see e.g. [6]–[11]) and bacteria [229].
Abbreviations used are: siRNA, short interfering RNA; shRNA, short hairpin RNA; FISH, fluorescence in situ hybridization; ChIP, chromatin immunoprecipitation; RNA-IP, immunoprecipitation of RNA associated with particular proteins.
There is also compelling genome-wide evidence that the large numbers of identified but as yet unstudied intronic, intergenic, and antisense ncRNAs have intrinsic indices of functionality (Table 2), as indicated by the following: (i) the conservation of their promoters, splice junctions, exons, predicted structures, genomic position, and expression patterns [2], [26]–[36]; (ii) their dynamic expression and alternative splicing during differentiation [13],[31],[32]; (iii) their altered expression or splicing patterns in cancer and other diseases [37]–[49]; (iv) their association with particular chromatin signatures that are indicative of actively transcribed genes [31],[32]; (v) their regulation by key morphogens and transcription factors [31],[32],[49],[50]; and (vi) their tissue- and cell-specific expression patterns and subcellular localization [16], [17], [19]–[22], [49], [51]–[56].
Table 2. Indices of the functionality of ncRNAs.
| Feature | References |
| Conservation of promoters | [2],[27],[32] |
| Conservation of splice junctions | [27] |
| Conservation of sequence | [26],[27],[32] |
| Conservation of genomic position | [31],[33],[34] |
| Conservation of secondary structure | [28]–[30] |
| Positive selection | [230] |
| Conservation of expression | [35],[36] |
| Dynamic expression and alternative splicing | [13],[31],[32] |
| Altered expression or splicing in cancer and other diseases | [37]–[49] |
| Association with particular chromatin signatures | [31],[32] |
| Regulation by morphogens and transcription factors | [31],[32],[49],[50] |
| Tissue- and cell-specific expression patterns | [16], [17], [19]–[22], [49], [51]–[56] |
| Specific subcellular localization | [19]–[22],[52],[56] |
Indeed, a recent study of over 1,300 mouse ncRNAs showed that almost half of them exhibit precise expression patterns in different parts of the brain, including different subregions of the hippocampus, olfactory bulb, neocortex, and cerebellum [52]. While many ncRNAs, like other regulatory sequences (see below), appear to be evolving quickly, including those with well-validated functions [26], most retain conserved patches within them [26],[32], and some may be under positive selection, especially in the brain [57]. At least some of these RNAs, including intronic RNAs, are precursors for small regulatory RNAs like microRNAs (miRNAs), small interfering RNAs (siRNAs), piwi-interacting RNAs (piRNAs), and small nucleolar RNAs (snoRNAs) [58]–[68]. Many miRNAs are highly conserved from nematodes to humans, whereas others are primate-specific, and their full repertoire remains to be determined [4],[69],[70].
It is widely accepted that animals have a relatively common set of protein-coding genes and that, notwithstanding lineage-specific innovations and splice variants, the primary basis of phenotypic, especially morphological, radiation and higher complexity has been the variation and expansion of the regulatory architecture that controls the deployment of these protein components and their isoforms during differentiation and development [71]. This regulatory architecture is generally more plastic than protein-coding sequences that are highly constrained by relatively strict structure-function relationships, which is reflected by the fact that regulatory sequences evolve at widely different rates [72]–[75]. These sequences range from promoter regions that have no recognizable sequence similarity yet direct orthologous patterns of gene expression between fishes and mammals [76] to highly conserved non-genic elements [77],[78], and “ultraconserved” sequences that have remained essentially unchanged over hundreds of millions of years of vertebrate evolution and appear to act as tissue-specific enhancers that regulate gene expression during development [79]–[82].
Regulatory sequences are also generally assumed to operate through their interactions with sequence-specific transcription factors and other regulatory proteins, but this assumption has been made in ignorance, until recently, of the extent of developmentally regulated transcription of ncRNAs from the genome, including many regions spanning enhancers and promoters (see, e.g., [45], [83]–[86]). The possibility is therefore that the genomes of mammals and other complex organisms encode a large repertoire of regulatory RNAs [4]. Indeed, the case has been made that a much higher degree of regulatory sophistication, aided by the co-option of the considerable powers of RNA to transmit sequence-specific information, was a prerequisite for the evolution of developmentally complex organisms [87],[88], and that many of these RNAs may be involved in the regulation of developmental processes, including the epigenetic trajectories that underpin them, for which there is increasing evidence [12],[89].
However, if these ncRNAs are functional and important in developmental and physiological processes, why have so few been identified in genetic screens to date? Here I outline the emerging evidence for ncRNA involvement in key molecular genetic phenomena and in specific functions and phenotypes. I also outline the expectational, perceptual, and practical factors that may collectively account for the low genetic visibility of individual ncRNAs. Awareness of these factors and the possibility that the structure of the genomic programming of complex organisms is different from our current understanding may lead to the increased recognition of ncRNAs in genetic analyses, assisted by the emerging fusion of genetics, genomics, and systems biology.
Phenotypic Impact
The ability to detect a relevant mutation or variation is dependent on the sensitivity of the phenotypic screen. Mutations in protein-coding sequences usually give severely compromised (i.e., obvious) phenotypes, whereas those in regulatory sequences often do not. Proteins are the key structural and functional analogue components of cells, and the loss of their function is often disastrous, leading in many cases to obvious defects, and in some cases to embryonic lethality. Mutations in generic transcription factors and other “regulatory” proteins are included, and their loss causes pleiotropic effects on gene expression at many loci and plays an important role in the molecular etiology of cancer [71],[90],[91]. This is in contrast to regulatory sequences, which, when damaged, may only affect a small part of the network, with more restricted and subtle consequences, often referred to as quantitative trait variations. Indeed the use of the word “mutation”, as opposed to “variation”, reflects an inherent bias in the identification of genetic factors that influence phenotype in animals, with those exhibiting strong effects understandably having taken precedence over those that do not, both perceptually and practically. Consistent with this, until recently, there were relatively few regulatory mutations identified among the catalogue of known human mutations that are associated with overt genetic disease.
There is, of course, a wide spectrum of effects of both coding and noncoding mutations (Figure 2), and there are exceptions to the rule in both directions. Loss-of-function mutations in some protein-coding genes have mild effects [92], as exemplified by as knockouts of the mammalian genes encoding calbindin D9k [93] and C/EBPdelta [94],[95], and the significant number of yeast genes that show no observable phenotype. Reciprocally, knockouts of some highly conserved miRNAs, many of which have multiple targets, give strong phenotypes [96]–[98], even though very few such genes have been identified in genetic screens in Caenorhabditis elegans and Drosophila, and none have been identified in mice, despite the intensity of such screens (see below). Moreover, to date, no naturally arising mutations have been discovered in the Xist gene in either humans or mice, despite the central role that this ncRNA plays in embryogenesis and in X-chromosome dosage control in females [99], possibly because such mutations are lethal.
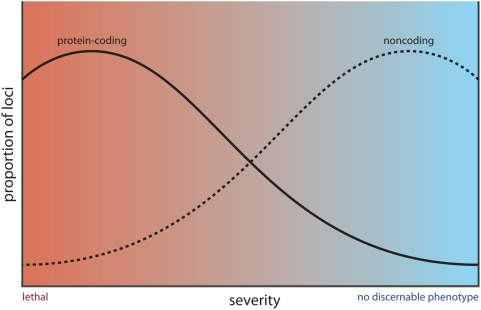
Figure 2. The contrasting effects of mutations in protein-coding and regulatory sequences.
A conceptual diagram of the spectrum of phenotypic effects of mutations in sequences encoding proteins and other analogue components of cells (continuous line) versus variations in non-coding sequences that specify regulatory interactions (dashed line).
On the other hand, the potential subtlety of mutations in ncRNAs, even when they are targeted by reverse genetics, is illustrated by the case of BC1, which is expressed in synaptodendritic domains of neurons in rodents. Knockout of this transcript produces no obvious physical or neurological abnormality, and the mutant mice were initially indistinguishable from wild type, but were subsequently found to have reduced exploratory behaviour and consequently a higher mortality in field experiments [100]. Thus this ncRNA causes a subtle behavioural phenotype that is invisible in the cage, and would escape detection in superficial forward genetic screens, but is almost certainly strongly disadvantageous in the wild. Similarly, deletions or insertions in some ultraconserved enhancers yield no discernable abnormality [101],[102], despite the fact that these sequences are clearly under intense selection [103], suggesting insensitivity of phenotypic screens in captivity or redundancy in the regulatory architecture (perhaps associated with developmental robustness) that we have yet to understand.
Monogenic Diseases and High Penetrance Mutations
The generally stronger effects of protein-coding mutations leads to a sampling bias, in that more severe phenotypes are not only more easily discerned but have also been more likely to attract further study, both in medical contexts and in model organisms. In medicine, mutations involving catastrophic component damage have been traditionally referred to as “monogenic diseases” and were the primary targets of study in the pioneering days of human genomics, just a decade or two ago, when the protein-coding genes underpinning cystic fibrosis, Huntington disease, and Duchenne muscular dystrophy, among others, were identified by positional mapping and cloning approaches. Thus, in humans, mutation mapping is still both a young science and extraordinarily difficult due to the sheer complexity of the genome, which naturally led to an initial focus on severe loss-of-function diseases that exhibit simple inheritance patterns with high penetrance (“single gene–large effect”) making them amenable to identification. This is now changing with the increasing availability of human genome sequences, and the application of genome-wide association (GWA) studies to the mapping of the genetic components of complex traits, wherein lies the majority of the health burden and the majority of the interesting phenotypic differences between individuals and species.
Complex Traits and GWA Studies
Indeed, functional classification of sequence variations that have been identified by GWA studies to be associated with complex traits, albeit still strongly focused on those of medical importance, shows that the vast majority of variations are located in noncoding regions [104],[105]. In most cases, the causative mutations have yet to be defined, and their mechanistic basis is unknown—especially whether they affect cis-acting binding sites for regulatory proteins or the function or expression of regulatory RNAs. One good candidate for the latter is the ncRNA ANRIL, which lies antisense to CDKN2A and traverses a noncoding region centromeric to CDKN2A, a region implicated in a range of complex diseases including cancer, type 2 diabetes, periodontitis, and coronary heart disease [106]–[112]. Perplexingly, however, those variants mapped by GWA studies to date account for only a small proportion of genetic variation in disease or quantitative traits [113]. These traits are clearly multifactorial and may be affected by rare variants with strong effects that have yet to be recognized.
The identification of the sequence changes that directly underpin quantitative trait variations has thus far been possible, or at least achieved, only in well-structured pedigrees in plants and animals. In the few cases where such quantitative trait loci (QTLs) have been mapped to completion, most have been found to be located in noncoding sequences, specifically: (i) regulatory sequences in promoters and distal enhancers (e.g., the “teosinte branched1” mutation affecting branching and inflorescence in maize [114]); (ii) 3′ untranslated regions (UTRs) (e.g., those underlying Tourette's syndrome [115], and muscular hypertrophy in sheep [116]; see also below); (iii) introns (e.g., a QTL affecting muscle growth in domestic pigs [117]); or (iv) intergenic sequences of unknown transcriptional status (e.g., the “callipyge” mutation causing posterior muscular hypertrophy in sheep [118]). The latter occurs in an imprinted locus and affects the expression of a number of protein-coding and ncRNA genes [119] associated with an unusual genetic phenomena termed “polar overdominance” [120], which may also occur in humans [121]. While these mutations are reasonably assumed to be regulatory in nature, their mechanistic basis has not been determined, although in the latter case, there is some evidence for the involvement of trans-acting miRNAs [119],[122]. In addition, linkage studies in a large family have recently identified the ncRNA AK023948 as a candidate susceptibility gene for papillary thyroid carcinoma [123].
Type of Mutation and Sensitivity of the Model System
The nature of the organism under study and the type of mutations also affect the outcome of genetic screens—relevant single-base mutations are not only harder to identify than insertions/deletions, especially in mammalian genomes and even in inbred mice, but also have milder effects on regulatory sequences.
Most mutations induced by ENU mutagenesis involve single-base changes, which are also, along with small indels and copy number variations, the most common type of natural variation in humans and other mammals, where few regulatory mutations have yet been identified. Whereas a nonsynonymous mutation in a protein-coding sequence can have severe effects on the structure and function of the protein, many regulatory sequences have loose consensus sequences, and variations in them, as noted already, may have subtle effects and go unnoticed, especially in superficial phenotypic screens.
On the other hand, insertions and deletions dominate mutational screens in Drosophila, and a large number map to noncoding intergenic and intronic regions. This is exemplified by the intensively studied bithorax complex, where there are not only mutations known in the coding sequences for the homeotic protein Ultrabithorax (Ubx), but also in upstream (bithoraxoid or bxd) and intronic (Contrabithorax or Cbx and anterobithorax or abx) sequences [124], which contain conserved blocks within them [125]–[127]. Such noncoding mutations are interpreted as affecting orthodox cis-acting enhancer sequences (i.e., those that bind cis-acting regulatory proteins), despite the fact that (for example) bxd mutations fall within a region that is transcribed during early embryogenesis into a complex set of short polyadenylated RNAs with no coding potential. These RNAs arise by alternative splicing of at least 11 exons derived from a 26-kb primary transcript [126]. Moreover, these regulatory regions involve interaction with Polycomb-group and Trithorax-group proteins, which are increasingly implicated as being directed to their sites of action by ncRNAs [7],[13],[128] (see below).
Similarly the iab regulatory elements of the bithorax complex that control the expression of abdominal-A and Abdominal-B, and consequently the identities of the 2nd–9th abdominal segments, are transcribed into ncRNAs in a spatially ordered pattern [129]. It has also recently been shown that 231 ncRNAs are expressed from the four human HOX loci in a spatially and temporally ordered progression along developmental axes, one of which (termed HOTAIR) from the HOXC locus controls expression of the HOXD locus in trans, as shown by siRNA-mediated knockdown experiments [13]. None of these ncRNAs has yet been specifically associated with a genetic variant in mammals, although in Drosophila there are many homeotic mutations that lie in regions encompassed by ncRNAs [12],[124], and Drosophila geneticists were clearly intrigued by them [130]. Moreover, as pointed out by Rinn et al. [13], the existence of such transacting regulatory ncRNAs may explain the observation that the deletion of the entire HOXC locus exhibits a milder phenotype than the deletion of individual HOXC genes [131].
Despite the now known importance of miRNAs in the control of gene expression [67],[132], only four miRNA loci have been identified in intense genetic screens in C. elegans and Drosophila, and none in mammals. This may be partly due to the fact that C. elegans is hermaphroditic and naturally driven to homozygosity in individual isolates, without the need for laborious back-crossing, and flies are routinely bred to homozygosity in mutational screens, making screening of recessive mutations far more efficient than in mice. There was also an element of serendipity, in that the first identified miRNA locus, lin-4, expressed a small RNA whose complementary sequence was present in multiple copies in the 3′ UTR of its target gene, lin-14, and thus was relatively easy to pinpoint [133],[134], which also applied in the subsequent case of let-7 [135].
Following the discovery of miRNAs, Drosophila mutants of uncertain provenance that mapped in gain-of-function screens to noncoding regions were re-analysed, and one that regulates growth [136], termed bantam, was identified to encode a miRNA [137]. The miRNAs let-7 [135] and lsy-6 [138] were also identified genetically in C. elegans, not in other organisms, despite the former being not only highly conserved in sequence and expression pattern throughout metazoan evolution [139], but also fundamental to normal and abnormal developmental processes [140]–[142], as are many other miRNAs [143]. On the other hand, lsy-6 is expressed in only a few cells [144], and has only rarely turned up in deep sequencing libraries. Subsequently most known miRNAs, of which there are hundreds in mammals, and later piRNAs, have been identified by biochemical not genetic means. In view of the lsy-6 example and the clearly incomplete sampling of the small RNA transcriptome, even using deep sequencing [70], there are likely to be many more.
Expectations and Interpretations
There has also been a strong expectation that mutations that have phenotypic effects will map to protein-coding genes or cis-regulatory elements that interact with regulatory proteins. The former has influenced the practical strategies for mutation searching, in terms of a focus on exon scanning of candidate genes (see below), and the latter has influenced the interpretation of regulatory variations, although in only a few cases has the mechanistic basis been determined [71],[145]. Some mutations map to gene “deserts” (see, e.g., [146]), and while it is conceivable that they affect distal enhancers (see, e.g., [147]), it is interesting and relevant to note that there is good evidence that enhancers and other regulatory sequences are transcribed into ncRNAs in the cells in which they are active [45], [83]–[86], and hence may act in part via ncRNAs.
Transvection and Locally Acting ncRNAs
Many loci, such as the bithorax complex referred to earlier, exhibit a genetic phenomenon called “transvection,” whereby a wild-type regulatory region upstream of a defective protein-coding sequence on one chromosome can rescue a relatively normal phenotype, when it is combined with a mutant regulatory region linked to a wild-type protein-coding sequence on the homologous chromosome (both of which give mutant phenotypes when homozygous) [130]. This phenomenon is well documented in Drosophila but appears to occur in most animals and has been interpreted as a physical cross-talk between functional cis-acting promoters or enhancers on one chromosome to engender transcription of adjacent protein-coding genes on the other, since the effect is usually pairing-dependent and lost when the regulatory and protein-coding sequences are separated to nonsyntenic positions in the genome [130],[148]. However, this is not always the case—at some loci, transvection between regulatory elements and protein-coding sequences can operate over large distances (even between different chromosomes) [149]–[152], suggesting the involvement of a trans-acting signal. Moreover, many promoter elements that exhibit transvection are transcribed into ncRNAs, and transvection is altered in Polycomb and zeste mutants [125]–[127],[130], indicating that epigenetic processes (which may be regulated by ncRNAs, see below) are involved. Taken together, these observations raise the possibility that transvection is mediated by trans-acting RNAs [153], in which case the observed cross-complementation may occur simply as a result of a compound heterozygosity between a regulatory ncRNA locus and a nearby protein-coding locus whose expression is controlled by it.
In support of this proposition, there is now rapidly emerging evidence that many ncRNAs derived from either same or opposite strands act locally to regulate the epigenetic status and expression of nearby protein-coding genes, often involving the recruitment of chromatin-activator or repressor complexes [7], [13]–[16], [89], [154]–[157], with sense-antisense pairs in some cases being the substrate for the generation of siRNAs [63]–[65],[68]. Moreover the many deletion studies of gene promoter regions to define regulatory sequences have almost always assumed, physically and mechanistically, that resultant changes to expression patterns are due to the loss of cis-acting protein binding sites rather than deletions in the same or opposite strand ncRNAs that frequently traverse and are expressed from the same region. The complexity of these relationships is illustrated by the examples of the ncRNA DLeu2 (deleted in lymphocytic leukemia 2), which has multiple splice variants and lies antisense to genes in a region deleted in various malignancies [158], and the ncRNA ANRIL referred to earlier. Thus the interpretation of the mechanisms by which such mutations operate remains not only an open question but a difficult problem to disentangle, given the complex interlacing and overlapping coding and noncoding transcripts, and splice variants thereof, that are expressed from many loci in different cells and tissues [2],[3].
Transinduction, Ectopic Expression and Gene Knockouts
RNA is also implicated in a curious genetic phenomenon called “transinduction,” whereby transient transfection of a β-globin gene induces transcription of the “locus control region” and intergenic regions at the chromosomal β-globin locus in non-erythroid cell lines. This effect is dependent on transcription of the globin gene from the transfected plasmid and its association with the endogenous β-globin locus, but not on protein expression, and therefore is RNA-mediated [83], although the responsible sequences have not been mapped. Indeed, the general assumption that mRNA is simply an intermediate between gene and protein, albeit with cis-acting regulatory elements, may be incorrect, and there may be a false dichotomy between coding and noncoding RNA [159]. This is indicated by the complexity of overlapping sense and antisense coding and noncoding transcripts from most genomic loci [2]–[5] and evidence that protein-coding sequences are under constraint not only at the amino acid sequence level, but also within their RNA sequence [160]. Moreover, given that many gene knockout studies concomitantly delete potential sources of regulatory ncRNAs such as introns (given that many miRNAs and all snoRNAs are sourced from introns) and antisense transcribed sequences, all aspects of the observed phenotypes cannot be unequivocally or solely ascribed to the loss of the protein without complementation studies or more precise deletions that are rarely done for reasons of technical difficulty.
Technical Limitations
The focus on protein-coding sequences has been reinforced by a practical problem, especially in mammals. Mutation mapping studies using whole-genome scanning techniques usually have not pinpointed the causative mutation or variation, and the affected region may encompass one megabase or more. Until recently, comparative sequencing of such regions was not feasible, and in any case it can be very difficult to sort the relevant polymorphism from the considerable background variation in huge intergenic and intronic regions, especially in outbred human populations. In most circumstances, understandably, investigators have resorted to analysing the most plausible candidate genes (recently including those expressing noncoding transcripts or ESTs [158],[161]) usually involving scanning of known exons (and sometimes the immediate 5′ flanking promoter sequences and UTRs) in the region by PCR amplification, in the hope that they can identify the causative mutation in these locations, which in turn are the ones that are then reported in the literature. However, there are many informal reports of mapping studies that have not identified such exonic mutations, and which consequently lie in abeyance, including (as noted already) the large number of GWA studies that have mapped disease-associated variations to noncoding, presumably regulatory, regions [104]. A reasonable strategy for searching for the relevant variations in these regions may be to focus on sequences that exhibit evolutionary conservation and/or whose expression is altered [162]. Conversely, reverse genetic screens looking for phenotypes associated with ncRNAs might target conserved blocks within them and focus on tissues where they are known to be expressed.
Mutations Affecting trans-Acting Functions of mRNAs
As noted above, regulatory mutations have been identified in the 3′ UTRs of mRNAs, such as those underlying Tourette's syndrome [115] and muscular hypertrophy in sheep [116], which appear to involve gain- or loss-of-function of miRNA binding sites. Interestingly, however, a number of other reported 3′ UTR mutations do not appear to act in cis to regulate the expression of the associated mRNA, as is normally assumed, but rather in trans as ncRNAs. For example the 3′ UTR of prohibitin (in the absence of the associated protein-coding sequences) can inhibit cell cycle progression in one complementation group of breast cancer–derived cells that is characterized by naturally occurring mutations in the 3′ UTR, indicating that these sequences are in fact functioning, in part, as trans-acting ncRNAs [163],[164]. Similarly, the oogenesis defect observed in Drosophila oskar null mutants is rescued by the oskar 3′ UTR alone [165].
A trans-acting function for mRNA sequences, both coding and noncoding, may be more general that expected. For example, the introduction of cancer-associated silent point-mutations in p53 mRNA alters its binding to the protein Mdm2, which in turn alters p53 expression and function [166]. The 3′ UTRs of troponin I, tropomyosin, and α-cardiac actin have been shown to reactivate muscle-specific promoters in a differentiation-defective myoblast mutant, enhance the differentiation of wild-type muscle cells, and suppress the proliferation of fibroblasts independently of their normally associated protein-coding sequences [167]. Similarly, the 3′ UTRs of tropomysin [168] and ribonucleotide reductase [169] can suppress tumour formation, and the 3′ UTR of the DM protein kinase gene, which is involved in myotonic dystrophy, inhibits the differentiation of C2C12 myoblasts [170]. Moreover, many 3′ UTRs in mouse appear to be expressed separately from their mRNAs in a developmentally regulated manner [171].
There other examples of mutations in sequences encoding 3′ UTRs that do not act via the UTR. A single nucleotide polymorphism that determines susceptibility to an autoimmune thyroid disease occurs both within the 3′ UTR of the ZFAT gene (zinc-finger gene in AITD susceptibility region) and also within the promoter of an antisense transcript (SAS-ZFAT), and increases the expression of ZFAT not through increasing mRNA stability, but by repressing the expression of the antisense transcript [172].
Regulation of Complex Genetic Processes by ncRNAs
Apart from the general presumption that most ncRNAs will be involved in regulation, variations in which will often have, individually, subtle effects on phenotype, there is, in fact, general evidence of their positive genetic signatures, as ncRNAs underpin most, if not all, complex genetic processes in the higher organisms. These include RNA interference-related phenomena such as co-suppression and transcriptional gene silencing [132], [173]–[176], as well as position effect variegation [177],[178], hybrid dysgenesis [179], parental imprinting, X-chromosome dosage compensation and allelic exclusion [180], germ cell reprogramming [181], and paramutation [182],[183], all of which involve epigenetic processes. Indeed, as noted already, there is increasing evidence that a major function of ncRNAs, both small and large, is the regulation of epigenetic memory through modifications to DNA and chromatin structure, involving the recruitment of DNA methyltransferases, histone-modifying enzymes, and chromatin remodelling complexes to their appropriate sites of action (including ncRNA genes themselves) in particular cells at particular stages of differentiation (for reviews, see [12],[89],[184]; also Table 1).
Examples of Mutations in ncRNAs
There are some known examples of mutations in ncRNAs, aside from those mentioned already, that give recognizable phenotypes or that are strongly implicated in altered phenotypic states. These include a triplet repeat expansion in the ncRNA SCA8, which causes the human neurodegenerative disease Spinocerebellar Ataxia 8 (which as a transgene can induce progressive retinal neurodegeneration in Drosophila) [39] and other examples of deleterious gain-of-function mutations in noncoding RNAs associated with diseases such as myotonic dystrophy [185], deletions encompassing ncRNA loci and alterations to ncRNA splicing patterns in various cancers [47],[106],[158],[161],[186],[187], and a SNP variant in an ncRNA MIAT that confers risk of myocardial infarction [188]. They also include many ncRNAs, including small nucleolar RNAs, that appear to be important in the mechanism of imprinting [189] and the molecular etiology of associated pathologies such as Prader-Willi syndrome [190],[191], some that are implicated as tumour suppressors [168],[192], or that are located at chromosomal translocation breakpoints associated with B-cell lymphoma [193] and schizophrenia [194]. It has also been shown that the translocation and induced expression of an antisense, long ncRNA can cause the epigenetic silencing of the adjacent α-globin gene, resulting in α-thalassemia [195].
Conclusion
There is not (yet) a huge catalogue of mutations in ncRNAs that have been shown to affect phenotype, compared to those in protein-coding sequences. However, on the assumption that most ncRNAs are regulatory and that most regulatory regions have yet to be assigned genetic signatures, it is no surprise that this may be the case. On the other hand, as screening for variations affecting complex traits becomes more sophisticated, it is reasonable to anticipate that many will map to, and affect the function of, ncRNAs. Certainly this possibility should be borne in mind in the interpretation of such variations and the consequent studies to define their mechanism of action. The functional analysis of ncRNAs is in its infancy, but in situ hybridization, genomic, and structural characteristics, and the perturbation of their expression by overexpression and siRNA-mediated knockdown are emerging as major tools (Tables 1 and 2). There seems little doubt that there is a hidden world of regulatory architecture underpinning the development of complex organisms that we have yet to explore, both genetically and functionally.
Acknowledgments
I thank Paulo Amaral, Grant Montgomery, and Manolis Dermitzakis for helpful comments on the manuscript; Paulo Amaral for assistance in the construction of Table 1; Ryan Taft for providing the analysis shown in Figure 1; and Tim Mercer for drawing the figures.
Footnotes
The author has declared that no competing interests exist.
The work was supported by the Australian Research Council (Fellowship Grant FF0561986), the University of Queensland, and the Queensland State Government. These agencies played no role in the preparation, review, or approval of the manuscript.
References
- 1.Taft RJ, Pheasant M, Mattick JS. The relationship between non-protein-coding DNA and eukaryotic complexity. Bioessays. 2007;29:288–299. doi: 10.1002/bies.20544. [DOI] [PubMed] [Google Scholar]
- 2.Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 3.Kapranov P, Drenkow J, Cheng J, Long J, Helt G, et al. Examples of the complex architecture of the human transcriptome revealed by RACE and high-density tiling arrays. Genome Res. 2005;15:987–997. doi: 10.1101/gr.3455305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet. 2006;15:R17–29. doi: 10.1093/hmg/ddl046. [DOI] [PubMed] [Google Scholar]
- 5.Kapranov P, Willingham AT, Gingeras TR. Genome-wide transcription and the implications for genomic organization. Nat Rev Genet. 2007;8:413–423. doi: 10.1038/nrg2083. [DOI] [PubMed] [Google Scholar]
- 6.Rajendra TK, Prasanth KV, Lakhotia SC. Male sterility associated with overexpression of the noncoding hsromega gene in cyst cells of testis of Drosophila melanogaster. J Genet. 2001;80:97–110. doi: 10.1007/BF02728335. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Elsner T, Gou D, Kremmer E, Sauer F. Noncoding RNAs of trithorax response elements recruit Drosophila Ash1 to Ultrabithorax. Science. 2006;311:1118–1123. doi: 10.1126/science.1117705. [DOI] [PubMed] [Google Scholar]
- 8.Hellwig S, Bass BL. A starvation-induced noncoding RNA modulates expression of Dicer-regulated genes. Proc Natl Acad Sci U S A. 2008;105:12897–12902. doi: 10.1073/pnas.0805118105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma J, Yan B, Qu Y, Qin F, Yang Y, et al. Zm401, a short-open reading-frame mRNA or noncoding RNA, is essential for tapetum and microspore development and can regulate the floret formation in maize. J Cell Biochem. 2008;105:136–146. doi: 10.1002/jcb.21807. [DOI] [PubMed] [Google Scholar]
- 10.Ben Amor B, Wirth S, Merchan F, Laporte P, d'Aubenton-Carafa Y, et al. Novel long non-protein coding RNAs involved in Arabidopsis differentiation and stress responses. Genome Res. 2009;19:57–69. doi: 10.1101/gr.080275.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camblong J, Iglesias N, Fickentscher C, Dieppois G, Stutz F. Antisense RNA stabilization induces transcriptional gene silencing via histone deacetylation in S. cerevisiae. Cell. 2007;131:706–717. doi: 10.1016/j.cell.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Amaral PP, Mattick JS. Noncoding RNA in development. Mamm Genome. 2008;19:454–492. doi: 10.1007/s00335-008-9136-7. [DOI] [PubMed] [Google Scholar]
- 13.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu W, Gius D, Onyango P, Muldoon-Jacobs K, Karp J, et al. Epigenetic silencing of tumour suppressor gene p15 by its antisense RNA. Nature. 2008;451:202–206. doi: 10.1038/nature06468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martianov I, Ramadass A, Serra Barros A, Chow N, Akoulitchev A. Repression of the human dihydrofolate reductase gene by a non-coding interfering transcript. Nature. 2007;445:666–670. doi: 10.1038/nature05519. [DOI] [PubMed] [Google Scholar]
- 16.Blin-Wakkach C, Lezot F, Ghoul-Mazgar S, Hotton D, Monteiro S, et al. Endogenous Msx1 antisense transcript: in vivo and in vitro evidences, structure, and potential involvement in skeleton development in mammals. Proc Natl Acad Sci U S A. 2001;98:7336–7341. doi: 10.1073/pnas.131497098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young TL, Matsuda T, Cepko CL. The noncoding RNA taurine upregulated gene 1 is required for differentiation of the murine retina. Curr Biol. 2005;15:501–512. doi: 10.1016/j.cub.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 18.Cano A, Nieto MA. Non-coding RNAs take centre stage in epithelial-to-mesenchymal transition. Trends Cell Biol. 2008;18:357–359. doi: 10.1016/j.tcb.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Sunwoo H, Dinger ME, Wilusz JE, Amaral PP, Mattick JS, et al. MEN ε/β nuclear-retained non-coding RNAs are up-regulated upon muscle differentiation and are essential components of paraspeckles. Genome Res. 2009;19:347–359. doi: 10.1101/gr.087775.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasaki YT, Ideue T, Sano M, Mituyama T, Hirose T. MENepsilon/beta noncoding RNAs are essential for structural integrity of nuclear paraspeckles. Proc Natl Acad Sci U S A. 2009;106:2525–2530. doi: 10.1073/pnas.0807899106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clemson CM, Hutchinson JN, Sara SA, Ensminger AW, Fox AH, et al. An architectural role for a nuclear noncoding RNA: NEAT1 RNA Is essential for the structure of paraspeckles. Mol Cell. 2009 doi: 10.1016/j.molcel.2009.01.026. epub ahead of print. doi:10.1016/j.molcel.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redrup L, Branco MR, Perdeaux ER, Krueger C, Lewis A, et al. The long noncoding RNA Kcnq1ot1 organises a lineage-specific nuclear domain for epigenetic gene silencing. Development. 2009;136:525–530. doi: 10.1242/dev.031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prasanth KV, Spector DL. Eukaryotic regulatory RNAs: an answer to the ‘genome complexity’ conundrum. Genes Dev. 2007;21:11–42. doi: 10.1101/gad.1484207. [DOI] [PubMed] [Google Scholar]
- 24.Louro R, Smirnova AS, Verjovski-Almeida S. Long intronic noncoding RNA transcription: expression noise or expression choice? Genomics. 2009;93:291–298. doi: 10.1016/j.ygeno.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 26.Pang KC, Frith MC, Mattick JS. Rapid evolution of noncoding RNAs: lack of conservation does not mean lack of function. Trends Genet. 2006;22:1–5. doi: 10.1016/j.tig.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Ponjavic J, Ponting CP, Lunter G. Functionality or transcriptional noise? Evidence for selection within long noncoding RNAs. Genome Res. 2007;17:556–565. doi: 10.1101/gr.6036807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Washietl S, Hofacker IL, Lukasser M, Huttenhofer A, Stadler PF. Mapping of conserved RNA secondary structures predicts thousands of functional noncoding RNAs in the human genome. Nat Biotechnol. 2005;23:1383–1390. doi: 10.1038/nbt1144. [DOI] [PubMed] [Google Scholar]
- 29.Torarinsson E, Sawera M, Havgaard JH, Fredholm M, Gorodkin J. Thousands of corresponding human and mouse genomic regions unalignable in primary sequence contain common RNA structure. Genome Res. 2006;16:885–889. doi: 10.1101/gr.5226606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torarinsson E, Yao Z, Wiklund ED, Bramsen JB, Hansen C, et al. Comparative genomics beyond sequence-based alignments: RNA structures in the ENCODE regions. Genome Res. 2008;18:242–251. doi: 10.1101/gr.6887408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dinger ME, Amaral PP, Mercer TR, Pang KC, Bruce SJ, et al. Long noncoding RNAs in mouse embryonic stem cell pluripotency and differentiation. Genome Res. 2008;18:1433–1445. doi: 10.1101/gr.078378.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guttman M, Amit I, Garber M, French C, Lin MF, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trinklein ND, Aldred SF, Hartman SJ, Schroeder DI, Otillar RP, et al. An abundance of bidirectional promoters in the human genome. Genome Res. 2004;14:62–66. doi: 10.1101/gr.1982804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engstrom PG, Suzuki H, Ninomiya N, Akalin A, Sessa L, et al. Complex loci in human and mouse genomes. PLoS Genet. 2006;2:e47. doi: 10.1371/journal.pgen.0020047. doi:10.1371/journal.pgen.0020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tupy JL, Bailey AM, Dailey G, Evans-Holm M, Siebel CW, et al. Identification of putative noncoding polyadenylated transcripts in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2005;102:5495–5500. doi: 10.1073/pnas.0501422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louro R, El-Jundi T, Nakaya HI, Reis EM, Verjovski-Almeida S. Conserved tissue expression signatures of intronic noncoding RNAs transcribed from human and mouse loci. Genomics. 2008;92:18–25. doi: 10.1016/j.ygeno.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Thrash-Bingham CA, Tartof KD. aHIF: a natural antisense transcript overexpressed in human renal cancer and during hypoxia. J Natl Cancer Inst. 1999;91:143–151. doi: 10.1093/jnci/91.2.143. [DOI] [PubMed] [Google Scholar]
- 38.Ji P, Diederichs S, Wang W, Boing S, Metzger R, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:6087–6097. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 39.Mutsuddi M, Marshall CM, Benzow KA, Koob MD, Rebay I. The spinocerebellar ataxia 8 noncoding RNA causes neurodegeneration and associates with staufen in Drosophila. Curr Biol. 2004;14:302–308. doi: 10.1016/j.cub.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 40.Reis EM, Nakaya HI, Louro R, Canavez FC, Flatschart AV, et al. Antisense intronic non-coding RNA levels correlate to the degree of tumor differentiation in prostate cancer. Oncogene. 2004;23:6684–6692. doi: 10.1038/sj.onc.1207880. [DOI] [PubMed] [Google Scholar]
- 41.Reis EM, Ojopi EP, Alberto FL, Rahal P, Tsukumo F, et al. Large-scale transcriptome analyses reveal new genetic marker candidates of head, neck, and thyroid cancer. Cancer Res. 2005;65:1693–1699. doi: 10.1158/0008-5472.CAN-04-3506. [DOI] [PubMed] [Google Scholar]
- 42.Sonkoly E, Bata-Csorgo Z, Pivarcsi A, Polyanka H, Kenderessy-Szabo A, et al. Identification and characterization of a novel, psoriasis susceptibility-related noncoding RNA gene, PRINS. J Biol Chem. 2005;280:24159–24167. doi: 10.1074/jbc.M501704200. [DOI] [PubMed] [Google Scholar]
- 43.Szymanski M, Barciszewska MZ, Erdmann VA, Barciszewski J. A new frontier for molecular medicine: noncoding RNAs. Biochim Biophys Acta. 2005;1756:65–75. doi: 10.1016/j.bbcan.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Angeloni D, ter Elst A, Wei MH, van der Veen AY, Braga EA, et al. Analysis of a new homozygous deletion in the tumor suppressor region at 3p12.3 reveals two novel intronic noncoding RNA genes. Genes Chromosomes Cancer. 2006;45:676–691. doi: 10.1002/gcc.20332. [DOI] [PubMed] [Google Scholar]
- 45.Calin GA, Liu CG, Ferracin M, Hyslop T, Spizzo R, et al. Ultraconserved regions encoding ncRNAs are altered in human leukemias and carcinomas. Cancer Cell. 2007;12:215–229. doi: 10.1016/j.ccr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 46.Pang KC, Stephen S, Dinger ME, Engstrom PG, Lenhard B, et al. RNAdb 2.0–an expanded database of mammalian non-coding RNAs. Nucleic Acids Res. 2007;35:D178–182. doi: 10.1093/nar/gkl926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christov CP, Trivier E, Krude T. Noncoding human Y RNAs are overexpressed in tumours and required for cell proliferation. Br J Cancer. 2008;98:981–988. doi: 10.1038/sj.bjc.6604254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez DS, Hoage TR, Pritchett JR, Ducharme-Smith AL, Halling ML, et al. Long, abundantly expressed non-coding transcripts are altered in cancer. Hum Mol Genet. 2008;17:642–655. doi: 10.1093/hmg/ddm336. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, Lian Z, Padden C, Gerstein MB, Rozowsky J, et al. A myelopoiesis-associated regulatory intergenic non-coding RNA transcript within the human HOXA cluster. Blood. 2009;113:2526–2534. doi: 10.1182/blood-2008-06-162164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cawley S, Bekiranov S, Ng HH, Kapranov P, Sekinger EA, et al. Unbiased mapping of transcription factor binding sites along human chromosomes 21 and 22 points to widespread regulation of noncoding RNAs. Cell. 2004;116:499–509. doi: 10.1016/s0092-8674(04)00127-8. [DOI] [PubMed] [Google Scholar]
- 51.Ravasi T, Suzuki H, Pang KC, Katayama S, Furuno M, et al. Experimental validation of the regulated expression of large numbers of non-coding RNAs from the mouse genome. Genome Res. 2006;16:11–19. doi: 10.1101/gr.4200206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mercer TR, Dinger ME, Sunkin SM, Mehler MF, Mattick JS. Specific expression of long noncoding RNAs in the mouse brain. Proc Natl Acad Sci U S A. 2008;105:716–721. doi: 10.1073/pnas.0706729105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kohtz JD, Fishell G. Developmental regulation of EVF-1, a novel non-coding RNA transcribed upstream of the mouse Dlx6 gene. Gene Expr Patterns. 2004;4:407–412. doi: 10.1016/j.modgep.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Inagaki S, Numata K, Kondo T, Tomita M, Yasuda K, et al. Identification and expression analysis of putative mRNA-like non-coding RNA in Drosophila. Genes Cells. 2005;10:1163–1173. doi: 10.1111/j.1365-2443.2005.00910.x. [DOI] [PubMed] [Google Scholar]
- 55.Brena C, Chipman AD, Minelli A, Akam M. Expression of trunk Hox genes in the centipede Strigamia maritima: sense and anti-sense transcripts. Evol Dev. 2006;8:252–265. doi: 10.1111/j.1525-142X.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 56.Sone M, Hayashi T, Tarui H, Agata K, Takeichi M, et al. The mRNA-like noncoding RNA Gomafu constitutes a novel nuclear domain in a subset of neurons. J Cell Sci. 2007;120:2498–2506. doi: 10.1242/jcs.009357. [DOI] [PubMed] [Google Scholar]
- 57.Pollard KS, Salama SR, King B, Kern AD, Dreszer T, et al. Forces shaping the fastest evolving regions in the human genome. PLoS Genet. 2006;2:e168. doi: 10.1371/journal.pgen.0020168. doi:10.1371/journal.pgen.0020168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14:1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aravin AA, Hannon GJ, Brennecke J. The Piwi-piRNA pathway provides an adaptive defense in the transposon arms race. Science. 2007;318:761–764. doi: 10.1126/science.1146484. [DOI] [PubMed] [Google Scholar]
- 60.Ruby JG, Jan CH, Bartel DP. Intronic microRNA precursors that bypass Drosha processing. Nature. 2007;448:83–86. doi: 10.1038/nature05983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okamura K, Hagen JW, Duan H, Tyler DM, Lai EC. The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell. 2007;130:89–100. doi: 10.1016/j.cell.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berezikov E, Chung WJ, Willis J, Cuppen E, Lai EC. Mammalian mirtron genes. Mol Cell. 2007;28:328–336. doi: 10.1016/j.molcel.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tam OH, Aravin AA, Stein P, Girard A, Murchison EP, et al. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature. 2008;453:534–538. doi: 10.1038/nature06904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watanabe T, Totoki Y, Toyoda A, Kaneda M, Kuramochi-Miyagawa S, et al. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature. 2008;453:539–543. doi: 10.1038/nature06908. [DOI] [PubMed] [Google Scholar]
- 65.Okamura K, Chung WJ, Ruby JG, Guo H, Bartel DP, et al. The Drosophila hairpin RNA pathway generates endogenous short interfering RNAs. Nature. 2008;453:803–806. doi: 10.1038/nature07015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klattenhoff C, Theurkauf W. Biogenesis and germline functions of piRNAs. Development. 2008;135:3–9. doi: 10.1242/dev.006486. [DOI] [PubMed] [Google Scholar]
- 67.Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9:219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 68.Ogawa Y, Sun BK, Lee JT. Intersection of the RNA interference and X-inactivation pathways. Science. 2008;320:1336–1341. doi: 10.1126/science.1157676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37:766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- 70.Berezikov E, van Tetering G, Verheul M, van de Belt J, van Laake L, et al. Many novel mammalian microRNA candidates identified by extensive cloning and RAKE analysis. Genome Res. 2006;16:1289–1298. doi: 10.1101/gr.5159906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carroll SB. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell. 2008;134:25–36. doi: 10.1016/j.cell.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 72.Smith NG, Brandstrom M, Ellegren H. Evidence for turnover of functional noncoding DNA in mammalian genome evolution. Genomics. 2004;84:806–813. doi: 10.1016/j.ygeno.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 73.Frith MC, Ponjavic J, Fredman D, Kai C, Kawai J, et al. Evolutionary turnover of mammalian transcription start sites. Genome Res. 2006;16:713–722. doi: 10.1101/gr.5031006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taylor MS, Kai C, Kawai J, Carninci P, Hayashizaki Y, et al. Heterotachy in mammalian promoter evolution. PLoS Genet. 2006;2:e30. doi: 10.1371/journal.pgen.0020030. doi:10.1371/journal.pgen.0020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pheasant M, Mattick JS. Raising the estimate of functional human sequences. Genome Res. 2007;17:1245–1253. doi: 10.1101/gr.6406307. [DOI] [PubMed] [Google Scholar]
- 76.Fisher S, Grice EA, Vinton RM, Bessling SL, McCallion AS. Conservation of RET regulatory function from human to zebrafish without sequence similarity. Science. 2006;312:276–279. doi: 10.1126/science.1124070. [DOI] [PubMed] [Google Scholar]
- 77.Dermitzakis ET, Reymond A, Lyle R, Scamuffa N, Ucla C, et al. Numerous potentially functional but non-genic conserved sequences on human chromosome 21. Nature. 2002;420:578–582. doi: 10.1038/nature01251. [DOI] [PubMed] [Google Scholar]
- 78.Dermitzakis ET, Reymond A, Scamuffa N, Ucla C, Kirkness E, et al. Evolutionary discrimination of mammalian conserved non-genic sequences (CNGs). Science. 2003;302:1033–1035. doi: 10.1126/science.1087047. [DOI] [PubMed] [Google Scholar]
- 79.Bejerano G, Pheasant M, Makunin I, Stephen S, Kent WJ, et al. Ultraconserved elements in the human genome. Science. 2004;304:1321–1325. doi: 10.1126/science.1098119. [DOI] [PubMed] [Google Scholar]
- 80.Stephen S, Pheasant M, Makunin IV, Mattick JS. Large-scale appearance of ultraconserved elements in tetrapod genomes and slowdown of the molecular clock. Mol Biol Evol. 2008;25:402–408. doi: 10.1093/molbev/msm268. [DOI] [PubMed] [Google Scholar]
- 81.Pennacchio LA, Ahituv N, Moses AM, Prabhakar S, Nobrega MA, et al. In vivo enhancer analysis of human conserved non-coding sequences. Nature. 2006;444:499–502. doi: 10.1038/nature05295. [DOI] [PubMed] [Google Scholar]
- 82.Visel A, Prabhakar S, Akiyama JA, Shoukry M, Lewis KD, et al. Ultraconservation identifies a small subset of extremely constrained developmental enhancers. Nat Genet. 2008;40:158–160. doi: 10.1038/ng.2007.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ashe HL, Monks J, Wijgerde M, Fraser P, Proudfoot NJ. Intergenic transcription and transinduction of the human beta-globin locus. Genes Dev. 1997;11:2494–2509. doi: 10.1101/gad.11.19.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ling J, Baibakov B, Pi W, Emerson BM, Tuan D. The HS2 enhancer of the beta-globin locus control region initiates synthesis of non-coding, polyadenylated RNAs independent of a cis-linked globin promoter. J Mol Biol. 2005;350:883–896. doi: 10.1016/j.jmb.2005.05.039. [DOI] [PubMed] [Google Scholar]
- 85.Lefevre P, Witham J, Lacroix CE, Cockerill PN, Bonifer C. The LPS-induced transcriptional upregulation of the chicken lysozyme locus involves CTCF eviction and noncoding RNA transcription. Mol Cell. 2008;32:129–139. doi: 10.1016/j.molcel.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lempradl A, Ringrose L. How does noncoding transcription regulate Hox genes? Bioessays. 2008;30:110–121. doi: 10.1002/bies.20704. [DOI] [PubMed] [Google Scholar]
- 87.Mattick JS. Challenging the dogma: the hidden layer of non-protein-coding RNAs in complex organisms. Bioessays. 2003;25:930–939. doi: 10.1002/bies.10332. [DOI] [PubMed] [Google Scholar]
- 88.Mattick JS. A new paradigm for developmental biology. J Exp Biol. 2007;210:1526–1547. doi: 10.1242/jeb.005017. [DOI] [PubMed] [Google Scholar]
- 89.Mattick JS, Amaral PP, Dinger ME, Mercer TR, Mehler MF. RNA regulation of epigenetic processes. Bioessays. 2009;31:51–59. doi: 10.1002/bies.080099. [DOI] [PubMed] [Google Scholar]
- 90.Liu ET. Functional genomics of cancer. Curr Opin Genet Dev. 2008;18:251–256. doi: 10.1016/j.gde.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 91.Tomasini R, Mak TW, Melino G. The impact of p53 and p73 on aneuploidy and cancer. Trends Cell Biol. 2008;18:244–252. doi: 10.1016/j.tcb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 92.Barbaric I, Miller G, Dear TN. Appearances can be deceiving: phenotypes of knockout mice. Brief Funct Genomic Proteomic. 2007;6:91–103. doi: 10.1093/bfgp/elm008. [DOI] [PubMed] [Google Scholar]
- 93.Kutuzova GD, Akhter S, Christakos S, Vanhooke J, Kimmel-Jehan C, et al. Calbindin D(9k) knockout mice are indistinguishable from wild-type mice in phenotype and serum calcium level. Proc Natl Acad Sci U S A. 2006;103:12377–12381. doi: 10.1073/pnas.0605252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- 95.Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129:303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 96.Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, et al. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 97.Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, et al. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 99.Ng K, Pullirsch D, Leeb M, Wutz A. Xist and the order of silencing. EMBO Rep. 2007;8:34–39. doi: 10.1038/sj.embor.7400871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lewejohann L, Skryabin BV, Sachser N, Prehn C, Heiduschka P, et al. Role of a neuronal small non-messenger RNA: behavioural alterations in BC1 RNA-deleted mice. Behav Brain Res. 2004;154:273–289. doi: 10.1016/j.bbr.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 101.Poulin F, Nobrega MA, Plajzer-Frick I, Holt A, Afzal V, et al. In vivo characterization of a vertebrate ultraconserved enhancer. Genomics. 2005;85:774–781. doi: 10.1016/j.ygeno.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 102.Ahituv N, Zhu Y, Visel A, Holt A, Afzal V, et al. Deletion of ultraconserved elements yields viable mice. PLoS Biol. 2007;5:e234. doi: 10.1371/journal.pbio.0050234. doi:10.1371/journal.pbio.0050234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Katzman S, Kern AD, Bejerano G, Fewell G, Fulton L, et al. Human genome ultraconserved elements are ultraselected. Science. 2007;317:915. doi: 10.1126/science.1142430. [DOI] [PubMed] [Google Scholar]
- 104.Hindorff LA, Junkins HA, Manolio TA. A catalog of published genome-wide association studies. 2008. Available at: www.genome.gov/26525384/
- 105.Manolio TA, Brooks LD, Collins FS. A HapMap harvest of insights into the genetics of common disease. J Clin Invest. 2008;118:1590–1605. doi: 10.1172/JCI34772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pasmant E, Laurendeau I, Heron D, Vidaud M, Vidaud D, et al. Characterization of a germ-line deletion, including the entire INK4/ARF locus, in a melanoma-neural system tumor family: identification of ANRIL, an antisense noncoding RNA whose expression coclusters with ARF. Cancer Res. 2007;67:3963–3969. doi: 10.1158/0008-5472.CAN-06-2004. [DOI] [PubMed] [Google Scholar]
- 107.Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McPherson R, Pertsemlidis A, Kavaslar N, Stewart A, Roberts R, et al. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–1491. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Helgadottir A, Thorleifsson G, Manolescu A, Gretarsdottir S, Blondal T, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 111.Broadbent HM, Peden JF, Lorkowski S, Goel A, Ongen H, et al. Susceptibility to coronary artery disease and diabetes is encoded by distinct, tightly linked SNPs in the ANRIL locus on chromosome 9p. Hum Mol Genet. 2008;17:806–814. doi: 10.1093/hmg/ddm352. [DOI] [PubMed] [Google Scholar]
- 112.Schaefer AS, Richter GM, Groessner-Schreiber B, Noack B, Nothnagel M, et al. Identification of a shared genetic susceptibility locus for coronary heart disease and periodontitis. PLoS Genet. 2009;5:e1000378. doi: 10.1371/journal.pgen.1000378. doi:10.1371/journal.pgen.1000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wray NR, Goddard ME, Visscher PM. Prediction of individual genetic risk of complex disease. Curr Opin Genet Dev. 2008;18:257–263. doi: 10.1016/j.gde.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 114.Clark RM, Wagler TN, Quijada P, Doebley J. A distant upstream enhancer at the maize domestication gene tb1 has pleiotropic effects on plant and inflorescent architecture. Nat Genet. 2006;38:594–597. doi: 10.1038/ng1784. [DOI] [PubMed] [Google Scholar]
- 115.Abelson JF, Kwan KY, O'Roak BJ, Baek DY, Stillman AA, et al. Sequence variants in SLITRK1 are associated with Tourette's syndrome. Science. 2005;310:317–320. doi: 10.1126/science.1116502. [DOI] [PubMed] [Google Scholar]
- 116.Clop A, Marcq F, Takeda H, Pirottin D, Tordoir X, et al. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet. 2006;38:813–818. doi: 10.1038/ng1810. [DOI] [PubMed] [Google Scholar]
- 117.Van Laere AS, Nguyen M, Braunschweig M, Nezer C, Collette C, et al. A regulatory mutation in IGF2 causes a major QTL effect on muscle growth in the pig. Nature. 2003;425:832–836. doi: 10.1038/nature02064. [DOI] [PubMed] [Google Scholar]
- 118.Smit M, Segers K, Carrascosa LG, Shay T, Baraldi F, et al. Mosaicism of Solid Gold supports the causality of a noncoding A-to-G transition in the determinism of the callipyge phenotype. Genetics. 2003;163:453–456. doi: 10.1093/genetics/163.1.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hagan JP, O'Neill BL, Stewart CL, Kozlov SV, Croce CM. At least ten genes define the imprinted Dlk1-Dio3 cluster on mouse chromosome 12qF1. PLoS ONE. 2009;4:e4352. doi: 10.1371/journal.pone.0004352. doi:10.1371/journal.pone.0004352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Georges M, Charlier C, Cockett N. The callipyge locus: evidence for the trans interaction of reciprocally imprinted genes. Trends Genet. 2003;19:248–252. doi: 10.1016/S0168-9525(03)00082-9. [DOI] [PubMed] [Google Scholar]
- 121.Wermter AK, Scherag A, Meyre D, Reichwald K, Durand E, et al. Preferential reciprocal transfer of paternal/maternal DLK1 alleles to obese children: first evidence of polar overdominance in humans. Eur J Hum Genet. 2008;16:1126–1134. doi: 10.1038/ejhg.2008.64. [DOI] [PubMed] [Google Scholar]
- 122.Davis E, Caiment F, Tordoir X, Cavaille J, Ferguson-Smith A, et al. RNAi-mediated allelic trans-interaction at the imprinted Rtl1/Peg11 locus. Curr Biol. 2005;15:743–749. doi: 10.1016/j.cub.2005.02.060. [DOI] [PubMed] [Google Scholar]
- 123.He H, Nagy R, Liyanarachchi S, Jiao H, Li W, et al. A susceptibility locus for papillary thyroid carcinoma on chromosome 8q24. Cancer Res. 2009;69:625–631. doi: 10.1158/0008-5472.CAN-08-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Duncan I. The bithorax complex. Annu Rev Genet. 1987;21:285–319. doi: 10.1146/annurev.ge.21.120187.001441. [DOI] [PubMed] [Google Scholar]
- 125.Akam ME, Martinez-Arias A. The distribution of Ultrabithorax transcripts in Drosophila embryos. Embo J. 1985;4:1689–1700. doi: 10.1002/j.1460-2075.1985.tb03838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lipshitz HD, Peattie DA, Hogness DS. Novel transcripts from the Ultrabithorax domain of the bithorax complex. Genes Dev. 1987;1:307–322. doi: 10.1101/gad.1.3.307. [DOI] [PubMed] [Google Scholar]
- 127.Petruk S, Sedkov Y, Riley KM, Hodgson J, Schweisguth F, et al. Transcription of bxd noncoding RNAs promoted by trithorax represses Ubx in cis by transcriptional interference. Cell. 2006;127:1209–1221. doi: 10.1016/j.cell.2006.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322:750–756. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sanchez-Herrero E, Akam M. Spatially ordered transcription of regulatory DNA in the bithorax complex of Drosophila. Development. 1989;107:321–329. doi: 10.1242/dev.107.2.321. [DOI] [PubMed] [Google Scholar]
- 130.Duncan IW. Transvection effects in Drosophila. Annu Rev Genet. 2002;36:521–556. doi: 10.1146/annurev.genet.36.060402.100441. [DOI] [PubMed] [Google Scholar]
- 131.Suemori H, Noguchi S. HoxC cluster genes are dispensable for overall body plan of mouse embryonic development. Dev Biol. 2000;220:333–342. doi: 10.1006/dbio.2000.9651. [DOI] [PubMed] [Google Scholar]
- 132.Vaucheret H. Post-transcriptional small RNA pathways in plants: mechanisms and regulations. Genes Dev. 2006;20:759–771. doi: 10.1101/gad.1410506. [DOI] [PubMed] [Google Scholar]
- 133.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 134.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 135.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 136.Hipfner DR, Weigmann K, Cohen SM. The bantam gene regulates Drosophila growth. Genetics. 2002;161:1527–1537. doi: 10.1093/genetics/161.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- 138.Sarin S, O'Meara MM, Flowers EB, Antonio C, Poole RJ, et al. Genetic screens for Caenorhabditis elegans mutants defective in left/right asymmetric neuronal fate specification. Genetics. 2007;176:2109–2130. doi: 10.1534/genetics.107.075648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408:86–89. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- 140.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 141.Yu F, Yao H, Zhu P, Zhang X, Pan Q, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 142.Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol. 2008 doi: 10.1016/j.tcb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 143.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 144.Johnston RJ, Hobert O. A microRNA controlling left/right neuronal asymmetry in Caenorhabditis elegans. Nature. 2003;426:845–849. doi: 10.1038/nature02255. [DOI] [PubMed] [Google Scholar]
- 145.De Gobbi M, Viprakasit V, Hughes JR, Fisher C, Buckle VJ, et al. A regulatory SNP causes a human genetic disease by creating a new transcriptional promoter. Science. 2006;312:1215–1217. doi: 10.1126/science.1126431. [DOI] [PubMed] [Google Scholar]
- 146.Libioulle C, Louis E, Hansoul S, Sandor C, Farnir F, et al. Novel Crohn disease locus identified by genome-wide association maps to a gene desert on 5p13.1 and modulates expression of PTGER4. PLoS Genet. 2007;3:e58. doi: 10.1371/journal.pgen.0030058. doi:10.1371/journal.pgen. 0030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Marcellini S, Simpson P. Two or four bristles: functional evolution of an enhancer of scute in Drosophilidae. PLoS Biol. 2006;4:e386. doi: 10.1371/journal.pbio.0040386. doi:10.1371/journal.pbio.0040386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sipos L, Mihaly J, Karch F, Schedl P, Gausz J, et al. Transvection in the Drosophila Abd-B domain: extensive upstream sequences are involved in anchoring distant cis-regulatory regions to the promoter. Genetics. 1998;149:1031–1050. doi: 10.1093/genetics/149.2.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hendrickson JE, Sakonju S. Cis and trans interactions between the iab regulatory regions and abdominal-A and abdominal-B in Drosophila melanogaster. Genetics. 1995;139:835–848. doi: 10.1093/genetics/139.2.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hopmann R, Duncan D, Duncan I. Transvection in the iab-5,6,7 region of the bithorax complex of Drosophila: homology independent interactions in trans. Genetics. 1995;139:815–833. doi: 10.1093/genetics/139.2.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Muller M, Hagstrom K, Gyurkovics H, Pirrotta V, Schedl P. The mcp element from the Drosophila melanogaster bithorax complex mediates long-distance regulatory interactions. Genetics. 1999;153:1333–1356. doi: 10.1093/genetics/153.3.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vazquez J, Muller M, Pirrotta V, Sedat JW. The Mcp element mediates stable long-range chromosome-chromosome interactions in Drosophila. Mol Biol Cell. 2006;17:2158–2165. doi: 10.1091/mbc.E06-01-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mattick JS, Gagen MJ. The evolution of controlled multitasked gene networks: the role of introns and other noncoding RNAs in the development of complex organisms. Mol Biol Evol. 2001;18:1611–1630. doi: 10.1093/oxfordjournals.molbev.a003951. [DOI] [PubMed] [Google Scholar]
- 154.Feng J, Bi C, Clark BS, Mady R, Shah P, et al. The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev. 2006;20:1470–1484. doi: 10.1101/gad.1416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pandey RR, Mondal T, Mohammad F, Enroth S, Redrup L, et al. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol Cell. 2008;32:232–246. doi: 10.1016/j.molcel.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 156.Nagano T, Mitchell JA, Sanz LA, Pauler FM, Ferguson-Smith AC, et al. The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science. 2008;322:1717–1720. doi: 10.1126/science.1163802. [DOI] [PubMed] [Google Scholar]
- 157.Terranova R, Yokobayashi S, Stadler MB, Otte AP, van Lohuizen M, et al. Polycomb group proteins Ezh2 and Rnf2 direct genomic contraction and imprinted repression in early mouse embryos. Dev Cell. 2008;15:1–12. doi: 10.1016/j.devcel.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 158.Corcoran MM, Hammarsund M, Zhu C, Lerner M, Kapanadze B, et al. DLEU2 encodes an antisense RNA for the putative bicistronic RFP2/LEU5 gene in humans and mouse. Genes Chromosomes Cancer. 2004;40:285–297. doi: 10.1002/gcc.20046. [DOI] [PubMed] [Google Scholar]
- 159.Dinger ME, Pang KC, Mercer TR, Mattick JS. Differentiating protein-coding and noncoding RNA: challenges and ambiguities. PLoS Comput Biol. 2008;4:e1000176. doi: 10.1371/journal.pcbi.1000176. doi:10.1371/journal.pcbi.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chamary JV, Parmley JL, Hurst LD. Hearing silence: non-neutral evolution at synonymous sites in mammals. Nat Rev Genet. 2006;7:98–108. doi: 10.1038/nrg1770. [DOI] [PubMed] [Google Scholar]
- 161.Davison EJ, Tarpey PS, Fiegler H, Tomlinson IP, Carter NP. Deletion at chromosome band 20p12.1 in colorectal cancer revealed by high resolution array comparative genomic hybridization. Genes Chromosomes Cancer. 2005;44:384–391. doi: 10.1002/gcc.20252. [DOI] [PubMed] [Google Scholar]
- 162.Dixon AL, Liang L, Moffatt MF, Chen W, Heath S, et al. A genome-wide association study of global gene expression. Nat Genet. 2007;39:1202–1207. doi: 10.1038/ng2109. [DOI] [PubMed] [Google Scholar]
- 163.Jupe ER, Liu XT, Kiehlbauch JL, McClung JK, Dell'Orco RT. Prohibitin in breast cancer cell lines: loss of antiproliferative activity is linked to 3′ untranslated region mutations. Cell Growth Differ. 1996;7:871–878. [PubMed] [Google Scholar]
- 164.Jupe ER, Liu XT, Kiehlbauch JL, McClung JK, Dell'Orco RT. The 3′ untranslated region of prohibitin and cellular immortalization. Exp Cell Res. 1996;224:128–135. doi: 10.1006/excr.1996.0120. [DOI] [PubMed] [Google Scholar]
- 165.Jenny A, Hachet O, Zavorszky P, Cyrklaff A, Weston MD, et al. A translation-independent role of oskar RNA in early Drosophila oogenesis. Development. 2006;133:2827–2833. doi: 10.1242/dev.02456. [DOI] [PubMed] [Google Scholar]
- 166.Candeias MM, Malbert-Colas L, Powell DJ, Daskalogianni C, Maslon MM, et al. p53 mRNA controls p53 activity by managing Mdm2 functions. Nat Cell Biol. 2008 doi: 10.1038/ncb1770. [DOI] [PubMed] [Google Scholar]
- 167.Rastinejad F, Blau HM. Genetic complementation reveals a novel regulatory role for 3′ untranslated regions in growth and differentiation. Cell. 1993;72:903–917. doi: 10.1016/0092-8674(93)90579-f. [DOI] [PubMed] [Google Scholar]
- 168.Rastinejad F, Conboy MJ, Rando TA, Blau HM. Tumor suppression by RNA from the 3′ untranslated region of alpha-tropomyosin. Cell. 1993;75:1107–1117. doi: 10.1016/0092-8674(93)90320-p. [DOI] [PubMed] [Google Scholar]
- 169.Fan H, Villegas C, Huang A, Wright JA. Suppression of malignancy by the 3′ untranslated regions of ribonucleotide reductase R1 and R2 messenger RNAs. Cancer Res. 1996;56:4366–4369. [PubMed] [Google Scholar]
- 170.Amack JD, Paguio AP, Mahadevan MS. Cis and trans effects of the myotonic dystrophy (DM) mutation in a cell culture model. Hum Mol Genet. 1999;8:1975–1984. doi: 10.1093/hmg/8.11.1975. [DOI] [PubMed] [Google Scholar]
- 171.Carninci P, Sandelin A, Lenhard B, Katayama S, Shimokawa K, et al. Genome-wide analysis of mammalian promoter architecture and evolution. Nat Genet. 2006;38:626–635. doi: 10.1038/ng1789. [DOI] [PubMed] [Google Scholar]
- 172.Shirasawa S, Harada H, Furugaki K, Akamizu T, Ishikawa N, et al. SNPs in the promoter of a B cell-specific antisense transcript, SAS-ZFAT, determine susceptibility to autoimmune thyroid disease. Hum Mol Genet. 2004;13:2221–2231. doi: 10.1093/hmg/ddh245. [DOI] [PubMed] [Google Scholar]
- 173.Mette MF, Aufsatz W, van Der Winden J, Matzke MA, Matzke AJ. Transcriptional silencing and promoter methylation triggered by double-stranded RNA. EMBO J. 2000;19:5194–5201. doi: 10.1093/emboj/19.19.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pal-Bhadra M, Bhadra U, Birchler JA. RNAi related mechanisms affect both transcriptional and posttranscriptional transgene silencing in Drosophila. Mol Cell. 2002;9:315–327. doi: 10.1016/s1097-2765(02)00440-9. [DOI] [PubMed] [Google Scholar]
- 175.Buhler M, Moazed D. Transcription and RNAi in heterochromatic gene silencing. Nat Struct Mol Biol. 2007;14:1041–1048. doi: 10.1038/nsmb1315. [DOI] [PubMed] [Google Scholar]
- 176.Matzke M, Kanno T, Huettel B, Daxinger L, Matzke AJ. Targets of RNA-directed DNA methylation. Curr Opin Plant Biol. 2007;10:512–519. doi: 10.1016/j.pbi.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 177.Pal-Bhadra M, Leibovitch BA, Gandhi SG, Rao M, Bhadra U, et al. Heterochromatic silencing and HP1 localization in Drosophila are dependent on the RNAi machinery. Science. 2004;303:669–672. doi: 10.1126/science.1092653. [DOI] [PubMed] [Google Scholar]
- 178.Singh J, Freeling M, Lisch D. A position effect on the heritability of epigenetic silencing. PLoS Genet. 2008;4:e1000216. doi: 10.1371/journal.pgen.1000216. doi:10.1371/journal.pgen.1000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Brennecke J, Malone CD, Aravin AA, Sachidanandam R, Stark A, et al. An epigenetic role for maternally inherited piRNAs in transposon silencing. Science. 2008;322:1387–1392. doi: 10.1126/science.1165171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yang PK, Kuroda MI. Noncoding RNAs and intranuclear positioning in monoallelic gene expression. Cell. 2007;128:777–786. doi: 10.1016/j.cell.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 181.Sasaki H, Matsui Y. Epigenetic events in mammalian germ-cell development: reprogramming and beyond. Nat Rev Genet. 2008;9:129–140. doi: 10.1038/nrg2295. [DOI] [PubMed] [Google Scholar]
- 182.Chandler VL. Paramutation: from maize to mice. Cell. 2007;128:641–645. doi: 10.1016/j.cell.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 183.Cuzin F, Grandjean V, Rassoulzadegan M. Inherited variation at the epigenetic level: paramutation from the plant to the mouse. Curr Opin Genet Dev. 2008;18:193–196. doi: 10.1016/j.gde.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 184.Costa FF. Non-coding RNAs, epigenetics and complexity. Gene. 2008;410:9–17. doi: 10.1016/j.gene.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 185.Osborne RJ, Thornton CA. RNA-dominant diseases. Hum Mol Genet. 2006;15:R162–169. doi: 10.1093/hmg/ddl181. [DOI] [PubMed] [Google Scholar]
- 186.Angeloni D, Ter Elst A, Wei MH, van der Veen AY, Braga EA, et al. Analysis of a new homozygous deletion in the tumor suppressor region at 3p12.3 reveals two novel intronic noncoding RNA genes. Genes Chromosomes Cancer. 2006;45:676–691. doi: 10.1002/gcc.20332. [DOI] [PubMed] [Google Scholar]
- 187.Dallosso AR, Hancock AL, Malik S, Salpekar A, King-Underwood L, et al. Alternately spliced WT1 antisense transcripts interact with WT1 sense RNA and show epigenetic and splicing defects in cancer. Rna. 2007;13:2287–2299. doi: 10.1261/rna.562907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ishii N, Ozaki K, Sato H, Mizuno H, Saito S, et al. Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J Hum Genet. 2006;51:1087–1099. doi: 10.1007/s10038-006-0070-9. [DOI] [PubMed] [Google Scholar]
- 189.Sleutels F, Zwart R, Barlow DP. The non-coding Air RNA is required for silencing autosomal imprinted genes. Nature. 2002;415:810–813. doi: 10.1038/415810a. [DOI] [PubMed] [Google Scholar]
- 190.Ding F, Prints Y, Dhar MS, Johnson DK, Garnacho-Montero C, et al. Lack of Pwcr1/MBII-85 snoRNA is critical for neonatal lethality in Prader-Willi syndrome mouse models. Mamm Genome. 2005;16:424–431. doi: 10.1007/s00335-005-2460-2. [DOI] [PubMed] [Google Scholar]
- 191.Sahoo T, del Gaudio D, German JR, Shinawi M, Peters SU, et al. Prader-Willi phenotype caused by paternal deficiency for the HBII-85 C/D box small nucleolar RNA cluster. Nat Genet. 2008;40:719–721. doi: 10.1038/ng.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhou Y, Zhong Y, Wang Y, Zhang X, Batista DL, et al. Activation of p53 by MEG3 non-coding RNA. J Biol Chem. 2007;282:24731–24742. doi: 10.1074/jbc.M702029200. [DOI] [PubMed] [Google Scholar]
- 193.Tanaka R, Satoh H, Moriyama M, Satoh K, Morishita Y, et al. Intronic U50 small-nucleolar-RNA (snoRNA) host gene of no protein-coding potential is mapped at the chromosome breakpoint t(3;6)(q27;q15) of human B-cell lymphoma. Genes Cells. 2000;5:277–287. doi: 10.1046/j.1365-2443.2000.00325.x. [DOI] [PubMed] [Google Scholar]
- 194.Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Human Mol Genet. 2000;9:1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- 195.Tufarelli C, Stanley JA, Garrick D, Sharpe JA, Ayyub H, et al. Transcription of antisense RNA leading to gene silencing and methylation as a novel cause of human genetic disease. Nat Genet. 2003;34:157–165. doi: 10.1038/ng1157. [DOI] [PubMed] [Google Scholar]
- 196.Seidl CI, Stricker SH, Barlow DP. The imprinted Air ncRNA is an atypical RNAPII transcript that evades splicing and escapes nuclear export. Embo J. 2006;25:3565–3575. doi: 10.1038/sj.emboj.7601245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, et al. Expression of a noncoding RNA is elevated in Alzheimer's disease and drives rapid feed-forward regulation of beta-secretase. Nat Med. 2008;14:723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lin D, Pestova TV, Hellen CU, Tiedge H. Translational control by a small RNA: dendritic BC1 RNA targets the eukaryotic initiation factor 4A helicase mechanism. Mol Cell Biol. 2008;28:3008–3019. doi: 10.1128/MCB.01800-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Takeda K, Ichijo H, Fujii M, Mochida Y, Saitoh M, et al. Identification of a novel bone morphogenetic protein-responsive gene that may function as a noncoding RNA. J Biol Chem. 1998;273:17079–17085. doi: 10.1074/jbc.273.27.17079. [DOI] [PubMed] [Google Scholar]
- 200.Wang X, Arai S, Song X, Reichart D, Du K, et al. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature. 2008;454:126–130. doi: 10.1038/nature06992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tsang WP, Wong TW, Cheung AH, Co CN, Kwok TT. Induction of drug resistance and transformation in human cancer cells by the noncoding RNA CUDR. Rna. 2007;13:890–898. doi: 10.1261/rna.359007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wagner LA, Christensen CJ, Dunn DM, Spangrude GJ, Georgelas A, et al. EGO, a novel, non-coding RNA gene, regulates eosinophil granule protein transcript expression. Blood. 2007;109:5191–5198. doi: 10.1182/blood-2006-06-027987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Brookheart RT, Michel CI, Listenberger LL, Ory DS, Schaffer JE. The non-coding RNA gadd7 is a regulator of lipid-induced oxidative and ER stress. J Biol Chem. 2009;284:7446–7454. doi: 10.1074/jbc.M806209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mourtada-Maarabouni M, Hedge VL, Kirkham L, Farzaneh F, Williams GT. Growth arrest in human T-cells is controlled by the non-coding RNA growth-arrest-specific transcript 5 (GAS5). J Cell Sci. 2008;121:939–946. doi: 10.1242/jcs.024646. [DOI] [PubMed] [Google Scholar]
- 205.Matouk IJ, DeGroot N, Mezan S, Ayesh S, Abu-lail R, et al. The H19 non-coding RNA is essential for human tumor growth. PLoS ONE. 2007;2:e845. doi: 10.1371/journal.pone.0000845. doi:10.1371/journal.pone.0000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gabory A, Ripoche MA, Yoshimizu T, Dandolo L. The H19 gene: regulation and function of a non-coding RNA. Cytogenet Genome Res. 2006;113:188–193. doi: 10.1159/000090831. [DOI] [PubMed] [Google Scholar]
- 207.Yoshimizu T, Miroglio A, Ripoche MA, Gabory A, Vernucci M, et al. The H19 locus acts in vivo as a tumor suppressor. Proc Natl Acad Sci U S A. 2008;105:12417–12422. doi: 10.1073/pnas.0801540105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shamovsky I, Ivannikov M, Kandel ES, Gershon D, Nudler E. RNA-mediated response to heat shock in mammalian cells. Nature. 2006;440:556–560. doi: 10.1038/nature04518. [DOI] [PubMed] [Google Scholar]
- 209.Mayer C, Schmitz KM, Li J, Grummt I, Santoro R. Intergenic transcripts regulate the epigenetic state of rRNA genes. Mol Cell. 2006;22:351–361. doi: 10.1016/j.molcel.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 210.Thakur N, Tiwari VK, Thomassin H, Pandey RR, Kanduri M, et al. An antisense RNA regulates the bidirectional silencing property of the Kcnq1 imprinting control region. Mol Cell Biol. 2004;24:7855–7862. doi: 10.1128/MCB.24.18.7855-7862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Imamura T, Yamamoto S, Ohgane J, Hattori N, Tanaka S, et al. Non-coding RNA directed DNA demethylation of Sphk1 CpG island. Biochem Biophys Res Commun. 2004;322:593–600. doi: 10.1016/j.bbrc.2004.07.159. [DOI] [PubMed] [Google Scholar]
- 212.Madamanchi NR, Hu ZY, Li F, Horaist C, Moon SK, et al. A noncoding RNA regulates human protease-activated receptor-1 gene during embryogenesis. Biochim Biophys Acta. 2002;1576:237–245. doi: 10.1016/s0167-4781(02)00308-1. [DOI] [PubMed] [Google Scholar]
- 213.Tochitani S, Hayashizaki Y. Nkx2.2 antisense RNA overexpression enhanced oligodendrocytic differentiation. Biochem Biophys Res Commun. 2008;372:691–696. doi: 10.1016/j.bbrc.2008.05.127. [DOI] [PubMed] [Google Scholar]
- 214.Willingham AT, Orth AP, Batalov S, Peters EC, Wen BG, et al. A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science. 2005;309:1570–1573. doi: 10.1126/science.1115901. [DOI] [PubMed] [Google Scholar]
- 215.Fu X, Ravindranath L, Tran N, Petrovics G, Srivastava S. Regulation of apoptosis by a prostate-specific and prostate cancer-associated noncoding gene, PCGEM1. DNA Cell Biol. 2006;25:135–141. doi: 10.1089/dna.2006.25.135. [DOI] [PubMed] [Google Scholar]
- 216.Ginger MR, Shore AN, Contreras A, Rijnkels M, Miller J, et al. A noncoding RNA is a potential marker of cell fate during mammary gland development. Proc Natl Acad Sci U S A. 2006;103:5781–5786. doi: 10.1073/pnas.0600745103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yan MD, Hong CC, Lai GM, Cheng AL, Lin YW, et al. Identification and characterization of a novel gene Saf transcribed from the opposite strand of Fas. Hum Mol Genet. 2005;14:1465–1474. doi: 10.1093/hmg/ddi156. [DOI] [PubMed] [Google Scholar]
- 218.Valgardsdottir R, Chiodi I, Giordano M, Cobianchi F, Riva S, et al. Structural and functional characterization of noncoding repetitive RNAs transcribed in stressed human cells. Mol Biol Cell. 2005;16:2597–2604. doi: 10.1091/mbc.E04-12-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Azzalin CM, Reichenbach P, Khoriauli L, Giulotto E, Lingner J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science. 2007;318:798–801. doi: 10.1126/science.1147182. [DOI] [PubMed] [Google Scholar]
- 220.Schoeftner S, Blasco MA. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nat Cell Biol. 2008;10:228–236. doi: 10.1038/ncb1685. [DOI] [PubMed] [Google Scholar]
- 221.Lee JT, Davidow LS, Warshawsky D. Tsix, a gene antisense to Xist at the X-inactivation centre. Nature Genet. 1999;21:400–404. doi: 10.1038/7734. [DOI] [PubMed] [Google Scholar]
- 222.Luikenhuis S, Wutz A, Jaenisch R. Antisense transcription through the Xist locus mediates Tsix function in embryonic stem cells. Mol Cell Biol. 2001;21:8512–8520. doi: 10.1128/MCB.21.24.8512-8520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sado T, Hoki Y, Sasaki H. Tsix silences Xist through modification of chromatin structure. Dev Cell. 2005;9:159–165. doi: 10.1016/j.devcel.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 224.Wang F, Li X, Xie X, Zhao L, Chen W. UCA1, a non-protein-coding RNA up-regulated in bladder carcinoma and embryo, influencing cell growth and promoting invasion. FEBS Lett. 2008;582:1919–1927. doi: 10.1016/j.febslet.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 225.Sim S, Weinberg DE, Fuchs G, Choi K, Chung J, et al. The subcellular distribution of an RNA quality control protein, the ro autoantigen, is regulated by noncoding y RNA binding. Mol Biol Cell. 2009;20:1555–1564. doi: 10.1091/mbc.E08-11-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Beltran M, Puig I, Pena C, Garcia JM, Alvarez AB, et al. A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial-mesenchymal transition. Genes Dev. 2008;22:756–769. doi: 10.1101/gad.455708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Komine Y, Nakamura K, Katsuki M, Yamamori T. Novel transcription factor zfh-5 is negatively regulated by its own antisense RNA in mouse brain. Mol Cell Neurosci. 2006;31:273–283. doi: 10.1016/j.mcn.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 228.Katayama S, Tomaru Y, Kasukawa T, Waki K, Nakanishi M, et al. Antisense transcription in the mammalian transcriptome. Science. 2005;309:1564–1566. doi: 10.1126/science.1112009. [DOI] [PubMed] [Google Scholar]
- 229.Toledo-Arana A, Repoila F, Cossart P. Small noncoding RNAs controlling pathogenesis. Curr Opin Microbiol. 2007;10:182–188. doi: 10.1016/j.mib.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 230.Pollard KS, Salama SR, Lambert N, Lambot MA, Coppens S, et al. An RNA gene expressed during cortical development evolved rapidly in humans. Nature. 2006;443:167–172. doi: 10.1038/nature05113. [DOI] [PubMed] [Google Scholar]