Summary
To mate, Candida albicans, must undergo homozygosis at the mating type like locus, MTL [1, 2], then switch from the white to opaque phenotype [3, 4]. Paradoxically, when opaque cells are transferred in vitro to 37 °C, the temperature of their animal host, they switch en masse to white [5–7], suggesting that their major niche may not be conducive to mating. It has been suggested that pheromones secreted by opaque cells of opposite mating type [8] or the hypoxic condition of host niches [9, 10] stabilize opaque cells. There is, however, an additional possibility, namely that CO2, which achieves levels in the host 100 times higher than in air [11–14], stabilizes the opaque phenotype. CO2 has been demonstrated to regulate the bud-hypha transition in C. albicans [15], expression of virulence genes in bacteria [16] and mating events in Cryptococcus neoformans [17]. Here we have tested the possibility that CO2 stabilizes the opaque phenotype. It is demonstrated that physiological levels of CO2 induce white-to-opaque switching and stabilize the opaque phenotype at 37 °C. It exerts this control equally under anaerobic and aerobic conditions. These results suggest that the high levels of CO2 in the host induce and stabilize the opaque phenotype, thus facilitating mating.
CO2 Stimulates White to Opaque Switching
The CO2 content of air is 0.03%, but in the animal host it ranges between 4.5 and 30% [11–14]. When white cells of five natural strains homozygous at the MTL locus (Table S1) were plated and incubated in air containing 5% CO2 at 25 °C, the frequency of switching, measured as the proportion of total colonies that are white with opaque regions or completely opaque, was 4 to 16 fold higher than in air (Figure 1A, B). When plated and incubated in air containing 20% CO2, the frequency was 10- to 105-fold higher than in air. CO2 also induced white-to-opaque switching at 37 °C (Figure S1). Variability existed among strains both in air and in the test concentrations of CO2, but switching was induced by high concentrations of CO2 in all strains (Figure 1B).
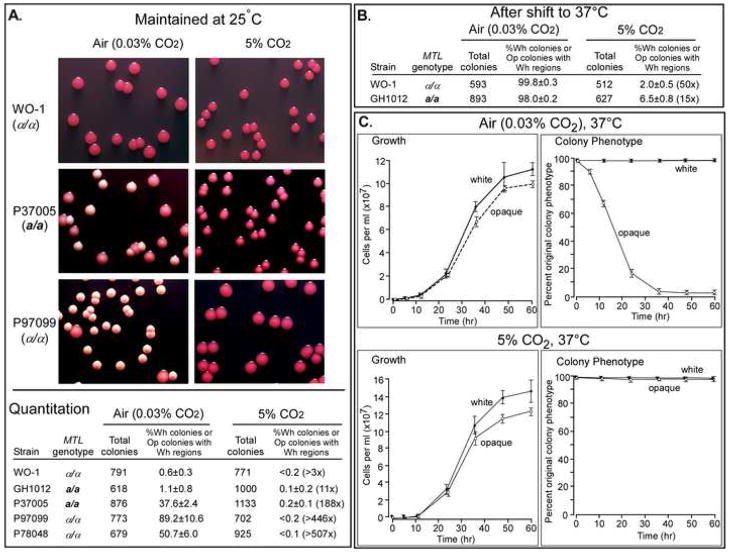
Figure 1.
High concentrations of CO2 induce switching from the white (Wh) to opaque (Op) phenotype in C. albicans. White cells of either α/α or a/a strains were plated on agar and then incubated in air, which contained 0.03% CO2, air (95%) containing 5% CO2, or air (80%) containing 20% CO2 (Experimental Procedures in Supplemental Data). The agar contained phloxine B which stained opaque colonies or opaque regions of white colonies red [28]
(A) Representative fields of colonies for one α/α (WO-1, FC4) and one a/a strain (GH1012).
(B) Quantitation of the frequency of switching measured as the proportion (%) of total colonies that were white with opaque regions or predominately opaque. The total number of colonies is the sum of three experiments. The “% Op colonies or Wh colonies with Op regions” represents the mean for the three experiments ± standard deviation. The fold difference with air is presented for the means at 5 and 20% CO2. The p-values calculated by the student’s two tailed T-test for switching frequencies in 5 and 20% CO2 compared to those in air were all <0.05.
CO2 Blocks Opaque to White Switching
When opaque cells of test strains were plated and incubated at 25 °C in air, two (WO-1, GH1012) exhibited moderate frequencies of switching, and three (P37005, P97099, P78048) high frequencies of switching (Figure 2A). In air containing 5% CO2, the frequency of switching by opaque cells of all five strains was reduced to low or negligible levels (Figure 2A). In addition, 5% CO2 blocked temperature-induced mass conversion of opaque to white [5–7] after a temperature shift from 25 ° to 37 °C (Figure 2B). At 37 °C in air, both white and opaque cells multiplied, but while white cells maintained their phenotype, opaque cells converted en masse to white (Figure 2C). In 5% CO2, however, both white and opaque cells multiplied and maintained their respective phenotypes (Figure 2C).
Figure 2.
CO2 stabilizes the opaque phenotype at 25 ° and 37 °C, and does not interfere with cell multiplication.
(A) Opaque cells of a/a and α/α cells were plated on agar and monitored for switching according to the general procedure outlined in the legend to Figure 1 and Experimental Procedures in Supplemental Data. The p-values calculated by the student’s two tailed T-test of the switching frequencies of P37005, P97099 and P78048 in 5% CO2 compared to those in air were <0.05.
(B) Opaque cells were plated at 37 °C and analyzed for switching to white, according to methods outlined in Experimental Procedures in Supplemental Data. The p-values calculated by the student’s two tailed T-test of WO-1 and GH1012 in 5% CO2 compared to those in air were <0.05.
(C) White or opaque cells were grown in liquid cultures at 37 °C in air or air containing 5% CO2, and monitored for cell number and cell phenotype (Experimental Procedures in Supplemental Data). Cell phenotype was monitored by plating aliquots at time intervals and counting the proportion of colonies with regions of alternative phenotype.
Phase-Specific Gene Expression
To test whether CO2-induced white-to-opaque switching was accompanied by associated changes in gene expression, two white-specific genes, WH11 [18] and EFG1 [19, 20], and two opaque-specific genes, OP4 [21] and WOR1 (TOS9) [22–24], were analyzed by northern blot hybridization. In air, the transcript levels of the two white-specific genes remained high, and the transcript levels of the two opaque-specific genes remained negligible (Figure 3). In 5 or 20% CO2, however, the transcript levels of the two white-specific genes decreased to negligible levels, while those of the two opaque-specific genes increased dramatically (Figure 3).
Figure 3.
CO2-induced switching from white to opaque is accompanied by down-regulation of white-specific and up-regulation of opaque-specific genes
Northern blot hybridization was performed for white- and opaque-specific genes in white cell cultures of strain WO-1 for 0.5, 1, 2 and 3 days on agar in air, air containing 5% CO2 or air containing 20% CO2. The ethidium bromide stained 18S ribosomal RNA bands are shown to demonstrate equal loading of lanes.
CO2 Facilitates Mating
Because CO2 induced white to opaque switching and stabilized the opaque phenotype, we predicted it would facilitate mating in white populations. To test this, crosses were performed in air and 5% CO2, by mixing white cells of strain WUM5A (α/α, ura3−) and MMY278 (a/Δα, ade2−), and white cells of strains CHY477 (Δa/α, ade2−) and 3UM5A (a/a, ura3−) (Table S1). 5% CO2 increased the mating efficiency in the two crosses by 583 and 395 fold, respectively (Table S3).
Roles of Carbonic Anhydrase, Adenylate Cyclase and Ras1
At low atmospheric CO2 levels, carbonic anhydrase (CA) catalyzes the interconversion of CO2 to carbonic acid (HCO3−), which is necessary for obtaining CO2 effects, presumably through HCO3− signaling; at high atmospheric CO2 levels, diffusion across the plasma membrane is high enough so that the uncatalyzed formation of HCO3− is sufficient [15]. In C. albicans, deletion of the gene for carbonic anhydrase, NCE103, blocks growth in air (0.03% CO2), but not in air supplemented with 5% CO2, and affects hypha induction at low but not high CO2 concentrations [15]. We tested whether carbonic anhydrase played a similar role in the induction of switching by CO2 by analyzing the null mutant nce103Δ (Table S1). Since nce103Δ cells did not grow in air [15], white cells of the mutant were first grown on agar in air containing 1% CO2, then plated on fresh agar plates and incubated in 1 or 5% CO2 to test for stimulation. While over 90% of the colonies formed in 1% CO2 (low CO2) by white cells of wild type and complemented strains contained opaque regions, only 22 and 19% of the colonies formed in 1% CO2 by white cells of strain nce103Δ contained opaque regions (Figure 4B). At 5% CO2 (high CO2), however, over 95% of the colonies formed by white cells of both control and mutant strains contained opaque regions (Figure 4B). Therefore, carbonic anhydrase was necessary for wild type levels of switching at low (1%) CO2, but it was not necessary for maximum levels at high (5%) CO2, which is similar to the effects described for CO2-induced filamentation [15].
Figure 4.
CO2 induction of switching from white to opaque in null mutants of carbonic anhydrase (nce103Δ), adenylate cyclase (cdc35Δ), Ras1 (ras1Δ), the master switch locus WOR1 (wor1Δ) and the transcription regulator Czf1 (czf1Δ)
(A) Agar cultures demonstrating that the nce103Δ mutant does not grow in air, but does grow in 5% CO2.
(B) Representative fields of colonies formed by white cells of parent strain GH1013 (Table S1) and nce103Δ in air containing 1 or 5% CO2. Fields of control strains nce103Δ+pACT-NCE103 (the complemented nce103Δ mutant) and nce103Δ +vector (nce103Δ transformed only with the vector) are also presented.
(C) The switching frequencies of the tested mutants, wild type controls and an a/α control, monitored according to methods outlined in the legend to Figure 1 and Experimental Procedures in Supplemental Data.
Klengel et al. [15] demonstrated that CO2 induced the bud-hypha transition by stimulating adenylyl cyclase. This cAMP-dependent pathway in turn required Ras1 [25]. We therefore tested the CO2 effect on switching in null mutants of the adenylyl cyclase gene (cdc35Δ) and RAS1 (ras1Δ) (Table S1). In both mutants, the frequency of switching was lower than that of the control strain in air (Figure 4C). In 1% CO2, switching increased in the two mutant strains, but to levels far below those of control cells (Figure 4C). At 20% CO2, however, switching was maximal, as in control cells (Figure 4C). Therefore, both adenylyl cyclase and Ras1, like carbonic anhydrase, enhanced switching in air and enhanced induction by low but not in high concentrations of CO2.
WOR1 (TOS9) and CZF1
Since the master switch locus WOR1 (TOS9) regulates the white-to-opaque transition [22–24], and overexpression of the transcription factor Czf1 promotes that switch [26], we analyzed the induction of switching by CO2 in the mutants wor1Δ and czf1Δ (Table S1). As expected, switching did not occur in air or at 20% CO2 in white cells of wor1Δ (Figure 4C). CO2 did, however, induce switching in white cells of czf1Δ, but the induced frequencies in 1 and 20% CO2 were lower than that of control cells, as was the basal frequency in air (Figure 4C). As expected, CO2 did not induce switching in a/α cells (Figure 4C).
CO2 and O2
Dumitru et al. [9] found that under anaerobic conditions at 37 °C, opaque cells switched to white at a lower frequency than under aerobic conditions, suggesting that hypoxia stabilized the opaque phenotype. However, opaque cells also divided at a slower rate under anaerobic conditions [9], and switching requires cell division [7, 18]. Therefore the observed reduction in frequency could be due to the decrease in growth rate. Ramirez-Zavala et al. [10] demonstrated that anaerobic conditions induced white cells to switch to opaque, and that induction was regulated by Czf1. Their method to remove O2, however, generated 18% CO2 in the culture jars, a concentration we found induce a maximal rate of switching (Figure 1) and a maximum level of phenotypic stabilization (Figure 2A). To address whether hypoxia alone can stimulate switching, white cells were plated and incubated in either N2 (99.97%) containing no O2 and 0.03% CO2 (the CO2 concentration in air) or N2 (99.47%) containing 0.5% O2 (a 40 fold reduction from that in air) and 0.03% CO2. Rather than stimulating switching, the frequency was reduced 8 and 12 fold, respectively, from that in air (Table S4). When white cells were plated and incubated in either N2 (90%) containing no O2 and 10% CO2, or N2 (89.75%) containing 0.5% O2 and 9.75% CO2, the frequencies of switching were 98.9 and 97.9%, respectively (Table S4). These results demonstrate that hypoxia does not induce white to opaque switching and that CO2 induces switching equally in the absence and presence of O2.
Conclusion
We have, therefore, presented evidence that exposure of white cells to the high levels of CO2 found in host niches induced switching from white to opaque, then maintained the opaque phenotype. These results provide an explanation for observations made twenty years ago that physiological temperatures caused mass conversion of opaque cells to the white phenotype [5–7, 18]. CO2 induced white to opaque switching equally well under anaerobic and aerobic conditions, the former also a characteristic of most host niches. Our results indicated that hypoxia was not an inducer of white-to-opaque switching. At low, but not high, concentrations of CO2, induction was selectively enhanced by carbonic anhydrase, adenylate cyclase and Ras1. Similar dependencies had been observed for CO2 induction of hypha formation [15], which may not be surprising given other similarities between the white-opaque transition and the bud-hypha transition [27]. At both low and high CO2 concentrations, the transcription factor Czf1 promoted the induction of switching. Czf1 had previously been shown to promote white-to-opaque switching [10, 26]. The observation that the basal rates of switching in air as well as the induced rates in low CO2 were depressed in the mutants nce103Δ, cdc35Δ and ras1Δ suggested that what has been referred to as “spontaneous” switching from white-to-opaque in air [5] may in fact represent induced switching by the low level of CO2 (0.03%). We therefore propose that in the host, it is the high level of CO2 that induces the white-to-opaque switch, then stabilizes the mating-competent opaque cell, thus facilitating mating.
Supplementary Material
Acknowledgments
The authors are indebted to Drs. Joachim Morschhäuser at the University of Würzburg, Germany, Alexander Johnson at the University of California, San Francisco, and Gerald R. Fink at Whitehead Institute for Biomedical Research, Massachusetts Institute of Technology for the generous gifts of plasmids and strains. The authors are also indebted to Dr. Karla Daniels for help in assembling the figures and Dr. Claude Pujol for comments in the manuscript. This research was supported by NIH grant AI2392 and the Developmental Studies Hybridoma Bank-Microbe at Iowa.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hull CM, Raisner RM, Johnson AD. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science. 2000;289:307–310. doi: 10.1126/science.289.5477.307. [DOI] [PubMed] [Google Scholar]
- 2.Magee BB, Magee PT. Induction of mating in Candida albicans by construction of MTLa and MTLα strains. Science. 2000;289:310–313. doi: 10.1126/science.289.5477.310. [DOI] [PubMed] [Google Scholar]
- 3.Miller MG, Johnson AD. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell. 2002;110:293–302. doi: 10.1016/s0092-8674(02)00837-1. [DOI] [PubMed] [Google Scholar]
- 4.Soll DR, Lockhart SR, Zhao R. Relationship between switching and mating in Candida albicans. Eukaryot Cell. 2003;2:390–397. doi: 10.1128/EC.2.3.390-397.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slutsky B, Staebell M, Anderson J, Risen L, Pfaller M, Soll DR. White-opaque transition”: a second high-frequency switching system in Candida albicans. J Bacteriol. 1987;169:189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soll DR. Mating-type locus homozygosis, phenotypic switching and mating: a unique sequence of dependencies in Candida albicans. Bioessays. 2004;26:10–20. doi: 10.1002/bies.10379. [DOI] [PubMed] [Google Scholar]
- 7.Rikkerink EH, Magee BB, Magee PT. Opaque-white phenotype transition: a programmed morphological transition in Candida albicans. J Bacteriol. 1988;170:895–899. doi: 10.1128/jb.170.2.895-899.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soll DR. Evolution of a mating system uniquely dependent upon switching and pathogenesis in Candida albicans. In: Baquero F, Nombela C, Cassell GH, Gutierrez-fuentes JA, editors. Evolutionary Biology of Bacterial and Fungal Pathogens. ASM Press; 2008. pp. 213–220. [Google Scholar]
- 9.Dumitru R, Navarathna DH, Semighini CP, Elowsky CG, Dumitru RV, Dignard D, Whiteway M, Atkin AL, Nickerson KW. In vivo and in vitro anaerobic mating in Candida albicans. Eukaryot Cell. 2007;6:465–472. doi: 10.1128/EC.00316-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramirez-Zavala B, Reuss O, Park YN, Ohlsen K, Morschhauser J. Environmental induction of white-opaque switching in Candida albicans. PLoS Pathog. 2008;4:e1000089. doi: 10.1371/journal.ppat.1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stenni B, Masson-Delmotte V, Johnsen S, Jouzel J, Longinelli A, Monnin E, Rothlisberger R, Selmo E. An oceanic cold reversal during the last deglaciation. Science. 2001;293:2074–2077. doi: 10.1126/science.1059702. [DOI] [PubMed] [Google Scholar]
- 12.Levitt MD, Bond JH., Jr Volume, composition, and source of intestinal gas. Gastroenterology. 1970;59:921–929. [PubMed] [Google Scholar]
- 13.Canan Avunduk. Manual of Gastroenterology: Diagnosis and Therapy. 3. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 14.Guyton AC, Hall JE. Textbook of Medical Physiology. Philadelphia, PA: W.B. Saunders; 2000. [Google Scholar]
- 15.Klengel T, Liang WJ, Chaloupka J, Ruoff C, Schroppel K, Naglik JR, Eckert SE, Mogensen EG, Haynes K, Tuite MF, et al. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol. 2005;15:2021–2026. doi: 10.1016/j.cub.2005.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmaster AR, Koehler TM. The anthrax toxin activator gene atxA is associated with CO2-enhanced non-toxin gene expression in Bacillus anthracis. Infect Immun. 1997;65:3091–3099. doi: 10.1128/iai.65.8.3091-3099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahn YS, Cox GM, Perfect JR, Heitman J. Carbonic anhydrase and CO2 sensing during Cryptococcus neoformans growth, differentiation, and virulence. Curr Biol. 2005;15:2013–2020. doi: 10.1016/j.cub.2005.09.047. [DOI] [PubMed] [Google Scholar]
- 18.Srikantha T, Soll DR. A white-specific gene in the white-opaque switching system of Candida albicans. Gene. 1993;131:53–60. doi: 10.1016/0378-1119(93)90668-s. [DOI] [PubMed] [Google Scholar]
- 19.Sonneborn A, Tebarth B, Ernst JF. Control of white-opaque phenotypic switching in Candida albicans by the Efg1p morphogenetic regulator. Infect Immun. 1999;67:4655–4660. doi: 10.1128/iai.67.9.4655-4660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srikantha T, Tsai LK, Daniels K, Soll DR. EFG1 null mutants of Candida albicans switch but cannot express the complete phenotype of white-phase budding cells. J Bacteriol. 2000;182:1580–1591. doi: 10.1128/jb.182.6.1580-1591.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrow B, Srikantha T, Anderson J, Soll DR. Coordinate regulation of two opaque-phase-specific genes during white-opaque switching in Candida albicans. Infect Immun. 1993;61:1823–1828. doi: 10.1128/iai.61.5.1823-1828.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zordan RE, Galgoczy DJ, Johnson AD. Epigenetic properties of white-opaque switching in Candida albicans are based on a self-sustaining transcriptional feedback loop. Proc Natl Acad Sci U S A. 2006;103:12807–12812. doi: 10.1073/pnas.0605138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang G, Wang H, Chou S, Nie X, Chen J, Liu H. Bistable expression of WOR1, a master regulator of white-opaque switching in Candida albicans. Proc Natl Acad Sci U S A. 2006;103:12813–12818. doi: 10.1073/pnas.0605270103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srikantha T, Borneman AR, Daniels KJ, Pujol C, Wu W, Seringhaus MR, Gerstein M, Yi S, Snyder M, Soll DR. TOS9 regulates white-opaque switching in Candida albicans. Eukaryot Cell. 2006;5:1674–1687. doi: 10.1128/EC.00252-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leberer E, Harcus D, Dignard D, Johnson L, Ushinsky S, Thomas DY, Schroppel K. Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans. Mol Microbiol. 2001;42:673–687. doi: 10.1046/j.1365-2958.2001.02672.x. [DOI] [PubMed] [Google Scholar]
- 26.Vinces MD, Kumamoto CA. The morphogenetic regulator Czf1p is a DNA-binding protein that regulates white opaque switching in Candida albicans. Microbiology. 2007;153:2877–2884. doi: 10.1099/mic.0.2007/005983-0. [DOI] [PubMed] [Google Scholar]
- 27.Anderson J, Cundiff L, Schnars B, Gao MX, Mackenzie I, Soll DR. Hypha formation in the white-opaque transition of Candida albicans. Infect Immun. 1989;57:458–467. doi: 10.1128/iai.57.2.458-467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson JM, Soll DR. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J Bacteriol. 1987;169:5579–5588. doi: 10.1128/jb.169.12.5579-5588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.