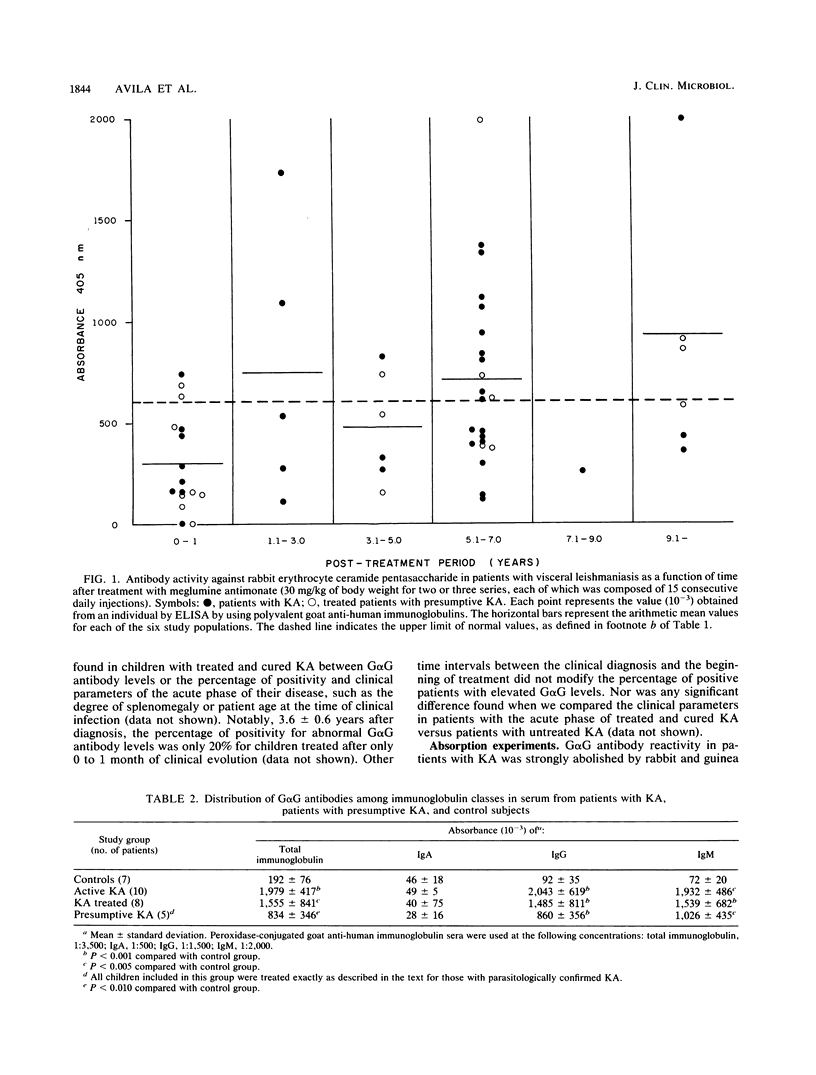
Abstract
Using rabbit erythrocyte-derived neutral glycosphingolipids enriched for a ceramide pentasaccharide as the antigen, we detected elevated anti-galactosyl-alpha(1-3)galactose (anti-G alpha G) antibody levels in 76% of children with active visceral leishmaniasis (kala-azar [KA]) and in 42% of clinically cured patients with KA who had been treated about 5 years previously with meglumine antimonate (30 mg/kg in a series of 15 daily injections). The long-term persistence of elevated G alpha G antibodies was also found in 56% of children living in the same geographic zone who, at the time of the initial clinical examination, had fever and evident splenomegaly with hyperglobulinemia but a negative bone marrow aspirate for leishmanial bodies. Five years after antimonate treatment, these clinically cured children with presumptive KA were studied serologically. Their mean G alpha G antibody values were slightly lower than those in patients with active KA but were still abnormal. Using different biochemical and immunological approaches, we found that elevated G alpha G antibodies present in patients with KA bound specifically to glycoconjugates with an alpha(1-3)-terminal galactose residue. G alpha G antibodies were mainly distributed between immunoglobulin classes G and M in patients with active KA and in antimonate-treated patients with clinically cured KA. The possibility of the existence of remnant living parasites or the persistence of inserted G alpha G epitopes in parasitized macrophages was proposed as a mechanism to explain the long-term persistence of abnormal G alpha G antibodies in patients apparently cured of KA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER S. LEISHMANIA. Adv Parasitol. 1964;2:35–96. doi: 10.1016/s0065-308x(08)60586-2. [DOI] [PubMed] [Google Scholar]
- Avila J. L., Bretaña A., Casanova M. A., Avila A., Rodríguez F. Trypanosoma cruzi: defined medium for continuous cultivation of virulent parasites. Exp Parasitol. 1979 Aug;48(1):27–35. doi: 10.1016/0014-4894(79)90051-1. [DOI] [PubMed] [Google Scholar]
- Avila J. L., Rojas M., Rieber M. Antibodies to laminin in American cutaneous leishmaniasis. Infect Immun. 1984 Jan;43(1):402–406. doi: 10.1128/iai.43.1.402-406.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J. L., Rojas M., Towbin H. Serological activity against galactosyl-alpha(1-3)galactose in sera from patients with several kinetoplastida infections. J Clin Microbiol. 1988 Jan;26(1):126–132. doi: 10.1128/jcm.26.1.126-132.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J. L., Rojas M., Velazquez-Avila G., von der Mark H., Timpl R. Antibodies to basement membrane protein nidogen in Chagas' disease and American cutaneous leishmaniasis. J Clin Microbiol. 1986 Nov;24(5):775–778. doi: 10.1128/jcm.24.5.775-778.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J. L., Rojas M., Velázquez-Avila G., Rieber M. Antibodies to basement membrane proteins nidogen and laminin in sera from streptococcal-related diseases and juvenile rheumatoid arthritis patients. Clin Exp Immunol. 1987 Dec;70(3):555–561. [PMC free article] [PubMed] [Google Scholar]
- Badaró R., Carvalho E. M., Rocha H., Queiroz A. C., Jones T. C. Leishmania donovani: an opportunistic microbe associated with progressive disease in three immunocompromised patients. Lancet. 1986 Mar 22;1(8482):647–649. doi: 10.1016/s0140-6736(86)91725-3. [DOI] [PubMed] [Google Scholar]
- Bryceson A. D., Chulay J. D., Ho M., Mugambii M., Were J. B., Muigai R., Chunge C., Gachihi G., Meme J., Anabwani G. Visceral leishmaniasis unresponsive to antimonial drugs. I. Clinical and immunological studies. Trans R Soc Trop Med Hyg. 1985;79(5):700–704. doi: 10.1016/0035-9203(85)90197-x. [DOI] [PubMed] [Google Scholar]
- Casali P., Lambert P. H. Purification of soluble immune complexes from serum using polymethylmetacrylate beads coated with conglutinin or C1q. Application to the analysis of the components of in vitro formed immune complexes and of immune complexes occurring in vivo during leishmaniasis. Clin Exp Immunol. 1979 Aug;37(2):295–309. [PMC free article] [PubMed] [Google Scholar]
- Chavas J., Guimarães Ferri R. Immunoglobulins in visceral leishmaniasis. Rev Inst Med Trop Sao Paulo. 1966 Sep-Oct;8(5):225–226. [PubMed] [Google Scholar]
- Davin J. C., Malaise M., Foidart J., Mahieu P. Anti-alpha-galactosyl antibodies and immune complexes in children with Henoch-Schönlein purpura or IgA nephropathy. Kidney Int. 1987 May;31(5):1132–1139. doi: 10.1038/ki.1987.119. [DOI] [PubMed] [Google Scholar]
- Duarte M. I., Silva M. R., Goto H., Nicodemo E. L., Amato Neto V. Interstitial nephritis in human kala-azar. Trans R Soc Trop Med Hyg. 1983;77(4):531–537. doi: 10.1016/0035-9203(83)90131-1. [DOI] [PubMed] [Google Scholar]
- Etienne-Decerf J., Malaise M., Mahieu P., Winand R. Elevated anti-alpha-galactosyl antibody titres. A marker of progression in autoimmune thyroid disorders and in endocrine ophthalmopathy? Acta Endocrinol (Copenh) 1987 May;115(1):67–74. doi: 10.1530/acta.0.1150067. [DOI] [PubMed] [Google Scholar]
- Galili U., Basbaum C. B., Shohet S. B., Buehler J., Macher B. A. Identification of erythrocyte Gal alpha 1-3Gal glycosphingolipids with a mouse monoclonal antibody, Gal-13. J Biol Chem. 1987 Apr 5;262(10):4683–4688. [PubMed] [Google Scholar]
- Galvão-Castro B., Sá Ferreira J. A., Marzochi K. F., Marzochi M. C., Coutinho S. G., Lambert P. H. Polyclonal B cell activation, circulating immune complexes and autoimmunity in human american visceral leishmaniasis. Clin Exp Immunol. 1984 Apr;56(1):58–66. [PMC free article] [PubMed] [Google Scholar]
- Ghose A. C., Haldar J. P., Pal S. C., Mishra B. P., Mishra K. K. Serological investigations on Indian kala-azar. Clin Exp Immunol. 1980 May;40(2):318–326. [PMC free article] [PubMed] [Google Scholar]
- Jahn A., Diesfeld H. J. Evaluation of a visually read ELISA for serodiagnosis and sero-epidemiological studies of kala-azar in the Baringo District, Kenya. Trans R Soc Trop Med Hyg. 1983;77(4):451–454. doi: 10.1016/0035-9203(83)90110-4. [DOI] [PubMed] [Google Scholar]
- Knobloch J., Mannweiler E. Development and persistence of antibodies to Entamoeba histolytica in patients with amebic liver abscess. Analysis of 216 cases. Am J Trop Med Hyg. 1983 Jul;32(4):727–732. doi: 10.4269/ajtmh.1983.32.727. [DOI] [PubMed] [Google Scholar]
- Levis W. R., Meeker H. C., Schuller-Levis G., Sersen E., Schwerer B. IgM and IgG antibodies to phenolic glycolipid I from Mycobacterium leprae in leprosy: insight into patient monitoring, erythema nodosum leprosum, and bacillary persistence. J Invest Dermatol. 1986 May;86(5):529–534. doi: 10.1111/1523-1747.ep12354963. [DOI] [PubMed] [Google Scholar]
- Libby P., Alroy J., Pereira M. E. A neuraminidase from Trypanosoma cruzi removes sialic acid from the surface of mammalian myocardial and endothelial cells. J Clin Invest. 1986 Jan;77(1):127–135. doi: 10.1172/JCI112266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANSON-BAHR P. E. Immunity in kala-azar. Trans R Soc Trop Med Hyg. 1961 Nov;55:550–555. doi: 10.1016/0035-9203(61)90078-5. [DOI] [PubMed] [Google Scholar]
- Ma D. D., Concannon A. J., Hayes J. Fatal leishmaniasis in renal-transport patient. Lancet. 1979 Aug 11;2(8137):311–312. doi: 10.1016/s0140-6736(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Maddox D. E., Shibata S., Goldstein I. J. Stimulated macrophages express a new glycoprotein receptor reactive with Griffonia simplicifolia I-B4 isolectin. Proc Natl Acad Sci U S A. 1982 Jan;79(1):166–170. doi: 10.1073/pnas.79.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonça-Previato L., Gorin P. A., Braga A. F., Scharfstein J., Previato J. O. Chemical structure and antigenic aspects of complexes obtained from epimastigotes of Trypanosoma cruzi. Biochemistry. 1983 Oct 11;22(21):4980–4987. doi: 10.1021/bi00290a016. [DOI] [PubMed] [Google Scholar]
- Neva F. A., Wyler D., Nash T. Cutaneous leishmaniasis--a case with persistent organisms after treatment in presence of normal immune response. Am J Trop Med Hyg. 1979 May;28(3):467–471. [PubMed] [Google Scholar]
- PIFANO F., SCORZA J. V. [Immunological aspects of Leishmania that parasite man, with special reference to Leishmania brasiliensis pifanoi, Medina & Romero, 1957]. Arch Venez Med Trop Parasitol Med. 1960 Dec;3:15–30. [PubMed] [Google Scholar]
- Palatnik C. B., Previato J. O., Gorin P. A., Mendonça Previato L. Partial chemical characterization of the carbohydrate moieties in Leishmania adleri glycoconjugates. Mol Biochem Parasitol. 1985 Jan;14(1):41–54. doi: 10.1016/0166-6851(85)90104-5. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T. Mechanism of lethal effect of human serum upon Leishmania donovani. J Immunol. 1980 Nov;125(5):2195–2201. [PubMed] [Google Scholar]
- Pontes De Carvalho L. C., Badaró R., Carvalho E. M., Lannes-Vieira J., Vinhaes L., Orge G., Marsochi M. C., Galvão-Castro B. Nature and incidence of erythrocyte-bound IgG and some aspects of the physiopathogenesis of anaemia in American visceral leishmaniasis. Clin Exp Immunol. 1986 Jun;64(3):495–502. [PMC free article] [PubMed] [Google Scholar]
- Szarfman A., Terranova V. P., Rennard S. I., Foidart J. M., de Fatima Lima M., Scheinman J. I., Martin G. R. Antibodies to laminin in Chagas' disease. J Exp Med. 1982 Apr 1;155(4):1161–1171. doi: 10.1084/jem.155.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Rosenfelder G., Wieslander J., Avila J. L., Rojas M., Szarfman A., Esser K., Nowack H., Timpl R. Circulating antibodies to mouse laminin in Chagas disease, American cutaneous leishmaniasis, and normal individuals recognize terminal galactosyl(alpha 1-3)-galactose epitopes. J Exp Med. 1987 Aug 1;166(2):419–432. doi: 10.1084/jem.166.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk J. L., Bryceson A. D. Immunological phenomena in leprosy and related diseases. Adv Immunol. 1971;13:209–266. doi: 10.1016/s0065-2776(08)60185-6. [DOI] [PubMed] [Google Scholar]
- Wijers D. J. A 10 years' study of kala-azar in Tharaka (Meru district, Kenya). II. Relapses. East Afr Med J. 1971 Oct;48(10):551–558. [PubMed] [Google Scholar]
- Williams K. M., Sacci J. B., Anthony R. L. Identification and recovery of Leishmania antigen displayed on the surface membrane of mouse peritoneal macrophages infected in vitro. J Immunol. 1986 Mar 1;136(5):1853–1858. [PubMed] [Google Scholar]


