Abstract
In a chemical mutagenesis screen, we identified the novel Scn8a8J allele of the gene encoding the neuronal voltage-gated sodium channel Nav1.6. The missense mutation V929F in this allele alters an evolutionarily conserved residue in the pore loop of domain 2 of Nav1.6. Electroencephalography (EEG) revealed well-defined spike-wave discharges (SWD), the hallmark of absence epilepsy, in Scn8a8J heterozygotes and in heterozygotes for two classical Scn8a alleles, Scn8amed (null) and Scn8amed-jo (missense). Mouse strain background had a significant effect on SWD, with mutants on the C3HeB/FeJ strain showing a higher incidence than on C57BL/6J. The abnormal EEG patterns in heterozygous mutant mice and the influence of genetic background on SWD make SCN8A an attractive candidate gene for common human absence epilepsy, a genetically complex disorder.
INTRODUCTION
The voltage-gated sodium channels include four genes with high expression levels in neurons of the central nervous system: SCN1A, which encodes Nav1.1, SCN2A encoding Nav1.2, SCN3A encoding Nav1.3 and SCN8A encoding Nav1.6. Voltage-gated sodium channels are responsible for initiation and propagation of transient depolarizing currents and play an important role in electrical signaling between cells. In patients with epilepsy, >300 mutations in SCN1A have been identified, the vast majority of which are de novo mutations in children with severe myoclonic epilepsy of infancy (1,2). A small number of mutations have been identified in SCN2A and SCN3A in other inherited epilepsies (1,3). Structure/function relationships of the mutated sodium channels remain unclear, and most of the mutations seem to affect multiple biophysical parameters. One frequent feature of pathogenic missense mutations is an increase in persistent sodium current that may facilitate premature and sustained firing.
To date, 13 mutant alleles of mouse Scn8a have been described, including seven spontaneous and four N-ethyl-N-nitrosourea (ENU)-induced alleles (4). Electrophysiological analyses of Scn8a mutant mice have revealed several unique features of Nav1.6 mediated sodium currents. This channel is responsible for an unusual conductance termed the resurgent current that flows during repolarization, and also contributes to persistent and subthreshold sodium currents in various types of neurons (5–9). Nav1.6 is localized to the axon initial segment and it is the major sodium channel at the nodes of Ranvier in adult myelinated axons (10,11).
Mice with Scn8a mutations often exhibit ataxic gait, consistent with the critical role of Nav1.6 in the repetitive firing of Purkinje cells. Tremor and loss of hind limb function are also common in homozygous Scn8a mutants, and null homozygotes do not survive beyond 3 weeks of age. Conditional inactivation of a floxed allele of Scn8a in both cerebellar Purkinje and granule cells is sufficient to produce ataxic gait and tremor, demonstrating the critical role of cerebellar Nav1.6 in normal locomotion (12). Although heterozygous mice with null mutations are not ataxic, they do exhibit subtle behavioral deficits (13). Furthermore, heterozygous patients in a human family segregating a null allele of SCN8A exhibited cognitive and behavioral deficits (14).
Absence epilepsy is a non-convulsive form of idiopathic generalized epilepsy that is characterized by recurrent seizures in patients without brain lesions. It often occurs in childhood and adolescence, with a prevalence of ∼10% among children with any type of epilepsy. Typical absence seizures are associated with 3–4 Hz spike-wave discharges (SWD) on the electroencephalogram (EEG) and are characterized by a brief loss of consciousness of abrupt onset and termination coincident with SWD. Although the general clinical features of absence seizures have been known for decades and a genetic etiology is suspected, a full understanding of the genetic basis of absence epilepsy, and molecular and cellular mechanisms have been elusive.
There are now several rodent models of absence epilepsy with genetic etiology, including polygenic models such as the WAG/Rij (15) and GAERS (16) rat strains and the inbred mouse strain A/J (17). The inbred mouse strain C3H/HeJ carries a mutation in Gria4, encoding an AMPA receptor subunit, which has a major effect on the generation of absence seizures (18). Mutations in each of the four major subunits of voltage-gated Ca2+ channels (19–22) and the knockout of Hcn2 encoding an Ih channel (23) have provided monogenic mouse models of recessively inherited absence epilepsy. The knockout of Cacna1g, encoding a T-type Ca2+ channel subunit, confers resistance to chemically and genetically induced absence seizures (24), consistent with the observation that dominant mutations in human CACNA1G can lead to absence epilepsy (25). A mutation in a splice variant of the T-type calcium channel gene Cacna1h has also been shown to contribute to the absence epilepsy phenotype in the GAERS rat model (26). These results underscore the central role of T-type Ca2+ channels in generating the normal thalamocortical oscillations that become impaired during absence seizures.
Absence seizures are also observed in a knock-in mouse model with the human R43Q point mutation in GABRG2, encoding a GABAA receptor subunit, which was identified in a family with a spectrum of generalized seizure phenotypes (27,28). Until now, the Gabrg2 R43Q knock-in mouse had been the only model of dominantly inherited absence epilepsy reported. Here we describe dominantly inherited absence epilepsy associated with three alleles of the sodium channel Scn8a and demonstrate that the severity of the phenotype is influenced by genetic background, making SCN8A an intriguing candidate gene for human absence epilepsy.
RESULTS
Origin of Scn8a8J
The new allele of Scn8a was initially detected in neurologically impaired animals from a standard three-generation ENU mutagenesis screen for recessive mutations carried out in the ReproGenomics facility at Jackson Laboratory. C57BL/6J (B6) mice were mutagenized and subsequently crossed to strain C3HeB/FeJ (FeJ) to generate potentially homozygous G3 animals (see http://reproductivegenomics.jax.org). In pedigree 596-16, 16 of 38 G3 mice were smaller than wild-type littermates and had impaired locomotor function with a weak and unsteady gait. Since neither parent was affected, the trait was presumed to be recessive. Homozygous mutants usually did not survive past 21 days, although a few survived as long as 7 weeks when left with the dams and fed with ground rather than solid grain pellets.
Genetic mapping and identification of the ENU-induced mutation in Scn8a
The recessive locomotor trait was mapped to telomeric chromosome 15 in a genome scan of 73 G3 and G3F1 mice including both affected and unaffected individuals (Fig. 1A). A survey of candidate genes located within the linked region identified the Scn8a gene at 100.7 Mb (Fig. 1A). Mutations of Scn8a are known to produce locomotor abnormalities (4). To determine whether line 596-16 carried a new allele of Scn8a, we carried out a complementation test by crossing unaffected carriers from line 596-16 with heterozygotes for the med allele of Scn8a, a null mutant (29). Among six litters with 38 offspring from the complementation cross, eight mice exhibited locomotor impairment at 3 weeks of age that was similar to the parental homozygous mice. This observed non-complementation is consistent with allelism of the new mutant with Scn8a.
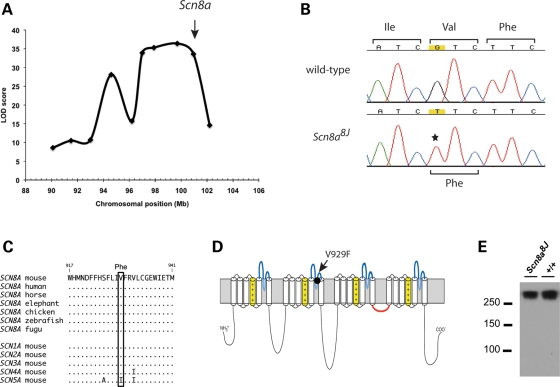
Figure 1.
Genetic mapping and identification of the Scn8a8J mutation. (A) LOD score plot of locomotor impairment in mice from G3 and G3F1 populations. The markers from left to right are D15Mit171 (90.1 Mb), D15Mit243 (93.1 Mb), D15Jmp29 (96.2 Mb), D15Mit161 (97.0 Mb), D15Mit43 (97.9 Mb), D15Mit14 (99.7 Mb), D15Mit246 (102.1 Mb) and D15Mit79 (103.4 Mb). Scn8a is located at 100.7 Mb (arrow). The maximum LOD score was 36.4 at 99.7 Mb. (B) Identification of the mutation (G>T) in a homozygote from the 596-16 pedigree. (C) Evolutionary conservation of residue valine 929 in eight vertebrate orthologs and five mouse paralogs of Scn8a. (D) Location of V929 in the ion pore region of domain 2. (E) Western blot of membrane protein from mutant and wild-type mouse brain. Lanes contained 75 µg of protein.
Identification of the Scn8a mutation
The 28 exons of Scn8a were amplified from affected mice and sequenced. We detected the mutation c.2785G>T in exon 13, which is predicted to change the amino acid valine 929 to phenylalanine (p.V929F) (Fig. 1B). Valine residue 929 is conserved in the vertebrate orthologs of Scn8a and in the neuronal paralogs Scn1a, Scn2a and Scn3a (Fig. 1C). This chemically non-conservative substitution is located in the pore region of domain 2 (Fig. 1D). Mutations in the pore regions of the sodium channel are frequently pathogenic in mouse and humans (30). Western blot analysis of brain membrane proteins demonstrated that Nav1.6 protein is present at normal levels in Scn8a8J homozygotes (Fig. 1E), suggesting that the V929F substitution may alter channel function.
SWD in Scn8a8J mutant mice
SWD were initially observed in two affected homozygous Scn8a8J mice from the G3 generation. Each had a very high incidence of SWD, with a burst frequency of 4–5 Hz (Supplementary Material, Fig. S1). Scn8a8J heterozygotes also exhibited prevalent SWD, with a burst frequency of 7–9 Hz, which is more typical of rodent models of absence epilepsy. The SWD were robust, with high amplitude, significant duration and high frequency, and were observed in most or all recording channels (Fig. 2A). SWD usually occurred between periods of locomotor activity. During episodes of SWD mice were immobile except for occasional whisker twitching, as ascertained in real-time and by video-EEG. Although the C3HeB/FeJ strain has a low incidence of SWD (18,31), in the present crosses there was a highly significant difference in the incidence of SWD between Scn8a8J heterozygotes and their wild-type littermates, indicating a major gene effect of Scn8a8J (Fig. 3, G3× C3HeB/FeJ; P = 7.0 × 10−5). The high incidence of SWD was retained after heterozygotes were backcrossed to strain C3HeB/FeJ for two more generations (Fig. 3, G3N2 and G3N3).
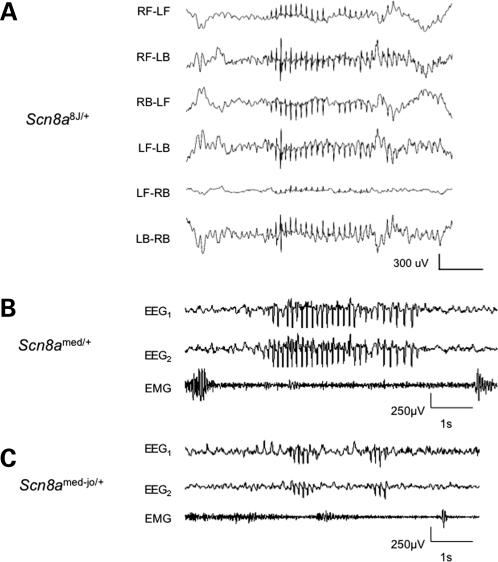
Figure 2.
Representative SWD in Scn8a mutants. (A) Traces correspond to six differential recordings from a Scn8a8J/+ mouse from each of four epidural electrodes; RF–LF (right front–left front), RF–LB (right front–left back), etc. carried out at Jackson Laboratory as described in Materials and Methods. SWD data from individual Scn8a8J/+ mice is shown in Figure 4. (B) and (C) Representative EEG traces from Scn8amed/+ and Scn8amed-jo/+ mutants, respectively, carried out at Emory University as described in Materials and Methods. EEG1 and EEG2 represent cortical activity from the right and left hemispheres, respectively. SWD are present in both hemispheres and are associated with lack of muscle movement.
Figure 3.
Effect of genetic background on incidence of SWD in heterozygous Scn8a8Jmice. Each point shows the number of SWD per hour for an individual mouse, in different genetic backgrounds. Heterozygous (het) genotypes are shown in filled symbols, wild-type (wt) in open symbols. The inset shows a plot of the average seizure frequency (±SE) in the four Scn8a8J genotypes. The average seizure frequency per hour (±SE) are: G3× C3HeB/FeJ: 132.1 ± 15.4 (het), 22.3 ± 4.3 (wt); G3N2 and G3N3: 104.6 ± 14.3 (het); (G3N3 X C57BL/6J)F1: 80.2 ± 12 (het), 0.8 ± .5 (wt); F1 X C57BL/6J: 60.6 ± 7.8 (het); C3HeB/FeJ–Scn8amed/+ 67.9 ± 2.5.
SWD in two additional Scn8a mutants
To determine whether the SWD in Scn8a8J heterozygotes was also present in other Scn8a mutants, we examined two classical alleles of Scn8a. Scn8amed is a loss-of-function allele (29), and Scn8amed-jo is a missense mutation that alters the voltage dependence of activation and inactivation (32). At Emory University, we examined 12 h (7 am to 7 pm) of continuous EEG data from six heterozygotes for Scn8amed/+ and Scn8amed-jo/+. Both alleles conferred high-amplitude SWD of variable duration accompanied by behavior arrest (Fig. 2B and C). The average number of SWD was significantly higher in the Scn8amed/+ mice compared with wild-type littermates (Scn8amed/+, 156 ± 28.7 SE (n = 6); wild-type, 31.8 ± 5.7 SE (n = 6), P = 0.002). However, the average incidence of SWD in Scn8amed-jo/+ mutants was significantly lower than in Scn8amed/+ mice (Scn8amed-jo/+, 20.7 ± 5.7 SE (n = 6), P = 0.0006), and we observed no SWD in their wild-type littermates. Because the two laboratories performing EEG analysis use electrode preparations with different sensitivities, it was difficult to directly compare the SWD incidence caused by the Scn8a8J allele with that by the two classical Scn8a alleles. However, analysis of four C3HeB/FeJ-Scn8amed/+ mutants at Jackson Laboratory revealed a slightly lower incidence of SWD than that of Scn8a8J (Fig. 3, far right; P = 0.05).
Reduction in SWD after treatment with ethosuximide
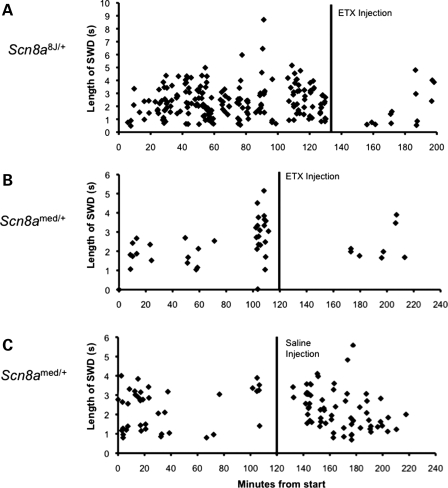
Ethosuximide (ETX) is often the first-line drug for the treatment of absence epilepsy in patients. It is quite selective in its anti-epileptic activity, being ineffective against convulsive and focal seizures. ETX has also been shown to reduce the frequency of SWD in rodent models of absence epilepsy (33). To further characterize the seizure phenotype of the Scn8a mutants, we evaluated the effect of acute ETX treatment on the frequency of SWD in heterozygous Scn8a8J (Fig. 4A) and Scn8amed (Fig. 4B) mutants. The administration of ETX resulted in fewer and shorter SWD episodes in both mutants, consistent with the classification of absence seizures. At Jackson Laboratory, three Scn8a8J/+ mutants had an average of 85.7 (±5.0 SE) SWD in the 60 min interval prior to ETX administration and 14.7 SWD (±5.8 SE) after ETX, a 6-fold decrease. At Emory, six Scn8amed/+ mutants averaged 15.1 (±3.96 SE) SWD in the 60 min interval prior to ETX administration and 1.4 (±0.47 SE) SWD in the 60 min after ETX administration, an 11-fold decrease. To control for the effect of handling, we also determined the frequency of SWD in Scn8amed/+ mice after the injection of saline (Fig. 4C). The frequency of SWD in six saline-injected mice increased 3-fold from 13 (±1.33 SE) SWD in the 60 min interval prior to saline injection to 34.6 (±10.9 SE) SWD in the 60 min interval after injection, suggesting that the ETX results are a conservative measure of the pharmacological effect.
Figure 4.
Effect of ETX treatment on SWD in Scn8a mutants. ETX treatment decreased the length and incidence of SWD. (A) Scn8a8J/+ examined at Jackson Laboratory. (B) Scn8amed/+ tested at Emory University. (C) Effect of the injection procedure on the incidence of SWD. Each diamond represents a single spike-wave discharge, and all of the SWD for a single mouse are shown in each panel. SWD were decreased following ETX administration and increased by the handling associated with saline injection. The average incidence before and after treatment as well as sample sizes are given in Results.
Genetic background influences the incidence of SWD in Scn8a8Jmice
In prior studies, SWD were not detected in the ENU-induced mutation Scn8anmf58, with the amino acid substitution L1404H in the pore region of Scn8a domain 3. Scn8anmf58 mice in those studies were generated and tested on the C57BL/6J background (30). In the present study, the fewest SWD were observed in Scn8amed-jo mice, also on the C57BL/6J background, whereas the other two alleles are on C3HeB/FeJ. To determine whether the C3HeB/FeJ background is an important factor in the incidence of Scn8a-associated SWD, we crossed G3N3 heterozygous Scn8a8J mice to strain C57BL/6J and tested the F1 hybrid heterozygotes (∼50% C3H-derived) and the first backcross (N2 generation; ∼25% C3H-derived; Fig. 3; G3N3, F1× C57BL/6J). The results, showing a linear difference over a 2-fold range between genotypes (Fig. 3 inset), suggest that SWD in Scn8a8J is enhanced by homozygosity for C3HeB/FeJ strain background alleles (P = 0.001) and suppressed by homozygosity for B6 alleles (P = 1.8 × 10−7).
Effect of different states of vigilance on the frequency of SWD
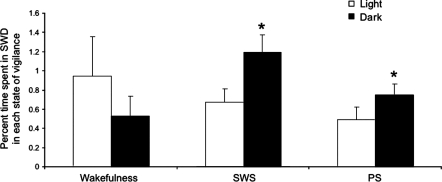
A relationship between the incidence of absence seizures and states of vigilance has been noted in humans and animals (34). To examine this relationship in Scn8amed/+ mutants, we analyzed 48 h of continuous EEG data and compared the average incidence of SWD in wakefulness, slow-wave sleep (SWS) and paradoxical sleep (PS) during the 12 h dark cycle with the 12 h light cycle. Scn8amed/+ mutants showed a similar frequency of SWD during wakefulness in the light and dark cycles (Fig. 5, P = 0.06). However, the frequency of SWD during SWS and PS was significantly higher during the dark cycle compared with the corresponding states of vigilance in the light cycle (Fig. 5, P = 0.03 for both comparisons).
Figure 5.
Relationship between SWD and states of vigilance in Scn8amed/+ mice. The percentage of time spent in SWD during wakefulness was similar in the light and dark cycles. A greater amount of time was spent in SWD during slow-wave sleep (SWS) and paradoxical sleep (PS) in the dark cycle when compared with the light cycle. The percentage of time spent in SWD during wakefulness, SWS and PS was compared between the light and dark cycles using the Wilcoxon signed-rank test implemented in SAS software. *, statistical difference between percentage time spent in SWD in light and dark cycles.
DISCUSSION
In this study, we show for the first time that heterozygous Scn8a mutant mice exhibit the hallmark of absence epilepsy—ETX-sensitive SWD with accompanying brief arrest of normal activity. We observed SWD in mice carrying three alleles: Scn8a8J, a new allele with a non-conservative amino acid substitution in the pore region of the channel and the well-characterized mutations Scn8amed, a null allele, and Scn8amed-jo, a missense mutation in an intracellular linker that causes altered voltage dependence. Although the functional impact of the Scn8a8J V929F amino acid substitution on Na+ channel activity is not yet known, the similar SWD phenotype of three different mutant alleles suggests a critical role for Scn8a in the regulation of thalamocortical function. We also noted that strain background has a strong effect on the incidence of SWD in Scn8a8J heterozygotes, with a higher incidence on the C3HeB/FeJ strain than on C57BL/6J. Future work will allow the mapping and identification of genetic modifiers that enhance or suppress SWD, which will be of interest for understanding absence epilepsy as a complex genetic trait.
The discovery of absence seizures in Scn8a mutants is somewhat surprising, because these mice have been studied for many years. However, systematic EEG analysis of Scn8a mutants was not part of the characterization of most of the previously described mutants. Human absence seizures are often overlooked, as well, because of their brevity and lack of post-ictal deficits. The lack of SWD in mice homozygous for the ENU-induced mutation Scn8anmf58 may be explained, in part, by their coisogenic C57BL/6J strain background (30). In the present study, Scn8a8J homozygotes on a predominantly C3HeB/FeJ background were identified during an unrelated phenotype screen, and EEG recording was carried out prior to the identification of the mutated gene. Scn8a8J homozygotes exhibited 4–5 Hz SWD, slower than those in Scn8a8J heterozygotes and most other rodent genetic models and closer to the 3–4 Hz burst frequency of human absence epilepsy. For example, a GEFS+ family with a GABRG2 R43Q substitution has 3–4 Hz SWD, but the corresponding mutant mice exhibit 7–9 Hz SWD that are typical of other rodent models (28). Our data demonstrate that factors in addition to species differences can influence SWD burst frequency.
Although the behavior of Scn8a heterozygous mutants is outwardly normal, systematic testing recently revealed behavioral abnormalities in mice heterozygous for a null allele of Scn8a on the C57BL/6J strain background (13). The null heterozygotes exhibited exaggerated conditioned fear, more pronounced avoidance of well-lit environments, and increased response to stress. The direction of the reported effects was consistent with the intermittent cessation of locomotor activity seen during SWD, but in control assays, the heterozygous mice did not differ from wild-type littermates with regard to locomotor activity in a familiar home cage, freezing prior to footshock or performance on the rotorod. As a direct test of the coincidence of behavioral abnormalities with absence seizures, EEG monitoring could be coupled with behavioral tasks in future studies of heterozygous null mice.
Rodent sleep architecture is defined by three different states of vigilance that are influenced by the environment. Sixty to seventy percent of the 12 h light cycle is composed of sleep, which is constantly fragmented by quiet wakefulness. During the 12 h dark cycle, rodents show large periods of active wakefulness that are interrupted by short periods of sleep. In humans and rodents with absence epilepsy, SWD show variable incidence depending on the levels of alertness associated with different states of vigilance (34). EEG analysis during a 48 h period showed that the incidence of SWD during wakefulness in Scn8amed/+ mutants was not statistically different between light and dark cycles. However, the frequency of SWD during SWS and PS was higher during the dark cycle when compared with the light cycle. During the dark cycle rodents experience light, highly fragmented sleep that facilitates SWD generation (35). In contrast, during the light cycle, mice experience longer periods of consolidated sleep, which is less likely to favor the generation of SWD (35).
An unexpected finding of this study was the observation of a relatively high incidence of SWD during PS. PS is characterized by a desynchronized EEG pattern, which tends to suppress spike-wave activity (36). Less than 20% of SWD in the WAG/Rij rat model of absence epilepsy occurs during PS (34). A recent study conducted in A/J mice demonstrated that only one out of a total of 13 mice exhibited SWD during PS (17). In contrast, we observed SWD in all Scn8amed/+ mutants during periods of PS. Furthermore, during the 48 h interval the percentage of time spent in SWD was comparable during wakefulness [0.7% (±0.2 SE)] and PS [0.6% (±0.1 SE)] (P = 0.7). This suggests that Scn8a dysfunction may alter the characteristics (e.g. waveform frequency) of PS. Additional studies will be required in order to more fully characterize the relationship between Scn8a dysfunction, sleep architecture and SWD.
In contrast to the higher incidence of absence seizures described above, Scn8amed and Scn8amed-jo heterozygotes exhibit increased thresholds for chemically induced convulsive seizures, and the Scn8amed-jo allele can rescue the low seizure threshold of heterozygous Scn1a knockout mice (37). Heterozygous Scn8a null mutants are also resistant to the initiation and development of kindling (38). This difference may be related to the involvement of different neuronal circuits in absence seizures (thalamocortical circuits) when compared with convulsive seizures. The cellular effect of sodium channel mutations varies in different populations of neurons and may be influenced by the overall channel composition of each neuronal type and by the timing characteristics of the circuit. For example, heterozygosity for a null allele of Scn1a has been shown to have a greater effect on bipolar inhibitory neurons of the hippocampus than on pyramidal neurons (39). Similarly, the pain-associated mutation L858H in Nav1.7 renders sensory neurons hyperexcitable and sympathetic neurons hypoexcitable (40).
The WAG/Rij rat strain exhibits a polygenic susceptibility to SWD. In these animals, Scn8a (Nav1.6) expression is upregulated in the somatosensory region of the cerebral cortex where SWD are initiated, but it is not clear whether this is a cause or a secondary consequence of the chronic seizure disorder (41). Independently, in a different rat model of absence epilepsy, GAERS, this cortical area was found to be sensitive to the suppression of SWD by local delivery of ETX (42). Since persistent sodium currents have been associated with neuronal burst firing, it could be suggested that overexpression of Scn8a could lead to excessive firing in cortical neurons, leading to hyperstimulation of the thalamic portion of the circuit as modeled previously (43). Our results indicate that reduced expression of Scn8a contributes to absence epilepsy, because heterozygosity for the null Scn8amed allele leads to SWD. The observed epileptogenic effects of both increased and decreased Scn8a may be accounted for by differences among neurons and synapses. For example, reduced Scn8a activity may directly increase burst firing of thalamic relay neurons, as suggested for other excitatory neurons, or enhance activity of inhibitory neurons of the reticular thalamic nucleus in a manner suggested for GluR4 mutant mice with SWD (18), producing prolonged inhibition and affect the timing of the thalamocortical oscillation. Further studies of Nav1.6 expression and function in thalamocortical areas will be necessary to reveal more about the pathophysiology of the SWD phenotype in Scn8a mutant mice.
The effect of genetic background on absence seizures in heterozygous mice offers a promising model for human absence epilepsy, which is likely to be inherited as a complex trait with dominant allelic behavior. In a human family with a SCN8A null allele, heterozygous carriers exhibited variable symptoms, including ataxia and cognitive impairment (14). This family did not undergo EEG analysis, but it is tempting to speculate that abnormal electrical discharges might contribute to their cognitive and behavioral abnormalities. Mutation screening in families with childhood or juvenile absence epilepsy would help evaluate the role of SCN8A in human absence epilepsy.
MATERIALS AND METHODS
Mice
The pedigree 596-16 carrying the Scn8a8J mutation was obtained from Jackson Laboratory's Reproductive Mutagenesis Program. C3HeB/FeJ (FeJ), C57BL/6J, C3HeB/FeJ-Scn8amed/J (Stock no. 003798) and C57BL/6J-Scn8amed-jo/J (Stock no. 003799) mice were originally obtained from Jackson Laboratory. All mice were housed in pathogen-free mouse facilities with a 12 h light/dark cycle. Food and water were available ad libitum. The Institutional Animal Care and Use Committees (IACUC) at Jackson Laboratory and Emory University approved all experimental protocols involving mice.
Genetic mapping
Genomic DNA from Scn8a8J mice was prepared from tail biopsies and initially genotyped by Jackson Laboratory's Genetic Fine Mapping Service using microsatellite markers. For generating the map position, data from telomeric chromosome 15 markers for G3 and G3F1 mice were combined, and the association between genotype and phenotype was tested using the computer program MAPMAKER/QTL. Affected mice were given a value of one and unaffected mice a value of zero. The physical chromosomal coordinates of each marker used to obtain the LOD score plot are shown in Figure 1.
Genotyping assays
The ENU-induced mutation Scn8a8J causes loss of a BbsI restriction site in exon 13. The Scn8a8J mutation was genotyped by amplification of a 258 bp genomic fragment using the primers 8J-F (5′ GGCCATCATTGTCTTCATCTTTGC) and 8J-R (5′ GAGAAGAAGACGTTGGTACTAACC). Digestion of the PCR product with BbsI generated two 129 bp fragments from the wild-type allele and a single 258 bp fragment from the Scn8a8J allele. PCR of genomic DNA was carried out in a 25 µl volume containing 1× GoTaq DNA polymerase buffer (Promega), 2.5 mm MgCl2, 0.2 mm dNTPs, 0.5 m primers and 1 U GoTaq DNA polymerase (Promega). Incubation at 94°C for 2 min was followed by 34 cycles of 94°C for 45 s, 60°C for 45 s and 72°C for 45 s, followed by 72°C for 10 min. Restriction enzyme–digested PCR products were separated on 2% agarose gels and stained with ethidium bromide.
Scn8amed/+ mice were genotyped as instructed by Jackson Laboratory (http://jaxmice.jax.org/pub-cgi/protocols/protocols.sh?objtype=protocol&protocol_id=454). Genotyping of the Scn8amed-jo/+ mutant was performed using primer pair 8aF (5′ ATGCCACAGAAGTGTCATTCC) and 8aR (5′ GGTATTTCCCAGCAAACAGGT) (32). PCR amplification was performed with one cycle at 94°C for 2 min and 40 cycles of 94°C for 30 s, 55°C for 30 s and 72°C for 1 min. The 213 bp PCR product was digested with MslI to produce fragments of 129, 75 and 9 bp from the wild-type allele, and 102, 75, 27 and 9 bp from the mutant allele.
Mutation detection
The exons and intron/exon boundaries of Scn8a were amplified from brain cDNA and genomic DNA prepared from affected 596-16 mice. Gel-purified PCR products were subjected to automated sequencing in the University of Michigan Sequencing Core.
Western blot
The membrane fraction was prepared from brain homogenates by centrifugation at 100 000g as previously described (44). Western blots loaded with 75 µg of membrane protein were hybridized with anti-Nav1.6 antiserum (1:200) from Sigma (S0438, lot 076K1101) as described (45).
EEG analysis
At Jackson Laboratory, Scn8a8J mutants 2 months of age or older were anesthetized with tribromoethanol (Avertin, 400 mg/kg i.p.). Small burr holes were drilled (1 mm anterior to bregma and 2 mm posterior to bregma) on both sides of the skull 2 mm lateral to the midline. EEG activity was measured by four Teflon-coated silver wires soldered onto a microconnector. The wires were placed between the dura and the brain and a dental cap was then applied. The mice were given a post-operative analgesic of carprofen (5 mg/kg subcutaneous) and a recovery period of at least 48 h before recordings. The mice were recorded for a 2 h period on two successive days using the Stellate Harmonie headbox, amplifier and software (Stellate, Inc.). SWD, the electroencephalographic feature of absence epilepsy, were defined by a rhythmic oscillation with a burst frequency of 7–9 Hz lasting at least 0.5 s, >2× background activity, observed in at least two of the six channels. For ETX treatment, on the day following their second standard EEG recording, mice were recorded for 90 min and then injected interperitoneally with 200 mg/kg of ETX (Sigma-Aldrich, Inc.). They were then recorded for a minimum of one additional hour.
At Emory University, 3- to 4-month-old Scn8amed/+ and Scn8amed-jo/+ male mice under deep isoflurane anesthesia were surgically implanted subdurally with four sterile 0-80x3/32 screw electrodes for EEG analysis. Two electrodes were placed on the right hemisphere above the frontal and parietal cortex (2 mm anterior to bregma and 1.2 mm lateral to the midline; and 1.5 mm posterior to bregma and 1.2 mm lateral to the midline, respectively). Contralaterally, the other two electrodes were placed on the left hemisphere above the parietal and visual cortex (0.5 mm posterior to bregma and 2.2 mm lateral to the midline; and 3.5 mm posterior to bregma and 2.2 mm lateral to the midline, respectively). Fine-wire electrodes were inserted into the left and right neck muscles for EMG acquisition. The animals were allowed to recover from surgery for 7 days. Amplified EEG and EMG signals and real-time video signals were collected and processed by Somnologica (Embla Medical, Reykjavik, Iceland) and Stellate Harmonie headbox, amplifier and software (version 6.1, Stellate, Inc.). Baseline EEG activity was recorded for 48 h in Scn8amed/+ mutants to evaluate the relationship between SWD and different states of vigilance. Each 10 s EEG episode was classified into three different states: wakefulness, SWS and PS. Wakefulness was defined as a low-voltage, high-frequency EEG with elevated and variable EMG. During SWS, the EEG signal increased in amplitude and decreased in frequency with the clear presence of high-amplitude delta waves (0.5–4 Hz), and the EMG signal displayed low regular muscular tone. PS was defined by the presence of regular theta waves (4.5–8 Hz) with lack of muscle tone with phasic bursts of varying duration and amplitude. Following 48 h of sleep/wake analysis, the same data were reanalyzed for number of SWD. SWD were defined by a rhythmic oscillation with a burst frequency of 7–9 Hz lasting at least 0.5 s, >2× background activity, observed in at least one channel. For each animal, the number of SWD observed in each state of vigilance during the light and dark cycles was divided by the total amount of time spent in each state of vigilance. For ETX treatment, the animals were first injected with saline solution (i.p., 10 ml/kg) to determine whether the handling and injection would alter the number of SWD. Then 48 h later the same animals were injected with ETX (i.p., 200 mg/kg, Sigma-Aldrich, Inc.). The number of SWD was quantified 60 min prior to injection and 60 min following injection of either saline or ETX.
SUPPLEMENTARY MATERIAL
FUNDING
Funding for the study was provided by NIH grants to W.N.F. (NS31348), V.A.L. (NS32801), M.H.M. (NS34509) and A.E. (NS046484). L.P. was supported by fellowships from AFIP and FAPESP (#07/50534-0). S.T. was supported by FAPESP (CEPID#98/14303-3).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Zhong-wei Zhang and Dr Greg Cox for critical reading of the manuscript, Carolyne Dunbar and Jonette Gilley for technical assistance and Cheryl Strauss for editorial assistance. We are grateful to Janice Pendola of the Reproductive Mutagenesis Program (supported by NIH grant HD42137) and the Genetic Fine Mapping Service at The Jackson Laboratory for their cited contributions to this work. We thank Dr Allan Levey, Dr David Rye and Dr Glenda Keating for facilitating the experiments conducted in Woodruff Memorial Research Building, Emory University.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Meisler M.H., Kearney J.A. Sodium channel mutations in epilepsy and other neurological disorders. J. Clin. Invest. 2005;115:2010–2017. doi: 10.1172/JCI25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kearney J.A., Meisler M.H. Encyclopedia of Basic Epilepsy Research. Elsevier; (in press) [Google Scholar]
- 3.Holland K.D., Kearney J.A., Glauser T.A., Buck G., Keddache M., Blankston J.R., Glaaser I.W., Kass R.S., Meisler M.H. Mutation of sodium channel SCN3A in a patient with cryptogenic pediatric partial epilepsy. Neurosci. Lett. 2008;433:65–70. doi: 10.1016/j.neulet.2007.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meisler M.H., Plummer N.W., Burgess D.L., Buchner D.A., Sprunger L.K. Allelic mutations of the sodium channel SCN8A reveal multiple cellular and physiological functions. Genetica. 2004;122:37–45. doi: 10.1007/s10709-004-1441-9. [DOI] [PubMed] [Google Scholar]
- 5.Raman I.M., Sprunger L.K., Meisler M.H., Bean B.P. Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron. 1997;19:881–891. doi: 10.1016/s0896-6273(00)80969-1. [DOI] [PubMed] [Google Scholar]
- 6.Maurice N., Tkatch T., Meisler M., Sprunger L.K., Surmeier D.J. D1/D5 dopamine receptor activation differentially modulates rapidly inactivating and persistent sodium currents in prefrontal cortex pyramidal neurons. J. Neurosci. 2001;21:2268–2277. doi: 10.1523/JNEUROSCI.21-07-02268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Do M.T., Bean B.P. Sodium currents in subthalamic nucleus neurons from Nav1.6-null mice. J. Neurophysiol. 2004;92:726–733. doi: 10.1152/jn.00186.2004. [DOI] [PubMed] [Google Scholar]
- 8.Cummins T.R., Dib-Hajj S.D., Herzog R.I., Waxman S.G. Nav1.6 channels generate resurgent sodium currents in spinal sensory neurons. FEBS Lett. 2005;579:2166–2170. doi: 10.1016/j.febslet.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Van Wart A., Matthews G. Impaired firing and cell-specific compensation in neurons lacking Nav1.6 sodium channels. J. Neurosci. 2006;26:7172–7180. doi: 10.1523/JNEUROSCI.1101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caldwell J.H., Schaller K.L., Lasher R.S., Peles E., Levinson S.R. Sodium channel Nav1.6 is localized at nodes of Ranvier, dentrites, and synapses. Proc. Natl Acad. Sci., USA. 2000;97:5616–5620. doi: 10.1073/pnas.090034797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Mart A., Trimmer J.S., Matthews G. Polarized distribution of ion channel within microdomains of the axon initial segment. J. Comp. Neurol. 2007;500:339–352. doi: 10.1002/cne.21173. [DOI] [PubMed] [Google Scholar]
- 12.Levin S.I., Khaliq Z.M., Aman T.K., Grieco T.M., Kearney J.A., Raman I.M., Meisler M.H. Impaired motor function in mice with cell-specific knockout of sodium channel Scn8a (Nav1.6) in cerebellar purkinje neurons and granule cells. J. Neurophysiol. 2006;96:785–793. doi: 10.1152/jn.01193.2005. [DOI] [PubMed] [Google Scholar]
- 13.McKinney B.C., Chow C.Y., Meisler M.H., Murphy G.G. Exaggerated emotional behavior in mice heterozygous null for the sodium channel Scn8a (Nav1.6) Genes Brain Behav. 2008;7:629–638. doi: 10.1111/j.1601-183X.2008.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trudeau M.M., Dalton J.C., Day J.W., Ranum L.P., Meisler M.H. Heterozygosity for a protein truncation mutation of sodium channel SCN8A in a patient with cerebellar atrophy, ataxia, and mental retardation. J. Med. Genet. 2006;43:527–530. doi: 10.1136/jmg.2005.035667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gauguier D., van Luijtelaar G., Bihoreau M.T., Wilder S.P., Godfrey R.F., Vossen J., Coenen A., Cox R.D. Chromosomal mapping of genetic loci controlling absence epilepsy phenotypes in the WAG/Rij rat. Epilepsia. 2004;45:908–915. doi: 10.1111/j.0013-9580.2004.13104.x. [DOI] [PubMed] [Google Scholar]
- 16.Rudolf G., Bihoreau M.T., Godfrey R.F., Wilder S.P., Cox R.D., Lathrop M., Marescaux C., Gauguier D. Polygenic control of idiopathic generalized epilepsy phenotypes in the genetic absence rats from Strasbourg (GAERS) Epilepsia. 2004;45:301–308. doi: 10.1111/j.0013-9580.2004.50303.x. [DOI] [PubMed] [Google Scholar]
- 17.Strohl K.P., Gallaugher L., Lynn A., Friedman L., Hill A., Singer J.B., Lander E.S., Nadeau J. Sleep-related epilepsy in the A/J mouse. Sleep. 2007;30:169–176. doi: 10.1093/sleep/30.2.169. [DOI] [PubMed] [Google Scholar]
- 18.Beyer B., Deleuze C., Letts V.A., Mahaffey C.L., Boumil R.M., Lew T.A., Huguenard J.R., Frankel W.N. Absence seizures in C3H/HeJ and knockout mice caused by mutation of the AMPA receptor subunit Gria4. Hum. Mol. Genet. 2008;17:1738–1749. doi: 10.1093/hmg/ddn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barclay J., Balaguero N., Mione M., Ackerman S.L., Letts V.A., Brodbeck J., Canti C., Meir A., Page K.M., Kusumi K., et al. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J. Neurosci. 2001;21:6095–6104. doi: 10.1523/JNEUROSCI.21-16-06095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgess D.L., Jones J.M., Meisler M.H., Noebels J.L. Mutation of the Ca2+ channel b subunit gene Cchb4 is associated with ataxia and seizures in the lethargic (lh) mouse. Cell. 1997;88:385–392. doi: 10.1016/s0092-8674(00)81877-2. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher C.F., Lutz C.M., O'sullivan T.N., Shaughnessy J.D., Jr, Hawkes R., Frankel W.N., Copeland N.G., Jenkins N.A. Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell. 1996;87:607–617. doi: 10.1016/s0092-8674(00)81381-1. [DOI] [PubMed] [Google Scholar]
- 22.Letts V.A., Felix R., Biddlecome G.H., Arikkath J., Mahaffey C.L., Valenzuela A., Bartlett F.S., II, Mori Y., Campbell K.P., Frankel W.N. The mouse stargazer gene encodes a neuronal Ca2+ channel gamma subunit. Nat. Genet. 1998;19:340–347. doi: 10.1038/1228. [DOI] [PubMed] [Google Scholar]
- 23.Ludwig A., Budde T., Stieber J., Moosmang S., Wahl C., Holthoff K., Langebartels A., Wotjak C., Munsch T., Zong X., et al. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO. J. 2003;22:216–224. doi: 10.1093/emboj/cdg032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim D., Song I., Keum S., Lee T., Jeong M.J., Kim S.S., McEnery M.W., Shin H.S. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking alpha1G T-type Ca2+ channels. Neuron. 2001;31:35–45. doi: 10.1016/s0896-6273(01)00343-9. [DOI] [PubMed] [Google Scholar]
- 25.Singh B., Monteil A., Bidaud I., Sugimoto Y., Suzuki T., Hamano S., Oguni H., Osawa M., Alonso M.E., Delgado-Escueta A.V., et al. Mutational analysis of CACNA1G in idiopathic generalized epilepsy. Mutation in brief #962. Online. Hum. Mutat. 2007;28:524–525. doi: 10.1002/humu.9491. [DOI] [PubMed] [Google Scholar]
- 26.Powell K.L., Cain S.M., Ng C., Sirdesai S., David L.S., Kyi M., Garcia E., Tyson J.R., Reid C.A., Bahlo M., et al. A Cav3.2 T-type calcium channel point mutation has splice-variant-specific effects on function and segregates with seizures expression in a polygenic rat model of absence epilepsy. Neurobiol. Dis. 2009;29:371–380. doi: 10.1523/JNEUROSCI.5295-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace R.H., Marini C., Petrou S., Harkin L.A., Bowser D.N., Panchal R.G., Williams D.A., Sutherland G.R., Mulley J.C., Scheffer I.E., et al. Mutant GABAA receptor γ2-subunit in childhood absence epilepsy and febrile seizures. Nat. Genet. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- 28.Tan H.O., Reid C.A., Single F.N., Davies P.J., Chiu C., Murphy S., Clarke A.L., Dibbens L., Krestel H., Mulley J.C., et al. Reduced cortical inhibition in a mouse model of familial childhood absence epilepsy. Proc. Natl Acad. Sci., USA. 2007;104:17536–17541. doi: 10.1073/pnas.0708440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohrman D.C., Harris J.B., Meisler M.H. Mutation detection in the med and medJ alleles of the sodium channel Scn8a. J. Biol. Chem. 1996;271:17576–17581. doi: 10.1074/jbc.271.29.17576. [DOI] [PubMed] [Google Scholar]
- 30.Buchner D.A., Seburn K.L., Frankel W.N., Meisler M.H. Three ENU-induced neurological mutations in the pore loop of sodium channel Scn8a (Na(v)1.6) and a genetically linked retinal mutation, rd13. Mamm. Genome. 2004;15:344–351. doi: 10.1007/s00335-004-2332-1. [DOI] [PubMed] [Google Scholar]
- 31.Frankel W.N., Beyer B., Maxwell C.R., Pretel S., Letts V.A., Siegel S.J. Development of a new genetic model for absence epilepsy: spike-wave seizures in C3H/He and backcross mice. J. Neurosci. 2005;25:3452–3458. doi: 10.1523/JNEUROSCI.0231-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohrman D.C., Smith M.R., Goldin A.L., Harris J., Meisler M.H. A missense mutation in the sodium channel Scn8a is responsible for cerebellar ataxia in the mouse mutant jolting. J. Neurosci. 1996;16:5993–5999. doi: 10.1523/JNEUROSCI.16-19-05993.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glauser T.A., Morita D.A., Wyllie E., editors. The Treatment of Epilepsy, Principles and Practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 34.Drinkenburg W.H., Coenen A.M., Vossen J.M., van Luijtelaar E.L. Spike-wake discharges and sleep-wake state in rats with absence epilepsy. Epilepsy Res. 1991;9:218–224. doi: 10.1016/0920-1211(91)90055-k. [DOI] [PubMed] [Google Scholar]
- 35.Coenen A.M., van Luijtelaar E.L. Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behav. Genet. 2003;33:635–655. doi: 10.1023/a:1026179013847. [DOI] [PubMed] [Google Scholar]
- 36.Coenen A.M., Drinkenburg W.H., Peeters B.W., Vossen J.M., van Luijtelaar E.L. Absence epilepsy and the level of vigilance in rats of the WAG/Rij strain. Neurosci. Biobehav. Rev. 1991;15:259–263. doi: 10.1016/s0149-7634(05)80005-3. [DOI] [PubMed] [Google Scholar]
- 37.Martin M.S., Tang B., Papale L.A., Yu F.H., Catterall W.A., Escayg A. The voltage-gated sodium channel Scn8a is a genetic modifier of severe myoclonic epilepsy of infancy. Hum. Mol. Genet. 2007;16:2892–2899. doi: 10.1093/hmg/ddm248. [DOI] [PubMed] [Google Scholar]
- 38.Blumenfeld H., Lampert A., Klein J.P., Mission J., Chen M.C., Rivera M., Dib-Hajj S., Brennan A.R., Hains B.C., Waxman S.G. Role of hippocampal sodium channel Nav1.6 in kindling epileptogenesis. Epilepsia. 2009;50:44–55. doi: 10.1111/j.1528-1167.2008.01710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu F.H., Mantegazza M., Westenbroek R.E., Robbins C.A., Kalume F., Burton K.A., Spain W.J., McKnight G.S., Scheuer T., Catterall W.A. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat. Neurosci. 2006;9:1142–1149. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- 40.Rush A.M., Dib-Hajj S.D., Liu S., Cummins T.R., Black J.A., Waxman S.G. A single sodium channel mutation produces hyper- or hypoexcitability in different types of neurons. Proc. Natl Acad. Sci. USA. 2006;103:8245–8250. doi: 10.1073/pnas.0602813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klein J.P., Khera D.S., Nersesyan H., Kimchi E.Y., Waxman S.G., Blumenfeld H. Dysregulation of sodium channel expression in cortical neurons in a rodent model of absence epilepsy. Brain Res. 2004;1000:102–109. doi: 10.1016/j.brainres.2003.11.051. [DOI] [PubMed] [Google Scholar]
- 42.Manning J.P., Richards D.A., Leresche N., Crunelli V., Bowery N.G. Cortical-area specific block of genetically determined absence seizures by ethosuximide. Neuroscience. 2004;123:5–9. doi: 10.1016/j.neuroscience.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 43.Blumenfeld H., McCormick D.A. Corticothalamic inputs control the pattern of activity generated in thalamocortical networks. J. Neurosci. 2000;20:5153–5162. doi: 10.1523/JNEUROSCI.20-13-05153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.West J.W., Scheuer T., Maechler L., Catterall W.A. Efficient expression of rat brain type IIA Na+ channel alpha subunits in a somatic cell line. Neuron. 1992;8:59–70. doi: 10.1016/0896-6273(92)90108-p. [DOI] [PubMed] [Google Scholar]
- 45.Levin S.I., Meisler M.H. Floxed allele for conditional inactivation of the voltage-gated sodium channel Scn8a (Nav1.6) Genesis. 2004;39:234–239. doi: 10.1002/gene.20050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.