Abstract
Approximately 10% of amyotrophic lateral sclerosis (ALS) cases are familial (FALS), and ∼25% of FALS cases are caused by mutations in Cu/Zn superoxide dismutase type 1 (SOD1). Mutant (MT) SOD1 is thought to be pathogenic because it misfolds and aggregates. A number of transgenic mice have been generated that express different MTSOD1s as transgenes and exhibit an ALS-like disease. Although one study found that overexpression of human wild-type (WT) SOD1 did not affect disease in G85R transgenic mice, more recent reports claim that overexpression of WTSOD1 in other MTSOD1 transgenic mice hastened disease, raising a possibility that the effect of WTSOD1 overexpression in this FALS mouse model is mutant-specific. In order to clarify this issue, we studied the effect of WTSOD1 overexpression in a G85R transgenic mouse that we recently generated. We found that G85R/WTSOD1 double transgenic mice had an acceleration of disease onset and shortened survival compared with G85R single transgenic mice; in addition, there was an earlier appearance of pathological and immunohistochemical abnormalities. The spinal cord insoluble fraction from G85R/WTSOD1 mice had evidence of G85R–WTSOD1 heterodimers and WTSOD1 homodimers (in addition to G85R homodimers) with intermolecular disulfide bond cross-linking. These studies suggest that WTSOD1 can be recruited into disease-associated aggregates by redox processes, providing an explanation for the accelerated disease seen in G85R mice following WTSOD1 overexpression, and suggesting the importance of incorrect disulfide-linked protein as key to MTSOD1 toxicity.
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by the selective loss of motor neurons (MNs). Approximately 10% of ALS cases are familial (known as FALS), and ∼25% of FALS cases are caused by mutations in Cu/Zn superoxide dismutase type 1 (SOD1) (1). There is convincing evidence that mutant (MT) SOD1 does not kill MNs because of a deficiency in dismutase activity, but because of a toxic gain of function or an augmentation of a normally present, non-dismutase activity of SOD1. This evidence partly comes from studies that demonstrate that neural cells from MTSOD1 FALS transgenic mice do not have decreased dismutase activity (2,3), and from studies of SOD1 knockout mice that show that a complete loss in SOD1 activity does not cause motor neuron disease (MND) (4). A number of proposals have been offered regarding the basis of MTSOD1 toxicity, including misfolding of the MTSOD1 leading to sequestration within MTSOD1 aggregates of proteins that are important in MN function and viability. Investigators have recently suggested that chemically modified forms of SOD1 may be involved in the pathogenesis of sporadic ALS in addition to FALS (5,6) and, therefore, that FALS and sporadic ALS have common pathways of MN death.
One question that arose in studies of the MTSOD1 FALS transgenic mouse model was whether overexpression of human wild-type (WT) SOD1 in these mice affected disease. There are conflicting reports regarding the effect of WTSOD1 overexpression on FALS transgenic mice. Bruijn et al. (7). reported that crossing human WTSOD1-overexpressing mice with G85R transgenic mice led to no change in the onset or duration of disease. In contrast, other investigators found that WTSOD1 overexpression hastened disease onset in the case of G93A and L126Z transgenic mice and converted an unaffected phenotype into an MND phenotype in the case of A4V transgenic mice. Interestingly, insoluble aggregates from endstage L126Z/WT double transgenic mice contained L126Z–WT heterodimers and WTSOD1 homodimers, in addition to L126Z homodimers, with intermolecular disulfide bonds (8,9). The finding of WTSOD1 as well as MTSOD1 within insoluble aggregates provided an explanation for the acceleration of disease following overexpression of WTSOD1 in MTSOD1 transgenic mice.
We questioned whether there were features specific to the G85R mutation that led to the differing results obtained by Bruijn et al. (7) compared with results obtained with other MTSOD1 transgenic mice (8,9). In order to investigate this issue, we compared the phenotype of G85R transgenic mice that we recently generated with that of G85R/WTSOD1 double transgenic mice. The G85R/WTSOD1 mice were of special interest with respect to the question as to whether insoluble aggregates from another MT/WTSOD1 double transgenic mouse, besides the L126Z/WTSOD1 mouse, contain homo- and heterodimers cross-linked with intermolecular disulfide bonds; these studies were made possible because G85R, like L126Z, has a different electrophoretic mobility than WTSOD1.
The present results demonstrate that WTSOD1 overexpression accelerates disease in a G85R mouse and also forms heterodimers and homodimers with intermolecular disulfide bonds that appear with disease progression. These findings have implications for our understanding of the toxicity of MTSOD1 and the treatment of FALS.
RESULTS
The preparation of G85R single transgenic mice and G85R/WTSOD1 double transgenic mice
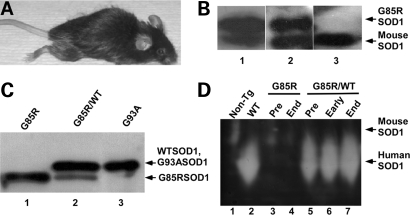
The floxed-G85R construct was inoculated into pronuclei, and tail DNA was analyzed by PCR to identify mice carrying the transgene (data not shown). G85R transgenic mice developed progressive paralysis culminating in death (Fig. 1A). The mice had evidence of G85R MTSOD1 protein in homogenates from the spinal cord (Fig. 1B, lane 1) and muscle (Fig. 1B, lane 2) on western blots (10), G85R SOD1 had a slower electrophoretic mobility than endogenous mouse SOD1 (Fig. 1B). The level of expression of G85R MTSOD1 in the spinal cord was ∼1.5× that of the endogenous mouse SOD1 (Fig. 1B, lane 1).
Figure 1.
Characterization of G85R and G85R/WTSOD1 transgenic mice. (A) A G85R transgenic mouse at ∼11 months of age. (B) Western blot of a spinal cord homogenate from a 6-month-old G85R mouse (lane 1), muscle homogenate from an 11-month-old G85R mouse (lane 2), and muscle homogenate from an 11-month-old non-transgenic littermate (lane 3) using an anti-SOD1 antibody that is directed against human as well as mouse SOD1. (C) Western blot using a human-specific anti-SOD1 antibody of a spinal cord homogenate from a G85R transgenic mouse (lane 1), a G85R/WTSOD1 transgenic mouse (lane 2) and a G93A transgenic mouse (lane 3). The arrows show that the G85R MTSOD1 (lane 1, lane 2—lower band) has a more rapid electrophoretic mobility than WTSOD1 (lane 2—upper band), which runs at the same mobility as G93A MTSOD1 (lane 3). Lane 2 contained a different amount of total protein in order to show the two bands and the ratio of G85R to WTSOD1. (D) G85R MTSOD1 has no SOD1 enzymatic activity. A protein extract (30 μg) from the spinal cord of a non-transgenic mouse (lane 1), WTSOD1 transgenic mouse (lane 2), a presymptomatic (lane 3) and endstage (lane 4) G85R single transgenic mouse, and a presymptomatic (lane 5), early disease phase (lane 6) and endstage (lane 7) G85R/WTSOD1 double transgenic mouse were electrophoresed on an SOD1 activity gel. Human SOD1 enzymatic activity is seen in the case of the WTSOD1 and G85R/WTSOD1 transgenic mice, but not the non-transgenic and G85R single transgenic mice.
To generate G85R/WTSOD1 double transgenic mice, G85R transgenic mice were crossed with WTSOD1 transgenic mice. Spinal cord homogenates from G85R/WTSOD1 mice had evidence of immunoreactive human SOD1 at two different electrophoretic mobilities on western blots (Fig. 1C, lane 2); one band, which was WTSOD1, had the same mobility as G93A MTSOD1 (Fig. 1C, lane 3), whereas the other band had a more rapid mobility that corresponded to G85R MTSOD1 (Fig. 1C, lane 1).
G85R SOD1 protein from the spinal cords of G85R transgenic mice had no superoxide dismutase enzymatic activity on an activity gel (Fig. 1D, lanes 3 and 4), in contrast to WTSOD1 protein (Fig. 1D, lane 2). Interestingly, the activity of the endogenous mouse SOD1 was no longer apparent in the spinal cord of the WTSOD1 single transgenic mouse (Fig. 1D, lane 2) and G85R/WTSOD1 double transgenic mice (Fig. 1D, lanes 5–7), presumably because the endogenous mouse SOD1 formed heterodimers with the very over-expressed human SOD1 in the single and double transgenic mice; because of the heterodimerization, the mouse SOD1 shifted its electrophoretic mobility and therefore became obscured by the large amount of human WTSOD1 activity. The endogenous mouse SOD1 activity appears to decrease in the case of the G85R single transgenic mice (Fig. 1D, lanes 3 and 4), presumably because some of the endogenous enzyme formed heterodimers with G85R, and is therefore no longer active.
WTSOD1 overexpression accelerates the onset of clinical disease in G85R transgenic mice
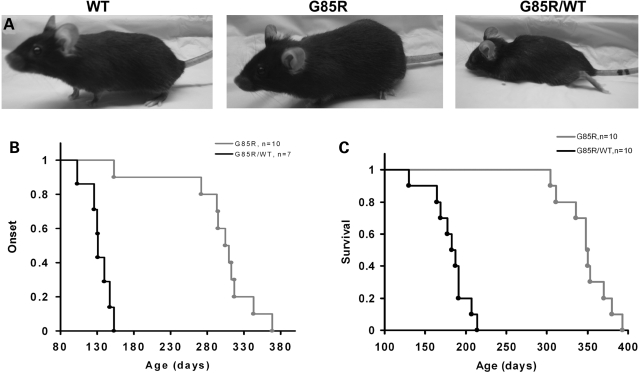
The G85R/WTSOD1 double transgenic mice had an earlier disease onset than G85R single transgenic mice (Fig. 2A and B). The disease onset, as defined by the beginning of weight loss, was 131.0 ± 16.4 days in double transgenic mice (n = 7) compared with 307.5 ± 30.8 days in littermate single transgenic mice (n = 10) (P < 0.001) (Fig. 2B). The mean survival was 185.0 ± 24.5 days in double transgenic mice (n = 10) compared with 349.0 ± 27.9 days in littermate single transgenic mice (n = 10) (P < 0.001) (Fig. 2C). The disease duration was not significantly different (P > 0.5) between the double (51.0 ± 13.3 days, n = 7) and single transgenic mice (40.5 ± 19.1 days, n = 10) (Fig. 2C), indicating that the decrease in survival in double transgenic mice was a result of the shortened incubation period and not related to any change in disease duration.
Figure 2.
Clinical disease in G85R and G85R/WTSOD1 transgenic mice. (A) The G85R/WTSOD1 mouse has clear hind limb weakness at ∼150 days compared with two littermates, one a WTSOD1 transgenic mouse and one a G85R single transgenic mouse. (B) The age of mice at the onset of disease in G85R versus G85R/WTSOD1 transgenic mice. A round mark shows the age of disease onset of individual mice at a particular age that is indicated in the x-axis. (C) Survival in G85R versus G85R/WTSOD1 transgenic mice.
WTSOD1 overexpression accelerates the appearance of pathological and immunohistochemical abnormalities in G85R transgenic mice
As noted above, G85R/WTSOD1 double mice had an earlier onset of clinical disease compared with G85R single transgenic mice, with a mean disease onset of 131 days. In order to compare the pathology in these mice, we examined the anterior horn of the lumbar spinal cord of G85R/WTSOD1 mice and WTSOD1 mice at 150 days with G85R mice at 150 and 350 days (Fig. 3).
Figure 3.
Neuropathological and immunohistochemical studies of the anterior horn of the spinal cord. The first three columns show WTSOD1, G85R and G85R/WTSOD1 mice at 150 days, whereas the fourth column shows G85R mice at 350 days with respect to Nissl staining (first row), CTB immunoreactivity (second row), GFAP immunoreactivity (third row) and SOD1 immunoreactivity (fourth row). The scale bar = 20 µm.
Nissl staining demonstrated a significant loss of MNs in G85R/WTSOD1 mice at 150 days that was not apparent in WTSOD1 mice and was not seen in G85R mice (except at the 350 days time point) (Fig. 3, top row). A loss of MN connections with muscle was identified by examining the lumbar spinal cord anterior horn 24 h after an intramuscular injection of cholera toxin subunit B (CTB), a neuronal tracer which only labels MNs that maintain functional projections with muscle. There was relatively little anti-CTB antibody staining of anterior horn cells of G85R/WTSOD1 mice at 150 days compared with WTSOD1 mice and G85R mice (Fig. 3, second row); at 350 days, a loss in CTB staining was apparent in G85R mice. Glial fibrillary acidic protein (GFAP) staining showed prominent astrocytosis in the G85R/WTSOD1 mice at 150 days that was not apparent in the WTSOD1 mice or the G85R mice (except at the 350 days time point) (Fig. 3, third row). SOD1-immunoreactive aggregates were seen in cells with MN morphology in the anterior horn of G85R/WTSOD1 mice at 150 days, but not in WTSOD1 mice or G85R mice (except at the 350 days time point) (Fig. 3, bottom row). These results indicate that the pathological and immunohistochemical hallmarks of disease were apparent much earlier in G85R/WTSOD1 double transgenic mice than G85R single transgenic mice.
WTSOD1 homodimers and G85R–WTSOD1 heterodimers with intermolecular disulfide bonds are present in the insoluble fraction of the spinal cord from affected G85R/WTSOD1 mice
As noted in Introduction, Deng et al. (8) and Furakawa et al. (9) identified WTSOD1 homodimers and MT–WT heterodimers in the insoluble mitochondrial fraction of the spinal cord of L126Z/WTSOD1 double transgenic mice; these studies were facilitated because of the difference in electrophoretic mobility of the truncated L126Z MTSOD1 compared with WTSOD1. The fact that G85R MTSOD1 also has a different mobility than WTSOD1 enabled us to carry out similar studies in the case of G85R/WTSOD1 double transgenic mice.
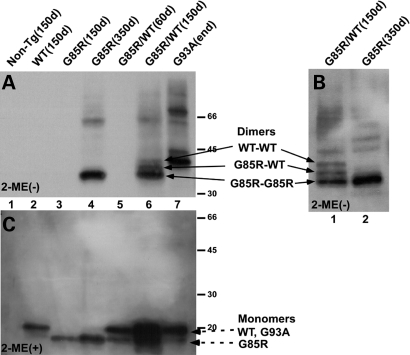
Spinal cords were homogenized in the presence of iodoacetamide (which reacts with and blocks free thiol groups in proteins, and therefore prevents artificial oxidative formation of the disulfide bond), and an insoluble fraction was obtained. Figure 4A shows a western blot with a human-specific anti-SOD1 antibody of the insoluble fraction in the absence of β-mercaptoethanol. No SOD1 protein from samples of the WTSOD1 transgenic mouse (Fig. 4A, lane 2) is visible in this panel because WTSOD1 dimers are not present and because WTSOD1 monomeric bands have been electrophoresed off the gel (in order to more clearly see the dimers from the other transgenic mice). In the case of the G85R and the G85R/WTSOD1 transgenic mice, SOD1 bands increased in amount with progression of disease (Fig. 4A, compare lane 4 with 3 and lane 6 with 5). When one compares the bands seen in the insoluble fraction from the G85R single transgenic mouse at 350 days with bands seen with the G85R/WTSOD1 double transgenic mouse at 150 days, two additional bands can be seen above and close to the predicted mobility of a G85R MTSOD1 dimer (Fig. 4A, lane 6, three arrows); these three protein species are also apparent in another gel of the spinal cord pellet from the same G85R/WTSOD1 double transgenic mouse (Fig. 4B, lane 1, three arrows). Interestingly, the uppermost of these three bands (Fig. 4A, lane 6; Fig. 4B, lane 1) corresponds to the mobility of the G93A dimer (Fig. 4A, lane 7), which is similar to the mobility of WTSOD1 (Fig. 1C, lane 2); this mobility and the absence of this band in homogenates from moribund G85R single transgenic mice (Fig. 4A, lane 4) suggest that this band represents WT–WTSOD1 homodimers. The band that is between the WT–WTSOD1 and G85R–G85R SOD1 homodimers is presumed to be WT–G85R SOD1 heterodimers (Fig. 4A, lane 6 and Fig. 4B, lane 1).
Figure 4.
SOD1 immunoreactivity on western blots of the insoluble fraction of the spinal cord. The insoluble fractions from the spinal cord of mice were electrophoresed on SDS–polyacrylamide gels in the absence (A and B) or the presence (C) of β-mercaptoethanol (2-ME), and then western blotted with an overlay of human-specific anti-SOD1 antibody. In (A and C): lane 1—non-transgenic, lane 2—WTSOD1 (150 days), lane 3—G85R (150 days), lane 4—G85R (350 days), lane 5—G85R/WTSOD1 (60 days), lane 6—G85R/WTSOD1 (150 days) and lane 7—G93A (endstage, ∼130 days) transgenic mice. (B): lane 1—G85R/WTSOD1 mouse (150 days), lane 2—G85R mouse (350 days). Note that the gel in (A) was run longer than (C) (and therefore the monomers were electrophoresed off the gel) in order to increase the separation of bands of the predicted size of the G85R–G85R SOD1 homodimer, G85R–WTSOD1 heterodimer and WT–WTSOD1 homodimer [see arrows in (A), lane 6]; these three bands are seen more clearly in (B, lane 1). The WT–WTSOD1 homodimer (A, lane 6) has the same mobility as the G93A–G93A SOD1 homodimer (A, lane 7). These dimers and multimers are not detected in (C). The monomers (dashed arrows) are seen in (C), with the WT and G93A protein species having a slower electrophoretic mobility than G85R.
Figure 4C shows a western blot with immunostaining of the same insoluble fractions as in Figure 4A, but in this case in the presence of β-mercaptoethanol. As expected, bands of two different mobilities were seen in the case of the G85R/WTSOD1 transgenic mouse, one corresponding to the mobility of the WTSOD1 (and G93A MTSOD1) monomer and one corresponding to the more rapid mobility of the G85R MTSOD1 monomer (dashed arrows). A monomeric SOD1 band was seen in the insoluble fraction of spinal cord homogenates from WTSOD1 transgenic mice (Fig. 4C, lane 2). The use of a human-specific anti-SOD1 antibody for immunostaining did not permit the detection of SOD1 in the case of the non-transgenic mouse (Fig. 4C, lane 1). Figure 4C had no evidence of dimers and multimers. The presence of dimeric protein species on a non-reducing denaturing gel (Fig. 4A, lanes 4, 6 and 7), but not following treatment with β-mercaptoethanol (Fig. 4C, lanes 4, 6 and 7) suggest that the dimer formation results from intermolecular disulfide bonds (since the native SOD1 dimers would have been disrupted under the conditions of the denaturing gel).
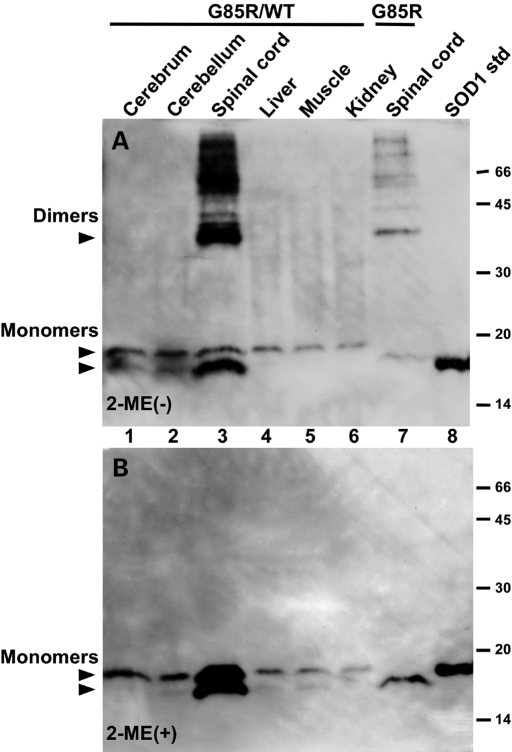
We investigated whether intermolecular disulfide bonds found in the insoluble fraction of the spinal cords of G85R and G85R/WTSOD1 transgenic mice are also present in other central nervous system (CNS) regions or non-neural tissues. Figure 5 shows western blots of the insoluble fraction from the spinal cord of G85R single transgenic mice and from the spinal cord and other tissues of G85R/WTSOD1 double transgenic mice; these samples were run in the absence (Fig. 5A) and the presence (Fig. 5B) of β-mercaptoethanol. As in the case of Figure 4, dimers and trimers were present when the insoluble fractions from the spinal cords of endstage G85R/WTSOD1 double transgenic mice (Fig. 5A, lane 3) and G85R single transgenic mice (Fig. 5A, lane 7) were electrophoresed under non-reducing denaturing conditions, but not in the presence of β-mercaptoethanol, indicating that these high molecular weight forms are cross-linked by intermolecular disulfide bonds. Interestingly, these cross-linked protein species make up a sizeable proportion of the SOD1 in the insoluble fraction from the spinal cord at endstage disease; i.e. virtually all of the SOD1 in the insoluble fraction from the spinal cord of endstage G85R single transgenic mice was cross-linked by intermolecular disulfide bonds (Fig. 5A, lane 7), whereas >50% of the SOD1 in the insoluble fraction from the spinal cord of G85R/WTSOD1 double transgenic mice was cross-linked by intermolecular disulfide bonds (Fig. 5A, lane 3). Of note, no high molecular weight forms were seen in the insoluble fraction from the cerebrum, cerebellum, liver, muscle or kidney of G85R/WTSOD1 double transgenic mice (Fig. 5A, lanes 1, 2, 4, 5 and 6 respectively). Figure 5 also demonstrates that when compared with other tissues, the insoluble fraction of the spinal cord from the G85R/WTSOD1 mouse has an increased amount of G85R and WTSOD1 (compare the amount of these two protein species in lane 3 versus the other lanes). There is also a relative increase of G85R versus WTSOD1 in the insoluble fraction of the spinal cord from the G85R/WTSOD1 mouse compared with the ratio of these SOD1 species in the insoluble fraction from other tissues (compare the ratio of G85R with WTSOD1 in Fig. 5B, lane 3 versus lane 1, 2, 4, 5, 6 and to the ratio seen in Fig. 1C, lane 2).
Figure 5.
SOD1 immunoreactivity on western blots of the insoluble fraction from varied tissues. An equal amount of protein of the insoluble fraction from the spinal cord and other tissues from a G85R/WTSOD1 double transgenic mouse (lanes 1–6) and from the spinal cord from a G85R single transgenic mouse (lane 7) was electrophoresed on SDS–polyacrylamide gels in the absence (A) and the presence (B) of β-mercaptoethanol (2-ME), and then western blotted with an overlay of human-specific anti-SOD1 antibody. In (A and B): lane 1—cerebrum, lane 2—cerebellum, lane 3—spinal cord, lane 4—liver, lane 5—muscle, lane 6—kidney, lane 7—spinal cord, lane 8—50 ng of SOD1 as a standard. Dimers that are present in (A) are disrupted and no longer detectable after β-mercaptoethanol treatment in (B). The positions of WT and G85R monomers in (A and B) are shown with arrowheads. The G85R/WTSOD1 double transgenic mouse was sacrificed at ∼160 days, whereas the G85R single transgenic mouse was sacrificed at ∼350 days.
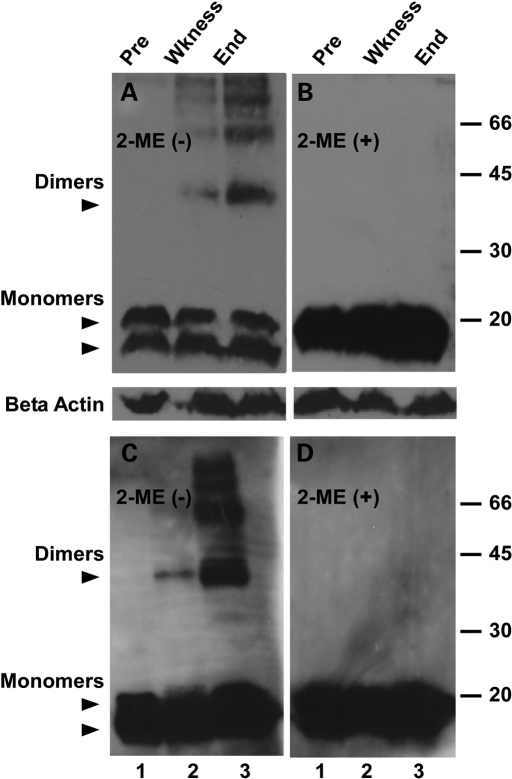
We questioned whether the level of G85R in the G85R/WT mouse increases during disease. Figure 6A shows that the amount of MTSOD1 increases over time in spinal cord homogenates (containing the soluble and insoluble fraction) of the G85R/WT mouse relative to WTSOD1 (Fig. 6A, lanes 1–3) and actin (Fig. 6A and B). The increase in G85R over time is also clear when one compares the ratio of G85R with WTSOD1 in Figure 1C, lane 2 (which shows a spinal cord homogenate of a 2-month-old G85R/WT mouse) with the ratio of G85R to WTSOD1 from G85R/WT mice at later times (Fig. 6A, lanes 2 and 3). These results suggest that G85R is being stabilized by the presence of the WTSOD1 as a result of cross-linking, aggregate formation and possibly incorporation into heterodimers. An increase over time of intermolecular disulfide cross-linking within the insoluble fraction of the spinal cord of the G85R/WT mouse is shown in Fig. 6C: high molecular weight bands in a non-reducing gel are not present in the presymptomatic stage (Fig. 6C, lane 1), but appear with the onset of weakness (Fig. 6C, lane 2), and become very prominent when the animal is completely paralyzed (Fig. 6C, lane 3). These high molecular weight bands are no longer detectable on the reducing gel in Figure 6D, indicating that they represent intermolecular disulfide bonds.
Figure 6.
SOD1 immunoreactivity on western blots of the spinal cords of G85R/WTSOD1 mice at different ages. An equal amount of protein of the homogenate (containing the soluble and insoluble fraction) (A and B) and insoluble fraction (C and D) of the spinal cords from two G85R/WTSOD1 mice were electrophoresed on SDS–polyacrylamide gels in the absence (A and C) and presence (B and D) of β-mercaptoethanol and then western blotted with an overlay of human-specific anti-SOD1 antibody (upper panels) or anti-actin antibody (lower panels of A and B). Lane 1—presymptomatic (pre), lane 2—onset of weakness (wkness), lane 3—complete paralysis (end). The location of the WT and G85R is designated by the upper and lower arrowheads, respectively.
DISCUSSION
The MTSOD1 FALS transgenic mouse has provided a key tool in studies of the pathogenesis and treatment of FALS and sporadic ALS. Despite all that has been learned from this model system, fundamental questions remain unanswered, such as the basis for the toxicity of MTSOD1 and for the selective vulnerability of MNs. The accelerated disease caused by WTSOD1 overexpression of MTSOD1 transgenic mice provides an opportunity to investigate the toxicity of MTSOD1. In some ways, these experiments (in which both human WT and MTSOD1 are expressed) mirror the situation in human FALS more authentically than in the case of the single transgenic MTSOD1 mouse (where human MTSOD1, but not human WTSOD1, is expressed); however, in other ways this situation in which there is very exaggerated overexpression of SOD1 is clearly very unphysiological.
There have been conflicting reports regarding the effect of overexpression of WTSOD1 on mice carrying MTSOD1 as a transgene. Bruijn et al. (7) failed to find a change in disease onset or duration when their G85R MTSOD1 transgenic mice were crossed with WTSOD1 transgenic mice [generated by Wong et al. (3)]. In contrast, Jaarsma et al. (11) found a significantly accelerated disease onset with no change in disease duration when G93A transgenic mice were crossed with WTSOD1 transgenic mice [generated by Gurney et al. (2)]. Deng et al. (8) similarly found that crossing G93A transgenic mice with WTSOD1 transgenic mice [generated by Gurney et al. (2)] hastened the onset of disease. These investigators also reported that the overexpression of WTSOD1 accelerated the onset of disease in L126Z transgenic mice and, in addition, converted the phenotype of A4V transgenic mice from unaffected to an ALS disease phenotype. One question that arose from these disparate results was whether the lack of effect seen following WTSOD1 overexpression in G85R transgenic mice (7) might be a result of a feature specific to the G85R mutation. This feature was unlikely to be related to the absence of SOD1 activity of the G85R mutation since WTSOD1 overexpression accelerated disease in the L126Z transgenic mouse (8) and since L126Z, like G85R, lacks SOD1 activity (12).
The results of the present study show that WTSOD1 overexpression in the G85R transgenic mouse that we generated leads to a shortened survival with acceleration of disease onset, neuropathology and SOD1 aggregate formation, but with no change in disease duration. These findings are similar to those obtained with other MTSOD1 transgenic mice (8,9,11). It is not clear why the results of Bruijn et al. (7) were at odds with the present findings, but we presume that the use of different G85R and WTSOD1 transgenic mice with different expression levels in the two studies affected the results. The WTSOD1 transgenic mouse used by Bruijn et al. expressed WTSOD1 in the spinal cord at 6× the level of the endogenous mouse SOD1 (7), whereas the WTSOD1 transgenic mouse that was used in the present study expressed WTSOD1 in the spinal cord at 10× the level of the endogenous mouse SOD1 (11). The G85R SOD1 transgenic mouse used by Bruijn et al. expressed G85R in the spinal cord at a level similar in amount to endogenous mouse SOD1 (13), whereas the G85R transgenic mouse used in the present study expressed G85R at 1.5× the level of the endogenous mouse SOD1 (Fig. 1B, lane 1). It may be that a certain level of SOD1 is necessary for disease to occur and that the amount of SOD1 in the double transgenic mice of Bruijn et al. (7) was insufficient. The enhanced level of human WT and G85R SOD1 expression in the present study may have enabled and fostered the incorrect disulfide bond cross-linking.
The availability of G85R/WTSOD1 double transgenic mice and the accelerated disease compared with that seen with G85R single transgenic mice provided an opportunity to examine the basis of MTSOD1 toxicity and to analyze the insoluble SOD1 aggregates. Human SOD1 is a homodimeric protein that contains four cysteines (C6, C57, C111 and C146) with an intramolecular disulfide bond between C57 and C146; this bond, along with binding to Cu/Zn ions, stabilizes and activates the enzyme and helps the protein to be maintained in its dimeric state. Remarkably, the intramolecular disulfide bond exists despite the very strongly reduced environment of the cytosol. Deng et al. (8) and Furakawa et al. (9) used the L126Z/WTSOD1 double transgenic mice to biochemically investigate the state of SOD1 in the insoluble aggregates and the possible inclusion of WTSOD1 in the aggregates. These studies were facilitated because L126Z, a truncated MTSOD1, has a different electrophoretic mobility than WTSOD1, and because of the availability of two different antibodies—one that specifically reacts to the carboxyl end of SOD1 (and therefore binds WTSOD1, but not the truncated L126Z MTSOD1) and another that reacts to both WT and MTSOD1. The investigations demonstrated that the insoluble mitochondrial fraction from the spinal cord of the double transgenic mice contained WT–WTSOD1 homodimers and L126Z–WTSOD1 heterodimers (as well as L126Z–L126Z SOD1 homodimers) that were linked by intermolecular disulfide bonds. The presence of intermolecular disulfide bonds in aggregates formed by MTSOD1 has also been reported by other investigators (14–16), but its importance remains controversial (17).
In the present study, we took advantage of the different electrophoretic mobility of G85R MTSOD1 and WTSOD1 to biochemically investigate the constituents of SOD1 aggregates in the G85R/WTSOD1 double transgenic mice, as previously performed in the case of the L126Z/WTSOD1 double transgenic mice. During disease progression, SOD1 immunoreactive dimers and multimers appeared in the insoluble fraction of homogenates from the spinal cord of G85R single and G85R/WTSOD1 double transgenic mice; these bands appeared at an earlier time in the spinal cord from G85R/WTSOD1 mice than from G85R single transgenic mice. The electrophoretic mobility of some of these bands corresponded to the predicted size of WT–WTSOD1 homodimers and G85R–WTSOD1 heterodimers (as well as G85R–G85R homodimers), suggesting that WTSOD1 participates in the formation of homo- and heterodimers in the spinal cord insoluble fraction from G85R/WTSOD1 double transgenic mice. The dimers and multimers were formed by intermolecular disulfide bonds since the bands disappeared under reducing conditions and not detected under the experimental denaturing conditions of the gel. It is possible that some of the multimeric bands that were seen on the gel also involved intermolecular disulfide bonds between G85R MTSOD1 and a non-SOD1 protein. These findings indicate that incorrect disulfide-linked proteins and WT–MT heterodimers are not a feature unique to L126Z/WTSOD1 mice and the L126Z mutant (which is a relatively unusual MTSOD1 without an intramolecular disulfide bond, because the truncated SOD1 eliminates Cys 146), and are therefore likely to be a general finding in MT/WTSOD1 double transgenic mice. Since the expression of MT and WTSOD1 is equal in cases of SOD-linked human FALS, one suspects that WT and MTSOD1 homo- and heterodimeric species cross-linked by intermolecular disulfide bonds are also present in human FALS.
The insoluble fraction of the spinal cord from endstage G85R/WTSOD1 double transgenic mice had a greater proportion of G85R to WTSOD1 when compared with the insoluble fractions from other tissues (and to the insoluble fraction from the spinal cord of double transgenic mice taken earlier in life) suggesting that the MTSOD1 preferentially aggregates in the spinal cord. In addition, most of the SOD1 in the insoluble fraction of the spinal cord from endstage G85R single transgenic mice—and an appreciable amount of the SOD1 in the insoluble fraction of the spinal cord from G85R/WTSOD1 double transgenic mice—had incorrect disulfide cross-links; additional studies (data not shown) showed that there was ∼300 μg/g wet weight (w/w) G85R and WTSOD1 in the soluble fraction and ≥320 μg/g w/w in the insoluble fraction of the spinal cord of the G85R/WT double transgenic mouse at endstage. The presence of these cross-linked proteins in the region of pathology (i.e. the spinal cord), but not in other tissues, as well as their association with clinical disease suggest that the cross-linking represents a toxic species. These cross-linked proteins may be toxic in many ways, including sequestration of proteins critical to MN function as well as disruption of axonal flow and mitochondrial function. We suspect that the overexpression of WTSOD1 in the G85R/WTSOD1 double transgenic mice stabilized G85R by increasing the amount of cross-linked and aggregated SOD1, and therefore led to an earlier onset of clinical disease.
How might intermolecular disulfide bonds form and why are they preferentially found in the insoluble fraction of the spinal cord? Oxidation of the monomeric form of SOD1 may lead to aberrant interactions between monomers or between monomers and other proteins, with cross-linking by means of intermolecular disulfide bonds that enhances stability and leads to the formation of SOD1 multimers. The importance of oxidation of SOD1 in the pathogenesis of ALS has been emphasized in the case of both FALS and sporadic ALS (18). MTSOD1s have a tendency to assume a reduced monomeric form (16), at least partly because of decreased metal binding of some of the different mutants (reviewed in 19). A reduced monomeric form of SOD1 that lacks metallation is the preferred SOD1 species for entry into mitochondria, where it may become oxidized. Banci et al. (20). have proposed that during the normal maturation process and before metal binding, SOD1 enters the mitochondria and is susceptible to oligomerization as a result of oxidation and the subsequent formation of intermolecular disulfide bond; MTSOD1 may be more susceptible to oliogmerization than WT because the free cysteines are more exposed or because the metallation process is slowed down. Oxidation of SOD1 may also occur during transit in the axon (21). Cross-linking may be prominent in the spinal cord because of the high concentration of intracellular SOD1 in MNs and because these cells may be under a heightened state of oxidative stress.
It is of interest that the overexpression of WTSOD1 in three MTSOD1 transgenic mice [G93A, L126Z, G85R (the present study)] led to an earlier disease onset with no prolongation in disease duration. On the basis of Cre/LoxP studies, the Cleveland laboratory found that disease onset in the G37R mouse seemed to be primarily related to the expression of the MTSOD1 in MNs, while expression of G37R MTSOD1 in microglia affected disease progression (22); we have found similar results in Cre/LoxP studies with the floxed-G85R mouse used in the present study (unpublished data). The results from these Cre/LoxP studies suggest that WTSOD1 overexpression in MTSOD1 transgenic mice may primarily affect the expression of MTSOD1 in MNs, perhaps because of the robust expression of MTSOD1 in this cell type.
One direction that is presently being pursued with respect to the treatment of MTSOD1-induced FALS is a knockdown of expression of both MT (along with WT) SOD1, either with anti-sense oligonucleotides or RNAi. Studies in MTSOD1 transgenic mice have shown a more prolonged disease onset as a result of this treatment (23). The findings of the present study bring up the possibility that this therapeutic approach may be efficacious both because of a removal of the WT as well as MTSOD1.
MATERIALS AND METHODS
Generation of the G85R mouse
A G85R genomic clone was generously provided by Dr David Borchelt. LoxP sites were engineered to flank the G85R SOD1 sequence because of plans to use this construct for unrelated studies of cell autonomy. The construct underwent standard micronucleus injection into C57BL/6J mice at University of Chicago, Transgenic Mouse Facility. Mouse tail DNA was analyzed by PCR for the presence of the transgene using the following primers: SOD1 forward 5′-CATAACTTCGTATAGCATACATTAT-3′ and SOD1 reverse 5′-CTTCTGCTCGAAATTGATGATGCCCT-3′.
Breeding
Mice carrying the floxed-G85R MTSOD1 as a transgene were crossed to mice carrying WTSOD1 (2), which had been backcrossed on to the C57BL/6J background. Tail DNA was PCR-amplified using primers flanking exon 4 of the human SOD1 transgenes: SOD1E4 forward 5′-AGTGGCATCAGCCCTAATCCATCT-3 and SOD1E4 reverse 5′-GCAAGTACAGTTTATCTGGATC-3′. The PCR products of 272 bp were agarose gel purified and then sequenced to determine whether mice carried both the human WT and G85R SOD1 genes.
Assessment of disease phenotype
Clinical assessment
Mice were weighed twice per week. As described by Boillee et al. (22): the onset of disease was defined as the peak weight before a decline; early disease was defined as the period from the peak weight until the loss of 10% of the maximal weight; and late disease was the time from this 10% loss in weight until death (when the mouse was unable to right itself within 20 s after being put on its back). Animals that could not right themselves within 20 s were euthanized as recommended by University of Chicago, Institutional Animal Care and Use Committee guidelines.
Pathology
Mice were anesthetized and perfused with ice-cold PBS, followed by 4% paraformaldehyde using a protocol approved by University of Chicago, Animal Resources Center. The pathology of the spinal cord was evaluated as previously described (24). Some mice were injected intramuscularly ∼24 h prior to sacrifice with CTB, as previously described (24).
Immunohistochemical evaluation
The expression pattern of SOD1 was determined by immunohistochemical staining using a rabbit human-specific anti-SOD1 antibody (24). The spinal cord was also examined with rabbit anti-GFAP antibody (1:1000, Chemicon, Temecula, CA, USA). The immunohistochemical antibody method has been previously published (24).
Western blots and activity gels
Western blots were carried out using previously published methods (25) with either a rabbit anti-SOD1 antibody that stains murine and human SOD1 (1:1000, Stressgen, BC, Canada) or a rabbit antibody raised against a peptide of SOD1 (amino acids 24–36) that only stains human SOD1 (8). In order to separate the G85R band from the mouse endogenous SOD1 and determine this ratio, muscle and spinal cord homogenates of G85R transgenic mice were subjected to 15% PAGE on a long gel for an extended period of time. The SOD1 enzymatic activity of spinal cord tissue from transgenic mice was determined by a gel assay, as previously published (26).
In some cases, homogenates of the spinal cord and other tissues were processed using a method similar to that previously published (8,9). Briefly, 15 mg of fresh tissue was homogenized in 200 µl homogenizing buffer (50 mm HEPES, 1 mm EDTA, 100 mm iodoacetamide), incubated at 37°C for 1 h, and then centrifuged at 20 000 g for 5 min. The pellet was washed three times with a washing buffer (50 mm HEPES, 1 mm EDTA, 100 mm iodoacetamide and 0.1% Nonidet P-40, pH 7.2) and centrifuged after each wash for 20 000 g for 10 min. The final pellet, which was designated the insoluble fraction, was dissolved in 200 µl in the final solution A [50 mm HEPES, 1 mm EDTA, 100 mm iodoacetamide and 2.5% sodium dodecyl sulfate (SDS), pH 7.2]. In some cases, a soluble and insoluble fraction, which was designated the homogenate, was obtained from the spinal cord; in these cases, the spinal cord was directly homogenized in solution A and then centrifuged 15 000 rpm for 10 min. Four microgram (unless otherwise noted) of the total protein of each sample was treated with or without β-mercaptoethanol before boiling and then loading on 15% SDS polyacrylamide gel for western blot analysis using a human-specific anti-SOD1 antibody. The amount of human SOD1 in the spinal cord was estimated by densitometry, as previously published (27). The quantification of band intensities was performed by densitometric analysis using Image J (National Institute of Mental Health, Bethesda, MD, USA).
Conflict of Interest statement. None declared.
FUNDING
This work was supported by ALS Association (#1211 to R.P.R.), Muscular Dystrophy Association (#4346 to R.P.R.) and National Institutes of Health (NS40308 to H.-X.D. and T.S., NS050641 to T.S. and NS046535 to T.S.).
REFERENCES
- 1.Bruijn L.I., Miller T.M., Cleveland D.W. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 2.Gurney M.E., Pu H., Chiu A.Y., Dal Canto M.C., Polchow C.Y., Alexander D.D., Caliendo J., Hentati A., Kwon Y.W., Deng H.X., et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 3.Wong P.C., Pardo C.A., Borchelt D.R., Lee M.K., Copeland N.G., Jenkins N.A., Sisodia S.S., Cleveland D.W., Price D.L. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 4.Reaume A.G., Elliott J.L., Hoffman E.K., Kowall N.W., Ferrante R.J., Siwek D.F., Wilcox H.M., Flood D.G., Beal M.F., Brown R.H., Jr, et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 5.Gruzman A., Wood W.L., Alpert E., Prasad M.D., Miller R.G., Rothstein J.D., Bowser R., Hamilton R., Wood T.D., Cleveland D.W., et al. Common molecular signature in SOD1 for both sporadic and familial amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. USA. 2007;104:12524–12529. doi: 10.1073/pnas.0705044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ezzi S.A., Urushitani M., Julien J.P. Wild-type superoxide dismutase acquires binding and toxic properties of ALS-linked mutant forms through oxidation. J. Neurochem. 2007;102:170–178. doi: 10.1111/j.1471-4159.2007.04531.x. [DOI] [PubMed] [Google Scholar]
- 7.Bruijn L.I., Houseweart M.K., Kato S., Anderson K.L., Anderson S.D., Ohama E., Reaume A.G., Scott R.W., Cleveland D.W. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998;281:1851–1854. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- 8.Deng H.X., Shi Y., Furukawa Y., Zhai H., Fu R., Liu E., Gorrie G.H., Khan M.S., Hung W.Y., Bigio E.H., et al. Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc. Natl Acad. Sci. USA. 2006;103:7142–7147. doi: 10.1073/pnas.0602046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furukawa Y., Fu R., Deng H.X., Siddique T., O’Halloran T.V. Disulfide cross-linked protein represents a significant fraction of ALS-associated Cu, Zn-superoxide dismutase aggregates in spinal cords of model mice. Proc. Natl Acad. Sci. USA. 2006;103:7148–7153. doi: 10.1073/pnas.0602048103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borchelt D.R., Lee M.K., Slunt H.S., Guarnieri M., Xu Z.S., Wong P.C., Brown R.H., Jr, Price D.L., Sisodia S.S., Cleveland D.W. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc. Natl Acad. Sci. USA. 1994;91:8292–8296. doi: 10.1073/pnas.91.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaarsma D., Haasdijk E.D., Grashorn J.A., Hawkins R., van Duijn W., Verspaget H.W., London J., Holstege J.C. Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1. Neurobiol. Dis. 2000;7:623–643. doi: 10.1006/nbdi.2000.0299. [DOI] [PubMed] [Google Scholar]
- 12.Ratovitski T., Corson L.B., Strain J., Wong P., Cleveland D.W., Culotta V.C., Borchelt D.R. Variation in the biochemical/biophysical properties of mutant superoxide dismutase 1 enzymes and the rate of disease progression in familial amyotrophic lateral sclerosis kindreds. Hum. Mol. Genet. 1999;8:1451–1460. doi: 10.1093/hmg/8.8.1451. [DOI] [PubMed] [Google Scholar]
- 13.Bruijn L.I., Becher M.W., Lee M.K., Anderson K.L., Jenkins N.A., Copeland N.G., Sisodia S.S., Rothstein J.D., Borchelt D.R., Price D.L., et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 14.Niwa J., Yamada S., Ishigaki S., Sone J., Takahashi M., Katsuno M., Tanaka F., Doyu M., Sobue G. Disulfide bond mediates aggregation, toxicity, and ubiquitylation of familial amyotrophic lateral sclerosis-linked mutant SOD1. J. Biol. Chem. 2007;282:28087–28095. doi: 10.1074/jbc.M704465200. [DOI] [PubMed] [Google Scholar]
- 15.Beers D.R., Henkel J.S., Xiao Q., Zhao W., Wang J., Yen A.A., Siklos L., McKercher S.R., Appel S.H. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. USA. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergemalm D., Jonsson P.A., Graffmo K.S., Andersen P.M., Brannstrom T., Rehnmark A., Marklund S.L. Overloading of stable and exclusion of unstable human superoxide dismutase-1 variants in mitochondria of murine amyotrophic lateral sclerosis models. J. Neurosci. 2006;26:4147–4154. doi: 10.1523/JNEUROSCI.5461-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karch C.M., Borchelt D.R. A limited role for disulfide cross-linking in the aggregation of mutant SOD1 linked to familial amyotrophic lateral sclerosis. J. Biol. Chem. 2008;283:13528–13537. doi: 10.1074/jbc.M800564200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kabashi E., Valdmanis P.N., Dion P., Rouleau G.A. Oxidized/misfolded superoxide dismutase-1: the cause of all amyotrophic lateral sclerosis? Ann. Neurol. 2007;62:553–559. doi: 10.1002/ana.21319. [DOI] [PubMed] [Google Scholar]
- 19.Tiwari A., Hayward L.J. Mutant SOD1 instability: implications for toxicity in amyotrophic lateral sclerosis. Neurodegener. Dis. 2005;2:115–127. doi: 10.1159/000089616. [DOI] [PubMed] [Google Scholar]
- 20.Banci L., Bertini I., Boca M., Girotto S., Martinelli M., Valentine J.S., Vieru M. SOD1 and amyotrophic lateral sclerosis: mutations and oligomerization. PLoS ONE. 2008;3:e1677. doi: 10.1371/journal.pone.0001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borchelt D.R., Wong P.C., Becher M.W., Pardo C.A., Lee M.K., Xu Z.S., Thinakaran G., Jenkins N.A., Copeland N.G., Sisodia S.S., et al. Axonal transport of mutant superoxide dismutase 1 and focal axonal abnormalities in the proximal axons of transgenic mice. Neurobiol. Dis. 1998;5:27–35. doi: 10.1006/nbdi.1998.0178. [DOI] [PubMed] [Google Scholar]
- 22.Boillee S., Vande Velde C., Cleveland D.W. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Miller T.M., Kim S.H., Yamanaka K., Hester M., Umapathi P., Arnson H., Rizo L., Mendell J.R., Gage F.H., Cleveland D.W., et al. Gene transfer demonstrates that muscle is not a primary target for non-cell-autonomous toxicity in familial amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. USA. 2006;103:19546–19551. doi: 10.1073/pnas.0609411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L.J., Lu Y.Y., Muramatsu S., Ikeguchi K., Fujimoto K., Okada T., Mizukami H., Matsushita T., Hanazono Y., Kume A., et al. Neuroprotective effects of glial cell line-derived neurotrophic factor mediated by an adeno-associated virus vector in a transgenic animal model of amyotrophic lateral sclerosis. J. Neurosci. 2002;22:6920–6928. doi: 10.1523/JNEUROSCI.22-16-06920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghadge G.D., Wang L., Sharma K., Monti A.L., Bindokas V., Stevens F.J., Roos R.P. Truncated wild-type SOD1 and FALS-linked mutant SOD1 cause neural cell death in the chick embryo spinal cord. Neurobiol. Dis. 2006;21:194–205. doi: 10.1016/j.nbd.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Subramaniam J.R., Lyons W.E., Liu J., Bartnikas T.B., Rothstein J., Price D.L., Cleveland D.W., Gitlin J.D., Wong P.C. Mutant SOD1 causes motor neuron disease independent of copper chaperone-mediated copper loading. Nat. Neurosci. 2002;5:301–307. doi: 10.1038/nn823. [DOI] [PubMed] [Google Scholar]
- 27.Pramatarova A., Laganiere J., Roussel J., Brisebois K., Rouleau G.A. Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment. J. Neurosci. 2001;21:3369–3374. doi: 10.1523/JNEUROSCI.21-10-03369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]