Abstract
Prokineticins are a pair of regulatory peptides that have been shown to play important roles in gastrointestinal motility, angiogenesis, circadian rhythms, and, recently, olfactory bulb neurogenesis. Prokineticins exert their functions via activation of two closely related G-protein-coupled receptors. Here we report a comprehensive mRNA distribution for both prokineticins (PK1 and PK2) and their receptors (PKR1 and PKR2) in the adult mouse brain with the use of in situ hybridization. PK2 mRNA is expressed in discrete regions of the brain, including suprachiasmatic nucleus, islands of Calleja and medial preoptic area, olfactory bulb, nucleus accumbens shell, hypothalamic arcuate nucleus, and amygdala. PK1 mRNA is expressed exclusively in the brainstem, with high abundance in the nucleus tractus solitarius. PKR2 mRNA is detected throughout the brain, with prominent expression in olfactory regions, cortex, thalamus and hypothalamus, septum and hippocampus, habenula, amygdala, nucleus tractus solitarius, and circumventricular organs such as subfornical organ, median eminence, and area postrema. PKR2 mRNA is also detected in mammillary nuclei, periaqueductal gray, and dorsal raphe. In contrast, PKR1 mRNA is found in fewer brain regions, with moderate expression in the olfactory regions, dentate gyrus, zona incerta, and dorsal motor vagal nucleus. Both PKR1 and PKR2 are also detected in olfactory ventricle and subventricular zone of the lateral ventricle, both of which are rich sources of neuronal precursors. These extensive expression patterns suggest that prokineticins may have a broad array of functions in the central nervous system, including circadian rhythm, neurogenesis, ingestive behavior, reproduction, and autonomic function.
Indexing terms: circadian rhythms, hypothalamus, feeding, neurogenesis, locomotor behavior, reproduction
Prokineticins (PKs) are a pair of novel regulatory peptides that were identified as the mammalian homologs of the frog secretion protein Bv8 and mamba intestinal toxin 1 (MIT1; Li et al., 2001). Mammalian PKs consist of two related cysteine-rich molecules with molecular weight of ~10 kDa and share over 40% amino acid identity with frog Bv8 and mamba MIT1. PKs and their nonmammalian counterparts contain 10 conserved cysteines with identical positions, which form disulfide bonds as essential structural motif for their activities (Bullock et al., 2004). The first N-terminal six amino acids (AVITGA) are also completely conserved among these species and have also been shown indispensable for the bioactivities of PKs (Bullock et al., 2004).
Within the last few years, multiple biological roles for PKs have been discovered. We originally identified PKs as specific and potent regulators of gastrointestinal smooth muscle contractions (Li et al., 2001). An independent group showed that PK1 (named endocrine gland-derived vascular endothelial growth factor; EG-VEGF) functions as a selective angiogenic mitogen for endothelial cells derived from endocrine organs (Le Couter et al., 2001). In the following year, we identified PK2 as a critical circadian output signaling molecule that regulates locomotor behavior (Cheng et al., 2002). Recently, we have also demonstrated that PK2 functions as a chemoattractant that is essential for olfactory bulb neurogenesis (Ng et al., 2005).
Pharmacological studies have indicated that PKs mediate their activities in GI smooth muscle cells by interacting with receptors belonging to the family of G-protein-coupled receptors (GPCR; Li et al., 2001). Molecular cloning has confirmed two very closely related GPCRs as functional receptors for PKs (Lin et al., 2002; Masuda et al., 2002; Soga et al., 2002). These receptors share over 80% identity at the nucleic acid sequence level. Previous RT-PCR and Northern blot analyses have shown that PK1 is more widely distributed in peripheral tissues than PK2. A similar peripheral distribution pattern has also been described for prokineticin receptor 1 (PKR1) and prokineticin receptor 2 (PKR2; Lin et al., 2002; Masuda et al., 2002; Soga et al., 2002).
In an attempt to understand the potential functional roles of PKs in the central nervous system, we have undertaken a comprehensive mapping of the distributions of mRNAs for both prokineticins (PK1 and PK2) and their receptors (PKR1 and PKR2) in the adult mouse brain by using in situ hybridization analysis. Our data indicate that both PKs and their receptors are expressed in the adult mouse brain, with PK2 and PKR2 more widely distributed. The regional expression patterns of the PK system suggest that these molecules may regulate a variety of physiological processes and behaviors, in addition to the known roles of PK2 in circadian rhythms and olfactory bulb neurogenesis.
MATERIALS AND METHODS
Tissue preparation
Tissues were obtained from five male adult C57Bl6 mice (Taconic Farms). Animals were housed under 12:12 hour light/dark cycle, with food and water available ad libitum. Animals were killed at different Zeitgeber times (ZT1 and ZT13) by cervical dislocation, and their brains were quickly removed and frozen in isopentane at −20°C for 30 seconds. This procedure was approved by our Institutional Animal Care and Use Committee and was consistent with Federal guidelines. Twenty-micrometer coronal sections were cut with a cryostat at −20°C. Serial sections were collected at 160-μm intervals and mounted onto Super-frost Plus slides. Tissue sections were fixed with 4% para-formaldehyde for 1 hour, followed by three 0.1 M phosphate buffer (PB) washes; air dried; and stored at −80°C until use.
Probes
Antisense and sense riboprobes containing the coding regions of mouse PK1 or PK2 were used, whereas the 3′ untranslated regions (UTR) of mouse PKR1 or PKR2 used as the coding regions of these receptors are over 80% identical at the nucleic acid sequence level. Antisense and sense riboprobes were generated as follows: cDNA fragments for PK1 (AF487281), PK2 (AF487280), PKR1 (AF487278, ~900 bp), and PKR2 (AF487279, ~1,000 bp) were subcloned into PCRII-TOPO vector. Antisense and sense probes for PK1 were generated by SP6 and T7 RNA polymerase, respectively. Antisense and sense probes for PK2, PKR1, and PKR2 were generated by T7 and SP6 RNA polymerase, respectively. Riboprobes were labeled with 35S-UTP, and unincorporated nucleotides were separated by using a G-50 Sephadex column (Roche, Indianapolis, IN). Each riboprobe was diluted to 1 × 107 cpm/ml in hybridization solution [50% formaldehyde, 10% dextran sulfate, 0.02% Ficoll, 0.02% polyvinylpyrolidone, 0.02% bovine serum albumin (BSA), 500 μg/ml tRNA, 10 mM dithiothreitol (DTT), 0.3 M NaCl, 10 mM Tris, pH 8.0, 1 mM EDTA, pH 8.0].
In situ hybridization
Tissue sections were processed for in situ hybridization as described (Winzer-Serhan et al., 1999). Briefly, sections were pretreated with proteinase K (1 μg/ml), acetylated, dehydrated, and air dried. Sections were hybridized with riboprobes (antisense or sense for each of the four probes, 1 × 107 cpm/ml) and incubated at 60°C for 18 hours, followed by RNAase (20 μg/ml) digestion, decreasing salinity washes, and a 30-minute high-stringency (68°C) wash. After dehydration and air drying, tissue sections were exposed to Kodak Biomax film for 3 days. Autoradiograms were developed and tissue sections were dipped in liquid Kodak NTB-2 emulsion. After 3 weeks of exposure, emulsion-dipped sections were developed, counterstained with cresyl violet, and coverslipped for qualitative analysis via transillumination microscopy.
Data evaluation and image processing
Distributions of PK1, PK2, PKR1, and PKR2 mRNA were qualitatively analyzed in autoradiograms and emulsion-dipped sections. Anatomical brain regions were identified with cresyl violet-stained sections by using brightfield analysis and comparisons with the mouse brain atlas (Paxinos and Franklin, 2004). To get an initial overview of cellular expression patterns, autoradiograms were first analyzed by using a video-based computerized image analysis system (MCID; Imaging Research, St. Catherine, Ontario, Canada). Specific hybridization signals in various brain regions were analyzed by comparing sections hybridized with antisense probe to those hybridized with sense probe. Hybridization signals in specific brain nuclei were qualitatively analyzed by using emulsion-dipped sections at high magnification by transillumination microscopy. The relative intensities of the mR-NAs in various brain regions were evaluated in the following manner and are listed in Table 1: ++++ represents very strong mRNA expression, +++ represents strong expression, ++ represents moderate expression, + represents widely scattered low expression, and − represents no expression. Images were taken under a transillumination microscope (Olympus BX50) with Spot camera software version 2.2.2 (Diagnostic Instruments, Sterling Heights, MI). Captured images were transferred to Adobe Photoshop 6.0 for figure preparation. Cropping, resizing, and minor adjustments to contrast and brightness were carried out in Photoshop.
TABLE 1.
Expression of Prokineticins and Prokineticin Receptors in the Adult Mouse Brain
| Brain regions | Abbreviation | PK1 | PK2 | PKR1 | PKR2 |
|---|---|---|---|---|---|
| Olfactory regions and ventricles | |||||
| Olfactory bulb | OB | − | +/++ | ++ | +++ |
| Ependyma and olfactory ventricle | E/OV | − | − | ++ | ++++ |
| Anterior olfactory nu. | AON | − | − | − | ++ |
| Lateral olfactory tract | LOT | − | − | − | + |
| Endopiriform nu. | DEn | − | − | − | + |
| Subventricular zone of lateral ventricle | SVZ | − | − | ++ | +++ |
| Cortex | |||||
| Piriform cortex | Pir | − | − | − | ++/+++ |
| Retrosplenial cortex, agranular/granular | RSA/RSG | − | − | − | + |
| Perirhinal cortex | PRh | − | − | − | ++ |
| Entorhinal cortex, medial | MEnt | − | − | − | ++ |
| Entorhinal cortex, lateral | LEnt | − | − | − | +/++ |
| Septum and hippocampus | |||||
| Lateral septum, dorsal | LSD | − | − | − | +++ |
| Lateral septum, intermediate | LSI | − | − | − | ++ |
| Lateral septum, ventral | LSV | − | − | 3 | + |
| CA1 | CA1 | − | − | − | ++ |
| CA2 | CA2 | − | − | − | ++ |
| CA3 | CA3 | − | − | ++ | ++ |
| Dentate gyrus | DG | − | − | ++ | + |
| Presubiculum | PrS | − | − | ± | + |
| Subiculum | S | − | − | ++ | ++/+++ |
| Basal ganglia | − | ||||
| Nucleus accumbens shell | AcbSH | − | ++/+++ | − | − |
| Ventral pallidum | VP | − | − | − | +/++ |
| Globus pallidus, lateral ventral | GP | − | − | + | ++/+++ |
| Islands of Callejia | ICj | − | +++ | − | |
| Amygdala | − | ||||
| Anterior cortical amygdala | ACO | − | − | − | ++ |
| Lateral amygdala | LA | − | − | − | +++ |
| Basomedial amygdala | BMA | − | − | − | +/++ |
| Medial amygdala | Me | − | ++ | − | ++++ |
| Amygdalohippocampal area | AHiPM | − | − | − | +++ |
| Amygdalopiriform transition area | APir | − | − | ++++ | |
| Thalamus | − | ||||
| Lateral dorsal thalamic nu. | LD | − | − | − | +/++ |
| Paraventricular thalamic nu. | PVT | − | − | − | ++++ |
| Paracentral nu. | PC | − | − | − | ++ |
| Central medial thalamic nu. | CM | − | − | − | ++/+++ |
| Intermediodorsal dorsal thalamic nu. | IMD | − | − | − | ++ |
| Lateral posterior thalamic nu. | LP | − | − | − | +/++ |
| Lateral habenula | LHb | − | − | ++++ | |
| Hypothalamus | |||||
| Medial preoptic area | MPA | − | +++ | − | − |
| Lateral preoptic area | LPA | − | − | − | + |
| Suprachiasmatic hypothalamic nu. | SCN | − | ++++ | − | ++++ |
| Paraventricular hypothalamic nu., dorsal cap | PaDC | − | − | − | +++ |
| Paraventricular hypothalamic nu. | Pa | − | − | − | +++ |
| Lateral hypothalamus | LH | − | − | − | + |
| Perifornical area | PeF | − | − | − | + |
| Arcuate nu. | Arc | − | ++ | +/++ | ++ |
| Dorsomedial hypothalamic nu. | DMH | − | − | − | ++/+++ |
| Premamillary nu. | PM | − | ± | − | − |
| Mammillary nu., medial, lateral | MM | − | − | ++ | ++/+++ |
| Zona incerta, caudal | ZI | − | − | +++ | − |
| Subfornical organ | SFO | − | − | − | ++/+++ |
| Median eminence | ME | − | − | − | ++ |
| Mesencephalon | |||||
| Superior colliculus | SC | − | − | − | + |
| Parabigeminal nu. | PBG | − | − | +/++ | − |
| Edinger3Westphal nu. | EW | − | +/++ | − | − |
| Periaqueductal gray, dorsomedial | DMPAG | − | − | − | ++/+++ |
| Periaqueductal gray, ventrolateral | VLPAG | − | − | + | + |
| Dorsal raphe | DR | − | − | − | +/++ |
| Brainstem | |||||
| Dorsal cochlear nu. | DCN | − | − | − | +/++ |
| Superficial glial zone of cochlear nu. | SGl | − | − | − | +/++ |
| Parabrachial nu., medial | PB | − | − | − | +/++ |
| Nucleus tractus solitarius | NTS | ++++ | − | − | ++/+++ |
| Dorsal motor nu., vagus | 10N | − | − | ++/+++ | − |
| Lateral reticular nu. | LRt | ++++ | − | − | − |
| Area postrema | AP | − | − | − | ++++ |
Nomenclature based on Paxinos and Franklin (2004). The relative densities of prokineticins and their receptors were evaluated as described in Materials and Methods.
RESULTS
Distribution of PK1 and PK2 mRNAs
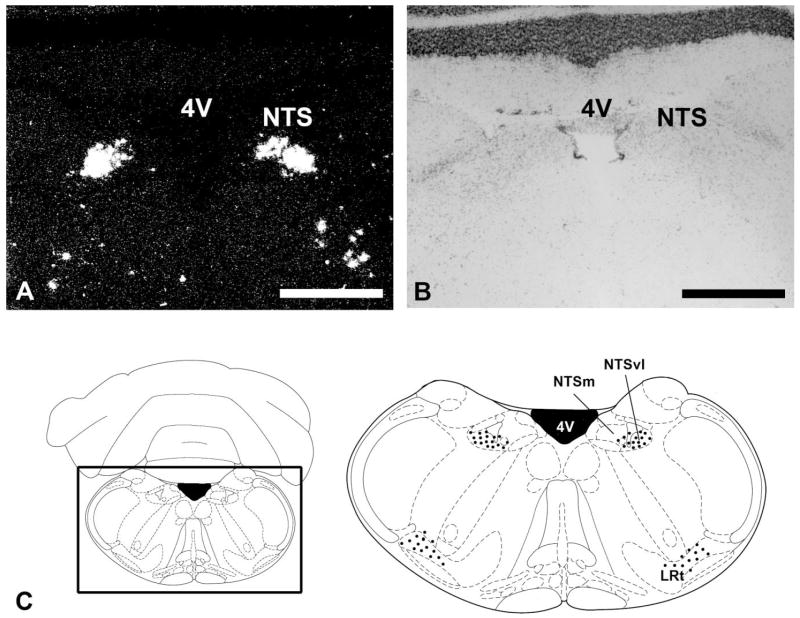
Both PK1 and PK2 are expressed in discrete regions of the mouse brain (Table 1). PK1 mRNA is detected exclusively in the brainstem (Fig. 1). There is high abundance of PK1 mRNA in the nucleus tractus solitarius (NTS), with densely labeled cells most concentrated in the ventrolateral division (Fig. 1A,C). In the reticular formation, clusters of PK1-positive cells are detected in the vicinity of the lateral reticular nucleus (Fig. 1A,C). Preliminary analyses indicate that these PK1-expressing cells are nonnoradrenergic (data not shown). No specific PK1 mRNA is detected in the forebrain.
Fig. 1.
Distribution of PK1 mRNA in the brainstem. A: Darkfield image of coronal brain section hybridized with PK1 antisense probe. Expression of PK1 mRNA in the nucleus tractus solitarius (NTS). B: Cresyl violet-counterstained section of the darkfield image in A. C: Diagram indicates the approximate location of the areas shown in A. The boxed area is enlarged at right to indicate PK1-expressing cells (black dots) in the NTS. NTSm, nucleus tractus solitarius, medial division; NTSvl, nucleus tractus solitarius, ventrolateral division. Scale bars = 1 mm.
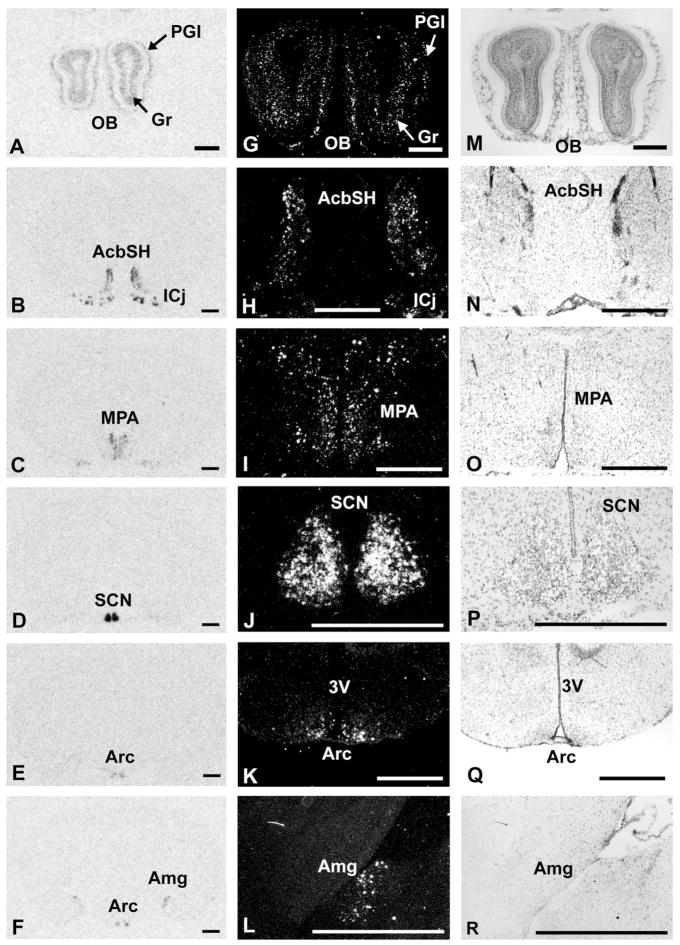
In contrast to PK1, PK2 mRNA is expressed in several forebrain regions (Table 1, Fig. 2). The highest expression of PK2 mRNA is found in the suprachiasmatic nucleus of the hypothalamus (Table 1, Fig. 2D,J), and this expression oscillates with high levels during the day and low or undetectable levels at night (Cheng et al., 2002). Dense clusters of PK2 mRNA are also found in the medial pre-optic area of the hypothalamus (Fig. 2C,I) and the islands of Calleja (Fig. 2B,H). Moderate levels of PK2 mRNA are also detected in the shell of the nucleus accumbens (Fig. 2B,H). In the medial amygdala, light to moderate levels of PK2-expressing cells are detected in the posterior dorsal region (Fig. 2F,L). Low to moderate expression of PK2 is observed in the arcuate nucleus of the hypothalamus (Fig. 2E,K) and the Edinger-Westphal nucleus (Table 1). A very low level of PK2 is also detected in the ventral part of the premammillary nucleus (Table 1). In the olfactory bulb, PK2 is moderately expressed in the granular and periglomerular layers (Fig. 2A,G).
Fig. 2.
Distribution of PK2 mRNA in the mouse brain. A–F: Autoradiographic images of coronal brain sections hybridized with PK2 antisense probe. Expression of PK2 mRNA in granule layer (Gr) and periglomerular layer (PGl) of olfactory bulb; AcbSH, nucleus accumbens shell; ICj, islands of Calleja; MPA, medial preoptic area; SCN, suprachiasmatic hypothalamic nucleus; Arc, arcuate hypothalamic nucleus; Me, medial amygdala. G–L: Darkfield images of the same sections illustrated in A–F under higher magnification. M–R: Cresyl violet-counterstained sections of the darkfield images illustrated in G–L. Scale bars = 1 mm.
Distribution of PKR1 and PKR2 mRNAs
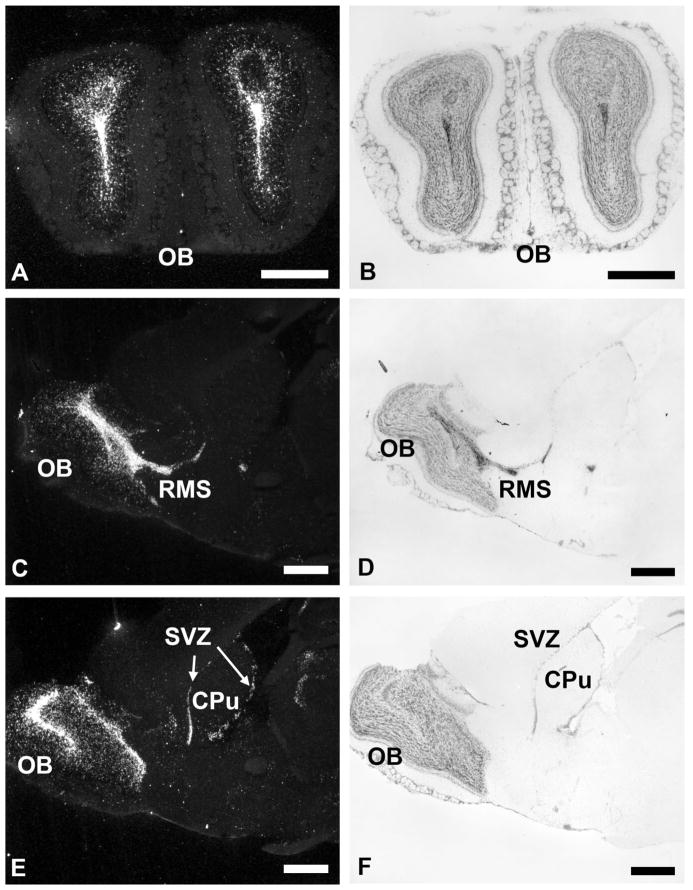
PKR1 and PKR2 mRNAs are detected throughout the brain (Table 1). The overall receptor distribution pattern indicates that PKR1 mRNA is expressed in limited regions at a low density, whereas PKR2 mRNA is highly and widely expressed throughout the adult mouse brain. Both PKR1 and PKR2 mRNAs are found in the olfactory ventricle and the subventricular zone of the lateral ventricle (Table 1, Figs. 3, 4). PKR1 mRNA is also detected in several olfactory regions, as well as other brain areas, including the islands of Calleja, hippocampus and dentate gyrus, zona incerta, and dorsal motor vagal nucleus (Fig. 4). PKR2 is prominently expressed throughout the brain, including thalamus and hypothalamus, septum and hippocampus, lateral habenula, amygdala, brainstem, and circumventricular organs (Table 1, see Figs. 5–7). Low to moderate levels of PKR2 mRNA are also detected in the periaqueductal gray and dorsal raphe nucleus (Table 1, Fig. 7). Detailed distributions of these receptors are described below.
Fig. 3.
Distribution of PKR2 mRNA in olfactory bulb and subventricular zone of the lateral ventricle. A,B: Darkfield and cresyl violet-counterstained coronal sections of the olfactory bulb (OB). C,D: Dark-field and cresyl violet-counterstained sagittal sections of the OB and the rostral migrating system (RMS). E,F: Darkfield and cresyl violet-counterstained sagittal sections of the OB and the subventricular zone of the lateral ventricle (SVZ). CPu, caudate putamen. Scale bars = 1 mm.
Fig. 4.
Distribution of PKR1 mRNA in the mouse brain. A–D: Autoradiographic images of PKR1 mRNA expression in ependyma/olfactory ventricle (E/OV); SVZ, subventricular zone of the lateral ventricle; DG, dentate gyrus; ZI, zona incerta; MM, mammillary nucleus; 10N, dorsal motor nucleus, vagus. E,F: Darkfield and cresyl violet-counterstained section of zona incerta shown in C, respectively. G,H: Darkfield and cresyl violet-counterstained section of 10N shown in D, respectively. AP, area postrema; CC, central canal. Scale bars = 1 mm.
Fig. 5.
Distribution of PKR2 mRNA in septum, amygdala, and hippocampus. A–C: Autoradiographic images of PKR2 mRNA in lateral septum (LS), paraventricular thalamic nucleus (PV), subfornical organ (SFO), suprachiasmatic nucleus (SCN), lateral habenula (LHb), lateral globus pallidus (LGP), and amygdala (Amg). D,E,G,H: Dark-field images at higher magnification of A–C. F,I: Cresyl violet-counterstained sections of E and H, respectively. J,L: Darkfield images of PKR2 mRNA in hippocampus (hpx) and amygdalohippocampal area (AHiPM). K: Cresyl violet-counterstained section of J. DMH, dorsomedial thalamic nucleus; MHb, medial habenula; DG, dentate gyrus. Scale bars = 1 mm.
Fig. 7.
Distribution of PKR2 mRNA in the mesencephalon and brainstem. A: Autoradiographic image of PKR2 mRNA in the rostral dorsomedial periaqueductal gray (DMPAG) above the sylvius aqueduct (Aq). B,C: Darkfield and cresyl violet-counterstained sections of the image shown in A, respectively. D: Darkfield image of PKR2 mRNA in caudal dorsomedial PAG (DMPAG), ventrolateral PAG (VL-PAG), and dorsal raphe (DR). E: Diagram depicting PKR2-expressing cells (black dots) in the PAG and DR. Darkfield (F) and cresyl violet-counterstained (G) section of PKR2 mRNA in area postrema (AP) and nucleus tractus solitarius (NTS). CC, central canal. Scale bars = 1 mm.
Olfactory regions and ventricles
High levels of PKR2 mRNA are detected in the olfactory bulb, particularly the ependyma and olfactory ventricle (Fig. 3A,C,E). Densely labeled PKR2 cells are also observed in the subventricular zone of the lateral ventricle (Fig. 3C,E). Moderate levels of PKR1 mRNA are found in these brain regions (Table 1, Fig. 4). Low levels of PKR2 mRNA are expressed as scattered cells in the lateral olfactory tract and endopiriform nucleus (Table 1).
Cortex, septum, and hippocampus
Although the mRNAs of both ligands (PK1 and PK2) and PKR1 are not detected in the cortex, moderate to high levels of PKR2 mRNA are found in several olfactory cortices (Table 1). In particular, discrete labeling of PKR2 mRNA is found in the piriform cortex, perirhinal cortex, and entorhinal cortex. PKR2 expression in the entorhinal cortex is higher in the medial part than in the lateral part. Low levels of PKR2 mRNA are also observed within the agranular and granular retrosplenial cortex (Table 1).
PKR2 mRNA is detected in most parts of the lateral septum, with the most intense labeling in the dorsal division (Fig. 5A,D). A few densely labeled cells are also de‘tected in the intermediate and ventral divisions of the lateral septum (Fig. 5D). In hippocampal formation, PKR2 mRNA is prominently expressed in the pyramidal layer of the CA1, CA2, and CA3 (Fig. 5J), and low expression of PKR2 is also detected in the dentate gyrus (Table 1). PKR1 mRNA is expressed in the hippocampus and the granule layer of the dentate gyrus (Fig. 4C). In the pre-subiculum and subiculum, both receptor mRNAs are detected, with PKR2 expressed at higher levels (Table 1).
Basal ganglia and amygdala
Whereas PK2 is expressed in the nucleus accumbens (Fig. 2B,H), neither PKR1 or PKR2 mRNA is detected in this region. Paralleling the expression of PK2, PKR1 mRNA is also lightly expressed in the islands of Calleja (Table 1). In the ventral pallidum, light to moderate expression of PKR2 is detected in the caudal part as a dispersed pattern (Table 1). In the globus pallidus, dense PKR2-positive cells are found in the lateral division (Fig. 5C,G).
In the amygdala, PKR2 mRNA is detected at high to moderate levels (Fig. 5C,G). The highest PKR2 mRNA expression is found in the amygdalohippocampal area (Fig. 5L). Strong PKR2 labeling is also detected in the amygdalopiriform transition area and the ventral part of the basomedial amygdala (Table 1), with more moderate expression in the lateral amygdala (Fig. 5C,G). Moderate levels of PKR2 are also detected in the anterior cortical amygdala (Fig. 6D). Low expression of PKR2 is also detected in the medial amygdala (Fig. 5C), the only amygdaloid nucleus that expresses PK2 (Fig. 2F,L).
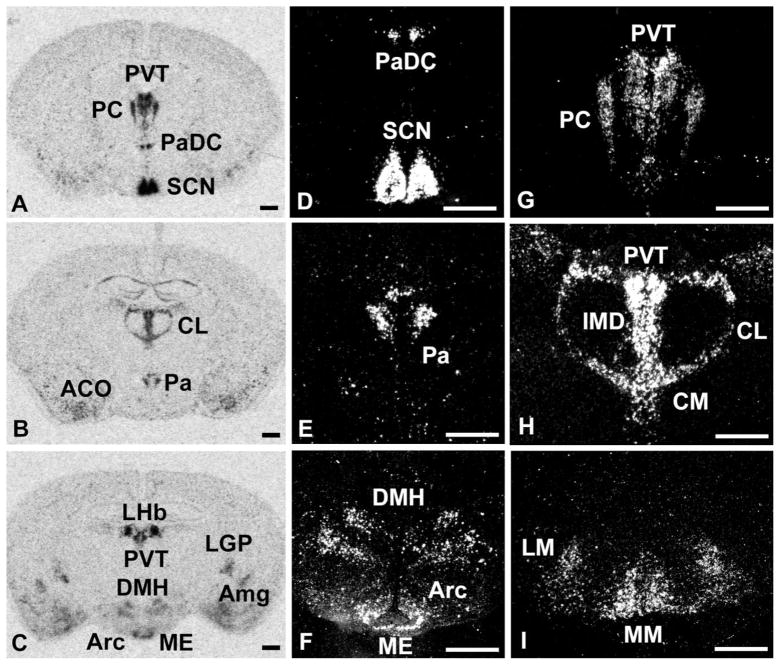
Fig. 6.
Distribution of PKR2 mRNA in thalamus and hypothalamus. A–C: Autoradiographic images of PKR2 mRNA expression in paraventricular thalamic nucleus (PVT); PC, paracentral thalamic nucleus (PC); paraventricular hypothalamic nucleus, dorsal cap (PaDC); suprachiasmatic nucleus (SCN); anterior cortical amygdala (ACO); central lateral thalamic nucleus (CL); paraventricular hypothalamic nucleus (Pa); interomediodorsal thalamic nucleus (IMD); central medial thalamic nucleus (CM); dorsal medial hypothalamic nucleus (DMH); arcuate nucleus (Arc); median eminence (ME); amygdala (Amg); lateral globus pallidus (LGP); medial mammilary nucleus (MM); and lateral mammilary nucleus (LM). D–I: Darkfield images at higher magnification of the same sections described in A–C. Scale bars = 1 mm.
Thalamus and hypothalamus
PKR2 mRNA is widely expressed in the thalamus and the hypothalamus (Fig. 6). In the thalamus, intense expression of PKR2 is detected in the paraventricular thalamic nucleus (Fig. 6A,G). Moderate levels of PKR2 mRNA are also seen in other midline thalamic nuclei, including the paracentral nucleus and central medial and intermediodorsal thalamic nucleus (Fig. 6A,B,G,H). Both the dorsal and the posterior thalamic nucleus also contain moderately PKR2-expressing cells, primarily in the lateral division (Table 1). PKR2 mRNA is also intensely expressed in a relay area of the limbic system, the lateral habenula (Fig. 5C,H). No specific PKR1 mRNA is detected in the thalamus.
In the hypothalamus, PKR2 mRNA is prominently expressed, with the highest labeling in the suprachiasmatic nucleus (SCN), including both the core and the shell subdivisions (Fig. 6A,D). Strong PKR2 expression is also found in the paraventricular hypothalamic nucleus, including the dorsal cap and the posterior divisions, and in the dorsomedial and arcuate nuclei (Fig. 6A–F). In the lateral preoptic area and the lateral hypothalamus, a few cells are detected with low PKR2 expression (Table 1). In circumventricular organs, such as the subfornical organ (Fig. 5B,E) and the median eminence (Fig. 6C,F), moderate levels of PKR2 mRNA are detected. PKR1 mRNA is detected in several hypothalamic nuclei, including the arcuate nucleus and the caudal division of the zona incerta (Fig. 4C,E). In the mammillary regions, both PKR1 and PKR2 mRNAs are expressed at moderate levels in the medial and lateral division of the mammillary nucleus (Table 1, Figs. 4C, 6I).
Mesencephalon
In the midbrain, PKR2 mRNA is detected in the periaqueductal gray (PAG; Fig. 7A–E). Rostrally, as the midbrain aqueduct emerges, a streak of densely labeled PKR2-positive neurons is detected dorsal to the aqueduct in an elongated triangular pattern, a region that is part of the dorsomedial PAG (Fig. 7A,B). These densely packed neurons appear to resemble cells entering or exiting the aqueduct; however, this expression pattern in the dorsomedial PAG has not been described previously. In the caudal part, where the aqueduct expands, larger PKR2-positive cells are still detected in the dorsomedial PAG, but with a dispersed expression pattern (Fig. 7D). A few PKR2-positive cells are also detected in the ventrolateral part of the PAG (Fig. 7D,E). Below the aqueduct, where the dorsal raphe nucleus emerges, moderate levels of PKR2 mRNA are also detected in the dorsal, ventral, and central divisions of the raphe nucleus (Fig. 7D,E). Low levels of PKR2 expression are detected in the superior colliculus. In the parabigeminal nucleus, moderate expression of PKR1 mRNA is observed (Table 1).
Brainstem
In the brainstem, PKR2-expressing cells are detected in the dorsal cochlear nucleus and superficial glial zone of the cochlear nucleus (Table 1). Light to moderate levels of PKR2 are also expressed in the medial part of the parabrachial nucleus. The most intense PKR2 expression in the brainstem is found in the NTS and the area postrema (Fig. 7F). Unlike the ventrolateral expression of PK1 in the NTS (Fig. 1A,C), PKR2-expressing cells in the NTS appear to be more dorsomedial (Fig. 7F). In the brainstem, specific PKR1 mRNA expression is detected only in the dorsal motor vagal nucleus (Fig. 4D,G).
DISCUSSION
In an attempt to elucidate further the potential functional roles for PKs, we have mapped the comprehensive anatomical distribution for PKs and their receptors in the adult mouse brain. The overall distribution pattern indicates that the ligands are expressed more discretely than the receptors. However, the present study elucidated the site of PK synthesis and not its axonal projections, so immunochemistry studies are required to confirm whether their terminal projections match the localization of the receptors. The widespread expression of the receptors, in particular PKR2, suggests that the PKs may regulate a broad array of functions, including circadian rhythms; olfactory-related functions such as olfactory bulb neurogenesis, ingestive behavior, and reproduction; and autonomic function. PK1 and PK2 can activate both receptors with similar affinity (Lin et al., 2002; Masuda et al., 2002; Soga et al., 2002), so we will discuss their potential functions based primarily on the localization of the receptors.
Functions in circadian rhythms
We have previously demonstrated that PK2 is a circadian output molecule from the SCN that regulates locomotor behavior (Cheng et al., 2002). The distribution pattern of PKR2 supports this role, insofar as PKR2 mRNAs are prominently expressed in the primary targets of the SCN output pathways (Fig. 8A; Cheng et al., 2002). Most notably, there is high expression of PKR2 in lateral septum, paraventricular thalamic nucleus, lateral habenula, and paraventricular and dorsomedial hypothalamic nucleus. Although it is not clear yet which of these SCN target areas regulates locomotor activity, the inhibitory effects of PK2 on locomotor behavior are likely to result from activation of one or more of these output targets, especially the paraventricular thalamic nucleus (Berendse et al., 1988; Buijs, 1996). Other PKR2-positive nuclei, such as lateral globus pallidus and ventral pallidum, might also mediate PK2’s effect on locomotion, insofar as these areas participate in the control of locomotor behavior (Swanson, 2000).
Fig. 8.
Diagrams for the possible regulatory pathway for PKs. A: Possible regulatory pathway for PK2 in circadian behavior. The receptors for PK2 are expressed in primary output targets of the suprachiasmatic nucleus (SCN). The projections from LHb-pineal gland or DMH-LC are possible pathways through which PK2 may regulate sleep/wake and arousal. B: Possible regulatory pathway of PK1 in autonomic function. PK1 mRNA is highly expressed in the nucleus tractus solitarius (NTS). The receptors for PK1 are localized in both ascending and descending pathways of the NTS. Shading represents areas where PK2 is expressed, and circled white areas represent areas where PKRs are expressed. Areas that are both shaded and circled represent areas where both PK2 and PKRs are expressed. Black arrows indicate direct projections, and gray arrows represent indirect projections.
The widespread expression of PKR2 in major SCN targets suggests that PK2 might also regulate other circadian-controlled processes. For example, PKR2 is expressed in several visual and sleep/wake-related nuclei. Low levels of PKR2 are detected in the superior colliculus (SC), a sensory nucleus important for visual navigation with outputs to motor centers to orient behaviors (Goodale et al, 1978; Sahibzada et al., 1986). SC has also been shown to mediate the effects of light on sleep and wakefulness (Miller et al., 1998; Moore et al., 2000). PKR1 is expressed in the parabigeminal nucleus, another visual-processing area that projects to the SC (Sefton and Dreher, 2004). High levels of PKR2 in the lateral habenula may represent another pathway in which PK2 can regulate melatonin secretions and sleep/wake through LHb-pineal projections (Fig. 8A; Zhao and Rusak, 2005). PKR2 expression is also detected in other nuclei important for sleep/wake regulation, such as lateral hypothalamus (Siegel, 1999; Salin-Pascual et al., 2001; Gerashchenko and Shiromani, 2004), perifornical region of the hypothalamus (Siegel, 1999; Willie et al., 2001), and dorsal raphe (Abrams et al., 2004). Although no specific PKR2 mRNA is detected in the locus coeruleus (LC), which is the primary nucleus that regulates arousal (Aston-Jones et al., 1999; Jones, 2003), it is possible that the circadian control of arousal is mediated through secondary projections. Although SCN lacks direct projections to LC, it has recently been demonstrated that SCN can regulate arousal through dorsomedial hypothalamus (DMH; Aston-Jones et al., 2001). Thus, PKR2 in DMH suggests that PK2 might partially regulate arousal or sleep/wake through SCN-DMH-LC projections (Fig. 8A). The expression of PKR2 in most known SCN output targets suggests that PK2 may act through these synaptic projections to regulate circadian-controlled processes, including locomotor behavior, feeding, sleep/wake, energy metabolism, and body temperature. However, it is also likely that some of these functions are noncircadian, in that other PK2-expressing areas clearly exist.
Functions in olfaction-associated behaviors
The widespread expression of PKR2 in olfactory regions, hypothalamus, and amygdala suggests a number of possible roles for PK2, in that PK2 mRNA is expressed in areas that project to these PKR2-positive regions. Some of the potential roles of PK2 include olfactory bulb neurogenesis, feeding/drinking behavior, and reproduction.
Neurogenesis of olfactory bulbs
PK2 is expressed in the mature granular and periglomerular layers of the olfactory bulb (OB), whereas its receptors (PKR1 and PKR2) are expressed in the immature ependyma and sub-ependymal layers of the olfactory ventricle (OV). Intense expression of the receptors is observed throughout the entire subventricular zone (SVZ) of the lateral ventricle. This complementary distribution pattern suggests a role of PK2 in neurogenesis, in that the OB is one of the main areas where adult neurogenesis occurs (Altman, 1969; Gage, 2000). New neurons are continuously generated in the OB from progenitor cells that reside in the SVZ of the lateral ventricle (Kaplan and Hinds, 1977; Lois and Alvarez-Buylla, 1994). We have recently demonstrated that PK2 serves as a chemotaxic signal for the migration of neuronal progenitors derived from the SVZ and is essential for the normal development of OB architecture (Ng et al., 2005). A recent report demonstrated that mice lacking the PKR2 gene also exhibited OB malformation, further illustrating the importance of PK2/PKR2 system in OB development (Matsumoto et al., 2006).
Ingestive behavior
Olfaction is essential for many behaviors, including ingestive behavior (Shipley et al., 2004). Our distribution analysis indicates that PKR2 is expressed in several hypothalamic regions, such as the paraventricular nucleus, arcuate nucleus, and dorsomedial hypothalamus, which are principal regions that regulate food intake (Kalra et al., 1999; Hillebrand et al., 2002). PKR1 is also expressed in the arcuate nucleus as well as in the zona incerta, another region implicated in ingestive behavior (Brown and Grossman, 1980). In the subfornical organ, the central brain region for thirst regulation (Gross, 1985), prominent PKR2-positive cells are detected. These receptor distribution patterns indicate that PK2 may regulate ingestive behavior such as feeding and drinking. This is consistent with the previously reported findings of frog Bv8 in these roles (Negri et al., 2004). Insofar as PKs are gut peptides that modulate the motility of gastrointestinal smooth muscles (Li et al., 2001), it is not surprising that PK2 might also regulate ingestive behaviors centrally. Indeed, Negri et al. (2004) have recently demonstrated that intracerebroventricular delivery of Bv8 modulates feeding/drinking in rats. Results from our laboratory also indicate that central delivery of PK2 inhibits food intake in rodents (unpublished observations). The role of PK2 in drinking is further supported by a recent study demonstrating that PK2 has an excitatory effect on neurons of the subfornical organ (Cottrell et al., 2004). Although the current target and pathway of Bv8/PK2 in regulating these ingestive behavior are still unclear, it is possible that this effect is related to the role of PK2 as a circadian output molecule, in that the SCN is known to regulate diverse physiological process, including feeding and drinking (Kalra et al., 1999), in which rodents consume most of their food and water during the dark period.
The expression of PKR2 in various limbic structures, such as the olfactory regions, amygdala, and hippocampal formation, suggests that PKs may be important in other aspects of ingestive behavior, such as the control of olfactory perception during food selection and olfactory memories. Moderate levels of PKR2 are detected in the main olfactory bulb and olfactory ventricle, anterior olfactory nucleus, and lateral olfactory tract. The olfactory amygdala, including anterior cortical amygdala, medial amygdala, basomedial amygdala, and amygdalopiriform and amygdalohippocampal area, also expresses prominent PKR2. These amygdaloid nuclei receive olfactory sensory input and project to the hypothalamus and hippocampus (Swanson and Petrovich, 1998; Petrovich et al., 2001; Shipley et al., 2004). It is interesting to note that medial amygdala is the only amygdaloid nucleus to express PK2, further implicating PK2 in feeding. PKR2 is expressed in several cortices, such as the piriform cortex and the entorhinal cortex, as well as the hippocampal formation and subiculum, all of which are important in learning and memory (McGaugh, 2000; Petrovich et al., 2001). Together, the expression patterns suggest that PKs may be involved in olfactory memory events specific to ingestive behavior. Furthermore, it is well known that the brain reward system is critical in mediating appetitive behavior (Kelley, 2004). PK2 is moderately expressed in the shell of nucleus accumbens, which has been shown to modulate feeding through projections to the lateral hypothalamus (Stratford and Kelley, 1999). PKR2 is also detected in the lateral hypothalamus, so this may be another pathway for regulatory control of feeding.
Reproductive behavior
One of the most important behaviors dependent on olfaction is reproduction (Shipley et al., 2004). High levels of PK2 mRNA are detected in the medial preoptic area (MPA), the principal brain region where most gonadotropin-releasing hormone (GnRH) neurons are localized (Lehman et al., 1986; Watanobe, 2002; Scott et al., 2003). MPA has strong reciprocal connections with the hypothalamus and the amygdala, including regions that mediate the neuroendocrine, autonomic, and somatomotor responses associated with reproduction and maternal behavior (Simerly, 2004). Among the extensive outputs of MPA, one of the extrahypothalamic projections reaches the dorsomedial and ventrolateral part of the PAG (Rizvi et al., 1992), where PKR2 receptors are expressed. PAG has been shown to participate in diverse functions, including analgesia, autonomic regulation, defensive behavior, and sexual behavior (Rizvi et al., 1992; Keay and Bandler, 2004). Several studies have demonstrated an important role of PAG in reproduction, in that lesions of the PAG block the MPA-induced activation of the urethrogenital reflex in male rats (Marson, 2004; Keay and Bandler, 2004). It is likely that PK2 participates in the regulation of sexual behavior and reproduction through this MPA-PAG pathway. PKR2 mRNA is also moderately expressed in the median eminence and arcuate nucleus, where GnRH axons terminate and release hormones into the portal circulation (Watanobe, 2002). The high levels of PK2 in MPA and the prominent expression of PKR2 in its output targets, such as the hypothalamus and the PAG, all suggest a role of PK2 in reproductive behaviors. However, insofar as PAG is also involved in analgesia, and previous studies have demonstrated a role of Bv8 in pain perception (Mollay et al., 1999; Negri et al., 2002), it is also possible that PKR2-positive cells in the PAG contribute to the nociceptive effect of PK2.
PK2 is expressed in other brain regions associated with reproduction, such as the premammillary nuclei and the islands of Calleja. Low levels of PK2 are detected in the ventral part of the premammillary nucleus, which has been implicated in reproductive behavior (Swanson, 2000). The islands of Calleja have also been implicated as the higher cerebral control of neuroendocrine events related to the reproductive system, in that these cells contain luteinizing-releasing hormone and estrogen binding sites (Fallon et al., 1983). A recent report demonstrated that mice lacking the PKR2 gene showed malformation of the reproductive system, highlighting the critical role of PKR2 in the maturation of reproductive organs, whereas PKR1-deficient mice retained normal sexual maturation (Matsumoto et al., 2006).
Functions in autonomic nervous system
Unlike PK2, which is expressed in several areas in the forebrain, PK1 mRNA is detectable only in the brainstem. In particular, intense PK1 expression is found in the NTS, the primary autonomic center that receives and integrates visceral information to regulate autonomic function (Saper, 2004). The PK1-expressing cells in the NTS are likely to regulate autonomic function through both ascending and descending pathways (Fig. 8B). The ventrolateral divison of the NTS, where densely labeled PK1 cells are mostly concentrated, sends descending projections to preganglionic cell groups in the brainstem, including the dorsal motor vagal nucleus, where moderate levels of PKR1 are expressed (Saper, 2004). Some of these descending projections also reach the spinal cord to regulate respiratory motor neurons and the sympathetic preganglionic column. PK1-expressing cells in the NTS might also send ascending projections to the forebrain, where high levels of PKR2 are expressed in the hypothalamus and amygdala (Saper, 2004). PKR1 is also detected in the medial parabrachial nucleus, a region that receives heavy input from the NTS and sends gustatory information to the dorsal thalamus and the limbic system (Lundy and Norgren, 2004). It is interesting to note that PKR2 is also expressed in the NTS, but in the dorsomedial division, suggesting that PK1 may also regulate local function through intra-NTS projections.
It appears that high levels of PKR2 are localized near ventricular areas or circumventricular organs. This includes the subfornical organ, median eminence, area postrema, SVZ of the lateral ventricle, and the cells above the sylvius aqueduct, which is part of the dorsomedial PAG. Most of these midline structures situated around the third and fourth ventricles contain fenestrated capillaries that permit blood-borne messengers to reach the brain without disrupting the blood– brain barrier (Oldfield and McKinley, 2004). This unique pattern of PKR2 distribution indicates that PKs may be involved in gating or modulating communications between the central and peripheral systems to regulate feeding and maintain autonomic homeostasis.
In summary, the comprehensive analysis of PKs and their receptor distributions reveals that PKs may regulate a broad array of functions in the central nervous system. These distribution patterns are consistent with some of the currently reported roles of PK2. The receptor distributions indicate that PK2 may act through synaptic connections; however, it is likely that PK2 also acts in a paracrine and/or autocrine fashion. The facts that PK2 is a secreted molecule and that its receptors are localized in circumventricular organs suggest that PKs might also be circulating hormones. Further studies are needed to identify new neurobiological functions for PKs and their receptors in the central nervous system.
Acknowledgments
National Institute of Mental Health; Grant number: R01 MH067753; Grant sponsor: PhRMA Foundation; Grant number: 32675 (to M.Y.C.).
We thank Kate Burton, Yiling Chen, and Alex Lee for discussions and technical assistance.
LITERATURE CITED
- Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomic and functional topography of the dorsal raphe nucleus. Ann N Y Acad Sci. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior fore-brain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433– 457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Voorn P, te Kortschot A, Groenewegen HJ. Nuclear origin of thalamic afferents of the ventral striatum determines their relation to patch/matrix configurations in enkephalin-immunoreactivity in the rat. J Chem Neuroanat. 1988;1:3–10. [PubMed] [Google Scholar]
- Brown B, Grossman SP. Evidence that nerve cell bodies in the zona incerta influence ingestive behavior. Brain Res Bull. 1980;5:593–597. doi: 10.1016/0361-9230(80)90266-x. [DOI] [PubMed] [Google Scholar]
- Buijs RM. The anatomical basis for the expression of circadian rhythms: the efferent projections of the suprachiasmatic nucleus. Prog Brain Res. 1996;111:229–240. doi: 10.1016/s0079-6123(08)60411-2. [DOI] [PubMed] [Google Scholar]
- Bullock CM, Li JD, Zhou QY. Structural determinants required for the bioactivities of prokineticins and identification of prokineticin receptor antagonists. Mol Pharmacol. 2004;65:582–588. doi: 10.1124/mol.65.3.582. [DOI] [PubMed] [Google Scholar]
- Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- Cottrell GT, Zhou QY, Ferguson AV. Prokineticin 2 modulates the excitability of subfornical organ neurons. J Neurosci. 2004;24:2375–2379. doi: 10.1523/JNEUROSCI.5187-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Loughlin SE, Ribak CE. The islands of Calleja complex of rat basal forebrain. III. Histochemical evidence for a striatopallidal system. J Comp Neurol. 1983;218:91–120. doi: 10.1002/cne.902180106. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Shiromani PJ. Different neuronal phenotypes in the lateral hypothalamus and their role in sleep and wakefulness. Mol Neurobiol. 2004;29:41–59. doi: 10.1385/MN:29:1:41. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Foreman NP, Milner AD. Visual orientation in the rat: a dissociation of deficits following cortical and collicular lesions. Exp Brain Res. 1978;31:445– 457. doi: 10.1007/BF00237301. [DOI] [PubMed] [Google Scholar]
- Gross PM. The subfornical organ as a model of neurohumoral integration. Brain Res Bull. 1985;15:65–70. doi: 10.1016/0361-9230(85)90062-0. [DOI] [PubMed] [Google Scholar]
- Hillebrand JJ, de Wied D, Adan RA. Neuropeptides, food intake and body weight regulation: a hypothalamic focus. Peptides. 2002;23:2283–2306. doi: 10.1016/s0196-9781(02)00269-3. [DOI] [PubMed] [Google Scholar]
- Jones BE. Arousal systems. Front Biosci. 2003;8:s438–s451. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Periaqueductal gray. In: Paxinos G, editor. The rat nervous system. 3. San Diego: Elsevier Academic Press; 2004. pp. 243–253. [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telm L, Frantz G, Rangell L, DeGuzman L, Keller GA, Peale F, Gurney A, Hillan KJ, Ferrara N. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412:877– 884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Robinson JE, Karsch FJ, Silverman AJ. Immunocytochemical localization of luteinizing hormone-releasing hormone (LHRH) pathways in the sheep brain during anestrus and the mid-luteal phase of the estrous cycle. J Comp Neurol. 1986;244:19–35. doi: 10.1002/cne.902440103. [DOI] [PubMed] [Google Scholar]
- Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol. 2001;59:692– 698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- Lin DC, Bullock CM, Ehlert FJ, Chen JL, Tian H, Zhou QY. Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. J Biol Chem. 2002;277:19276–19280. doi: 10.1074/jbc.M202139200. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lundy RF, Norgren R. Gustatory system. In: Paxinos G, editor. The rat nervous system. 3. San Diego: Elsevier Academic Press; 2004. pp. 891–900. [Google Scholar]
- Marson L. Lesions of the periaqueductal gray block the medial preoptic area-induced activation of the urethrogenital reflex in male rats. Neurosci Lett. 2004;367:278–282. doi: 10.1016/j.neulet.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Takatsu Y, Terao Y, Kumano S, Ishibashi Y, Suenaga M, Abe M, Fukusumi S, Watanabe T, Shintani Y, Yamada T, Hinuma S, Inatomi N, Ohtaki T, Onda H, Fujino M. Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G-protein-coupled receptors. Biochem Biophys Res Commun. 2002;293:396–402. doi: 10.1016/S0006-291X(02)00239-5. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, Matsuo A, Ishii H, Kobori M, Katoh M, Matsushime H, Furuichi K, Shigeyoshi Y. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci U S A. 2006;103:4140– 4145. doi: 10.1073/pnas.0508881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Memory—a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Miller AM, Obermeyer WH, Behan M, Benca RM. The superior colliculus-pretectum mediates the direct effects of light on sleep. Proc Natl Acad Sci U S A. 1998;95:8957– 8962. doi: 10.1073/pnas.95.15.8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollay C, Wechselberger C, Mignogna G, Negri L, Melchiorri P, Barra D, Kreil G. Bv8, a small protein from frog skin and its homologue from snake venom induce hyperalgesia in rats. Eur J Pharmacol. 1999;374:189–196. doi: 10.1016/s0014-2999(99)00229-0. [DOI] [PubMed] [Google Scholar]
- Moore RY, Weis R, Moga MM. Efferent projections of the intergeniculate leaflet and the ventral lateral geniculate nucleus in the rat. J Comp Neurol. 2000;420:398– 418. doi: 10.1002/(sici)1096-9861(20000508)420:3<398::aid-cne9>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Negri L, Lattanzi R, Giannini E, Metere A, Colucci M, Barra D, Kreil G, Melchiorri P. Nociceptive sensitization by the secretory protein Bv8. Br J Pharmacol. 2002;137:1147–1154. doi: 10.1038/sj.bjp.0704995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri L, Lattanzi R, Giannini E, De Felice M, Colucci A, Melchiorri P. Bv8, the amphibian homologue of the mammalian prokineticins, modulates ingestive behaviour in rats. Br J Pharmacol. 2004;142:181–191. doi: 10.1038/sj.bjp.0705686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science. 2005;308:1923–1927. doi: 10.1126/science.1112103. [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, McKinley MJ. Circumventricular organs. In: Paxinos G, editor. The rat nervous system. 3. San Diego: Elsevier Academic Press; 2004. pp. 389–401. [Google Scholar]
- Paxinos G, Franklin DBJ. The mouse brain in stereotaxic coordinates. San Diego: Elsevier Science; 2004. [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Shipley MT. Reciprocal connections between the medial preoptic area and the midbrain periaqueductal gray in rat: a WGA-HRP and PHA-L study. J Comp Neurol. 1992;315:1–15. doi: 10.1002/cne.903150102. [DOI] [PubMed] [Google Scholar]
- Sahibzada N, Dean P, Redgrave P. Movements resembling orientation or avoidance elicited by electrical stimulation of the superior colliculus in rats. J Neurosci. 1986;6:723–733. doi: 10.1523/JNEUROSCI.06-03-00723.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin-Pascual R, Gerashchenko D, Greco M, Blanco-Centurion C, Shiromani PJ. Hypothalamic regulation of sleep. Neuropsychopharmacology. 2001;25(5 Suppl):S21–S27. doi: 10.1016/S0893-133X(01)00318-9. [DOI] [PubMed] [Google Scholar]
- Saper CB. Central autonomic system. In: Paxinos G, editor. The rat nervous system. 3. San Diego: Elsevier Academic Press; 2004. pp. 761–765. [Google Scholar]
- Scott CJ, Clarke IJ, Tilbrook AJ. Neuronal inputs from the hypothalamus and brain stem to the medial preoptic area of the ram: neurochemical correlates and comparison to the ewe. Biol Reprod. 2003;68:1119–1133. doi: 10.1095/biolreprod.102.010595. [DOI] [PubMed] [Google Scholar]
- Sefton A, Dreher B. Visual system. In: Paxinos G, editor. The rat nervous system. 3. San Diego: Elsevier Academic Press; 2004. pp. 1121–1122. [Google Scholar]
- Shipley MT, Ennis M, Puche AC. Olfactory saystem. In: Paxinos G, editor. The rat nervous system. 3. San Diego: Elsevier Academic Press; 2004. [Google Scholar]
- Siegel JM. Narcolepsy: a key role for hypocretins (orexins) Cell. 1999;98:409– 412. doi: 10.1016/s0092-8674(00)81969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB. Anatomical substrates of hypothalamic integration. In: Paxinos G, editor. The rat nervous system. 3. San Diego: Elsevier Academic Press; 2004. pp. 342–345. [Google Scholar]
- Soga T, Matsumoto S, Oda T, Saito T, Hiyama H, Takasaki J, Kamohara M, Ohishi T, Matsushime H, Furuichi K. Molecular cloning and characterization of prokineticin receptors. Biochim Biophys Acta. 2002;1579:173–179. doi: 10.1016/s0167-4781(02)00546-8. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19:11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Watanobe H. Leptin directly acts within the hypothalamus to stimulate gonadotropin-releasing hormone secretion in vivo in rats. J Physiol. 2002;545:255–268. doi: 10.1113/jphysiol.2002.023895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429– 458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Broide RS, Chen Y, Leslie FM. Highly sensitive radioactive in situ hybridization using full length hydrolyzed riboprobes to detect alpha 2 adrenoceptor subtype mRNAs in adult and developing rat brain. Brain Res Brain Res Protoc. 1999;3:229–241. doi: 10.1016/s1385-299x(98)00043-9. [DOI] [PubMed] [Google Scholar]
- Zhao H, Rusak B. Circadian firing-rate rhythms and light responses of rat habenular nucleus neurons in vivo and in vitro. Neuroscience. 2005;132:519–528. doi: 10.1016/j.neuroscience.2005.01.012. [DOI] [PubMed] [Google Scholar]