Abstract
Secreted peptides play broad regulatory roles in brain function and elsewhere in the body. Prokineticins are a pair of newly identified regulatory peptides that signal through two highly homologous G protein–coupled receptors. Prokineticins possess a unique structural motif of five disulfide bonds and a completely conserved N-terminal hexapeptide sequence that is essential to biological activity. Diverse biological functions, including roles in development and cell differentiation, have been assigned to the prokineticins. A network of genes, subject to various transcriptional factors, may functionally converge on the prokineticins as regulatory targets.
Introduction
Regulatory peptides play broad roles in the integration of brain functions and body physiology. Snake venoms and skin secretions from frogs are rich sources of biologically active regulatory peptides. The history of the prokineticins (PKs) can be traced back to more than twenty years ago, when a cysteine-rich seventy-seven–residue peptide was purified from the venom of the black mamba (1). This peptide was later named mamba intestinal toxin 1 (MIT1) for its ability to stimulate the contraction of gastrointestinal smooth muscle (2, 3). A highly homologous frog protein with similar contractile activity was subsequently isolated from Bombina variegata and named Bv8 (4). Mammalian homologs of Bv8 and MIT1 were subsequently identified by three groups and have become known as the prokineticins (5–7); receptors for PKs were cloned soon thereafter (8–10). Mature human PK1 and PK2 contain 86 and 81 amino acids, respectively, and are among the largest ligands known for G protein–coupled receptors (GPCRs). Over the last few years, a spectrum of biological functions, ranging from development to adult physiology, have been assigned to this new family of regulatory peptides (Figure 1) [for review see (11–13)].
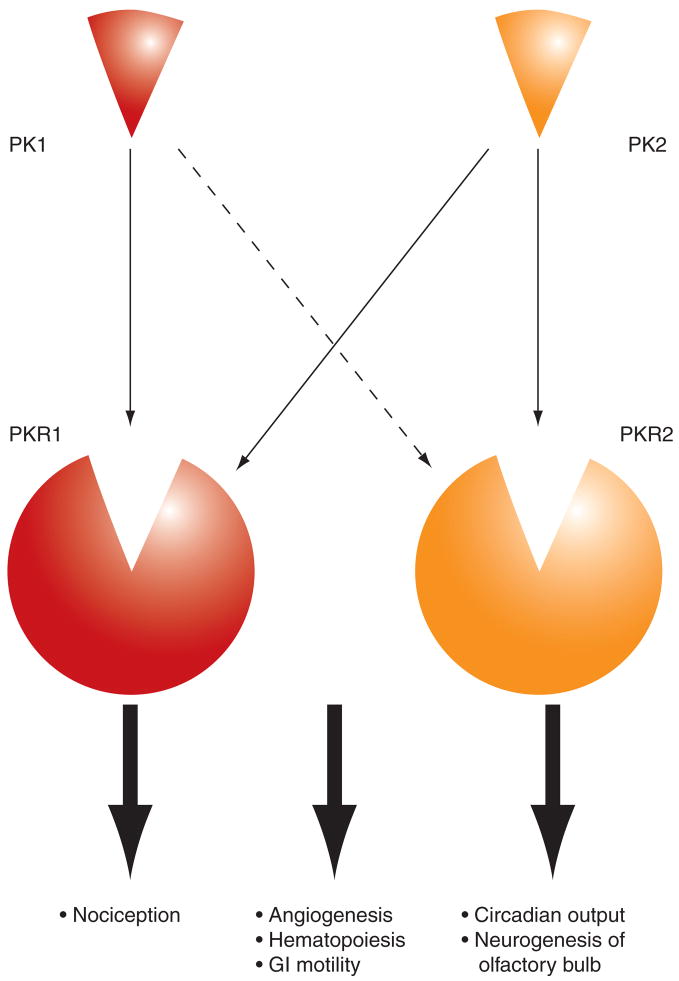
Figure 1. Biological functions of prokineticins.
Prokineticins (PK1 and PK2) are a pair of cysteine-rich secreted molecules that exert their functions via activation of two G protein–coupled receptors (PKR1 and PKR2). Prokineticins are versatile molecules that regulate multiple biological processes, with apparent selectivity of ligand/receptor pairing. In particular, PK2/PKR2 signaling is critical for circadian clock output and neurogenesis of the olfactory bulb (OB), whereas the PK2/PKR1 pair plays a dominant role in pain perception. Prokineticins also regulate other processes, including gastrointestinal smooth muscle motility, angiogenesis, and hematopoiesis, where the involvement of ligand/receptor pairing is largely unexplored. The dashed arrow implies that PK1 and PKR2 may form a complex in unidentified functions.
Prokineticin Signaling
Two G protein–coupled receptors, with an unusually high degree of sequence similarity, have been cloned as receptors for PKs (8–10). PK receptor 1 (PKR1) and PK receptor 2 (PKR2), encoded within distinct chromosomes in both mouse and human, share about 85% amino acid identity (8). In cultured cells that express PKR1 or PKR2 exogenously, recombinant PKs activate both receptors with similar agonist potency (8). This apparent non-selectivity of ligand/receptor activation implies that the availability of ligands (PK1 or PK2) and receptors (PKR1 or PKR2) is likely to determine which possible signaling pair is involved for particular physiological or behavioral process.
Multiple signaling pathways are activated by PKRs. In PKR-transfected neuronal and specific endothelial cell lines, the activation of PKRs stimulates calcium mobilization, phosphoinositol turnover, and activation of Akt kinase and mitogen-activated protein kinase (MAPK) (8–10, 14, 15). Whereas the stimulation of calcium mobilization upon receptor activation is dependent on Gq, activation of the MAPK pathway is pertussis toxin–sensitive, suggesting that PKRs may also couple to G proteins such as Gi. In dorsal root ganglion neurons, PKRs activate transient receptor potential vanilloid 1 (TRPV1) channels, and this signaling pathway is likely to involve the activation of protein kinase C (16).
PKR1 is widely distributed in the periphery, including the gastrointestinal system, lungs, and blood system (8–10). Although both PKR1 and PKR2 are expressed in the gastrointestinal system, PKR1 is more abundantly expressed in the intestine, suggesting that it may be the major receptor mediating the actions of PKs in the regulation of gastrointestinal motility. In situ analysis indicates that PKR2 is the dominant receptor in the adult brain, with particularly high expression of PKR2 in the hypothalamus, the olfactory ventricular regions, and the limbic system (17–19). Reverse transcriptase–polymerase chain reaction studies also indicate that PKRs are expressed in various endocrine tissues, including thyroid, pituitary, adrenal gland, testis, and ovary (8–10).
Structural Elements Required for the Bioactivities of Prokineticins
PK1 and PK2 share about 45% amino acid identity with each other as well as with their non-mammalian counterparts (6). Human and mouse PK-encoding genes contain three exons, with the first exon encoding a signal peptide and the first five amino acids, and exons 2 and 3 encoding the cysteine-rich domains (20). PKs and their vertebrate anologs exhibit the complete conservation of the first six N-terminal amino acids (i.e., AVITGA) and the ten cysteine residues predicted to form five disulfide bonds (Figure 2) (6, 21). The structural elements essential for the function of PKs have been investigated by deletion, insertion, and substitution mutations (20), as well as proteolytic fragmentation (22). Any disruption of the N-terminal hexapeptide sequence renders human PK1 inactive (20), whereas deletion of the N-terminal alanine residue of frog Bv8 results in significant but not complete loss of biological activity (22). Although residue changes in the C terminus tend to be somewhat tolerable, studies with chimeric proteins have demonstrated the critical role of the cysteine-rich domains. For example, a chimeric protein consisting of the human PK1 hexapeptide fused to Dickkopf 4, which conserves the ten cysteine residues, is not functional, whereas the cysteine-rich domains of PK1 and PK2 are interchangeable (20). Intriguingly, two N-terminal mutant PKs, with either substitution or addition of only a single amino acid, are antagonists of wild-type PK activity (20), further indicating the importance of the N-terminal six residues in binding to PKRs. A splice variant of PK2 has been identified that contains an extra twenty-one–residue insertion and is active, although with a much lower potency (20). It has been suggested that this longer PK2 variant may undergo proteolytic cleavage within the twenty-one–residue sequence (23); however, the biological function of this PK2 splicing variant is still unclear.
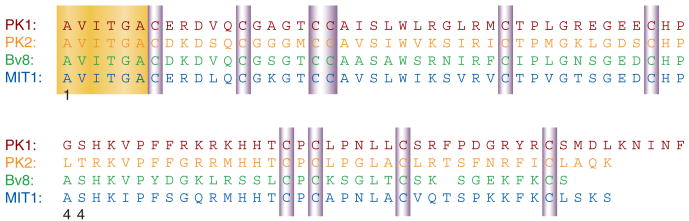
Figure 2. Amino acid sequences of human PKs and their analogs from frog and snake.
The sequences of human PK1 (red) and PK2 (orange) are compared to that of frog (green) and snake (blue). The conserved N-terminal hexapeptide is highlighted in yellow. Ten conservative cysteine residues are highlighted in purple. Adapted from (6).
Prokineticins as Regulators of Smooth Muscle Contractility
The motility of the gastrointestinal tract is regulated by classical neurotransmitters, neuropeptides, and circulating hormones. Recombinant PKs potently stimulate the contraction of guinea pig ileum (6), which accounts for the name “prokineticin.” This contractile activity is similar to that of the non-mammalian homologs (i.e., MIT1 and Bv8) on similar preparations (3, 4). The physiology and pharmacology of PKs on gastrointestinal smooth muscle cells have not been elaborated, but in vivo studies have demonstrated that both PKs stimulate gastrointestinal transit (US patent filing US2004/0162238A1; C. Owyang, personal communication), and thus the effects of PKs are propulsive. Ligand binding studies and function studies with sodium channel blockers had revealed the presence of high-affinity receptors for PKs in the smooth muscle cells of the small intestine (6). Recently, the role of PKs in gastric and colonic contractility has also been investigated (24–26). In one report, PK2 was found to increase the emptying of a liquid meal from rat stomach (26), although this observation has been called into question (24). In colons, PK1 was found to suppress giant contractions of the circular muscle, likely via the release of nitric oxide (25). This study also revealed that PKR1 is expressed on myenteric plexus neurons and colocalizes with a small subset of neurons that express nitric oxide synthase. Thus, PK may regulate gastrointestinal motility directly, by activating smooth muscle cells, and indirectly, by modulating the activities of enteric neurons.
Prokineticins and Pain Perception
A number of studies have implicated PK signaling in nociception. Intraplantar injections of PK2 and Bv8 cause strong and localized hyperalgesia by reducing nociceptive thresholds to thermal and mechanical stimuli (4, 27). Systemic injection of Bv8 also induces hyperalgesia to tactile and thermal stimuli (27). The hyperalgesia caused by Bv8 is likely due to the sensitization of TRPV1 in primary dorsal root ganglia (DRG) neurons (16). TRPV1 is an excitatory ion channel that is critical for the detection and integration of pain-producing chemical and thermal stimuli by primary sensory neurons (28). Both PKR1 and PKR2 mRNA are expressed in the DRG and the spinal cord (27). Functional assays indicate that PK2 and Bv8 are able to mobilize calcium in cultured rat DRG neurons (27, 29). These functional studies are consistent with results of colocalization experiments that indicate that the majority of PKR-positive DRG neurons also express TRPV1 (29). Mice lacking PKR1 were recently reported to exhibit impaired pain perception to various stimuli, including noxious heat, mechanical stimuli, capsaicin, and protons (30). Thus, PKR1 is implicated as the dominant PK receptor that exerts a tonic activation of TRPV1 in DRG neurons. The reduced response of TRPV1-deficient mice to Bv8 (30) further indicates the critical role of TRPV1 in the downstream signaling of PKR1 in pain perception.
Patterns of protein expression indicate that PK2 might be the major ligand for PKR1 in nociception, especially in inflammatory pain. PK2 is highly expressed in peripheral blood cells, notably in monocytes, neutrophils, and dendritic cells. PK2 has been identified as a monocyte chemoattractant (31, 32). PK2 may be released into the sites of inflammation by neutrophils, thereby activating macrophages and inducing the release of proinflammatory cytokines such as interleukin-1 and interleukin-12 (33). Genetic studies with PK2-deficient mice confirm the critical involvement of PK2 in acute and inflammatory pain (29). PK2-deficient mice display strong reduction in nociception induced by thermal and chemical stimuli. Thus, PK2–PKR1 seems a dominant ligand–receptor pair in the regulation of pain sensation.
Regulatory Function of PK2 in Circadian Clock Output
The suprachiasmatic nucleus (SCN) in the anterior hypothalamus is the primary mammalian circadian clock that drives daily rhythms of diverse physiology and behavior, including the sleep/wake cycle, blood pressure, and energy metabolism (34). Whereas our understanding of the molecular mechanism for the circadian clockwork per se has advanced considerably (34, 35), much less is known about the mechanisms by which circadian information is transmitted to control physiology and behavior. In an attempt to study the function of PKs in the central nervous system, we have mapped their distribution, as well as that of their receptors, in the mouse brain. In particular, the observation that levels of PK2 mRNA in the SCN display dramatic circadian rhythmicity under light/dark and constant dark conditions has suggested a potential regulatory function for PK2 in the circadian clock (17). Subsequently, multiple lines of evidence have corroborated the role of PK2 as a prominent output molecule for the SCN circadian clock. In vitro studies have revealed that PK2 is the product of a first-order clock-controlled gene, the expression of which is controlled by E-boxes under the regulation of the CLOCK–BMAL1 transactivation complex (17). Correlative in vivo studies have confirmed that the clock-controlled PK2-encoding gene lies downstream of Clock and Bmal1. In mutant mice lacking a functional clockwork, including clock mutant and Cry1−/−Cry2−/− mice, the PK2 mRNA oscillation in the SCN is abolished (17). Moreover, the cycling of PK2 mRNA levels in the SCN was shown to adapt faster to the delay of light cycles than to the advance of light cycles (36), which is in keeping with the well-known asymmetry of the circadian clock. The differential rates of adaptation of PK2 rhythm to the delay and the advance of light cycles, moreover, are consistent with the respective rates of behavioral and physiological adaptation observed in animals and humans (37). Furthermore, PKR2 is expressed in virtually all known primary SCN targets (17, 19), indicating that these SCN targets can respond to the oscillatory PK2 signal from the SCN. In addition, intracerebroventricular delivery of recombinant PK2 suppresses nocturnal wheel-running (17) and feeding behaviors (38) that normally occur when endogenous PK2 levels are minimal.
Recently, direct genetic evidence for the role of PK2 in the control of circadian rhythmicity has been reported. In a transgenic model of Huntington’s disease, a correlation between increased daytime locomotor activity and reduced SCN expression of PK2 was observed (39). The characterization of PK2-deficient mice has further demonstrated the critical role of PK2 for the maintenance of robust circadian rhythms (40, 41). Under both normal light/dark and constant dark conditions, the reduction of circadian locomotor rhythmicity in PK2-null mice is apparent. PK2-null mice in fact display significantly reduced rhythmicity for a variety of physiological and behavioral parameters measured, including sleep/wake cycle, body temperature, circulating glucocorticoid and glucose levels, as well as the expression of peripheral clock genes. The fact that PK2-null mice otherwise manifest essentially normal oscillations in clockwork genes in the SCN suggests that PK2, as an output molecule, has no major effect on the core clockwork in the SCN.
In the absence of PK2, the control of the SCN over diverse circadian processes can be seen to be weakened. This weakening of SCN control is more fully appreciated when PK2-null mice are subjected to a schedule of restricted feeding (RF) during daytime, which is known to entrain circadian locomotor rhythms independent of the SCN (42, 43). In response to daytime RF, rodents become active before food is made available, a phenomenon called food anticipatory activity. During RF, the “light-entrained oscillator” (i.e., the SCN) thus appears to become subordinate to a “food-entrained oscillator” in the control of activity and physiological events (44, 45). Significantly, PK2-null mice display significantly higher food anticipatory activity (40) than their wild-type counterparts under daytime RF, suggesting that PK2 contributes to the SCN-controlled rhythmicity of locomotor activity.
Recent studies have shown that PK2 mediates SCN regulation of diverse circadian rhythms through a neurophysiological mechanism. First, PK2 exerts an excitatory effect on the neurons that express the PK2 receptor (46), revealing a possible link between the oscillation of PK2 levels and neuronal firing rates. Subsequently, the neurophysiological effect of SCN-derived PK2 on the paraventricular nucleus (PVN), a critical target of SCN projections, has been investigated. The PVN of the hypothalamus is involved in the regulation of endocrine rhythms and oscillation of autonomic nervous system. As PKR2 is expressed in the PVN, PK2 may regulate glucocorticoid level through this pathway. Yuill et al. have demonstrated that PK2 was able to excite PVN neurons of both parvocellular and magnocellular branches (47). Importantly, a peptide-based PK2 receptor antagonist was able to decrease the basal activity of parvocellular neurons in hypothalamus slices (including SCN and PVN) only during light phase, when PK2 is highly expressed in the SCN (47). Thus, endogenous SCN-derived PK2 excites the parvocellular branch of PVN neurons in a phase-dependent manner.
The molecular rhythm of PK2 in the SCN of a diurnal rodent has been described (48). Similar to the oscillation pattern observed in nocturnal mouse (17) and rat species (49), PK2 mRNA in the SCN of diurnal Arvicanthis niloticus is rhythmically expressed, with peak levels in the morning hours and negligible levels during night phase (48). Thus, the phase of PK2 expression in the SCN of diurnal rodents is the same as that of nocturnal rodents, consistent with a growing body of evidence suggesting that the key to diurnality lies downstream of the SCN circadian clock.
Functions in Angiogenesis and the Reproductive System
During the last decade, significant progress has been made in the molecular mechanisms that regulate vascular growth in crucial biological processes including development, tumorigenesis, reproduction, and wound healing. In a library of secreted molecules, LeCouter et al. attributed PK1 with the capability of inducing proliferation of primary bovine adrenal cortex–derived capillary endothelial (ACE) cells (7). Additional evidence for the angiogenic effects of PK1 also exists (7, 15, 50). In particular, the delivery of PK1 to the ovary elicits potent angiogenesis and cyst formation, whereas PK1 delivery does not elicit angiogenesis in the cornea or skeletal muscles. The promoter of the PK1-encoding gene possesses putative binding sites for hypoxia-inducing factor 1, and the expression of PK1 is indeed induced by hypoxia, presumably an essential property for an angiogenic factor. PK1 has since been hypothesized as the first example of a tissue-specific angiogenic factor (12), although its angiogenic effect may be more broad than originally claimed (51). It should also be noted that PK1 is upregulated in certain disease conditions, including malignancy (52–54). In all in vitro angiogenic assays, PK2 behaves similarly to PK1: it induces proliferation, survival, and migration of ACE cells, and its expression is hypoxia-inducible (50). In testis, PK2 likely plays a dominant role, as it is highly expressed in the organ, and adenoviral delivery of PK2 into mouse testis results in a potent angiogenic response (50).
Because placenta and testis are the highest expression sites of PK1 and PK2, and given the crucial role of angiogenesis in reproductive functions such as development of the corpus luteum and placentation, several groups have focused on the role of PKs in reproductive organs. A possible paracrine role for the PKs and their receptors in the vascular function of the endometrium and placenta has been suggested by expression analyses. Battersby et al. have shown that the expression of PK1 but not PK2 is elevated during the secretory phase of the menstrual cycle, and PK1 is induced by treatment with progesterone (55). In human placenta, the expression pattern of PK1 appears complementary to that of VEGF, such that PK1 is mainly localized to the syncytiotrophoblast layer, whereas VEGF is relatively abundant in the cytotrophoblast and extravillous trophoblast cells (56). In cultured trophoblast cells, both PK1 and PKR1 mRNAs are regulated by hypoxia (56). It has recently been shown that PK1 is localized in the human corpus luteum, where its expression rises throughout the luteal phase, peaking during the mid to late luteal phase; PK2 expression remains low in the corpus luteum (57–60). PK1 mRNA localizes predominantly to granulosa-derived cells of the corpus luteum, and human chorionic gonadotropin induces PK1 mRNA in vitro (57). In PKR2-null mice, the ovary mainly contains undeveloped follicles and no apparent corpora lutea (49). Spermatogenesis is also arrested in PKR2-null mice (49), although it is not clear whether spermatogenic dysfunction is secondary to effects on angiogenesis.
Although it is possible that reproductive dysfunction arises from the local effects of deficiencies in PK1 or PK2 within the sex organs, more global effects on the reproductive hormonal axis must also be considered. For example, disruption in the morphogenesis of the olfactory bulb (OB), normally required for the proper migration of gonadotropin-releasing hormone (GnRH) neurons from nasal cavity to hypothalamus (61), can severely disrupt development and maturation. In fact, PKR2-null mice exhibit anosmia and hypogonadotropic hypogonadism, hallmarks of human Kallmann syndrome (49). Immunohistochemical analysis reveals that GnRH neurons are absent in the hypothalamus of these animals. Similar phenotypes are also observed in PK2-deficient mice (N Pitteloud, C Zhang, D Pignatelli, J-D Li, T Raivio, L Cole, L Plummer, E Dickson-Jackobson, P Mellon, Q-Y Zhou, W Crowley, Jr). PK2/PKR2 signaling pathways thus appear to be critical for both OB ontogenesis and GnRH neuron migration (18, 62). Indeed, a recent report has indicated that PK2 and PKR2 are candidate genes for human Kallman syndrome (63).
Role of Prokineticins in the Development and Function of Blood Cells
Since PKs and their receptors are expressed in bone marrow and other hematopoietic organs (6), a possible role of PK1 and PK2 in hematopoiesis has been suspected. Dorsch et al. report that PK1 greatly supports the differentiation of mouse and human bone marrow cells into the monocyte/macrophage lineage (32). PK2 exerts similar effects on the monocyte lineage (31), and it promotes the survival and differentiation of granulocytic lineages in cultures of human and mouse hematopoietic stem cells. Detailed expression analyses indicate that both PKR1 and PKR2 are expressed in hematopoietic stem cells (31). These studies imply an evolutionarily conserved role for PKs, particularly PK2, in hematopoiesis (64).
As both PKR1 and PKR2 are also expressed in mature blood cell types, including lymphocytes, their roles may extend beyond development per se to affect cell functionality. Human peripheral blood monocytes respond to PK1 by undergoing changes in morphology and expression levels of cytokines or cytokine receptors (32). In particular, monocytes treated with PK1 manifest elevated interleukin-12 and tumor necrosis factor α levels and produce less interleukin-10 in response to lipopolysaccharide. PK1 may thus prime monocytes by enhancing a proinflammatory response to pathogens. These observations are interesting in the context of the sites of PK1 expression: PK1 is constitutively expressed in the gonad and adrenal glands, and can also be found in B cells, T cells, and in inflamed tissues (7, 32). It is possible that the main site of PK1 action is on circulating immature monocytes, such that PK1 could act as a systemic regulator of myeloid development and/or monocyte-derived cell responses. Expression of PK1 in inflamed tissues suggests it may modify macrophage function locally. Intriguingly, PK2 (along with PKR1 and PKR2) is expressed in macrophages (33), and it is also detected in dendritic cells and neutrophils (31). PK2 and Bv8 induce chemotaxis of macrophages with very high potency (31, 33). These results indicate that PK1 and PK2 may regulate monocyte activation. Interestingly, PK2 is also highly expressed in infiltrating cells at sites of inflammation, predominantly in neutrophils, and the expression of PK2, PKR1, and PKR2 are induced in monocytes upon exposure to lipopolysaccharide (31). These observations further indicate that PK2 may function as a chemoattractant for granulocytes and monocytes. PKs are thus likely hematopoietic cytokines that promote survival and modulate the innate and the adaptive immune systems.
Function of PK2 in Neurogenesis
In mammals, neurogenesis occurs mainly during embryonic and early postnatal stages; however, neurogenesis persists in certain regions of adult mammalian brains, including the OB and the dentate gyrus of the hippocampus (65, 66). New neurons are continuously generated in the OB from progenitor cells that reside in the subventricular zone (SVZ) of the lateral ventricle, where they proliferate and migrate through the rostral migratory stream (RMS) to the OB to form granular and periglomerular cells (67–69). Although this SVZ–OB migratory pathway and the timing of neurogenesis have been well documented, the molecular mechanisms of adult neurogenesis are not well understood. It has recently been demonstrated that PK2 functions as a chemoattractant for SVZ-derived neuronal progenitors, and this PK2 signal is essential for the normal development of the OB architecture (18). PK2 is expressed in the mature granular and periglomerular layers of the OB, whereas its receptors (PKR1 and PKR2) are expressed in the immature ependyma and subependymal layers of the olfactory ventricle (18, 19). In vitro migration assays indicate that PK2 can stimulate migration of neuronal progenitors from the RMS in both adult and postnatal rats, and this migration is directional and can be inhibited by PK antagonist (18). The critical role of PK2 in OB development is demonstrated by genetic studies. PK2-deficient mice undergo abnormal development of the OB, including dramatic reduction in OB size and the loss of normal OB layer architecture (18). Although both PKR1 and PKR2 are expressed in the immature ependyma and subependymal layers of the olfactory ventricle, genetic analysis indicates that PKR2 but not PKR1 is a critical receptor for OB development (62). PKR2-null mice exhibit similar abnormal development of the OB, whereas as PKR1-deficient mice are not affected in this way. These genetic studies reveal that PK2 and PKR2 comprise a dominant signaling pair for OB neurogenesis.
The normalcy of OB development in mice bearing mutations in Clock and Bmal1 (20, 70) indicates that PK2 is likely under the control of transcriptional factors other than CLOCK and BMAL1 in regulating neurogenesis of the OB. In the OB, the PK2-encoding gene is likely to be a functional target of the E-box specific basic Helix-Loop-Helix (bHLH) factors, such as MASH1 and Ngn1 (i.e., NEUROG1), that have been shown as crucial regulators of neurogenesis (71). During development, MASH1 and Ngn1 regulate several important steps of neurogenesis, including the commitment of stem cells to neuronal and glial lineages, the specification of neuronal subtype identities, and neuronal migration. The overlapping expression of PK2 with Ngn1 and MASH1 in the OB, along with corroborating defects in OB neurogenesis in PK2-deficient (i.e., Prok2−/−) and Mash1−/− mice (72), indicate that PK2 is likely a critical downstream target gene of these bHLH transcriptional factors in regulating OB neurogenesis.
The PK2/PKR2 signaling pair is interesting in that it thus plays a critical role in seemingly distinct biological processes—OB neurogenesis (in embryonic development) and circadian clock output (in adult physiology). Different families of bHLH transcriptional factors, in turn, may regulate PK2 function in the different processes (Figure 3). It is clear that bHLH transcriptional factors MASH1, HIF, and ClOCK/Bmal1 also have other target genes. This gene network of transcriptional control may strengthen our growing appreciation that the evolutionary complexity of mammalian physiology reflects not a sheer increase in the quantity of genes (73), but rather a more sophisticated interplay of gene regulatory mechanisms.
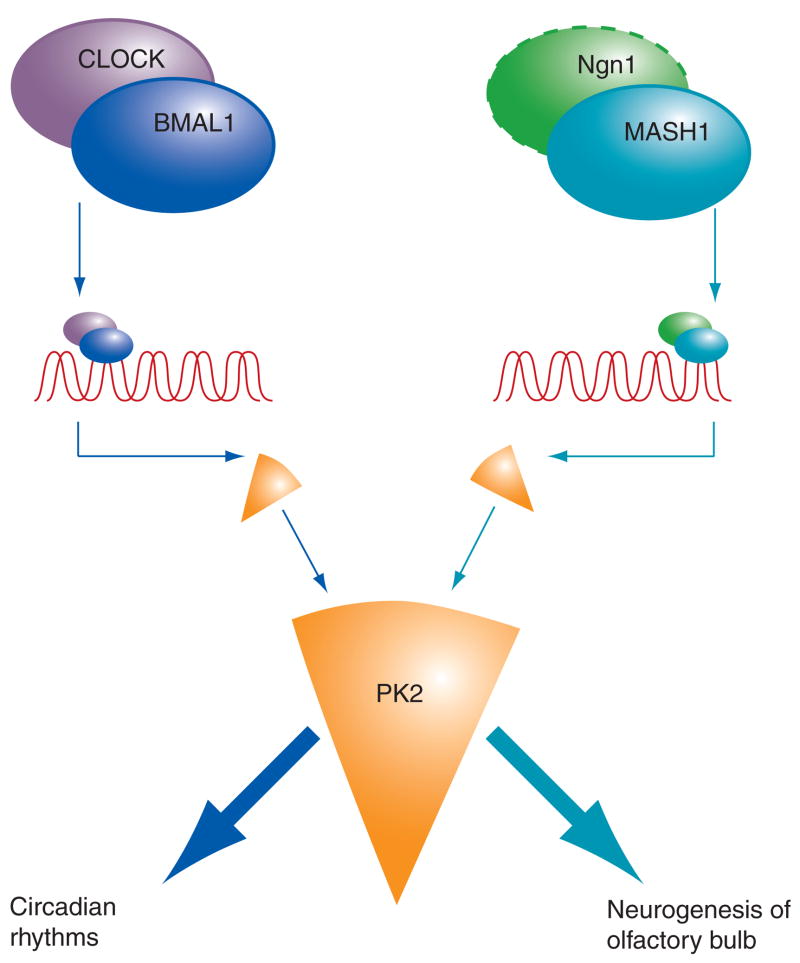
Figure 3. Transcriptional regulation of prokineticin 2.
The promoter of the PK2-encoding gene, in both human and mouse, contains multiple E-box sequences (CACGTG) that are targeted by basic helix-loop-helix (bHLH) transcription factors (ovals; CLOCK/BMAL1 and Ngn1/MASH1 dimers are schematized here). The expression of PK2 (orange) is a common functional target for both sets of transcriptional factors, although distinct biological processes are ultimately affected, depending the particular transcriptional machinery that is recruited (indicated by different shades of blue arrows). PK2 is also induced by hypoxia (as is PK1), and thus HIF-1 (hypoxia-inducing factor, also a bHLH transcription factor) may also regulate the functionality of PK2. (The dashed outline around Ngn1 is intended to indicate the speculative nature of its specific transcriptional role in this instance. See text for details.)
Summary and Perspectives
Over the last few years, PKs have become appreciated as signaling peptides that regulate diverse functions, ranging from embryonic development to circadian regulation of adult physiology. These diverse functions may be classified into two general categories: cell differentiation/migration and cell excitability. The regulatory function of PKs in gastrointestinal smooth muscle, circadian clock output, and pain perception relates to the enhanced cell excitability via activation of PKRs, whereas the function of PKs in angiogenesis, hematopoiesis, and neurogenesis is linked to the effect of PKs in regulating cell differentiation and motility. Another emerging theme is that PK2 expression is a possible common functional target for different upstream transcriptional factors in regulating different biological processes. No doubt, our current knowledge of PK function will expand considerably over the course of the next several years.
Acknowledgments
Research in author’s laboratory is supported by grants from NIH and UC Discovery program. The author is a co-founder of Kinexis, Inc. and a member of its scientific advisory board. Kinexis is working to develop therapeutics, in part based on prokineticin targeting, for the treatment of circadian disorders, obesity, and dementia.
Biography
Qun-Yong Zhou, PhD, is Associate Professor of Pharmacology at the University of California Medical School in Irvine. E-mail qzhou@uci.edu; fax 949-824-4855.

References
- 1.Joubert FJ, Strydom DJ. Snake venom. The amino acid sequence of protein A from Dendroaspis polylepis polylepis (black mamba) venom. Hoppe Seylers Z Physiol Chem. 1980;361:1787–1794. doi: 10.1515/bchm2.1980.361.2.1787. [DOI] [PubMed] [Google Scholar]
- 2.Schweitz H, Bidard JN, Lazdunski M. Purification and pharmacological characterization of peptide toxins from the black mamba (Dendroaspis polylepis) venom. Toxicon. 1990;28:847–856. doi: 10.1016/s0041-0101(09)80007-x. [DOI] [PubMed] [Google Scholar]
- 3.Schweitz H, Pacaud P, Diochot S, Moinier D, Lazdunski M. MIT(1), a black mamba toxin with a new and highly potent activity on intestinal contraction. FEBS Lett. 1999;461:183–188. doi: 10.1016/s0014-5793(99)01459-3. [DOI] [PubMed] [Google Scholar]
- 4.Mollay C, Wechselberger C, Mignogna G, Negri L, Melchiorri P, Barra D, Kreil G. Bv8, a small protein from frog skin and its homologue from snake venom induce hyperalgesia in rats. Eur J Pharmacol. 1999;374:189–196. doi: 10.1016/s0014-2999(99)00229-0. [DOI] [PubMed] [Google Scholar]
- 5.Wechselberger C, Puglisi R, Engel E, Lepperdinger G, Boitani C, Kreil G. The mammalian homologues of frog Bv8 are mainly expressed in spermatocytes. FEBS Lett. 1999;462:177–181. doi: 10.1016/s0014-5793(99)01473-8. [DOI] [PubMed] [Google Scholar]
- 6.Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol. 2001;59:692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- 7.LeCouter J, Kowalski J, Foster J, et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412:877–884. doi: 10.1038/35091000. The authors identify the angiogenic role of PK1 for the endothelial cells derived from endocrine organs. [DOI] [PubMed] [Google Scholar]
- 8.Lin DC, Bullock CM, Ehlert FJ, Chen JL, Tian H, Zhou QY. Identification and molecular characterization of two closely related G protein–coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. J Biol Chem. 2002;277:19276–19280. doi: 10.1074/jbc.M202139200. [DOI] [PubMed] [Google Scholar]
- 9.Masuda Y, Takatsu Y, Terao Y, et al. Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G protein–coupled receptors. Biochem Biophys Res Commun. 2002;293:396–402. doi: 10.1016/S0006-291X(02)00239-5. [DOI] [PubMed] [Google Scholar]
- 10.Soga T, Matsumoto S, Oda T, et al. Molecular cloning and characterization of prokineticin receptors. Biochim Biophys Acta. 2002;1579:173–179. doi: 10.1016/s0167-4781(02)00546-8. [DOI] [PubMed] [Google Scholar]
- 11.Kaser A, Winklmayr M, Lepperdinger G, Kreil G. The AVIT protein family. Secreted cysteine-rich vertebrate proteins with diverse functions. EMBO Rep. 2003;4:469–73. doi: 10.1038/sj.embor.embor830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LeCouter J, Ferrara N. EG-VEGF and Bv8. A novel family of tissue-selective mediators of angiogenesis, endothelial phenotype, and function. Trends Cardiovasc Med. 2003;13:276–282. doi: 10.1016/s1050-1738(03)00110-5. [DOI] [PubMed] [Google Scholar]
- 13.Zhou QY, Cheng MY. Prokineticin 2 and circadian clock output. FEBS Lett. 2005;272:5703–5709. doi: 10.1111/j.1742-4658.2005.04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melchiorri D, Bruno V, Besong G, et al. The mammalian homologue of the novel peptide Bv8 is expressed in the central nervous system and supports neuronal survival by activating the MAP kinase/PI-3-kinase pathways. Eur J Neurosci. 2001;13:1694–1702. doi: 10.1046/j.1460-9568.2001.01549.x. [DOI] [PubMed] [Google Scholar]
- 15.Lin R, LeCouter J, Kowalski J, Ferrara N. Characterization of endocrine gland-derived vascular endothelial growth factor signaling in adrenal cortex capillary endothelial cells. J Biol Chem. 2002;277:8724–8729. doi: 10.1074/jbc.M110594200. [DOI] [PubMed] [Google Scholar]
- 16.Vellani V, Colucci M, Lattanzi R, Giannini E, Negri L, Melchiorri P, McNaughton PA. Sensitization of transient receptor potential vanilloid 1 by the prokineticin receptor agonist Bv8. J Neurosci. 2006;26:5109–5116. doi: 10.1523/JNEUROSCI.3870-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–410. doi: 10.1038/417405a. The authors identify PK2 as an output signal for the SCN circadian clock, the first known CNS function of prokineticins. [DOI] [PubMed] [Google Scholar]
- 18.Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science. 2005;308:1923–1927. doi: 10.1126/science.1112103. The authors demonstrate that PK2, functioning as a migratory chemical guide, is required for neurogenesis of olfactory bulb. [DOI] [PubMed] [Google Scholar]
- 19.Cheng MY, Leslie FM, Zhou QY. Expression of prokineticins and their receptors in the adult mouse brain. J Comp Neurol. 2006;498:796–809. doi: 10.1002/cne.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bullock CM, Li JD, Zhou QY. Structural determinants required for the bioactivities of prokineticins and identification of prokineticin receptor antagonists. Mol Pharmacol. 2004;65:582–588. doi: 10.1124/mol.65.3.582. [DOI] [PubMed] [Google Scholar]
- 21.Boisbouvier J, Albrand JP, Blackledge M, Jaquinod M, Schweitz H, Lazdunski M, Marion D. A structural homologue of colipase in black mamba venom revealed by NMR floating disulphide bridge analysis. J Mol Biol. 1998;283:205–219. doi: 10.1006/jmbi.1998.2057. [DOI] [PubMed] [Google Scholar]
- 22.Negri L, Lattanzi R, Giannini E, et al. Biological activities of Bv8 analogues. Br J Pharmacol. 2005;146:625–632. doi: 10.1038/sj.bjp.0706376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Kuei C, Sutton S, Wilson S, Yu J, Kamme F, Mazur C, Lovenberg TW, Liu C. Identification and Pharmacological Characterization of prokineticin 2{beta} as a selective ligand for prokineticin receptor 1. Mol Pharmacol. 2005;67:2070–2076. doi: 10.1124/mol.105.011619. [DOI] [PubMed] [Google Scholar]
- 24.Bassil AK, Dass NB, Murray CD, Muir A, Sanger GJ. Prokineticin-2, motilin, ghrelin and metoclopramide: Prokinetic utility in mouse stomach and colon. Eur J Pharmacol. 2005;524:138–144. doi: 10.1016/j.ejphar.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Hoogerwerf WA. Prokineticin 1 inhibits spontaneous giant contractions in the murine proximal colon through nitric oxide release. Neurogastroenterol Motil. 2006;18:455–463. doi: 10.1111/j.1365-2982.2006.00776.x. [DOI] [PubMed] [Google Scholar]
- 26.Lewis KE. Prokineticin-2, a potential novel protein therapeutic increases post-operative gastric and intestinal motility in rats. Gastroenterology. 126(Suppl 2):A641–2004. [Google Scholar]
- 27.Negri L, Lattanzi R, Giannini E, Metere A, Colucci M, Barra D, Kreil G, Melchiorri P. Nociceptive sensitization by the secretory protein Bv8. Br J Pharmacol. 2002;137:1147–1154. doi: 10.1038/sj.bjp.0704995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caterina MJ, Julius D. The vanilloid receptor: A molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 29.Hu WP, Li JD, Zhang C, Luo G, Amadesi S, Bunnett N, Zhou QY. Impaired pain sensation in mice lacking the prokineticin 2. Mol Pain. 2(35) doi: 10.1186/1744-8069-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Negri L, Lattanzi R, Giannini E, et al. Impaired nociception and inflammatory pain sensation in mice lacking the prokineticin receptor PKR1: Focus on interaction between PKR1 and the capsaicin receptor TRPV1 in pain behavior. J Neurosci. 2006;26:6716–6727. doi: 10.1523/JNEUROSCI.5403-05.2006. The authors use knock-out mice to demonstrate that PKR1 mediates the pain sensitizing effect of prokineticins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeCouter J, Zlot C, Tejada M, Peale F, Ferrara N. Bv8 and endocrine gland-derived vascular endothelial growth factor stimulate hematopoiesis and hematopoietic cell mobilization. Proc Natl Acad Sci USA. 2004;101:16813–16818. doi: 10.1073/pnas.0407697101. The authors demonstrate the role of PK2 for hematopoiesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorsch M, Qiu Y, Soler D, Frank N, Duong T, Goodearl A, O’Neil S, Lora J, Fraser CC. PK1/EG-VEGF induces monocyte differentiation and activation. J Leukoc Biol. 2005;78:426–434. doi: 10.1189/jlb.0205061. [DOI] [PubMed] [Google Scholar]
- 33.Martucci C, Franchi S, Giannini E, Tian H, Melchiorri P, Negri L, Sacerdote P. Bv8, the amphibian homologue of the mammalian prokineticins, induces a proinflammatory phenotype of mouse macrophages. Br J Pharmacol. 2006;147:225–234. doi: 10.1038/sj.bjp.0706467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 35.Lowrey PL, Takahashi JS. Mammalian circadian biology: Elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng MY, Bittman EL, Hattar S, Zhou QY. Regulation of prokineticin 2 expression by light and the circadian clock. BMC Neurosci. 2005;6:17–27. doi: 10.1186/1471-2202-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamazaki S, Numano R, Abe M, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 38.Negri L, Lattanzi R, Giannini E, De Felice M, Colucci A, Melchiorri P. Bv8, the amphibian homologue of the mammalian prokineticins, modulates ingestive behaviour in rats. Br J Pharmacol. 2004;142:181–191. doi: 10.1038/sj.bjp.0705686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morton AJ, Wood NI, Hastings MH, Hurelbrink C, Barker RA, Maywood ES. Disintegration of the sleep-wake cycle and circadian timing in Huntington’s disease. J Neurosci. 2005;25:157–163. doi: 10.1523/JNEUROSCI.3842-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li JD, Hu WP, Boehmer LN, Cheng MY, Lee AG, Jilek A, Siegel JM, Zhou QY. Attenuated circadian rhythms in mice lack the prokineticin 2 gene. J Neurosci. 2006;26:11615–11623. doi: 10.1523/JNEUROSCI.3679-06.2006. The authors use knock-out mice to demonstrate that PK2 is a transmitter of circadian rhythmicity from the SCN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu WP, Li JD, Zhang C, Boehmer L, Siegel JM, Zhou QY. Altered circadian and homeostatic sleep regulation in prokineticins 2-deficient mice. Sleep. (in press) [PMC free article] [PubMed] [Google Scholar]
- 42.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 44.Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, McKnight SL. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science. 2003;301:379–383. doi: 10.1126/science.1082795. [DOI] [PubMed] [Google Scholar]
- 45.Pitts S, Perone E, Silver R. Food-entrained circadian rhythms are sustained in arrhythmic Clk/Clk mutant mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R57–67. doi: 10.1152/ajpregu.00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cottrell GT, Zhou QY, Ferguson AV. Prokineticin 2 modulates the excitability of subfornical organ neurons. J Neurosci. 2004;24:2375–2379. doi: 10.1523/JNEUROSCI.5187-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuill EA, Ferri CC, Zhou QY, Ferguson AV. Prokineticin 2 depolarizes paraventricular nucleus neurons: Cellular correlates for circadian regulation of autonomic function. (Unpublished results) [Google Scholar]
- 48.Lambert CM, Machida KK, Smale L, Nunez AA, Weaver DR. Analysis of the prokineticin 2 system in a diurnal rodent, the unstriped nile grass rat (Arvicanthis niloticus) J Biol Rhythms. 2005;20:206–218. doi: 10.1177/0748730405275135. [DOI] [PubMed] [Google Scholar]
- 49.Masumoto KH, Nagano M, Takashima N, Hayasaka N, Hiyama H, Matsumoto S, Inouye ST, Shigeyoshi Y. Distinct localization of prokineticin 2 and prokineticin receptor 2 mRNAs in the rat suprachiasmatic nucleus. Eur J Neurosci. 2006;23:2959–2970. doi: 10.1111/j.1460-9568.2006.04834.x. [DOI] [PubMed] [Google Scholar]
- 50.LeCouter J, Lin R, Tejada M, Frantz G, Peale F, Hillan KJ, Ferrara N. The endocrine-gland-derived VEGF homologue Bv8 promotes angiogenesis in the testis: Localization of Bv8 receptors to endothelial cells. Proc Natl Acad Sci USA. 2003;100:2685–2690. doi: 10.1073/pnas.0337667100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka N, Ikawa M, Mata NL, Verma IM. Choroidal neovascularization in transgenic mice expressing prokineticin 1: An animal model for age-related macular degeneration. Mol Ther. 2006;13:609–616. doi: 10.1016/j.ymthe.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 52.Ferrara N, Frantz G, LeCouter J, Dillard-Telm L, Pham T, Draksharapu A, Giordano T, Peale F. Differential expression of the angiogenic factor genes vascular endothelial growth factor (VEGF) and endocrine gland-derived VEGF in normal and polycystic human ovaries. Am J Pathol. 2003;162:1881–1893. doi: 10.1016/S0002-9440(10)64322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L, Yang N, Conejo-Garcia JR, et al. Expression of endocrine gland-derived vascular endothelial growth factor in ovarian carcinoma. Clin Cancer Res. 2003;9:264–272. [PubMed] [Google Scholar]
- 54.Pasquali D, Rossi V, Staibano S, et al. The endocrine-gland-derived vascular endothelial growth factor (EG-VEGF)/prokineticin 1 and 2 and receptor expression in human prostate: Up-regulation of EG-VEGF/prokineticin 1 with malignancy. Endocrinol. 2006;147:4245–4251. doi: 10.1210/en.2006-0614. [DOI] [PubMed] [Google Scholar]
- 55.Battersby S, Critchley HO, Morgan K, Millar RP, Jabbour HN. Expression and regulation of the prokineticins (endocrine gland-derived vascular endothelial growth factor and Bv8) and their receptors in the human endometrium across the menstrual cycle. J Clin Endocrinol Metab. 2004;89:2463–2469. doi: 10.1210/jc.2003-032012. [DOI] [PubMed] [Google Scholar]
- 56.Hoffmann P, Feige JJ, Alfaidy N. Expression and oxygen regulation of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 and its receptors in human placenta during early pregnancy. Endocrinol. 2006;147:1675–1684. doi: 10.1210/en.2005-0912. [DOI] [PubMed] [Google Scholar]
- 57.Fraser HM, Bell J, Wilson H, Taylor PD, Morgan K, Anderson RA, Duncan WC. Localization and quantification of cyclic changes in the expression of endocrine gland vascular endothelial growth factor in the human corpus luteum. J Clin Endocrinol Metab. 2005;90:427–434. doi: 10.1210/jc.2004-0843. [DOI] [PubMed] [Google Scholar]
- 58.Kisliouk T, Levy N, Hurwitz A, Meidan R. Presence and regulation of endocrine gland vascular endothelial growth factor/prokineticin-1 and its receptors in ovarian cells. J Clin Endocrinol Metab. 2003;88:3700–3707. doi: 10.1210/jc.2003-030492. [DOI] [PubMed] [Google Scholar]
- 59.Kisliouk T, Podlovni H, Meidan R. Unique expression and regulatory mechanisms of EG-VEGF/prokineticin-1 and its receptors in the corpus luteum. Ann Anat. 2005;187:529–537. doi: 10.1016/j.aanat.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 60.Kisliouk T, Podlovni H, Spanel-Borowski K, Ovadia O, Zhou QY, Meidan R. Prokineticins (endocrine gland-derived vascular endothelial growth factor and Bv8) in the bovine ovary: Expression and role as mitogens in survival factors for corpus luteum–derived endothelial cells. Endocrinology. 2005;146:3950–3958. doi: 10.1210/en.2005-0297. [DOI] [PubMed] [Google Scholar]
- 61.Wierman ME, Pawlowski JE, Allen MP, Xu M, Linseman DA, Nielsen-Preiss S. Molecular mechanisms of gonadotropin-releasing hormone neuronal migration. Trends Endocrinol Metab. 2004;15:96–102. doi: 10.1016/j.tem.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 62.Masumoto S, Yamazaki C, Masumoto KH, et al. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci USA. 2006;103:4140–4145. doi: 10.1073/pnas.0508881103. The authors use knock-out mice to show that PKR2 mediates the neurogenic effect of PK2 in olfactory bulb development and also demonstrate that PKR2 is required for normal reproductive function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dode C, Teixeira L, Levilliers J, et al. Kallmann syndrome: Mutations in the genes encoding prokineticin 2 and prokineticin receptor 2. PLoS Genet. 1906;2:e175. doi: 10.1371/journal.pgen.0020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soderhall I, Kim YA, Jiravanichpaisal P, Lee SY, Soderhall K. An ancient role for a prokineticin domain in invertebrate hematopoiesis. J Immunol. 2005;174:6153–6160. doi: 10.4049/jimmunol.174.10.6153. [DOI] [PubMed] [Google Scholar]
- 65.Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 66.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 67.Kaplan MS, Hinds JW. Neurogenesis in the adult rat: Electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 68.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 69.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 70.Vitaterna MH, King DP, Chang AM, et al. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- 72.Murray RC, Navi D, Fesenko J, Lander AD, Calof AL. Widespread defects in the primary olfactory pathway caused by loss of Mash1 function. J Neurosci. 2003;23:1769–1780. doi: 10.1523/JNEUROSCI.23-05-01769.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]