Abstract
Circadian timing from the suprachiasmatic nucleus (SCN) is a critical component of sleep regulation. Animal lesion and genetic studies have indicated an essential interaction between the circadian signals and the homeostatic processes that regulate sleep. Here we summarize the biological functions of prokineticins, a pair of newly discovered regulatory proteins, with focus on the circadian function of prokineticin 2 (PK2) and its potential role in sleep-wake regulation. PK2 has been shown as a candidate SCN output molecule that regulates circadian locomotor behavior. The PK2 molecular rhythm in the SCN is predominantly controlled by the circadian transcriptional/translational loops, but also regulated directly by light. The receptor for PK2 is expressed in the primary SCN output targets that regulate circadian behavior including sleep-wake. The depolarizing effect of PK2 on neurons that express PK2 receptor may represent a possible mechanism for the regulatory role of PK2 in circadian rhythms.
Keywords: circadian, G-protein coupled receptor, locomotor, PK2, prokineticin, secretory protein, sleep wakefulness, suprachiasmatic nucleus
Introduction
The regulation of sleep has been modeled as a two-process system consisting of a homeostatic process and a circadian timing process, which together determine the propensity, length, and incidence of episodes and intensity of sleep [1]. The circadian control of sleep is evident because the sleep-wake cycle continues on a near 24 h basis even when the environment is devoid of any timing cues [2,3]. Early studies in rats with lesions of the suprachiasmatic nucleus (SCN) demonstrated that the control of normal circadian expression of the sleep-wake cycle is mediated by the SCN clock; however, the duration of sleep was unaffected in SCN-lesioned animals [4–6]. In addition, recovery time after sleep deprivation in these SCN-lesioned animals was unchanged [7–9]. Thus, SCN circadian timing of sleep has been suggested to be independent of the homeostatic process. However, SCN lesion studies in squirrel monkeys have indicated an essential interaction between circadian and homeostatic processes because complete lesions of the monkey SCN produced both a loss of circadian timing and a 4 h increase in daily sleep time [10]. This finding led to the ‘opponent process’ model, which suggests that the SCN clock produces a ‘wakefulness-promoting’ signal that enhances wakefulness in diurnal animals and thereby actively opposes the homeostatic tendency for sleep [10]. Results from the clock mutant mice (Clk−/−) appear to be consistent with the hypothesis that the circadian clock has major affects on the homeostatic control of sleep. In particular, Clk−/− mutant mice sleep 2 h less than wildtype mice daily, indicating that the reduced circadian signaling in these mutant mice suppresses total sleep time [11]. Cryptochrome double knockout mice (Cry1−/−Cry2−/−) exhibited an increase in nonrapid eye movement sleep (NREMS) time, NREMS consolidation and electroencephalogram delta power during NREMS under basal and constant dark conditions [12], further supporting the importance of these core clock genes in homeostatic sleep regulation as well as circadian rhythmicity. Thus, circadian timing signals appear to promote sleep in nocturnal rodents. Similar observations have been observed with SCN-lesioned animals [13], indicating that transitions into REM sleep are facilitated by the SCN during rest phase. Thus, it appears that SCN signals actively promote REM sleep during light phase. Although it remains unclear how much the circadian clock contributes to sleep homeostasis, it appears that the SCN circadian timing system is critical in homeostatic sleep regulation, in addition to its control in sleep-wake rhythms.
Prokineticins (PK1 and PK2) are a pair of cysteine-rich secreted proteins that regulate diverse biological processes via activation of two cognate G-protein coupled receptors [14–16]. In this review, we summarize the biological function of PKs, with a focus on the function of PK2 in circadian rhythms.
Functional roles of prokineticins in noncircadian processes
The first function associated with prokineticins was the stimulation of gastrointestinal (GI) smooth muscle contraction. Schweitz and coworkers purified mamba intestinal toxin 1 from snake venom and demonstrated that it potently stimulated contraction of the guinea pig ileum [17,18]. A protein (Bv8) of similar size secreted from frog skin had also been purified and demonstrated to cause potent contractions of GI smooth muscles [19]. In the search for the mammalian homologs of these molecules, we isolated and characterized two human cDNAs, and named them ‘prokineticins’ to reflect their potent actions on GI contractility [20]. Prokineticins and their nonmammalian counterparts exhibit conservation in their amino acid sequences including the first six amino acids in the N-terminal domain (AVITGA) and the 10 cysteine residues predicted to form five pairs of disulfide bonds [20] (Fig. 1). Using recombinant human PKs, we demonstrated that these mammalian proteins also stimulate contractions of GI smooth muscle with similar potency. GTPγS binding studies revealed that the receptors for PKs belong to the family of G-protein coupled receptors (GPCR) [20]. Three independent groups subsequently identified two closely related GPCRs for PKs, prokineticin receptor 1 (PKR1) and prokineticin receptor 2 (PKR2) [14–16]. Activation of these receptors leads to mobilization of calcium, phosphoinositide hydrolysis, and activation of the mitogen-activated protein kinase and protein kinase B pathways [14–16].
Fig. 1.

Structural sequences of prokineticins and their non-mammalian counterparts. Prokineticins are two secreted human proteins that contain about 80 amino acids, with characteristic signal peptide sequence and 10 cysteine residues. The diagram displays the amino acid sequences of prokineticin 1 (A), prokineticin 2 (B), frog Bv8 (C), and partial sequence of mamba intestinal toxin 1 (D). Signal peptides are underlined. Ten conservative cysteine residues are marked (*). Arrows indicate the splice sites for introns. (Figure adapted from Li et al. [20]).
Around the time when PKs were identified as potent GI spasmogens, an independent group discovered an angiogenic mitogen while screening a library of human secreted molecules for their ability to induce proliferation in primary bovine adrenal cortex-derived capillary endothelial cells. Due to its tissue specific angiogenic nature, this molecule was named endocrine gland-derived vascular endothelial growth factor (EG-VEGF) [21]. Delivery of EG-VEGF to the ovary elicited potent angiogenesis and cyst formation. Sequence analysis revealed that EG-VEGF is the same molecule as PK1 [20,21]. Following this initial finding with PK1, it was demonstrated that PK2 also possess a similar angiogenic effect [22]. This angiogenic effect is likely to be mediated through PK receptors because both PK receptors are expressed in the vascular endothelial cells of the adrenal and testis [22]. As the process of angiogenesis is crucial for many processes including reproductive functions, several groups have further examined the role of PKs in angiogenesis of reproductive organs [23–25].
Because PKs and their receptors are expressed in bone marrow and other hematopoietic organs [20], it is not surprising that a possible role for PKs in hematopoiesis was reported [26,27]. Both PK1 and PK2 induce monocyte migration and increase the number of colony forming units, both granulocytic and monocytic, in hematopoietic stem cells. Systemic injection of PK2 also increases the number of immune cells and promotes their survival against 5-fluorouracil induced injury [26]. This suggests that PK2 may function as a monocyte chemoattractant and a hematopoietic cytokine that promotes survival and modulates the innate and the adaptive immune systems.
Central administration of Bv8, the frog homolog of PK2, has been shown to regulate feeding behavior and nociception in rodents. The expression of PKR2 in areas such as the arcuate nucleus, paraventricular hypothalamic nucleus and subfornical organ suggests that PK2 may play a role in feeding and drinking [28–30]. Negri et al. showed that intracerebroventricular delivery of Bv8 suppressed diurnal and nocturnal feeding, as well as deprivation-induced and neuropeptide Y-stimulated feeding [31]. They also showed that microinjection of Bv8 into the subfornical organ stimulated drinking. A possible central effect of PKs in nociception was first reported by Mollay et al. who observed that administration of Bv8 into the rat brain induced a marked hyperalgesic response [32]. A further study by Negri et al. demonstrated that intravenous, subcutaneous and intrathecal injections of Bv8 all produced intense systemic nociceptive sensitization to mechanical and thermal stimuli applied to tail and paws [32]. Both PKR1 and PKR2 mRNA are expressed in the spinal cord, in addition to dorsal root ganglia, and Bv8 can bind to these PK receptors with high affinity [32]. These data suggest that Bv8 or its mammalian homologs play a role in nociception, possibly by sensitizing central or peripheral nociceptors via activation of PK receptors in the spinal cord or dorsal root ganglia.
Function of PK2 in circadian rhythms
The SCN in the anterior hypothalamus is the primary mammalian circadian pacemaker that regulates daily rhythms of physiology and behavior, including sleep-wake cycle [33–35]. While the understanding of the molecular mechanism of the circadian clockwork has emerged in the last decade [34–37], little is known about the mechanism by which the circadian clock transmits timing information to control physiology and behavior. In an attempt to study the function of PKs in the central nervous system, we have mapped a comprehensive mRNA distribution of the PKs and their receptors in the adult mouse brain. Our initial characterization revealed that PK2 mRNA is highly expressed in the SCN in a rhythmic fashion, with high levels during the day and low or undetectable levels at night [28]. This PK2 oscillation in the SCN is maintained in animals under normal 12 h light:12 h dark as well as under constant darkness, indicating that this PK2 oscillation is driven by the endogenous circadian clockwork [28]. In situ hybridization revealed that the receptor for PK2 (PKR2) is expressed in primary SCN output target areas, further suggesting the role of PK2 in circadian rhythms.
Subsequent molecular and behavioral studies demonstrated that PK2 is a candidate output signaling molecule for circadian locomotor rhythms [28]. As Clock and Bmal1 are the positive elements of the clockwork, these transcription factors heterodimerize and bind an CACGTG sequence known as an E-box to activate transcription of other clock or clock-controlled genes [34,35]. Both the human and mouse PK2 promoter contains four E-boxes within the first 2 kb upstream of the transcription start site [28]. In vitro transcription assays indicate that PK2 transcription is tightly regulated by clockwork gene products through activation of the E-boxes residing in its proximal promoter. These in vitro findings were validated in vivo, as PK2 mRNA expression in the SCN is completely absent or blunted in mutant mice lacking functional clockwork, including Clk−/− mice and Cry1−/−Cry2−/− mice. To elucidate whether PK2 plays a role in circadian-regulated behavior, we tested the effects of PK2 on locomotor wheel running activity, a behavioral readout that closely mimics the sleep-wake rhythm. Intracerebroventricular delivery of PK2 into the lateral ventricle during subjective night, when endogenous PK2 is low, inhibited the nocturnal wheel running activity of rats [28]. Taken together, our results indicate that PK2 is a candidate output molecule that transmits circadian behavioral rhythms from the SCN clock.
Because nocturnal light pulses can rapidly induce PK2 expression in the SCN, we investigated the possibility that PK2 is under the dual regulation of both light and the core clockwork. Using a common model for jet lag, we examined how PK2 responds to abrupt shifts of light/dark cycles (phase delays or phase advances) [38]. We observed that PK2 expression exhibits transients in response to phase advances but entrains rapidly to phase delays. Interestingly, this differential pattern of PK2 expression during phase shifts is consistent with the circadian phenomenon that animals and humans adjust rapidly to time delays but slowly to advances [39,40]. These studies provide additional evidence for PK2 as an output signaling molecule from the SCN. Interestingly, we also observed that light can directly regulate PK2 in the absence of functional circadian clockwork. Studies with Cry1−/−Cry2−/− mice revealed that a light-regulated low amplitude PK2 rhythm persists in these mice that lack functional circadian oscillators [38]. Collectively, our data suggest that PK2 is controlled by two mechanisms, dominantly by the circadian oscillator, and directly by light. Perhaps PK2 evolved before the circadian timing system became internalized in the brain.
While the direct genetic analysis of PK2 deficiency on circadian rhythm has yet to be determined, Morton et al. have observed a correlation between increased daytime activity and reduced SCN expression of PK2 molecular rhythm in transgenic mice expressing a mutant Huntington’s gene [41]. This finding suggests that the reduced PK2 rhythm may contribute to the sleep disturbances and abnormal circadian behavior seen in this strain of transgenic mice. Recently, the molecular rhythm of PK2 in the SCN of a diurnal rodent (Arvicanthis niloticus) has been reported [42]. Similar to the oscillation pattern observed in mouse, PK2 mRNA in the SCN of this diurnal species was also rhythmically expressed, with peak levels in the morning and trough levels in the evening [42]. Thus, the phase of PK2 expression in the SCN of diurnal rodent is similar to that of nocturnal rodents, which is consistent with a growing body of evidence suggesting that the key to diurnality lies downstream of the SCN circadian clock.
The wide distribution of PKR2 in primary SCN output targets suggests that PK2 may regulate a number of circadian clock controlled physiological processes and behaviors in addition to circadian locomotor behavior. Most notably, PK2 is likely to play a role in sleep-wake regulation, as PKR2 is expressed in several visual and sleep-wake related nuclei [43]. PKR2 expression is detected in the superior colliculus, a sensory nucleus crucial for visual navigation, which sends efferent fibers to motor centers that orient behavior [44,45]. The superior colliculus has also been shown to mediate the effects of light on sleep and wakefulness [46]. PKR2 expression is also detected in other nuclei important for sleep-wake regulation [43], such as the lateral hypothalamus [47–49], the perifornical region of the hypothalamus [47,50] and dorsal raphe [51]. A high level of PKR2 is also detected in the lateral habenula (LHb), an important relay nucleus that is a primary output target of the SCN. The connection of the LHb with the raphe, preoptic nuclei and the pineal gland suggests its role in the regulation of sleep and arousal [52]. LHb projects directly to the pineal gland and has been shown to mediate the firing rate of pineal cells [53]. This SCN-LHb-pineal projection may represent another pathway by which PK2 can regulate melatonin secretion and sleep-wake cycle [52]. Although no specific PKR2 mRNA is detected in the locus coeruleus (LC), the primary nucleus that regulates arousal [54,55], it is possible that the circadian control of arousal is mediated through secondary projections. Although the SCN lacks direct projections to LC, it has recently been demonstrated that the SCN can regulate arousal through dorsomedial hypothalamus (DMH) [56]. The presence of PKR2 in DMH suggests that PK2 may regulate arousal or the sleep-wake cycle through SCN-DMH-LC projections. These expression patterns of PKR2 in most known SCN output targets strongly implicate PK2 in the regulation of the sleep-wake cycle.
Recent studies have indicated a possible neurophysiological mechanism for the regulatory role of PK2 in the brain, including its role in circadian rhythms [57] (E. A. Yuill, C. C. Ferri, Q.-Y. Zhou & A. V. Ferguson, unpublished results). Using dissociated neurons of the subfornical organ (SFO), it has been demonstrated that PK2 activates and depolarizes SFO neurons and inhibits delayed rectifier potassium currents [57]. Subsequent studies indicated that PK2 also has an excitatory effect on different populations of the paraventricular hypothalamic neurons (PVN) (E. A. Yuill, C. C. Ferri, Q.-Y. Zhou & A. V. Ferguson, unpublished results). The excitatory effect of PK2 on parvocellular PVN neurons is maintained under tetrototoxin, but is blocked by the addition of a PK receptor antagonist (A1MPK1), demonstrating that this effect is directly and specifically mediated by the PK receptor (E. A. Yuill, C. C. Ferri, Q.-Y. Zhou & A. V. Ferguson, unpublished results). Because PK2 is highly expressed in the SCN and there is a direct efferent projection from the SCN to PVN [58,59], it is likely that PK2 may exert a circadian regulation on these parvocellular neuroendocrine and autonomic neurons. Although the source of PK2 that modulates the SFO is unclear, it is conceivable that PK2 from the SCN may diffuse through CSF. As the SFO and the PVN are principal brain regions controlling autonomic and neuroendocrine function [29,30], these findings demonstrate that PK2 has a neuromodulatory role in the CNS and identify these sites as potential targets for circadian regulation of autonomic function.
Summary
Over the last few years, PKs have evolved as a pair of signaling peptides that regulate diverse functions, including circadian rhythms, gastrointestinal motility, angiogenesis, hematopoiesis and ingestive behavior (Fig. 2). It is interesting to note that these diverse functions of PKs may be classified into two general categories: cell excitability and cell motility. It appears that the regulatory function of PKs in GI smooth muscle, circadian clock output and ingestive behaviors may be related to the activation of PK receptors in target cells that leads to enhanced cell excitability, whereas the function of PKs in angiogenesis, hematopoiesis and neurodevelopment seems to be more closely linked to the effect of PKs in modulating cell motility and/or differentiation. Without a doubt, more exciting functions for this pair of regulatory proteins are emerging.
Fig. 2.
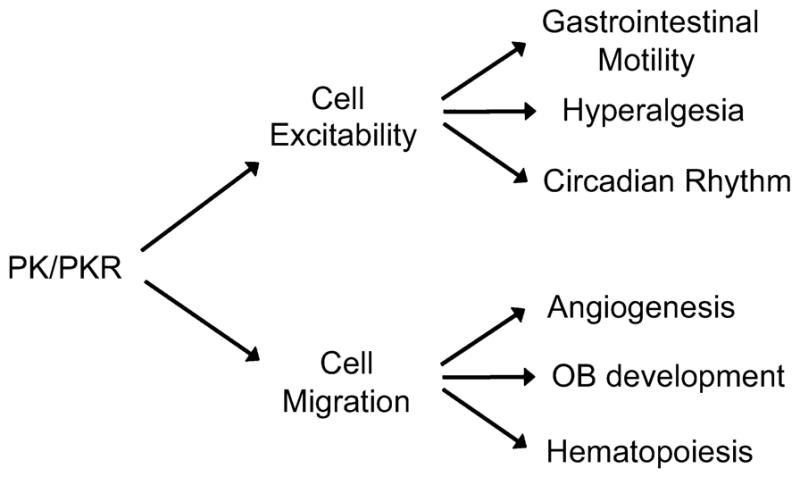
Current biological functions of prokineticins (PKs). Activation of prokineticin receptors (PKRs) eventually leads to cell excitability or cell migration. The current known functions of PKs appear to fall into these two general categories. The functions of PKs in gastrointestinal motility, hyperalgesia and circadian rhythm appear to be related to the activation of PKRs in the target cells that leads to enhanced cell excitability, whereas PKs role in angiogenesis, olfactory bulb (OB) development and hematopoiesis seem to be more closely linked to the ability of PKs to induce cell proliferation, migration and/or differentiation.
Acknowledgments
Research in the authors’ lab is supported by an NIH grant and a UC Discovery grant. M.Y.C. is partially supported by PHRMA Foundation.
Abbreviations
- Clk
clock
- Cry
cryptochrome
- DMH
dorsomedial hypothalamus
- EG-VEGF
endocrine gland-derived vascular endothelial growth factor
- GI
gastrointestinal
- GPCR
G-protein coupled receptor
- LC
locus coeruleus
- LHb
lateral habenula
- NREMS
nonrapid eye movement sleep
- PK
prokineticin
- PKR
prokineticin receptor
- PVN
paraventricular hypothalamic neurons
- REM
rapid eye movement
- SCN
suprachiasmatic nucleus
- SFO
subfornical organ
References
- 1.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 2.Czeisler CA, Weitzman E, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: its duration and organization depend on its circadian phase. Science. 1980;210:1264–1267. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- 3.Zulley J, Wever R, Aschoff J. The dependence of onset and duration of sleep on th circadian rhythm of rectal temperature. Pflugers Arch. 1981;391:314–318. doi: 10.1007/BF00581514. [DOI] [PubMed] [Google Scholar]
- 4.Mouret J, Coindet J, Debilly G, Chouvet G. Suprachiasmatic nuclei lesions in the rat: alterations in sleep circadian rhythms. Electroencephalogr Clin Neurophysiol. 1978;45:402–408. doi: 10.1016/0013-4694(78)90191-8. [DOI] [PubMed] [Google Scholar]
- 5.Eastman CI, Mistlberger RE, Rechtschaffen A. Suprachiasmatic nuclei lesions eliminate circadian temperature and sleep rhythms in the rat. Physiol Behav. 1984;32:357–368. doi: 10.1016/0031-9384(84)90248-8. [DOI] [PubMed] [Google Scholar]
- 6.Mistlberger RE, Bergmann BM, Rechtschaffen A. Relationships among wake episode lengths, contiguous sleep episode lengths, and electroencephalographic delta waves in rats with suprachiasmatic nuclei lesions. Sleep. 1987;10:12–24. [PubMed] [Google Scholar]
- 7.Mistlberger RE, Bergmann BM, Waldenar W, Rechtschaffen A. Recovery sleep following sleep deprivation in intact and suprachiasmatic nuclei-lesioned rats. Sleep. 1983;6:217–233. doi: 10.1093/sleep/6.3.217. [DOI] [PubMed] [Google Scholar]
- 8.Tobler I, Borbely AA, Groos G. The effect of sleep deprivation on sleep in rats with suprachiasmatic lesions. Neurosci Lett. 1983;42:49–54. doi: 10.1016/0304-3940(83)90420-2. [DOI] [PubMed] [Google Scholar]
- 9.Borbely AA, Achermann P, Trachsel L, Tobler I. Sleep initiation and initial sleep intensity: interactions of homeostatic and circadian mechanisms. J Biol Rhythms. 1989;4:149–160. [PubMed] [Google Scholar]
- 10.Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J Neurosci. 1993;13:1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naylor E, Bergmann BM, Krauski K, Zee PC, Takahashi JS, Vitaterna MH, Turek FW. The circadian clock mutation alters sleep homeostasis in the mouse. J Neurosci. 2000;20:8138–8143. doi: 10.1523/JNEUROSCI.20-21-08138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wisor JP, O’Hara BF, Terao A, Selby CP, Kilduff TS, Sancar A, Edgar DM, Franken P. A role for cryptochromes in sleep regulation. BMC Neurosci. 2002;3:20. doi: 10.1186/1471-2202-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wurts SW, Edgar DM. Circadian and homeostatic control of rapid eye movement (REM) sleep: promotion of REM tendency by the suprachiasmatic nucleus. J Neurosci. 2000;20:4300–4310. doi: 10.1523/JNEUROSCI.20-11-04300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin DC, Bullock CM, Ehlert FJ, Chen JL, Tian H, Zhou QY. Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. J Biol Chem. 2002;277:19276– 19280. doi: 10.1074/jbc.M202139200. [DOI] [PubMed] [Google Scholar]
- 15.Masuda Y, Takatsu Y, Terao Y, Kumano S, Ishibashi Y, Suenaga M, Abe M, Fukusumi S, Watanabe T, Shintani Y, et al. Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G-protein-coupled receptors. Biochem Biophys Res Commun. 2002;293:396–402. doi: 10.1016/S0006-291X(02)00239-5. [DOI] [PubMed] [Google Scholar]
- 16.Soga T, Matsumoto S, Oda T, Saito T, Hiyama H, Takasaki J, Kamohara M, Ohishi T, Matsushime H, Furuichi K. Molecular cloning and characterization of prokineticin receptors. Biochim Biophys Acta. 2002;1579:173–179. doi: 10.1016/s0167-4781(02)00546-8. [DOI] [PubMed] [Google Scholar]
- 17.Schweitz H, Bidard JN, Lazdunski M. Purification and pharmacological characterization of peptide toxins from the black mamba (Dendroaspis polylepis) venom. Toxicon. 1990;28:847–856. doi: 10.1016/s0041-0101(09)80007-x. [DOI] [PubMed] [Google Scholar]
- 18.Schweitz H, Pacaud P, Diochot S, Moinier D, Lazdunski M. MIT(1), a black mamba toxin with a new and highly potent activity on intestinal contraction. FEBS Lett. 1999;461:183–188. doi: 10.1016/s0014-5793(99)01459-3. [DOI] [PubMed] [Google Scholar]
- 19.Mollay C, Wechselberger C, Mignogna G, Negri L, Melchiorri P, Barra D, Kreil G. Bv8, a small protein from frog skin and its homologue from snake venom induce hyperalgesia in rats. Eur J Pharmacol. 1999;374:189–196. doi: 10.1016/s0014-2999(99)00229-0. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol. 2001;59:692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- 21.LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telm L, Frantz G, Rangell L, DeGuzman L, Keller GA, et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412:877–884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- 22.LeCouter J, Lin R, Tejada M, Frantz G, Peale F, Hillan KJ, Ferrara N. The endocrine-gland-derived VEGF homologue Bv8 promotes angiogenesis in the testis: Localization of Bv8 receptors to endothelial cells. Proc Natl Acad Sci USA. 2003;100:2685–2690. doi: 10.1073/pnas.0337667100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kisliouk T, Levy N, Hurwitz A, Meidan R. Presence and regulation of endocrine gland vascular endothelial growth factor/prokineticin-1 and its receptors in ovarian cells. J Clin Endocrinol Metab. 2003;88:3700–3707. doi: 10.1210/jc.2003-030492. [DOI] [PubMed] [Google Scholar]
- 24.Battersby S, Critchley HO, Morgan K, Millar RP, Jabbour HN. Expression and regulation of the prokineticins (endocrine gland-derived vascular endothelial growth factor and Bv8) and their receptors in the human endometrium across the menstrual cycle. J Clin Endocrinol Metab. 2004;89:2463–2469. doi: 10.1210/jc.2003-032012. [DOI] [PubMed] [Google Scholar]
- 25.Fraser HM, Bell J, Wilson H, Taylor PD, Morgan K, Anderson RA, Duncan WC. Localization and quantification of cyclic changes in the expression of endocrine gland vascular endothelial growth factor in the human corpus luteum. J Clin Endocrinol Metab. 2005;90:427–434. doi: 10.1210/jc.2004-0843. [DOI] [PubMed] [Google Scholar]
- 26.LeCouter J, Zlot C, Tejada M, Peale F, Ferrara N. Bv8 and endocrine gland-derived vascular endothelial growth factor stimulate hematopoiesis and hematopoietic cell mobilization. Proc Natl Acad Sci USA. 2004;101:16813–16818. doi: 10.1073/pnas.0407697101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorsch M, Qiu Y, Soler D, Frank N, Duong T, Good-earl A, O’Neil S, Lora J, Fraser CC. PK1/EG-VEGF induces monocyte differentiation and activation. J Leukoc Biol. 2005;78:426–434. doi: 10.1189/jlb.0205061. [DOI] [PubMed] [Google Scholar]
- 28.Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- 29.Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- 30.Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- 31.Negri L, Lattanzi R, Giannini E, De Felice M, Colucci A, Melchiorri P. Bv8, the amphibian homologue of the mammalian prokineticins, modulates ingestive behaviour in rats. Br J Pharmacol. 2004;142:181–191. doi: 10.1038/sj.bjp.0705686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Negri L, Lattanzi R, Giannini E, Metere A, Colucci M, Barra D, Kreil G, Melchiorri P. Nociceptive sensitization by the secretory protein Bv8. Br J Pharmacol. 2002;137:1147–1154. doi: 10.1038/sj.bjp.0704995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore RY. Circadian rhythms: basic neurobiology and clinical applications. Annu Rev Med. 1997;48:253–266. doi: 10.1146/annurev.med.48.1.253. [DOI] [PubMed] [Google Scholar]
- 34.Allada R, Emery P, Takahashi JS, Rosbash M. Stopping time: the genetics of fly and mouse circadian clocks. Annu Rev Neurosci. 2001;24:1091–1119. doi: 10.1146/annurev.neuro.24.1.1091. [DOI] [PubMed] [Google Scholar]
- 35.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 36.Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 37.Hastings MH. Circadian clockwork: two loops are better than one. Nat Rev Neurosci. 2000;1:143–146. doi: 10.1038/35039080. [DOI] [PubMed] [Google Scholar]
- 38.Cheng MY, Bittman EL, Hattar S, Zhou QY. Regulation of prokineticin 2 expression by light and the circadian clock. BMC Neurosci. 2005;6:17. doi: 10.1186/1471-2202-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 40.Reddy AB, Field MD, Maywood ES, Hastings MH. Differential resynchronisation of circadian clock gene expression within the suprachiasmatic nuclei of mice subjected to experimental jet lag. J Neurosci. 2002;22:7326–7330. doi: 10.1523/JNEUROSCI.22-17-07326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morton AJ, Wood NI, Hastings MH, Hurelbrink C, Barker RA, Maywood ES. Disintegration of the sleep-wake cycle and circadian timing in Huntington’s disease. J Neurosci. 2005;25:157–163. doi: 10.1523/JNEUROSCI.3842-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lambert CM, Machida KK, Smale L, Nunez AA, Weaver DR. Analysis of the Prokineticin 2 System in a Diurnal Rodent, the Unstriped Nile Grass Rat (Arvicanthis niloticus) J Biol Rhythms. 2005;20:206–218. doi: 10.1177/0748730405275135. [DOI] [PubMed] [Google Scholar]
- 43.Cheng MY, Leslie FM, Zhou QY. Expression of prokineticins and their receptors in the adult mouse brain. J Comp Neurol. doi: 10.1002/cne.21087. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodale MA, Foreman NP, Milner AD. Visual orientation in the rat: a dissociation of deficits following cortical and collicular lesions. Exp Brain Res. 1978;31:445–457. doi: 10.1007/BF00237301. [DOI] [PubMed] [Google Scholar]
- 45.Sahibzada N, Dean P, Redgrave P. Movements resembling orientation or avoidance elicited by electrical stimulation of the superior colliculus in rats. J Neurosci. 1986;6:723–733. doi: 10.1523/JNEUROSCI.06-03-00723.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller AM, Obermeyer WH, Behan M, Benca RM. The superior colliculus-pretectum mediates the direct effects of light on sleep. Proc Natl Acad Sci USA. 1998;95:8957–8962. doi: 10.1073/pnas.95.15.8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siegel JM. Narcolepsy: a key role for hypocretins (orexins) Cell. 1999;98:409–412. doi: 10.1016/s0092-8674(00)81969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salin-Pascual R, Gerashchenko D, Greco M, Blanco- Centurion C, Shiromani PJ. Hypothalamic regulation of sleep. Neuropsychopharmacology. 2001;25:S21–S27. doi: 10.1016/S0893-133X(01)00318-9. [DOI] [PubMed] [Google Scholar]
- 49.Gerashchenko D, Shiromani PJ. Different neuronal phenotypes in the lateral hypothalamus and their role in sleep and wakefulness. Mol Neurobiol. 2004;29:41–59. doi: 10.1385/MN:29:1:41. [DOI] [PubMed] [Google Scholar]
- 50.Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429– 458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- 51.Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomic and functional topography of the dorsal raphe nucleus. Ann N Y Acad Sci. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- 52.Zhao H, Rusak B. Circadian firing-rate rhythms and light responses of rat habenular nucleus neurons in vivo and in vitro. Neuroscience. 2005;132:519–528. doi: 10.1016/j.neuroscience.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 53.Semm P, Schneider T, Vollrath L. Morphological and electrophysiological evidence for habenular influence on the guinea-pig pineal gland. J Neural Transm. 1981;50:247–266. doi: 10.1007/BF01249146. [DOI] [PubMed] [Google Scholar]
- 54.Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- 55.Jones BE. Arousal systems. Front Biosci. 2003;8:S438–S451. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- 56.Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 57.Cottrell GT, Zhou QY, Ferguson AV. Prokineticin 2 modulates the excitability of subfornical organ neurons. J Neurosci. 2004;24:2375–2379. doi: 10.1523/JNEUROSCI.5187-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buijs RM. The anatomical basis for the expression of circadian rhythms: the efferent projections of the suprachiasmatic nucleus. Prog Brain Res. 1996;111:229–240. doi: 10.1016/s0079-6123(08)60411-2. [DOI] [PubMed] [Google Scholar]
- 59.Kalsbeek A, Buijs RM. Output pathways of the mammalian suprachiasmatic nucleus: coding circadian time by transmitter selection and specific targeting. Cell Tissue Res. 2002;309:109–118. doi: 10.1007/s00441-002-0577-0. [DOI] [PubMed] [Google Scholar]


