Abstract
The mRNA encoding a putative human enzyme named Epidermal Retinol Dehydrogenase 2 (RDH-E2) was found to be significantly elevated in psoriatic skin (Matsuzaka et al., BBRC 297 (2002) 1171–1180). This finding led the authors to propose that RDH-E2 may be involved in the pathogenesis of psoriasis through its potential role in retinoic acid biosynthesis and stimulation of keratinocyte proliferation. However, enzymatic activity for RDH-E2 has never been demonstrated. RDH-E2 is a member of the short-chain dehydrogenase/reductase (SDR) superfamily of proteins, and is most closely related to the group of SDRs comprised of both NAD+- and NADP+-dependent enzymes with activities toward retinoid and steroid substrates. In this study, we began the characterization of RDH-E2 protein in order to determine whether it might play a role in retinoic acid biosynthesis. The results of this study show that, similarly to other SDR-type retinol dehydrogenases, RDH-E2 appears to be associated with the membranes of endoplasmic reticulum. Furthermore, RDH-E2 expressed in Sf9 insect cells as a fusion to the C-terminal His6-tag and purified using Ni2+-affinity chromatography recognizes all-trans-retinol and all-trans-retinaldehyde as substrates and exhibits a strong preference for NAD+/NADH as cofactors. Specific activity of RDH-E2 toward all-trans-retinoids is much lower than that of other retinoid-active SDRs, such as human RoDH4 or RDH10. The preference for NAD+ suggests that RDH-E2 is likely to function in the oxidative direction in vivo, further supporting its potential role in the oxidation of retinol to retinaldehyde for retinoic acid biosynthesis in human keratinocytes.
Keywords: retinol, dehydrogenase, epidermal, human
1. Introduction
The cDNA for RDH-E2 was originally cloned by Matsuzaka et al. as a result of their search for novel psoriasis-associated genes [1]. The authors carried out microarray analysis of the gene expression in the affected and unaffected skin of patients with psoriasis, and the normal skin of healthy individuals, and identified several genes that displayed increased expression in psoriatic skin. One of these genes was found to encode a previously unrecognized member of the short-chain dehydrogenase/reductase (SDR) superfamily of proteins [1]. Based on its sequence similarity to retinal SDR2 (retSDR2, also known as pan1b, 17beta-HSD11) [2–4], the novel gene was named human epidermal retinol dehydrogenase 2 (RDH-E2) [1]. Real-time quantitative RT-PCR confirmed that the expression level of the RDH-E2 gene in psoriatic tissues was at least threefold higher than that in healthy skin [1].
Psoriasis vulgaris is a chronic autoimmune inflammatory skin disease characterized by hyperproliferation of keratinocytes [5]. Hyperproliferation of keratinocytes may be induced by retinoic acid [6], the activating ligand of nuclear transcription factors, retinoic acid receptors [7]. Therefore, the authors proposed that RDH-E2 may be involved in the pathogenesis of psoriasis through its potential role in retinoic acid biosynthesis. However, RDH-E2 protein has never been shown to possess an enzymatic activity and recognize retinoids as substrates. To determine whether RDH-E2 could play a role in retinoic acid biosynthesis, we initiated the characterization of its substrate and cofactor specificity. Here, we provide the first evidence that RDH-E2 might function as a retinol dehydrogenase.
2. Experimental procedures
2.1. Construction of expression vectors
A cDNA clone encoding RDH-E2 was obtained from the American Type Culture Collection (Manassas, VA, USA) (ATCC clone #7998610, IMAGE collection #5752917). For expression in eukaryotic cells, the full-length RDH-E2 cDNA in ATCC clone was PCR-amplified using forward primer 5′- AGC GAA TTC ATG TCT TTC AAC CTG CAA TCA T -3′ (EcoRI restriction site underlined) and reverse primer 5′- TCT CTC GAG CTT CTT CTT TTG GTC AAC -3′ (XhoI restriction site underlined). The PCR product was gel-purified, cleaved with restriction endonucleases EcoRI and XhoI, and cloned into the corresponding sites of eukaryotic expression vector pCMV-Tag4a in-frame with the C-terminal FLAG-tag. Fidelity of the expression construct was verified by sequencing.
For expression in insect Sf9 cells, RDH-E2 cDNA was PCR-amplified using primers 5′- AGC TCT AGA ATG TCT TTC AAC CTG CAA TCA T -3′ (XbaI restriction site underlined) and 5′- GTT GCG GCC GCG AGC TTC TTC TTT TGG TCA ACA -3′ (NotI restriction site underlined). The PCR product was gel-purified, cleaved with restriction endonucleases XbaI and NotI, and cloned into the corresponding sites of a modified baculovirus transfer vector pVL1393 described previously [8], in-frame with the C-terminal His6-tag. Recombinant baculovirus was produced by co-transfection of Sf9 cells with the transfer vector and the linearized Sapphire Baculovirus DNA (Orbigen Inc., San Diego, CA), according to the manufacturer’s instructions.
2.2. Analysis of recombinant RDH-E2 expressed in human cells
Human embryonic kidney 293 (HEK293) cells (American Type Culture Collection, Manassas, VA) were cultured in minimal essential medium containing 10% horse serum and penicillin/streptomycin at 37 °C with 5% CO2. Cells were plated in 35 mm dishes and transiently transfected with an expression construct for hRDH-E2 in pCMV-tag 4A vector containing FLAG-tag using Lipofectamine (Invitrogen, Carlsbad, CA) or with empty vector, according to the manufacturer’s protocol.
Twenty-four hours after transfection, RDH-E2-transfected and mock-transfected cells were analyzed for retinoid and steroid metabolism. For detection of retinoid activities, the cells were incubated with either 10 μM all-trans-retinaldehyde or 20 μM all-trans-retinol (Sigma-Aldrich) for 3 h or 24 h, respectively. Media and cells were collected under reduced light. Retinoids were extracted into hexane and separated by normal phase high performance liquid chromatography (HPLC) using Waters Alliance Separation Module and 2996 Photodiode Array Detector. Peaks were identified by comparison to retention times of retinoid standards and evaluation of wavelength maxima and quantified as described previously [9].
For analysis of steroid activities, HEK293 cells were seeded into 24-well plates. On the next day, cell culture medium in each well was replaced with 0.45 ml of fresh medium and steroids were added from the 10 μM BSA-solubilized stocks to the final concentration of 1 μM. The amount of radiolabeled steroids was 2 μCi per well. Incubations were carried out for 3 h. Steroids were extracted from the media as described previously [10]. Dried residue after extraction was dissolved in 40 μl of dichloromethane and loaded onto thin-layer chromatography (TLC) plates. The plates were developed in 80:20 toluene:acetone mobile phase, dried, and the distribution of radioactivity was analyzed by PhosphoImager after an overnight exposure of TLC plates containing 3H-labeled steroids to PhosphoImager tritium screen [10].
2.3. Expression of recombinant RDH-E2 in insect cells
Sf9 cells were infected with recombinant virus at a virus/cell ratio of 10:1 and incubated at 28 °C for 4 days. Sf9 cells were harvested by centrifugation at 5,000 g for 10 min and resuspended in 50 mM Tris-HCl, pH 7.4, supplemented with protease inhibitors aprotinin (2 μg/ml), leupeptin (2 μg/ml), pepstatin A (1 μg/ml), phenylmethylsulfonyl fluoride (PMSF) (5 mM), EDTA (0.1 mM), and dithiothreitol (1 mM). The cell suspension was homogenized using a French press mini-cell at 800 psi. PMSF was added immediately after homogenization. For analysis of RDH-E2 subcellular localization, unbroken cells and cellular debris were removed by centrifugation at 1000 g for 10 min. Nuclei and mitochondria were isolated by centrifugation at 3,000 g for 10 min and at 10,000 g for 35 min, respectively. Microsomes were pelleted by centrifugation at 105,000 g for 1.5 h and resuspended in 90 mM potassium phosphate, pH 7.4, 40 mM KCl plus 0.1 mM EDTA, 1 mM dithiothreitol, and 20% glycerol. Protein concentration was determined by Lowry using bovine serum albumin as a standard [11].
2.4. Purification of RDH-E2–His6
Sf9 cell homogenates obtained as described above were solubilized using 15 mM detergent 1,2-diheptanoyl-sn-glycero-3-phosphocholine (DHPC) (Avanti Polar Lipids, Alabaster, AL) for 30 min on ice with continuous vortexing followed by centrifugation at 105,000g for 1 h to remove insoluble material. Then, 5 mM imidazole was added to the soluble fraction, and the extract was incubated with Ni2+-NTA resin (Qiagen Inc.) in a batch mode for 30 min on ice according to the manufacturer’s instructions. The resin was washed with 120–150 bed volumes of buffer containing 50 mM potassium phosphate, 300 mM KCl, 10 mM imidazole, and 10% glycerol. RDH-E2–His6 was eluted with a stepwise gradient of 50–500 mM imidazole in the same buffer. Fractions were analyzed by electrophoresis in 12% polyacrylamide gel in the presence of sodium dodecyl sulfate (SDS-PAGE). To remove imidazole, the buffer in the eluate was exchanged for 90 mM potassium phosphate, pH 7.4, 40 mM KCl plus 10% glycerol using Centriplus concentrators (Millipore Corp., Billerica, MA).
2.5. HPLC Analysis of purified RDH-E2 activity
Catalytic activity of purified RDH-E2 toward retinoids was assayed in 90 mM potassium phosphate, pH 7.4, and 40 mM KCl (reaction buffer) at 37 °C in siliconized glass tubes. Stock solutions of all-trans-retinol or all-trans-retinaldehyde (Sigma-Aldrich, St. Louis, MO) were prepared in ethanol. Ethanol-dissolved retinoids were solubilized in the reaction buffer by 10-min sonication in the presence of equimolar delipidated bovine serum albumin. The 500-μl reactions were started by the addition of cofactor, carried out for 15 min at 37 °C, and terminated by the addition of 1 ml of cold methanol. Reaction products were extracted twice with 2 ml of hexane, and hexane was evaporated under nitrogen flow. Retinoids were dissolved in 200 μl of hexane. Samples were analyzed at 350 nm using a Waters 2996 Photodiode Array Detector. Peaks were identified and quantified as described previously [9].
2.6. Immunofluorescence analysis
HEK293 cells transfected with RDH-E2/pCMV-Tag4a (100,000 cells) were seeded in Lab-Tek II Chamber Slide System (Thermo Fisher Scientific, Rochester, NY). The slides were fixed in 4% paraformaldehyde for 10 min and then permeabilized with phosphate buffered saline (PBS) supplemented with 0.5% Triton X-100 for 10 min at room temperature. To stain for RDH-E2, the slides were incubated with monoclonal anti-FLAG M2 antibodies (Sigma, St. Louis, MO) diluted in goat serum (1:500) for 1 h at 37 °C. After incubation, the slides were washed three times for 5 min with PBS plus 1% Tween-20. For immunofluorescence assays, RDH-E2 was stained using Fluorescein (FITC)-conjugated AffiniPure Goat Anti-Mouse IgG (1:1,000 dilution, Jackson ImmunoResearch, West Grove, PA) at 37 °C for 1 h. After incubation, the slides were washed as described above. Nuclei were stained using 4′,6-diamidino-2-phenylindole (DAPI) for 3 min, quickly rinsed with the washing solution, air-dried, and mounted in Antifade (0.1 mM p-phenylenediamine in 100% glycerol; the pH was adjusted to 8.0 with a carbonate buffer containing 4 mM Na2CO3 and 46 mM NaHCO3).
For concanavalin A staining, 25 μg/ml concanavalin A diluted in PBS was added to the slide with RDH-E2-transfected HEK293 cells after permeabilization. Incubation was performed at 37 °C for 45 min. The slides were rinsed, and DAPI staining was followed as described above. All images were acquired using Olympus AX70 fluorescence microscope with a 100X objective lens and Speicher filters (Chroma, Rockingham, VT), and a Zeiss Axiocam digital camera (Thornwood, NY). Processing and assembly were accomplished with Photoshop (Adobe Systems, Mountain View, CA).
2.7. Western Blot Analysis
Protein samples were subjected to 12 % SDS-PAGE and transferred to Hybond-P membrane (Amersham Biosciences, Piscataway, NJ). The blot was incubated with anti-His monoclonal antibody (Clontech Mountain View, CA) at a 1:3,000 dilution or anti-FLAG polyclonal antibody (Sigma-Aldrich, St. Louis, MO) at a 1:1,250 dilution. RDH-E2 was visualized using the chemiluminescence detection system (Pierce, Rockford, IL).
3. Results
3.1. Expression and characterization of recombinant hRDH-E2 in human cells
To determine whether RDH-E2 is catalytically active, the recombinant protein was initially expressed in HEK293 cells as a fusion to the C-terminal FLAG tag. Analysis of the transfected cells by immunofluorescence revealed the presence of RDH-E2/FLAG in ~30% of the cells (Fig. 1). RDH-E2 protein exhibited a punctuate localization pattern, surrounding the nucleus (Fig. 1 B). This pattern was similar to that observed for cells stained with fluorescently labeled concanavalin A (Fig. 1 C), suggesting that RDH-E2 is associated with endoplasmic reticulum.
Figure 1. Immunofluorescence analysis of RDH-E2 expression.
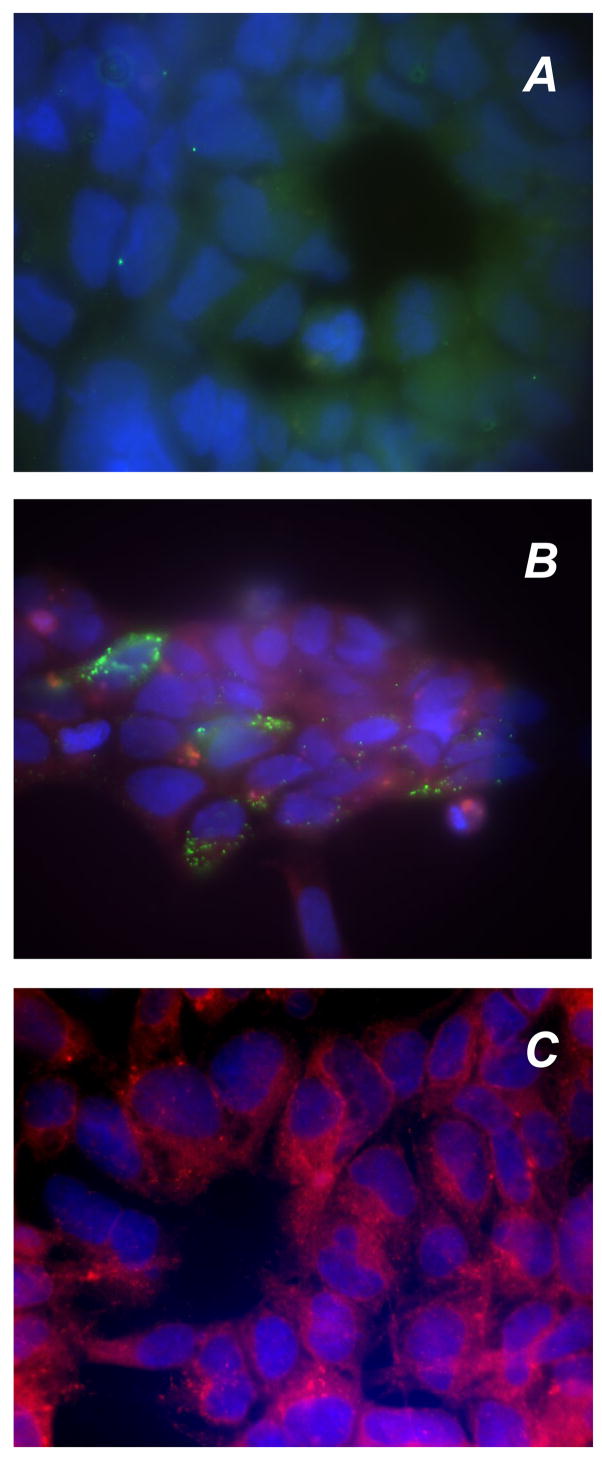
HEK293 cells were transfected with either empty vector (A) or RDH-E2 expression vector (B) and incubated with anti-FLAG antibodies. RDH-E2 expression was visualized using FITC-conjugated antibodies. Endoplasmic reticulum of HEK293 cells was visualized by staining with FITC-conjugated concanavalin A (C).
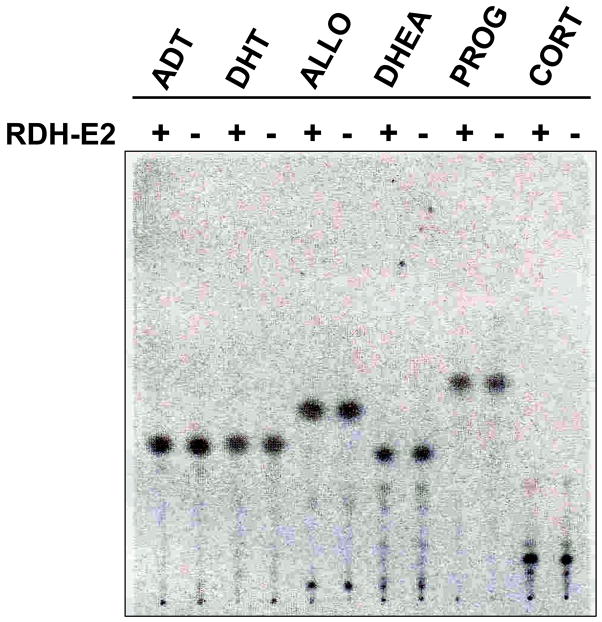
Having confirmed the expression of RDH-E2 in HEK293 cells, we analyzed the cells for activity toward steroid and retinoid substrates. RDH-E2–transfected and mock–transfected cells were incubated with tritiated 3α-hydroxy-5α-androstan-17-one (androsterone); 17β-hydroxy-5α-androstan-3-one (dihydrotestosterone); 3α-hydroxy-5α-pregnan-20-one (or allopregnanolone); 3β-hydroxy-5-androsten-17-one (dehydroepiandrosterone); 4-pregnen-3,20-dione (progesterone) and 4-pregnen-11β,21-diol-3,20-dione (corticosterone). In addition, 1,3,5(10)-estratrien-3,17β-diol (estradiol) was tested in a separate experiment (data not shown). Thin-layer chromatography of steroids extracted from the cells did not reveal any new forms of steroids (Fig. 2), indicating that there was no detectable enzymatic activity toward the functional groups in positions 3, 11, 17, 20, or 21 of the above steroids in either RDH-E2–transfected or mock–transfected cells after 3 hours of incubation. Similarly, there was no difference in the metabolism of all-trans-retinol or all-trans-retinaldehyde in RDH-E2–transfected cells versus mock–transfected cells (data not shown). These results suggested that either RDH-E2 was not active toward indicated retinoids and steroids or that the specific activity of RDH-E2 was very low. Western blot analysis using FLAG tag antibodies failed to detect RDH-E2 in the total cell lysate (data not shown), suggesting low levels of RDH-E2 protein. The low level of protein expression could be in part responsible for the lack of detectable RDH-E2 activity in the cells.
Figure 2. Assays of steroid oxidoreductive activities of RDH-E2.
HEK293 cells transfected with expression vector for RDH-E2 (+) or empty vector (−) were incubated with tritiated androsterone (ADT), dihydrotestosterone (DHT), allopregnanolone (ALLO), dehydroepiandrosterone (DHEA); progesterone (PROG), or corticosterone (CORT).
3.2. Expression of recombinant RDH-E2 in insect cells
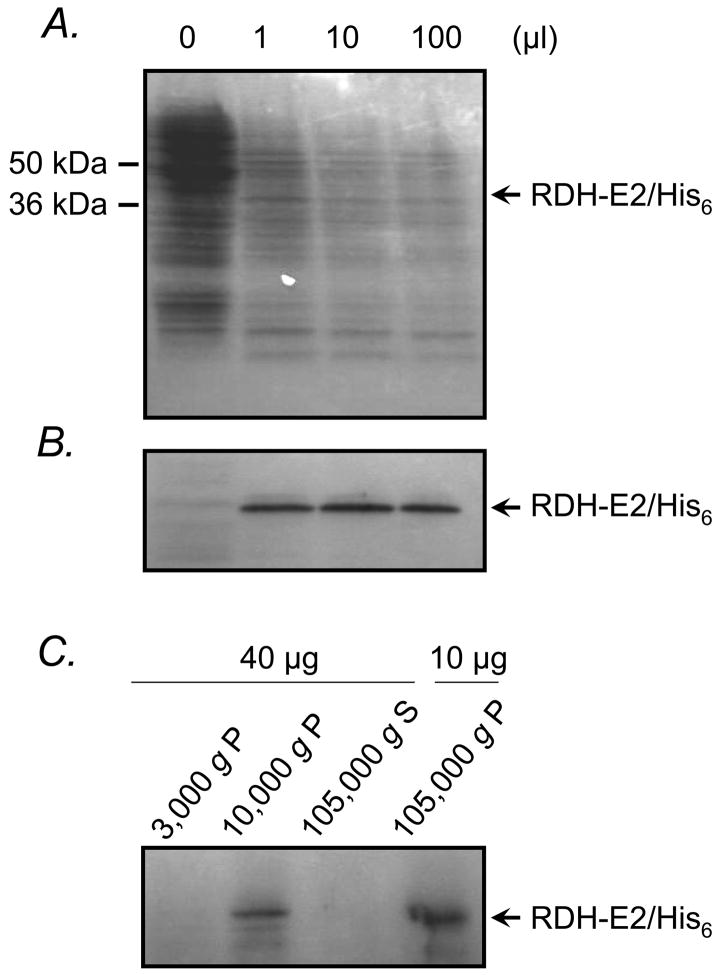
To obtain sufficient amount of purified RDH-E2 protein for in vitro activity assays, we constructed a vector encoding a His6-tagged RDH-E2 for expression using the baculovirus system. Titration of Sf9 cells with different doses of recombinant baculovirus resulted in the appearance of a single protein band of expected molecular mass that was not present in uninfected cells (Fig. 3 A). The identity of this protein band was confirmed by Western blot analysis using His-tag antibodies (Fig. 3 B). Subcellular localization of RDH-E2 in Sf9 cells was determined by Western blot analysis of cellular fractions obtained by differential centrifugation. Most of the RDH-E2 protein was associated with microsomal membranes although some amount of the protein was also detected in the mitochondrial fraction (Fig. 3 C). Coupled in vitro transcription/translation analysis of RDH-E2 in the presence or absence of microsomes did not reveal any post-transcriptional modifications of the protein (data not shown).
Figure 3. Expression and subcellular localization of RDH-E2 in Sf9 cells.
Sf9 cells (105) were titrated with recombinant baculovirus carrying the expression construct for RDH-E2/His6. SDS-PAGE analysis of cell lysates revealed the presence of a new protein band of expected molecular mass in baculovirus infected cells (A). This band was recognized by antibodies against His-tag (B). Fractionation of Sf9 cells by differential centrifugation followed by Western blot analysis showed that RDH-E2 was localized in the 105,000 g pellet and 10,000 g pellet fractions of the cells (C). P, pellet; S, supernatant.
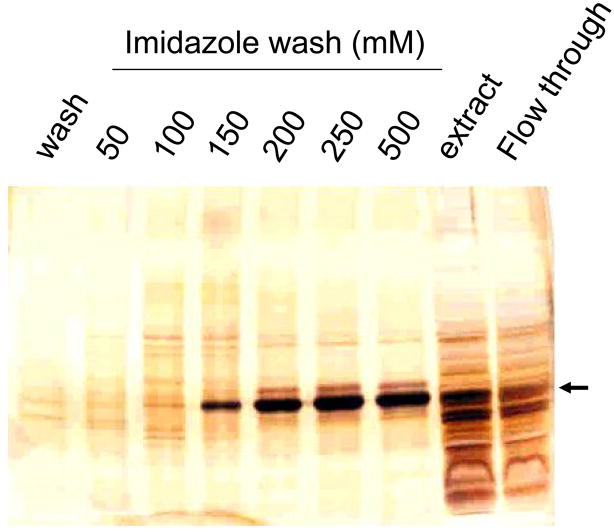
3.3. Purification and characterization of recombinant RDH-E2
RDH-E2 protein was purified using Ni2+-affinity chromatography (Fig. 4). The yield of the protein from nine T175 flasks of infected Sf9 cells was ~2500 μg. Purified RDH-E2 was assayed for activity toward all-trans-retinol and all-trans-retinaldehyde in the presence of NAD(P)+ or NAD(P)H. At least 40 micrograms of purified RDH-E2 were required to detect the activity of RDH-E2 toward retinoids. The enzyme was active in both the oxidative and reductive directions and exhibited a strong preference for non-phosphorylated nicotinamide dinucleotides as cofactors. With all-trans-retinol and NAD+, RDH-E2 activity was ~0.06 nmol·min−1mg−1. The activity toward all-trans-retinaldehyde in the presence of NADH was ~2-fold lower (Fig. 5 A). No activity was detected with 11-cis-retinol or 11-cis-retinaldehyde as substrates with either NAD+/NADH or NADP+/NADPH.
Figure 4. Purification of RDH-E2/His6.
The protein was purified using Ni2+-affinity chromatography. Fractions were analyzed by SDS-PAGE followed by silver staining.
Figure 5. Activity assays of purified RDH-E2/His6.
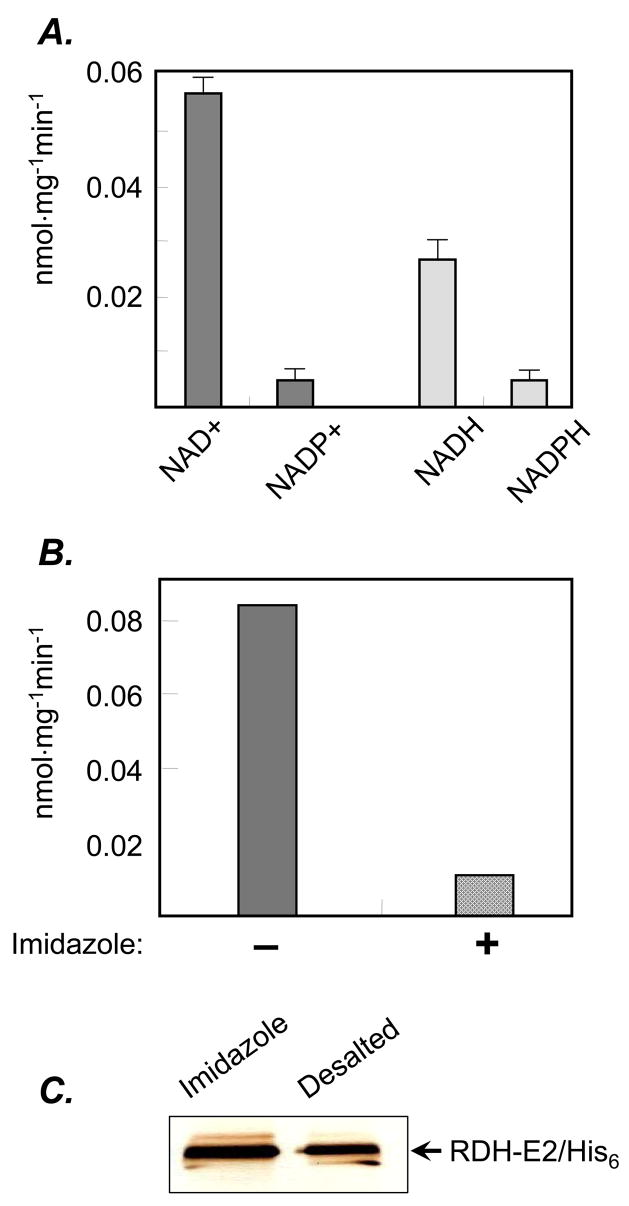
Forty to eighty micrograms of purified RDH-E2 were incubated with all-trans-retinol (dark grey) in the presence of NAD(P)+ or with all-trans-retinaldehyde (light grey) in the presence of NAD(P)H (A). The effect of additional imidazole on RDH-E2 was tested by comparing its activity toward all-trans-retinol in the absence (dark grey) or presence (patterned dark grey) of 200 mM imidazole added directly to the reaction mixture (B). RDH-E2/His6 fraction eluted with 250 mM imidazole off of the Ni2+-affinity resin was desalted using gel-filtration column. The protein was not lost as a result of desalting, as shown by SDS-PAGE (C), while the activity decreased significantly.
Increasing the amount of imidazole in the reaction mixture with purified RDH-E2 from 25 mM to 225 mM was found to significantly decrease RDH-E2 activity (Fig. 5 B), suggesting that imidazole was inhibitory. However, gel-filtration of RDH-E2 preparation to remove imidazole resulted in significant loss of activity (~90%) despite nearly complete recovery of the protein (Fig. 5 C), suggesting that RDH-E2 is sensitive to desalting. Thus, the low activity of purified RDH-E2 could be, at least in part, due to its inhibition by imidazole and sensitivity of the enzyme to buffer composition.
4. Discussion
The properties of RDH-E2 are of special interest because this protein may have a role in the progression of psoriatic skin disease [1, 12]. As shown in this study, RDH-E2 appears to be predominantly associated with the membranes of endoplasmic reticulum. According to various algorithms for prediction of transmembrane segments, at least three transmembrane helices can be detected in the amino acid sequence of RDH-E2, suggesting that it is an integral membrane protein. RDH-E2 is not glycosylated, consistent with the lack of N-glycosylation consensus motifs, and it does not undergo any post-translational modifications in the presence of microsomes.
Purified RDH-E2 possesses catalytic activity; furthermore, it is active toward all-trans-retinol and all-trans-retinaldehyde. However, RDH-E2 is much less active than other SDR-type microsomal retinoid oxidoreductases. For example, the activity of the recently characterized RDH10 can be estimated at 13 nmol·min−1·mg−1 based on the amount of recombinant protein in Sf9 microsomes and the activity of the microsomal RDH10 preparation [13]. This is two orders of magnitude greater than the activity of purified RDH-E2 determined in this study. However, as we have established, imidazole present in the elution buffer significantly inhibits RDH-E2 activity; thus, it is possible that a different expression and purification system that does not employ imidazole may produce a more active RDH-E2.
The preference of RDH-E2 for NAD+/NADH as cofactors is consistent with the current understanding of the structural determinants of SDR cofactor specificity [14]. As discussed in our previous manuscript [13], structure-function analysis of SDRs strongly suggests that their cofactor specificity is determined by the presence of aspartate residue in the βαβ motif at the beginning of the Rossmann fold. This residue directly contributes to NAD+ binding by forming two hydrogen bonds with the 2′ and 3′ hydroxyl groups of ribose. At the same time, aspartate takes up the space that would be occupied by the phosphoryl group of NADP+, thereby creating sterical hindrance and charge repulsion mechanism for the exclusion of NADP+. Alignment of the cofactor-binding motif of RDH-E2 with those of other NAD+-preferring SDRs clearly shows that the corresponding position in RDH-E2 is occupied by Asp-71 [13], indicating that RDH-E2 is an NAD(H)-preferring enzyme, in agreement with our experimental data. The preference of RDH-E2 for NAD(H) suggests that this enzyme will function in the oxidative direction in vivo, supporting its role in the oxidation of retinol to retinaldehyde for retinoic acid biosynthesis. However, the final determination of whether RDH-E2 contributes to retinoic acid biosynthesis in health or disease to any significant extent will require additional experimentation.
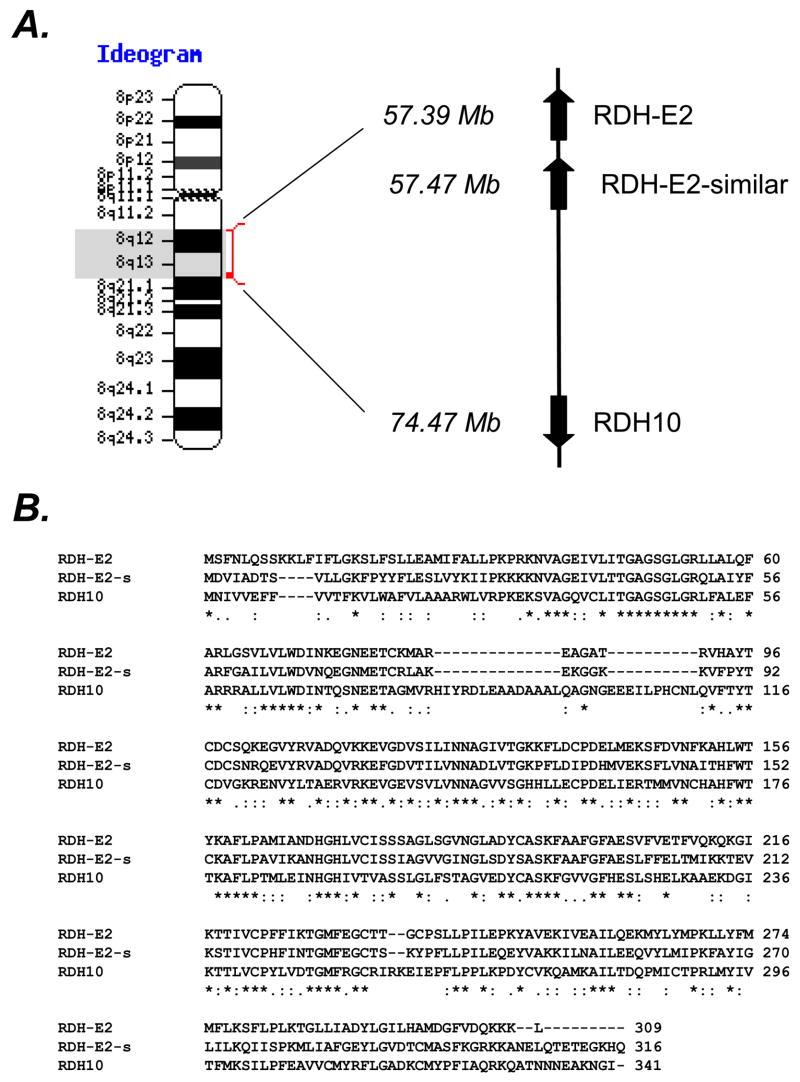
RDH-E2 shares the greatest sequence identity (~43%) with the only SDR (RDH10) that was proven to be physiologically relevant for retinoic acid biosynthesis in human cells and in mice embryos [13, 15]. Interestingly, the genes encoding RDH-E2 and RDH10 are located on the same chromosome 8 within 20 Mb (Fig. 6 A), potentially suggesting common evolutionary origins. Furthermore, right next to RDH-E2 gene is located a gene encoding an open reading frame of 316 amino acids with ~60% sequence identity to RDH-E2 (XP_498284). Transcripts for the mouse ortholog of RDH-E2–similar gene (NT_039258.7|Mm4_39298_37), which shares 78% sequence identity with the human RDH-E2–similar gene, are found in mouse tongue, skin, and embryonic tissue (http://www.ncbi.nlm.nih.gov/UniGene/ESTProfileViewer.cgi?uglist =Mm.298546), indicating that RDH-E2 homolog is not a pseudogene. Characterization of RDH-E2 homolog in addition to RDH-E2 might provide a better understanding of the substrates and physiological roles of these two related proteins.
Figure 6. Identification of RDH-E2-similar protein.
The gene encoding putative RDH-E2-similar (RDH-E2-s) protein was found to be localized next to RDH-E2 gene on chromosome 8 (A). Protein sequence alignment revealed that RDH-E2-similar is seven amino acids longer than RDH-E2 and has an Asp residue in the position (a.a. 67, bold) that determines cofactor specificity, suggesting a preference for NAD+ as a cofactor.
Acknowledgments
This work was supported by the National Institute on Alcohol Abuse and Alcoholism Grant AA12153.
Abbreviations
- RDH-E2
Epidermal Retinol Dehydrogenase 2
- SDR
short-chain dehydrogenase/reductase
- RoDH4
retinol dehydrogenase 4
- RDH10
retinol dehydrogenase 10
- DHPC
1,2-diheptanoyl-sn-glycero-3-phosphocholine
- DAPI
4′,6-diamidino-2-phenylindole
- FITC
fluorescein
Footnotes
Conflict of Interest statement. The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matsuzaka Y, Okamoto K, Tsuji H, Mabuchi T, Ozawa A, Tamiya G, Inoko H. Identification of the hRDH-E2 gene, a novel member of the SDR family, and its increased expression in psoriatic lesion. Biochem Biophys Res Commun. 2002;297:1171–1180. doi: 10.1016/s0006-291x(02)02344-6. [DOI] [PubMed] [Google Scholar]
- 2.Li KX, Smith RE, Krozowski ZS. Cloning and expression of a novel tissue specific 17beta-hydroxysteroid dehydrogenase. Endocr Res. 1998;24:663–667. doi: 10.3109/07435809809032667. [DOI] [PubMed] [Google Scholar]
- 3.Haeseleer F, Palczewski K. Short-chain dehydrogenases reductases in retina. Methods Enzymol. 2000;316:372–383. doi: 10.1016/s0076-6879(00)16736-9. [DOI] [PubMed] [Google Scholar]
- 4.Brereton P, Suzuki T, Sasano H, Li K, Duarte C, Obeyesekere V, Haeseleer F, Palczewski K, Smith I, Komesaroff P, Krozowski Z. Pan1b (17betaHSD11)-enzymatic activity and distribution in the lung. Mol Cell Endocrinol. 2001;171:111–117. doi: 10.1016/s0303-7207(00)00417-2. [DOI] [PubMed] [Google Scholar]
- 5.Stem RS. Psoriasis. Lancet. 1997;350:349–353. doi: 10.1016/S0140-6736(97)05257-4. [DOI] [PubMed] [Google Scholar]
- 6.Kang S, Duell EA, Fisher GJ, Datta SC, Wang ZQ, Reddy AP, Tavakkol A, Yi JY, Griffiths CEM, Elder JT, Voorhees JJ. Application of retinol to human skin in vivo induces epidermal hyperplasia and cellular retinoid binding proteins characteristic of retinoic acid but without measurable retinoic acid levels or irritation. J Invest Dermatol. 1995;105:549–556. doi: 10.1111/1523-1747.ep12323445. [DOI] [PubMed] [Google Scholar]
- 7.Saitou M, Sugai S, Tanaka T, Shimouchi K, Fuchs W, Narumiya S, Kakizuka A. Inhibition of skin development by targeted expression of a dominant-negative retinoic acid receptor. Nature. 1995;374:159–162. doi: 10.1038/374159a0. [DOI] [PubMed] [Google Scholar]
- 8.Belyaeva OV, Stetsenko AV, Nelson PS, Kedishvili NY. Properties of short-chain dehydrogenase/reductase RalR1: characterization of purified enzyme, its orientation in the microsomal membrane, and distribution in human tissues and cell lines. Biochemistry. 2003;42:14838–14845. doi: 10.1021/bi035288u. [DOI] [PubMed] [Google Scholar]
- 9.Belyaeva OV, Korkina OV, Stetsenko AV, Kim T, Nelson PS, Kedishvili NY. Biochemical properties of purified human retinol dehydrogenase 12 (RDH12): catalytic efficiency toward retinoids and C9 aldehydes and effects of cellular retinol-binding protein type I (CRBPI) and cellular retinaldehyde-binding protein (CRALBP) on the oxidation and reduction of retinoids. Biochemistry. 2005;44:7035–7047. doi: 10.1021/bi050226k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chetyrkin SV, Hu J, Gough WH, Dumaual N, Kedishvili NY. Further characterization of human microsomal 3alpha-hydroxysteroid dehydrogenase. Arch Biochem Biophys. 2001;386:1–10. doi: 10.1006/abbi.2000.2203. [DOI] [PubMed] [Google Scholar]
- 11.Lowry OH, Rosebrough NH, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 12.Matsuzaka Y, Okamoto K, Yoshikawa Y, Takaki A, Oka A, Mabuchi T, Iizuka M, Ozawa A, Tamiya G, Kulski JK, Inoko H. hRDH-E2 gene polymorphisms, variable transcriptional start sites, and psoriasis. Mamm Genome. 2004;15:668–675. doi: 10.1007/s00335-004-2349-5. [DOI] [PubMed] [Google Scholar]
- 13.Belyaeva OV, Johnson MP, Kedishvili NY. Kinetic analysis of human enzyme RDH10 defines the characteristics of a physiologically relevant retinol dehydrogenase. J Biol Chem. 2008;283:20299–20308. doi: 10.1074/jbc.M800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kallberg Y, Oppermann U, Jörnvall H, Persson B. Eur J Biochem. 2002;269:4409–4417. doi: 10.1046/j.1432-1033.2002.03130.x. [DOI] [PubMed] [Google Scholar]
- 15.Sandell LL, Sanderson BW, Moiseyev G, Johnson T, Mushegian A, Young K, Rey JP, Ma JX, Staehling-Hampton K, Trainor PA. Genes Dev. 2007;21:1113–1124. doi: 10.1101/gad.1533407. [DOI] [PMC free article] [PubMed] [Google Scholar]