Abstract
Purpose
Dysmenorrhea affects quality of life and contributes to absenteeism from school and work diminishing opportunities for successful psychosocial and cognitive development during adolescence. In adults, depression, anxiety, and smoking have an impact on menstrual cycles and dysmenorrhea. Associations between these potential problems have not been examined in adolescents. The purpose of this study was to examine relationships between depressive symptoms and anxiety with menstrual symptoms. Smoking was examined as a moderator of this relationship.
Methods
This study enrolled 154 post-menarcheal girls from a sample of 207 girls age 11, 13, 15, and 17 years [M = 15.4 years (± 1.9)]. Self-reported measures included the Menstrual Symptom Questionnaire (MSQ), Children’s Depression Inventory (CDI), State-Trait Anxiety Inventory, and smoking behavior. Generalized linear regression modeled MSQ outcomes separately for depressive symptoms and anxiety.
Results
More depressive symptoms/anxiety were related to higher numbers of menstrual symptoms (r = 0.23–0.44, p < .05). Smoking status (ever) was related to higher MSQ scores. Moderating effects of smoking and depressive symptoms or anxiety on menstrual symptoms were consistent across most MSQ factors where effects were stronger in never smokers.
Conclusion
This is the first study in adolescents showing smoking status and depressive symptoms/anxiety are related to menstrual symptoms and that the impact of depressive symptoms/anxiety on menstrual symptoms is stronger in never smokers. The dynamic and complex nature of smoking, moods, and dysmenorrhea cannot be disentangled without longitudinal analyses. Efforts to reduce menstrual symptoms should begin at a young gynecological age and include consideration of mood and smoking status.
Menstrual disorders have a major impact on health and societal costs worldwide. Dysmenorrhea specifically is a leading gynecologic complaint, resulting in a significant number of both work and school absences (1, 2). In fact, it is estimated that over 600 million hours are lost from work each year due to dysmenorrhea (3). In adolescents, absenteeism from school or work due to dysmenorrhea ranged from 14% (1) to 51% (4) of girls and decreased participation in school-related functions ranged from 29% to 50% (5). In those with severe dysmenorrhea, 50% missed school (1). Such absences diminish opportunities for successful educational, psychosocial, and cognitive development during the critical period of adolescent growth.
There is a dearth of studies on symptoms that adolescents experience during menstruation. Earlier literature reported that primary dysmenorrhea is less common in the first two to three years post-menarche. However, once ovulation begins and becomes established, dysmenorrhea becomes more common (1). Recent reports have narrowed the focus of menstrual symptoms to primarily premenstrual syndromes (6),(7),(8). Virtually absent are studies of symptoms that occur throughout menstruation (e.g., dysmenorrhea), particularly in adolescents. Emotional and behavior problems may exacerbate menstrual cycle problems and dysmenorrhea. For example in adults, depression and/or anxiety symptoms (9),(10),(11), or smoking (4),(12),(13),(14) are reported to have an impact on menstrual cycle function and dysmenorrhea. Specifically, the risk of dysmenorrhea was nearly twice as high in women smoking 10–30 cigarettes per day and this risk increased as the duration of smoking increased (14). There was also an increased amount of bleeding daily and an increased duration of dysmenorrhea in smokers (12). To our knowledge, examination of these factors in relation to menstrual symptoms in adolescents has received little attention. Since menarche occurs later in puberty and during the pubertal years there are increases in depressive symptoms and or anxiety,(15),(16) and smoking behavior,(12),(17) it is crucial to examine the association of these problems with menstrual symptoms when evaluating the etiology of menstrual problems. Thus, the primary purpose of this study was to: a) describe differences in self reported menstrual symptoms in adolescents in relation to smoking behavior, b) examine the relationship of depressive symptoms and anxiety with menstrual symptoms and, c) examine whether smoking moderates the relationship between depressive symptoms/anxiety and menstrual symptoms.
PARTICIPANTS AND METHODS
Our study enrolled 154 post-menarcheal girls who were drawn from a larger study of 207 adolescent girls in age cohorts of 11, 13, 15, and 17 years. Girls were enrolled into a smoking experience category based on a modified version of five defined levels(18) indicating smoking experience from “not even a puff” to daily smoking. The overall study was a cross-sequential design (19) which examined the relationship between psychological symptoms and smoking on reproductive and bone health of adolescent girls across three years. This paper focuses on cross sectional analyses at Time 1.
Recruitment was from an urban teen health center and the general community of a large Midwestern city. Exclusion criteria included: 1) pregnancy or breast feeding within 6 months, 2) primary amenorrhea (> 16 years), 3) secondary amenorrhea (< 6 cycles/year), 4) body mass index (BMI) < 5th percentile or weight > 300 pounds, 5) medication/medical disorder influencing bone health, and 6) psychological disabilities impairing comprehension or compliance. The study was approved by the Institutional Review Board of a medical center. Parents provided informed consent and adolescents provided assent.
Girls had a mean age of 15.4 years (± 1.9) and were Caucasian (55.3%), African American (40%) or mixed race/other (4.7%). Demographic and physical characteristics of the sample appear in Table 1. Girls enrolled in the study did not differ from those who were screened by questionnaire and not enrolled on the variables of race/ethnicity, smoking history, or physical activity level. Those who were not enrolled after the screen were either ineligible or chose not to participate. They were younger, had lower weight and BMI, and were taking fewer prescription medications than enrolled participants.
Table 1.
Descriptive statistics (frequencies, means ± standard deviations) for demographic, physical and behavioral characteristics of post-menarcheal girls
| Chronologic Age (yr) | 15.4 ± 1.9 |
| Age at Menarche (yr) | 12.2 ± 1.9 |
| Gynecological Age (yr) | 3.11+/− 1.9 |
| Race: Caucasian | 83 (55.3%) |
| African American | 60 (40.0%) |
| Other | 7 (4.7%) |
| Tanner Breast Stage: 3 | 3 (2.0%) |
| 4 | 16 (10.8%) |
| 5 | 129 (87.2%) |
| Regularly took medication for dysmenorrhea (Yes) | 56 (36.8%) |
| Took hormonal contraceptives in past 12 months (Yes) | 50 (32.5%) |
| Smoking Status: Ever | 91 (60.7%) |
| Never | 59 (39.3%) |
Note: the sample includes 154 postmenarcheal girls.
Numbers do not always add up indicating missing data of 1–6 cases.
Participants came to the General Clinic Research Center at an urban children’s hospital for their assessments. After consent and assent were obtained, a physical exam was conducted for Tanner staging for breast and pubic hair development by an adolescent medicine physician or nurse practitioner. Medication and menstrual histories were also obtained at this time. Blood samples were drawn and questionnaires were completed.
Girls completed the Menstrual Symptom Questionnaire (MSQ)(20) which includes 24 items that are rated as 1 = “never” to 5 = “always”. Previous research has found support for 2 factor and 5 factor solutions of the items. The 2 factors each have 12 items which are summed to provide a score on Spasmodic and Congestive dysmenorrhea. Items in the Spasmodic factor generally reflect symptoms occurring during menstruation not unlike spasms similar to labor pains while items in the Congestive Factor generally reflect symptoms or moods in the premenstrual phase (20), (21). The (possible) range of scores was 12–52 for both the Spasmodic factor and the Congestive factor. The two factor solution was used because the MSQ has not been validated with younger adolescents and although two factors have not been replicated in young adult samples they could very well provide useful distinctions by which to examine dysmenorrhea in adolescents. However, due to the lack of replication of the two factor solution other factor structures were considered. Overall there is not a consensus regarding a clear number of factors for the MSQ, thus we chose the factor solution which we deemed strongest. Although Webster (22) reported seven factors in their paper, two of the factors only contained two items leading to those factors being underidentified. This was recognized by Stephenson and colleagues (23) who also used only five of the seven factors Webster reported in their paper. This supported our decision to use the five psychometrically sound factors that Webster reported (22). The five factors were: menstrual pain, premenstrual negative affect, premenstrual water retention, premenstrual pain, and menstrual back pain. In the present sample the alpha reliabilities ranged from .68 to .90 for the MSQ factors. Both the two and five factor solutions were used in the analysis. In all cases a higher score indicates more symptoms.
Symptoms of depression were determined by the 27-item self-report Children’s Depression Inventory (CDI) (24). The range of possible scores was 0 to 54. Reliability was high at 0.90. Trait anxiety was determined by the State-Trait Anxiety Inventory (STAI) (25) for those age 12 and above or the child version for the age 11 year cohort (STAIC) (26). Trait anxiety included 20 items and is thought to represent more stable individual differences than state anxiety and it represents how the person generally feels (25). Trait scale items were summed and converted to T scores based on age distributions. Reliability coefficients were high (α = 0.86–0.91). T-scores with a mean of 50 and a standard deviation of 10 were used for both depression and anxiety scores.
Smoking behavior was determined by questionnaire and asked if girls had ever smoked tobacco. Response options included, “never” or one of four categories ranging from “even a puff or two” to daily smoking. Since our complete sample was not enrolled at the point of these analyses, frequency distributions of some of the various smoking categories were low and thus did not allow for a multilevel categorical variable of smoking. For example, the fourth category included 2% of the sample and the highest category had approximately 15%. For the current analyses smoking was defined as “ever” versus “never” at the first visit. Table 2 reports the descriptive statistics for key variables.
Table 2.
Means (± SD) of Menstrual Symptoms Questionnaire factors and depression and anxiety for all menarcheal girls and for “never” and “ever” smokers
| Total Group | Never (n = 58) | Ever (n = 91)** | |
|---|---|---|---|
| MSQ-Sum (24 items) | 51.8 ± 16.5 | 43.4 ± 15.5 | 56.9 ± 15.2 |
| MSQ – C (12 items) | 24.6 ± 8.3 | 20.4 ± 7.3 | 27.3 ± 8.0 |
| MSQ – S (12 items) | 26.8 ± 8.7 | 22.5 ± 8.1 | 29.5 ± 8.2 |
| F1: Menstrual Pain (7 items) | 17.7 ± 6.3 | 15.2 ± 6.6 | 19.0 ± 5.7 |
| F2: Premenstrual Negative Affect (4 items) | 9.1 ± 3.5 | 7.6 ± 3.1 | 10.0 ± 3.4 |
| F3: Premenstrual Water Retention (5 items) | 9.7 ± 3.9 | 7.9 ± 2.9 | 10.9 ± 4.0 |
| F4: Premenstrual Pain (5 items) | 10.9 ± 4.6 | 9.4 ± 4.3 | 11.0 ± 4.5 |
| F5: Menstrual Back Pain (2 items) | 3.9 ± 2.2 | 2.9 ± 1.7 | 4.5 ± 2.3 |
| CDI – T-Score | 47.0 ± 11.6 | 42.2 ± 8.8 | 50.3 ± 12.1 |
| Trait Anxiety – T-Score | 48.3 ± 10.0 | 43.4 ± 7.8 | 51.2 ± 10.1 |
Note: There were significant (p < .01) group differences (Never vs. Ever) for smoking behavior on all variables. Standard Deviation (SD); Menstrual Symptom Questionnaire (MSQ); Factor (F); Congestive (C); Spasmodic (S). A high score = more symptoms for the MSQ. Children’s Depression Inventory (CDI); T-Score = mean of 50 and SD of 10
The covariates considered in our statistical analyses were: a) gynecological age computed from the date of the visit and self-report of age at menarche; b) breast stage; c) self-report of hormonal contraceptive use and/or d) use of medication for menstrual discomfort (prescription or over-the-counter) as ascertained by asking “Do you take any medications to help how you feel during your period?”.
The internal consistency of the MSQ factors (both 2 factor and 5 factor models) were examined using Cronbach’s alpha. Student T-test and correlation analyses were used to examine the association between MSQ factor scores and the smoking, depressive and anxiety symptoms, as well as a set of selected covariates [socioeconomic status (SES), chronological age, gynecological age, race, pubertal status via physical exam, medications for dysmenorrhea, and hormonal contraceptive use]. The covariates were selected based on the current literature. For example, gynecological age (1) and use of medications taken to relieve dysmenorrhea (27) and hormonal contraceptives have all been reported to be negatively associated with dysmenorrhea; age, SES, and race, although demographic variables, are routinely controlled for in clinical studies and have biological plausibility for associations with anxiety/depression, smoking, and menstrual symptoms. Linear regression analyses were used to evaluate the effects of smoking, depressive symptoms, anxiety, and their interactions (moderating effects), after adjusting for covariates for each of the MSQ factor scores. Multivariate regression analyses then were used to examine the overall effect on MSQ factor scores. Analyses were conducted separately for depressive symptoms and trait anxiety, in order to examine their independent relationship to menstrual symptoms.
RESULTS
Descriptive statistics on the MSQ factor scores, depressive symptoms and anxiety are reported for the total sample and then stratified by smoking status in Table 2. Student T-tests suggest that compared to never smokers, girls who were ever smokers had significantly higher MSQ factor scores, depressive symptoms and trait anxiety scores. Ever smokers had significantly higher (p < .01) MSQ sum and MSQ Spasmodic scores than never-smokers, and remained significant after adjusting for covariates and depressive or anxiety symptoms. However, differences between smoking groups in MSQ Congestive scores became non-significant after adjusting for covariates and depressive and anxiety symptoms. Multivariate analysis with the two-factor model and the MSQ sum showed that smoking had significant effects in both models: for depressive symptoms: F=4.74 (2,118), p ≤ .01 and for trait anxiety F=3.66 (2,118), p ≤ .05.
Correlation analyses between the MSQ factor scores and depressive symptoms and trait anxiety suggested that there was a significant positive relationship in most cases (p ≤ .05). (See Table 3). More reported menstrual symptoms were related to higher depressive symptoms and higher trait anxiety.
Table 3.
Correlations between the Menstrual Symptom Questionnaire (MSQ) Factors and depressive symptoms and anxiety in post-menarcheal girls
| Menstrual Symptom Factor Scores | CDI | Trait Anxiety |
|---|---|---|
| F1: Menstrual Pain | .29** | .28** |
| F2: Premenstrual Negative Affect | .44*** | .46*** |
| F3: Premenstrual Water Retention | .27** | .23* |
| F4: Premenstrual Pain | .34*** | .28** |
| F5: Menstrual Back Pain | .30** | .27** |
| MSQ-Congestive | .37*** | .37*** |
| MSQ-Spasmodic | .32** | .27*** |
Note: CDI = Children’s Depression Inventory; F = Menstrual Symptom Questionnaire Factor;
p ≤ .05;
p ≤ .01;
p ≤ .001
Moderating effects of smoking on the relationship between depressive symptoms/anxiety and menstrual symptoms: Testing three models
MSQ Sum Score
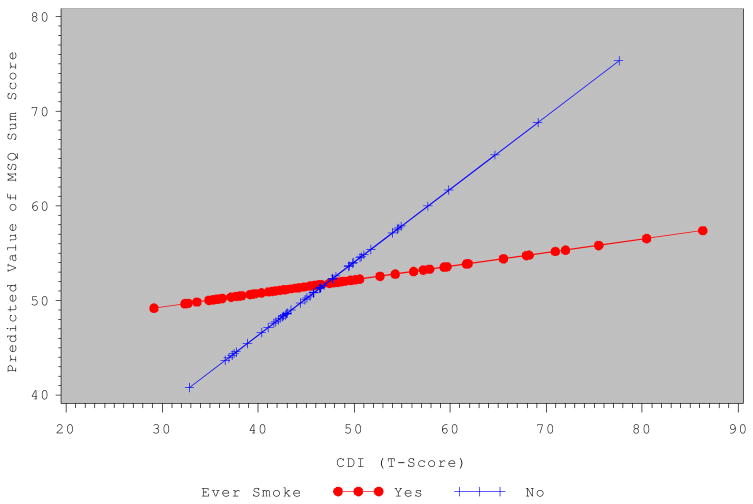
To determine whether smoking moderates the relationship between depressive symptoms/anxiety and menstrual symptom factor scores, linear regression analyses were conducted. For the MSQ sum score, ever smokers had significantly higher scores than never-smokers, and remained significant after adjusting for covariates and depressive symptoms, or trait anxiety. In addition, there was a significant depressive symptoms by smoking interaction effect (Beta = 0.52; p ≤ .05). Figure 1 shows the predicted value of the MSQ sum scores by the CDI T-scores for the ever smoker vs. never smoker groups, where the effect of depressive symptoms on the MSQ sum was stronger in the never-smoker compared to the ever smoker group. Similarly, for the trait anxiety model the anxiety by smoking interaction was significant where the MSQ sum was stronger in the never-smoker compared to the ever smoker group (Beta = 0.59, p ≤ .05).
Figure 1.
Predicted value of the Menstrual Symptom Questionnaire (MSQ) Sum Score by Children’s Depression Inventory (CDI) T-Score for the Ever Smoker group (Yes) and the Never Smoker Group (No). T-scores can be interpreted as a mean of 50 and standard deviation of 10. Analyses controlled for a) gynecological age, b) pubertal stage-breast, c) self-report of hormone contraceptive use, and d) whether medications were taken to relieve menstrual discomfort.
The two factor model for the MSQ
For the two factor model, no significant depressive symptoms by smoking interaction or trait anxiety by smoking interaction effects were found for the MSQ-Congestive factor. For the MSQ-Spasmodic factor, ever smokers had significantly higher scores than never-smokers, and that difference remained significant after adjusting for covariates and depressive or trait anxiety symptoms. In addition, a significant depressive symptoms by smoking interaction (Beta = 0.35; p ≤ .01) effect, as well as trait anxiety by smoking (Beta = 0.37; p ≤ .05) interaction effect was found. Post hoc tests showed that the effect of depressive symptoms and trait anxiety on menstrual symptoms was stronger in the never smoker compared to the ever smoker group.
The five factor model for the MSQ
In the MSQ-5 Factor model there was a significant interaction effect of smoking status by depressive symptoms for Factor 1 (menstrual pain) (Beta = 0.22; p ≤ .05) and Factor 4 (pre-menstrual pain) (Beta = 0.17, p ≤ .05). The effect of depressive symptoms on Factor 1 and 4 was stronger in the never smoker group compared with the ever smoker group. Similarly, there was a significant interaction of smoking status by trait anxiety for Factor 1 (Beta = 0.23; p ≤ .05) where the effect of trait anxiety on Factor 1 was stronger in the never smoker group. (Figures of all significant interactions described above are similar to Figure 1). No other significant interactions were noted on any of the other factors for depressive symptoms or trait anxiety by smoking status.
DISCUSSION
In spite of the significant health and economic consequences of dysmenorrhea in adolescent girls, studies of menstrual symptoms and mood variations in adolescents seldom appear in the recent literature. Premenstrual syndrome or premenstrual dysphoric disorder primarily is the focus in today’s literature when considering emotions and problem behaviors,,(6),(7),. Past studies have demonstrated associations between negative emotions and dysmenorrhea (9),(10),(11), and between smoking and dysmenorrhea. (4),(12),(13),(14) However, to our knowledge, this is the first study in adolescents to examine depressive and anxiety symptoms as well as smoking behavior, and their relationship to menstrual symptoms.
The first aim was to describe self reported characteristics of adolescent menstrual symptoms in relation to smoking behavior. The findings document adolescents who smoke also report having more menstrual symptoms. These results held even after controlling for key covariates. Similarly, the Australian Longitudinal Study on Women’s Health showed that 18–23 year old current smokers and ex-smokers had an increased risk of menstrual symptoms compared to non-smokers (13). Importantly, menstrual symptoms increased in those with a younger age onset of smoking and as cigarette consumption increased. In other studies, smokers had a longer duration of dysmenorrhea,(12) and smoking increased the risk of dysmenorrhea, which increased as cigarette consumption became higher (14). Smokers also reported more pain with menses or pain that required medication or time off from work than nonsmokers (28). Only two studies were found in younger adolescents where dysmenorrhea was positively associated with smoking (29),(30) whereas smoking was negatively correlated with dysmenorrhea in 19 year olds (31). Given our findings that adolescent girls who smoke had more menstrual symptoms and the earlier findings of menstrual problems in adult women who smoke, one may want to consider the possibility that smoking may influence adolescent menstrual cycles. Smoking cessation efforts that inform adolescents of this possible relationship may contribute to less smoking in adolescents. Future research will need to confirm such findings in a longitudinal fashion and to examine causation.
The second and third aims of the study were to establish the relation between depressive and anxiety symptoms and menstrual symptoms and if the relation was moderated by smoking. Our study is the first to report that smoking status and depressive symptoms/anxiety are associated with menstrual symptoms in a positive direction and that the impact of depressive symptoms and anxiety is stronger in those who have not had smoking experience. In previous studies depression and anxiety in college age women were positively associated with total menstrual pain using the modified MSQ. Our correlations in the total sample support these previous findings. The fact that both depression and anxiety show a significant relationship with the MSQ may be due to the fact that the two affective variables may have overlapping or associated features.
One novel aspect of our findings is that depressive symptoms and anxiety were more strongly related to higher numbers of menstrual symptoms in the never smoker group than in the ever smoker group of adolescents. This finding initially is counterintuitive. Using Figure 1 it appears that the predicted MSQ Sum scores are relatively equal across the range of depressive symptom scores for the ever smoker group. It may be that this group self-medicates with smoking or other substances as well as pain medication. For example, some individuals smoke to reduce depressive symptoms (32). The girls in the smoker group were also older and may have had easier access to medications for dysmenorrhea at home or at school. Smokers also were more likely to use hormonal contraceptives (27.7% vs. 3.8% of oral contraceptive use; p ≤ .0001; 49.5% vs. 8.5% of any hormonal contraceptives in the past 12 months, p <.0001) which may improve dysmenorrhea as reported in some studies (23), but not others (22). We did however, control for medication use and hormonal contraceptive use in the statistical model. An alternative hypothesis is that dysmenorrhea may be less severe in smokers, as shown by relative stability of dysmenorrhea across depressive symptoms, due to decreased prostaglandin synthesis (33). This however, cannot be tested in our study. Prostaglandins primarily are responsible for symptoms associated with primary dysmenorrhea and substances found in cigarettes (i.e., nicotine, acrolein) may antagonize prostaglandin synthesis.
Additionally, Figure 1 shows a wider discrepancy in menstrual symptoms by smoking group at the higher end of the depressive symptom distribution. It may be that depressive symptoms in the never smoker group, magnify the intensity of menstrual symptoms (i.e., depression drives menstrual symptoms) whereas low levels of depressive symptoms have no impact on menstrual symptoms allowing smoking to have a greater impact on menstrual symptoms (smoking status drives menstrual symptoms). Longitudinal analyses will be needed to test this hypothesis. In brief, our results show that both depressive symptoms and trait anxiety are significantly correlated with all MSQ factors in a positive direction. Further, there was consistency in the interactions of depressive symptoms and/or anxiety with smoking with the MSQ-sum as well as the MSQ-Spasmodic and Factor 1 (menstrual pain) factors. Significant associations were seen with all MSQ factors with depressive symptoms and anxiety and moderating effects of smoking were seen with many of the factors. However, not all literature supports the use of the two factor model of the MSQ. Future research will need to validate previously defined factor solutions and examine whether other factors exist for the MSQ in this younger age group. Our study examined the same factors that were reported in the literature in order to compare our younger adolescent sample with the results from the standard sample of college age and older women.
An additional reason that some of our findings are contrary to the literature may be related to the variable reflecting smoking behavior. First, although smoking status in studies similar to the current study frequently use a self-report variable, the variable may contain a margin of error. However, in our study girls were asked their smoking history at the screen and then again at their first visit. The kappa coefficient of these two time points was very high (k = .89). The time interval between the two interviews ranged from just a few days to as long as several weeks, thus “disagreement” could also reflect a transition to a higher stage of smoking. If that is the case, our kappa coefficient underestimates agreement. Other studies have shown high reliability in 11 and 12 year olds. Second, dichotomizing smoking status also may limit understanding the full impact of smoking on menstrual symptoms in this age. However, by using a dichotomous variable we minimized the possibility of misclassification of smoking status since any thing more than a puff or two was categorized as “ever smoker”.
Our findings are strengthened by the fact that the study includes a more normative sample of girls with respect to dysmenorrhea; that is girls were not enrolled based on their dysmenorrhea status. We had a range of menstrual symptoms reported but fewer at the high end of severe dysmenorrhea. Earlier studies only enrolled girls who reported dysmenorrhea (20). Our study also controlled for potentially important confounding variables such as gynecological age, pubertal status, hormonal contraceptive use, and use of medications for dysmenorrhea. Additionally, the sample is relatively large and includes a significant proportion of minorities. However, since our minority population is primarily African American the study cannot be generalized to other minority populations including Hispanics.
This study provides a step towards further understanding menstrual symptoms in adolescent girls and the association that smoking behavior and depressive symptoms or anxiety may have in this academic and socially costly problem. The mechanisms no doubt are complex and dynamic thus calling for more complex models in longitudinal analyses. For example, there is literature that supports that smoking is a risk for future depression,(34),(35) and that smoking in adolescence is associated with anxiety (36),(37). But the causal direction is not known (38). Other studies show that high depressive symptoms in adolescent non-smokers was not predictive of future smoking,(35) or opposing findings (39). Similarly, the literature on the association of smoking and dysmenorrhea is not always in a consistent direction (31) and thus differences must be considered.
Knowing that both smoking and mood are associated with menstrual symptoms has clinical relevance to those providing care for adolescents. Previous research has demonstrated adolescent smokers have thought about or tried quitting (40). Health care providers may specifically counsel the adolescent female about the mental and functional benefits of smoking cessation, which may be more applicable to their daily life than discussion about future risks of lung cancer and coronary artery disease (atherosclerosis). Earlier research has stressed that more immediate risks from smoking, such as menstrual symptoms, (compared to chronic or long term effects of smoking) may improve the relevance of anti-smoking campaigns focusing on this younger age group (13). Finally, the findings indicate that the relation between depression or anxiety and menstrual pain begins early in the post-menarcheal years. Therefore, it may be important for providers to give special attention to dysmenorrhea in young adolescents who have depressive symptoms or anxiety or who smoke, as well. In turn, quality of life may be improved and there may be fewer days of school or work absence.
In conclusion, this study shows that moods and smoking are associated with menstrual problems even in young girls. Our finding that girls who have depressive or anxiety symptoms and who smoke may have lower menstrual distress should be accompanied by a note of caution given that the study does not establish causality. Further, the dynamic and complex nature of smoking, moods, and dysmenorrhea cannot be disentangled in a cross-sectional study. Future studies will need to focus on more detailed reports of smoking and the longitudinal changes that occur in the relationships examined in this study. Overall, the findings indicate that efforts to prevent or reduce menstrual symptoms should begin at a younger gynecological age than previously anticipated and that mood and smoking status should be considered.
Acknowledgments
Financial support was from a grant received by the first author (R01DA16402). The study was also supported by USPHS GCRC Grant #M01 RR 08084 from the National Center for Research Resources, NIH. No other support was received by any of the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klein JR, Litt IF. Epidemiology of Adolescent Dysmenorrhea. Pediatrics. 1981;68(5):661–4. [PubMed] [Google Scholar]
- 2.Hillen TI, Grbavac SL, Johnston PJ, Straton JA, Keogh JM. Primary dysmenorrhea in young Western Australian women: prevalence, impact, and knowledge of treatment. Journal of Adolescent Health. 1999;25(1):40–5. doi: 10.1016/s1054-139x(98)00147-5. [DOI] [PubMed] [Google Scholar]
- 3.Dawood MY. Dysmenorrhea. The Journal of Reproductive Medicine. 1985;30(3):154–67. [PubMed] [Google Scholar]
- 4.Sundell G, Milsom I, Andersch B. Factors influencing the prevalence and severity of dysmenorrhoea in young women. British Journal of Obstetrics and Gynecology. 1990;97:588–94. doi: 10.1111/j.1471-0528.1990.tb02545.x. [DOI] [PubMed] [Google Scholar]
- 5.Banikarim C, Chacko MR, Kelder SH. Prevalence and impact of dysmenorrhea on Hispanic female adolescents. Archives of Pediatric Adolescent Medicine. 2000;154(12):1226–9. doi: 10.1001/archpedi.154.12.1226. [DOI] [PubMed] [Google Scholar]
- 6.Endicott J. History, evolution, and diagnosis of premenstrual dysphoric disorder. J Clin Psychiatry. 2000;61(12):5–8. [PubMed] [Google Scholar]
- 7.Ross LE, Steiner M. A biopsychosocial approach to premenstrual dysphoric disorder. Psychiatric Clinics of North America. 2003;26(3):529–46. doi: 10.1016/s0193-953x(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 8.Vichnin M, Freeman EW, Lin H, Hillman J, Bui S. Premenstrual syndrome (PMS) in adolescents: severity and impairment. Journal of Pediatric and Adolescent Gynecology. 2006;19(6):397–402. doi: 10.1016/j.jpag.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Harlow BL, Lee CS, Otto MW, Spiegelman D, Cramer DW. Early life menstrual characteristics and pregnancy experiences among women with and without major depression: the Harvard study of moods and cycles. Journal of Affective Disorders. 2004;79:167–76. doi: 10.1016/S0165-0327(02)00459-7. [DOI] [PubMed] [Google Scholar]
- 10.Strine TW, Chapman DP. Menstrual-Related Problems and Psychological Distress among Women in the United States. J Women’s Health. 2005;14(4):316–23. doi: 10.1089/jwh.2005.14.316. [DOI] [PubMed] [Google Scholar]
- 11.Sigmon ST, Dorhofer DM, Rohan KJ, Boulard NE. The impact of anxiety sensitivity, bodily expectations, and cultural beliefs on menstrual symptom reporting: a test of the menstrual reactivity hypothesis. Journal of Anxiety Disorders. 2000;14(6):615–33. doi: 10.1016/s0887-6185(00)00054-2. [DOI] [PubMed] [Google Scholar]
- 12.Hornsby PP, Wilcox AJ, Weinberg CR. Cigarette smoking and disturbance of menstrual function. Epidemiology. 1998;9(2):193–8. [PubMed] [Google Scholar]
- 13.Mishra GD, Dobson AJ, Schofield MJ. Cigarette smoking, menstrual symptoms and miscarriage among young women. Aust NZJ. 2000;24(4):413–20. doi: 10.1111/j.1467-842x.2000.tb01604.x. [DOI] [PubMed] [Google Scholar]
- 14.Parazzini F, Tozzi L, Mezzopane R, Luchini L, Marchini M, Fedele L. Cigarette smoking, alcohol consumption, and risk of primary dysmenorrhea. Epidemiology. 1994;5(number4):469–72. doi: 10.1097/00001648-199407000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Patton GC, Hibbert M, Rosier MJ, Carlin JB, Caust J, Bowes G. Is Smoking Associated With Depression and Anxiety in Teenagers? Am J of Public Health. 1996;86(number2):225–30. doi: 10.2105/ajph.86.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and Development of Psychiatric Disorders in Childhood and Adolescence. Archives of General Psychiatry. 2003;60:837–44. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- 17.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national results on adolescent drug use: Overview of key findings, 2006. Bethesda, MD: National Institute on Drug Abuse; 2007. (NIH Publication No. 07–6202) [Google Scholar]
- 18.Mayhew KP, Flay BR, Mott JA. Stages in the Development of Adolescent Smoking. Drug and Alcohol Dependence. 2000;59(Suppl 1):S61–S81. doi: 10.1016/s0376-8716(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki Y, Raudenbush SW. Tests for linkage of multiple cohorts in an accelerated longitudinal design. Psychological Methods. 2000;5(1):44–63. doi: 10.1037/1082-989x.5.1.44. [DOI] [PubMed] [Google Scholar]
- 20.Chesney MA, Tasto DL. The Development Of The Menstrual Symptom Questionnaire. Behavior Research & Therapy. 1975;13:237–44. doi: 10.1016/0005-7967(75)90028-5. [DOI] [PubMed] [Google Scholar]
- 21.Dalton K. The Menstrual Cycle. Pantheon Books; New York, NY: 1969. [Google Scholar]
- 22.Webster SK, Martin HG, Uchalik D, Gannon L. The Menstrual Symptom Questionnaire and Spasmodic/Congestive Dysmenorrhea: Measurement of an Invalid Construct. Journal of Behavioral Medicine. 1979;2(1):1–19. doi: 10.1007/BF00846559. [DOI] [PubMed] [Google Scholar]
- 23.Stephenson LA, Denney DR, Aberger EW. Factor structure of the Menstrual Symptom Questionnaire: relationship to oral contraceptives, neuroticism and life stress. Behaviour Research and Therapy. 1983;21(2):129–35. doi: 10.1016/0005-7967(83)90157-2. [DOI] [PubMed] [Google Scholar]
- 24.Kovacs M. Children’s Depression Inventory (CDI) Manual. North Tonawanda, NY: Multi-Health Systems, Inc.; 1992. [Google Scholar]
- 25.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Consulting Psychologists Press, Inc.; 1983. [Google Scholar]
- 26.Spielberger CD, Edwards CD, Lushene RE, Montuori J, Platzek D. STAIC Preliminary Manual. Palo Alto, CA: Consulting Psychologists Press; 1973. [Google Scholar]
- 27.Marjoribanks J, Proctor ML, Farquhar C. Nonsteroidal anti-inflammatory drugs for primary dysmenorrhoea. Cochrane database of systematic reviews (Online) 2003;(4):CD001751. doi: 10.1002/14651858.CD001751. [DOI] [PubMed] [Google Scholar]
- 28.Kritz-Silverstein D, Wingard DL, Garland FC. The Association of Behavior and Lifestyle Factors with Menstrual Symptoms. Journal of Women’s Health & Gender-Based Medicine. 1999;8(9):1185–93. doi: 10.1089/jwh.1.1999.8.1185. [DOI] [PubMed] [Google Scholar]
- 29.Teperi J, Rimpela M. Menstrual Pain, Health and Behaviour in Girls. Social Sciences Medicine. 1989;29(2):163–9. doi: 10.1016/0277-9536(89)90164-0. [DOI] [PubMed] [Google Scholar]
- 30.Charlton A, While D. Smoking and menstrual problems in 16-year-olds. JRSM. 1996;89(4):193–5. doi: 10.1177/014107689608900405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersch B, Milsom I. An epidemiologic study of young women with dysmenorrhea. American Journal of Obstetrics and Gynecology. 1982;144(6):655–60. doi: 10.1016/0002-9378(82)90433-1. [DOI] [PubMed] [Google Scholar]
- 32.Kendler KS, Neale MC, Maclean CJ, Heath AC, Eaves LJ, Kessler RC. Smoking and major depression: A causal analysis. Archives of General Psychiatry. 1993;50(1):36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- 33.Backon J. Negative correlation of cigarette smoking and dysmenorrhea: reduced prostaglandin synthesis due to beta-endorphin, nicotine, or acrolein antagonism. Med Hypo. 1989;28(3):213–41. doi: 10.1016/0306-9877(89)90054-6. [DOI] [PubMed] [Google Scholar]
- 34.Breslau N, Novak SP, Kessler RC. Daily smoking and the subsequent onset of psychiatric disorders. Psychology Medicine. 2004;34(2):323–33. doi: 10.1017/s0033291703008869. [DOI] [PubMed] [Google Scholar]
- 35.Goodman E, Capitman J. Depressive symptoms and cigarette smoking among teens. Pediatrics. 2000;106(4):748–55. doi: 10.1542/peds.106.4.748. [DOI] [PubMed] [Google Scholar]
- 36.Brook JS, Cohen P, Brook DW. Longitudinal study of co-occurring psychiatric disorders and substance use. J Am Acad Child Adolesc Psychiatry. 1998;37:322–30. doi: 10.1097/00004583-199803000-00018. [DOI] [PubMed] [Google Scholar]
- 37.Johnson JG, Cohen P, Pine DS, Klein DF, Kasen S, Brook JS. Association Between Cigarette Smoking and Anxiety Disorders During Adolescence and Early Adulthood. JAMA. 2000;284(18):2348–51. doi: 10.1001/jama.284.18.2348. [DOI] [PubMed] [Google Scholar]
- 38.Morissette SB, Tull MT, Gulliver SB, Kamholz BW, Zimering RT. Anxiety, anxiety disorders, tobacco use, and nicotine: a critical review of interrelationships. Psychological Bulletin. 2007;133(2):245–72. doi: 10.1037/0033-2909.133.2.245. [DOI] [PubMed] [Google Scholar]
- 39.Covey LS, Tam D. Depressive mood, the single-parent home, and adolescent cigarette smoking. Am J of Public Health. 1990;80(11):1330–3. doi: 10.2105/ajph.80.11.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.U.S. Department of Health and Human Services. Preventing tobacco use among young people: A report of the Surgeon General. Atlanta, Georgia: U.S. Dept. of HHS, CDC, NCCDPHP, Office on Smoking and Health; 1994. [Google Scholar]