Abstract
Early theories of species diversity proposed that communities at equilibrium are saturated with species. However, experiments in plant communities suggest that many communities are unsaturated and species richness can be increased by adding propagules of new species. We experimentally tested for community saturation and measured the effects of propagule supply on community structure in a benthic marine system. We manipulated propagule supply (arrival of individuals of numerous species) of mobile grazers in experimental mesocosms over multiple generations and, unlike previous tests, we examined the cascading effects of propagule supply on prey (macroalgae) biomass. We found little evidence for saturation, despite the absence of processes such as disturbance and predation that are thought to alleviate saturation in nature. Increasing propagule supply increased the total number of species and made rare species more abundant. Perhaps surprisingly, given the strong effect of propagule supply on species richness, supply-related changes in body size and composition suggest that competitive interactions remained important. Grazer supply also had strong cascading effects on primary production, possibly because of dietary complementarity modified by territorial behavior. Our results indicate that propagule supply can directly influence the diversity and composition of communities of mobile animals. Furthermore, the supply of consumer propagules can have strong indirect effects on prey and fundamental ecosystem properties.
Keywords: biodiversity, propagule limitation, saturation
Elton (1) argued >50 years ago that “the number of different kinds of animals that can live together in an area of uniform type rapidly reaches a saturation point.” Just as saturated liquids contain as much solute as can be dissolved without precipitation, saturated communities are thought to include the maximum number of species that coexist without local extinction. Classical niche theory invokes resource partitioning and interspecific competition as processes that act in concert to keep species richness at a saturation point (2). Under this niche-based definition, resources are underutilized in unsaturated communities, allowing new species to colonize and persist until resident species monopolize all available resources. Theory predicts that when species attempt to colonize an already-saturated community, there is an unsustainable amount of overlap in resource use; inferior competitors will be driven extinct, returning the community to the saturation point. Thus defined, species diversity at saturation is the stable equilibrium point to which communities are naturally attracted. Whether or not communities are likely to ever reach saturation remains an unresolved, yet fundamental ecological question (1, 3–5) with direct consequences for our understanding of important phenomena, such as species invasions and climate-driven range expansions (6).
A community is predicted to be saturated given sufficient homogeneity of resources in space and time (4) and in the absence of external sources of mortality that weaken competition, e.g., disturbance or predation (7). Even under these stringent conditions, a community will be open to colonization at equilibrium if it is isolated from propagules of novel species (8). A propagule is the ecologically relevant unit of dispersal, defined as a colonizing organism or vegetative structure capable of establishing a self-sustaining population. Depending on a species' life history, a propagule can be a pregnant female, a mating pair, seeds, or spores. Propagule limitation occurs if species that could coexist in a locality are absent because propagules do not arrive at that locality in sufficient numbers, resulting in unsaturated communities (9).
Community saturation can be tested directly by increasing propagule supply experimentally, i.e., by increasing or decreasing the number of potential colonists arriving at suitable habitat (10). Because supply is augmented and propagule limitation is relaxed, a subsequent increase in richness indicates that the local community was not saturated. In contrast, failure to colonize or the competitive displacement of resident species indicates that richness was not limited by propagule supply and the community may have been saturated. Propagule-addition experiments in terrestrial plant communities suggest that propagule limitation is widespread and many communities are naturally unsaturated (10–13), although most relevant research has been focused at the population level (14, 15). Populations below carrying capacity can respond to increased propagule supply with increases in population size. Thus, population-level “saturation” occurs when supplying additional propagules does not increase a species' abundance, whereas community-level saturation requires that species richness remains the same when propagules are added.
Virtually all community-level propagule addition experiments have focused on terrestrial plants (refs. 11–13, 16, and 17 but see ref. 18), thus the generality of these propagule supply experiments to other trophic levels or other systems remains unclear. However, decades of research have demonstrated that propagule supply structures many marine populations (19–23) and can influence composition of marine communities (24). Research on rocky shores suggests that propagule supply correlates with changes in community structure and mediates interspecific interactions strength (25–27) but covarying changes in environmental conditions make inference about community saturation impossible. Despite empirical evidence that propagule supply influences marine community structure and theoretical analyses suggesting that propagule supply may determine diversity in some marine communities (9, 28, 29), the potential for saturation at the community level remains to be tested experimentally in a marine system.
When propagule supply determines diversity and species' relative abundances, effects of supply are likely to propagate through the ecosystem. Experimental changes in diversity have predictable effects on ecosystem-level properties such as resource-use efficiency and total community biomass (30). Therefore, supply-driven changes in diversity could also influence ecosystem functioning. Evidence from plant communities supports this prediction; increasing propagule supply increased abundance, percentage cover (13, 16), and biomass (31) in manipulated communities. Yet, it remains unclear how alleviating propagule limitation among consumers will affect lower trophic levels and ecosystem properties. Theoretical evidence suggests that plant biomass is strongly influenced by the rate of herbivore propagule supply and that the outcome of plant–herbivore interactions depends on relative supply rates (32). Increasing rates of propagule supply could increase rates of consumption via at least 3 mechanisms: (i) by increasing the probability that a highly efficient grazer will establish a population (a sampling effect), (ii) by increasing grazer complementary resource use via increases in species richness (33), or (iii) through facilitative interactions (34).
We manipulated the propagule supply of mobile marine mesograzers (35) in experimental communities to test for propagule limitation and local saturation of species richness and to measure the effects of propagule supply on trophic interactions and ecosystem properties. Specifically, we tested (i) whether species richness was saturated in a model community of mobile marine grazers, (ii) if saturation depended on a persistent source of propagules, and (iii) whether grazer propagule supply has cascading effects on functioning at lower trophic levels. We used communities of mobile grazers consisting primarily of crustaceans and mollusks feeding on a combination of macroalgae, microalgae, and algal detritus (36) that disperse as juveniles and adults via drifting and rafting. Our experiment integrates several research questions by examining fundamental constraints on local species richness in a model multitrophic system open to immigration and emigration. Spatial models have revealed that recurrent immigration can affect the stability of population sizes and consumer–prey interactions (37, 38), suggesting that a persistent supply of grazer propagules may have different effects than a single addition of propagules. We addressed this idea by concurrently manipulating the magnitude and frequency of propagule supply to the mesograzer community.
Results
Supply Effects on Diversity and Composition.
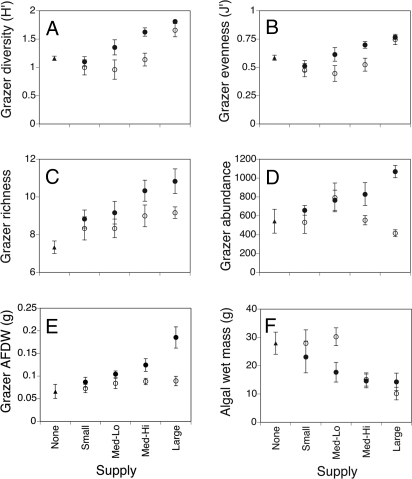
Final grazer diversity depended on the volume of propagules added (hereafter, magnitude) and whether or not additions occurred once or recurrently (frequency). Increasing the magnitude of propagule supply had a strong positive effect on grazer Shannon–Weiner diversity (Fig. 1A) explaining nearly 40% of observed variation (Table 1). In protected post hoc comparisons, communities receiving the greatest number of propagules had significantly higher diversity than all other treatments (P < 0.05 for each of the following: large vs. medium-high, large vs. medium-low, large vs. small). Grazer diversity was also higher in treatments receiving propagule additions weekly versus a single time, although the additional frequency effect was weaker than the effect of magnitude (Table 1). Interestingly, the lack of significant interaction term indicates that the positive effects of supply magnitude did not depend on a continuous supply of propagules. The effect of propagule supply on diversity was caused by concurrent increases in both the representation of less abundant species (i.e., greater evenness; Fig. 1B) and species richness (Fig. 1C). Communities receiving multiple propagule additions had more individuals than those receiving a single addition and this effect was greatest in high-magnitude treatments (Fig. 1D and Table 1).
Fig. 1.
Effects of propagule suppy on grazer community structure and ecosystem properties (means ± SE). Magnitude treatments are indicated along x axis. Frequency treatments are indicated by: ●, multiple; ○, single. Controls in which supply was not augmented are indicated by ▴. (A) Grazer Shannon–Weiner diversity. (B) Grazer Pielou's coefficient of evenness. (C) Grazer species richness. (D) Total abundance of grazer individuals. (E) Grazer ash-free dry weight (AFDW). (F) Algal wet mass.
Table 1.
Analysis of variance results
| Response | Effect | df | F | P value | ɷ2 |
|---|---|---|---|---|---|
| Grazer diversity | Supply size | 3,40 | 13.9 | <0.0001 | 39.2 |
| Supply frequency | 1,40 | 12.2 | <0.01 | 11.3 | |
| Size × frequency | 3,40 | 1.31 | NS | ||
| Grazer richness | Supply size | 3,40 | 3.2 | <0.05 | 11.1 |
| Supply frequency | 1,40 | 7.9 | <0.01 | 11.6 | |
| Size × frequency | 3,40 | 0.5 | NS | ||
| Grazer evenness | Supply size | 3,40 | 10.4 | <0.0001 | 35.7 |
| Supply frequency | 1,40 | 7.6 | <0.01 | 6.7 | |
| Size × frequency | 3,40 | 1.3 | NS | ||
| Grazer abundance | Supply size | 3,40 | 1.4 | NS | |
| Supply frequency | 1,40 | 14.5 | <0.001 | 12.0 | |
| Size × frequency | 3,40 | 4.7 | <0.01 | 8.3 | |
| Algal biomass | Supply size | 3,40 | 6.8 | <0.001 | 25.5 |
| Supply frequency | 1,40 | 1.8 | NS | ||
| Size × frequency | 3,40 | 2.0 | NS | ||
| Grazer biomass | Supply size | 3,40 | 7.48 | <0.001 | 19.8 |
| Supply frequency | 1,40 | 21.7 | <0.0001 | 21.0 | |
| Size × frequency | 3,40 | 4.37 | <0.01 | 10.3 |
Effects of propagule supply magnitude and frequency on grazer Shannon–Weiner diversity, species richness, Pielou's evenness, abundance, and biomass, and algal production. NS, not significant.
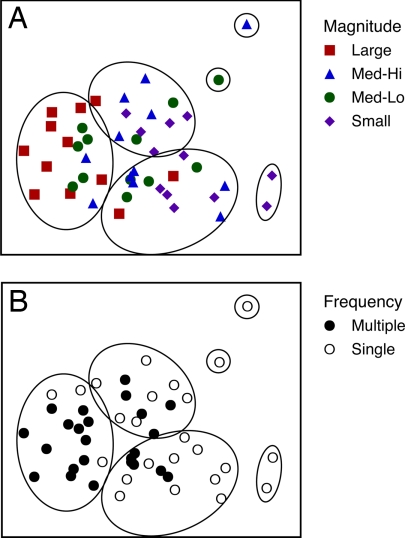
Multivariate analysis of similarities (ANOSIM) in species composition among treatments indicated that supply also influenced species identity and relative abundances (ANOSIM: magnitude, global R = 0.25, P < 0.001; frequency, global R = 0.35, P < 0.001; Fig. 2). Composition in the smallest-magnitude treatments differed from composition in medium–high and large supply treatments (protected posthoc comparisons; small vs. medium–high: R = 0.33, P < 0.003; small vs. large: R = 0.64, P = 0.001). These results were reinforced by hierarchical cluster analysis; at 80% similarity, communities receiving the least propagules formed groups distinct from the majority of communities receiving the greatest volume of propagules (Fig. 2A). These composition differences among supply treatments were driven by rare species (i.e., those with abundance <10% of total community abundance).
Fig. 2.
Nonmetric ordination of experimental communities based on species identity and abundance. Ordination derived from MDS analysis of Bray–Curtis similarities (stress = 0.2). Samples enclosed within circles have >80% similarity in composition (see Methods). (A) Communities coded by supply magnitude treatment. (B) Communities coded by supply frequency treatment.
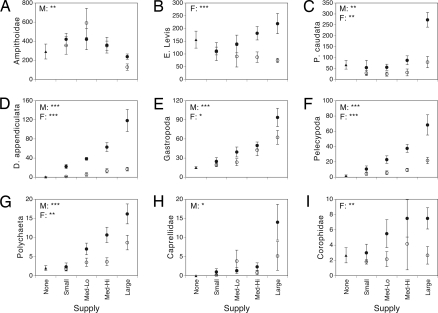
In addition to overall differences in grazer community composition, propagule supply had significant population-level effects. Nine species were absent in no-addition control communities despite the large number of individuals present in that treatment, indicating strong effects of propagule limitation on species composition (Fig. 1C). Other abundant species tended to increase in population size as the magnitude and frequency of propagule supply increased (Fig. 3). In contrast, 2 rare species, Jassa falcata and Microprotopus raneyi, were absent only in large supply treatments. The most abundant taxon, ampithoid amphipods, was significantly less abundant in large-magnitude supply treatments than in all other propagule magnitude treatments (Fig. 3 and Table S1; Tukey's HSD, P < 0.05). This reduction in abundance had no significant effect on total ampithoid biomass (magnitude: F3,16 = 0.008, P > 0.99; frequency: F1,16 = 0.0008, P > 0.97; magnitude × frequency: F3,16 = 1.06, P > 0.39) because increasing the magnitude of propagule supply significantly increased the proportion of large-bodied ampithoids (ANOSIM: magnitude, global R = 0.20, P < 0.05; Fig. S2). However, supply did not significantly affect body size of 3 other abundant species, Dulichiella appendiculata, Elasmopus levis, or Paracerceis caudata. There was a marginally significant positive effect of supply magnitude on the proportion of gravid (i.e., egg-bearing) ampithoids (magnitude: F3,40 = 2.5, P = 0.07; frequency: F1,40 = 0.004, P > 0.95; magnitude × frequency: F3,40 = 0.52, P > 0.65).
Fig. 3.
Effect of propagule supply on individual species' abundances (means ± SE). Results from 2-factor ANOVA (factors = magnitude and frequency; n = 6) performed on log-transformed abundances indicated by asterisks (*, P < 0.05; **, P < 0.01; **, P < 0.001). Only significant tests are shown. There were no significant magnitude × frequency interactions. Magnitude treatments are indicated along x axis. Frequency treatments are indicated by ●, multiple; ○, single. Controls in which supply was not augmented are indicated by ▴.
Ecosystem Properties.
Grazer propagule supply affected ecosystem properties at multiple trophic levels. Adding grazer propagules increased the final biomass of grazers, especially in those treatments receiving the largest and most frequent additions (Fig. 1E and Table 1). Grazer supply also had cascading effects on primary production via changes in grazing intensity (Fig. 1F). Final macroalgal biomass decreased with increasing magnitude of propagule supply; however, frequency of additions had no significant effect (Table 1).
Discussion
Despite conditions that favored competitive exclusion, we found no evidence for saturation of grazer species richness or diversity; both strongly depended on the magnitude and frequency of propagule supply, indicating propagule limitation at the community level. For species richness and diversity, comparison of communities receiving propagules at different frequencies (i.e., single vs. multiple) suggests that our communities were open to new species throughout our experiment. The effect of recurrent propagule additions remained constant and positive across the gradient of diversity represented in the single treatments (SI Text), as indicated by the lack of a significant interaction between supply magnitude and frequency treatments (Fig. 1 A and B). In other words, initially high supply magnitude did not affect invasibility by subsequently added propagules. In contrast, increased frequency of propagule additions had a disproportionate effect on grazer abundance and biomass in communities receiving the greatest magnitude of propagule supply (Fig. 1 D and F), possibly because of the stabilizing effects of recurrent immigration on population dynamics at high immigration rates (37). These results demonstrate that the abundance and species richness of this experimental grazer community were propagule-limited.
Relieving propagule limitation caused significant changes in identity and relative abundances of grazer species. Similar to studies in terrestrial plant communities, changes in community composition were largely driven by the absence of rare species in the no-addition controls (16). In our communities, 4 rare species (each <10% total abundance) were absent in control and small-magnitude supply treatments either because they were not strongly represented in randomly drawn additions or because colonization rates might not have exceeded rates of stochastic extinction. Of these, 3 species, Melita dentata, Gammarus mucronatus, and Lembos smithi, were also absent in field samples (see Methods), suggesting that they are locally rare. Interestingly, results from our multidimensional scaling (MDS) analysis (Fig. 2) suggest that whether propagule limitation is relieved by an increase in the number propagules arriving or by more frequent arrivals the impact on community composition is essentially the same.
By altering grazer species richness, composition, and abundance, grazer propagule supply indirectly affected primary production (Fig. 1E). Algal standing stock declined with the magnitude of grazer propagule supply, but was not affected by supply frequency. Several mechanisms might explain the effects of grazer propagule supply on algal biomass. It could be caused by increased per-capita consumption driven by a shift from small- to large-bodied individuals (Fig. S2); however, supply only affected the body size of ampithoid amphipods and not the 3 other most abundant species whose abundance decreased with increasing supply (Fig. 3). Alternatively, grazer assemblages may have consumed more algae in high-supply treatments because these assemblages were more diverse. Negative effects of consumer diversity on prey or resource abundances are well-documented (30, 33, 39, 40) in experimental manipulations of species identity and richness. Despite strong effects of frequency on total grazer abundance and richness (Fig. 1 C and D), supply frequency did not affect algal biomass in the largest magnitude treatments. We speculate that this may be caused by antagonistic interactions among grazers that limit algal consumption. An important group of grazers, ampithoid amphipods, construct and inhabit tubes that provide substrate for epiphytic algae. In the largest-magnitude treatments, our results suggest that there was strong intraspecific competition among ampithoids (see next section). Because of territorial behavior and interference competition, grazers may not have had access to algae growing on tubes, preventing algal biomass from dropping below ≈10 g of wet mass (Fig. 1F). Based on these results, we surmise that mobile grazer behavior may modify positive effects of species richness on resource use efficiency.
Propagule Limitation in Mobile Grazers.
Methodologically, we gave every opportunity for competitive exclusion to occur; the communities persisted for multiple generations in an environment free of disturbance and predation. Still, our communities were unsaturated with species. There are at least 3 possible explanations. First, there may not have been enough time for species to be driven extinct by competitive exclusion. In other words, we may be observing oversaturated communities that have not yet reached equilibrium. However, if time increased the probability of saturation, we would have expected to see that the effects of supply on diversity and richness were weaker in communities receiving a single addition (i.e., communities with the greatest time between propagule additions and the end of the experiment). This was not the case; supply effects did not vary significantly with the frequency of propagule additions (nonsignificant magnitude × frequency interactions; see Results). The behavior and life histories of mobile mesograzers also suggest that our study was of appropriate duration. Dispersal occurs rapidly and extensively in this guild; studies of mesograzer colonization observed a daily turnover rate of 30% of resident individuals in natural seagrass habitats (41). High mobility, combined with an experimental design that allowed emigration from mesocoms (and thus allowed both exploitative and interference competition to occur) suggest that competitive dynamics should occur rapidly in this system (42).
A second possibility is that mobility and habitat selection behavior among these grazers increases the likelihood of propagule limitation because species leave suboptimal habitats that could nevertheless support viable populations. Stream insects have been observed to abandon habitats and enter the water column in association with poor food quality (43); this behavioral response to resource limitation could weaken competition and prevent saturation. Ampithoid amphipods, the numerically dominant taxa in all treatments, were least abundant in communities receiving the most propagules (Fig. 3A). As population sizes decreased, there was a concurrent shift to larger-bodied individuals (Fig. S2) such that total ampithoid biomass did not vary with propagule supply. This result may be caused by self-thinning via density-dependent mortality, as observed in sessile plant populations (44); however, it is more likely that shifts in abundance and size structure are driven by emigration to avoid strong intraspecific competition for food or tube-building space. As propagule supply increased there was also a trend toward greater representation of gravid ampithoid females (15.2% gravid in small-magnitude treatments vs. 21.4% gravid in large-magnitude treatments, P > 0.07). Previous work suggests that juvenile and small adult amphipods are more likely to abandon habitats than mature adults because smaller individuals are poor competitors (45). Together, our results suggest that increasing propagule supply may drive strong intraspecific competition and emigration from areas with unfavorable resources rather than interspecific competition that could lead to competitive exclusion and saturation.
A third possibility is that natural variability in propagule supply on short time scales keeps these communities unsaturated. In marine systems, natural variability in propagule supply can have dramatic impacts on resident communities (19–21, 23). However, experimental tests have focused predominantly on population-level effects of propagule limitation at a particular life stage (i.e., recruitment or settlement). Further, among marine and terrestrial experiments there is usually a strong seasonal component to dispersal (e.g., when seeds or larvae are produced) and the period of dispersal is short relative to species generation times. Unlike plants and sessile marine species, for which dispersal is a single, predictable event in an individual's life, our experimental organisms may disperse multiple times, multiple dispersal events occur per generation, and there are several generations per season (46). Because propagules are arriving on time scales similar to (or even shorter than) those of demographic processes, propagule supply strongly influences local community dynamics (47). In other words, the equilibrial dynamics necessary for saturation to occur may be prevented in a community with near-constant dispersal. As a consequence, we would expect to see similar unsaturated patterns in other systems where demographic rates are comparable to dispersal rates.
It is important to recognize that in an unsaturated community not all species will be able to colonize successfully. Stochastic niche theory, which integrates the effects of propagule supply into a niche-based model of community assembly, predicts that the majority of propagules reaching a community will ultimately fail to produce viable populations because of demographic stochasticity (48). Several rare species found in samples of our propagule additions were not found in any of our experimental communities. These results are consistent with the only other work manipulating propagule supply in a community of mobile animals, in which communities were not saturated but several added species were unable to invade (18). Alternatively, novel colonists in propagule additions may exclude some resident species but still increase overall species diversity if the number of successful species is greater than the number of species lost. Although our experimental communities did not reach an apparent richness limit, 2 species were absent only in large-magnitude supply treatments. Species released from propagule limitation via experimentally increased supply may have suppressed competitive subordinates. Resource availability may also have affected establishment (48). Algal biomass was low in communities receiving the greatest supply (Fig. 1F) and these conditions may have been insufficient to sustain populations of Jassa or Microprotopus. Our results emphasize that lack of saturation does not infer lack of competition or population regulation in our communities, but instead that competitive exclusion does not decrease or limit species diversity.
Conclusions
Our experiment suggests that propagule limitation occurs even in relatively open systems in which dispersal occurs frequently and rapidly. We also found strong cascading effects of propagule supply on lower trophic levels, which had not been documented previously to our knowledge. Our results suggest that supply alters ecosystem functioning by increasing consumption of resources and that these effects are strong enough to persist despite emigration. Additionally, our findings reinforce the idea that population regulation and competition can shape unsaturated communities via changes in composition without limiting species richness and that propagule limitation does not preclude density-dependent interactions (9). These results emphasize the role propagule supply plays in maintaining diverse communities and suggest that supply effects at one trophic level may cascade throughout food webs.
Methods
Experimental Design.
All experiments were conducted at the University of North Carolina's Institute of Marine Sciences in Morehead City. In July 2004 we established 54 4L flow-through mesocosms supplied with gravel-filtered seawater from Bogue Sound, NC and shaded them to reproduce field light conditions. We manipulated magnitude (4 levels of magnitude: small, medium–low, medium–high, large) and frequency of grazer propagule additions (2 levels of frequency: single and multiple) in a fully factorial design. The experiment was performed in flow-through mesocosms to control potentially confounding factors such as sampling scale, habitat complexity, and flow regime and to ensure homogeneity of resources. We also included control mesocosms in which no additional grazers were added to developing communities. Six mesocosms were randomly assigned to each of the 9 treatments and every mesocosm included an artificial seagrass mimic made of frayed polypropelene (49). All mimics were preconditioned with seawater filtered by a 100-μm filter to prevent epifaunal colonization for 3 days preceding the experiment; this allowed epiphytic algal propagules to settle and provide food for grazers. Grazers were collected from nearby habitats, added to a large holding tank, and added to the experimental mesocosms according to the assigned treatments by volume (i.e., medium–low, medium–high, and large treatments received 2, 4, and 8 times the volume of grazers added to small treatments, respectively). Samples of propagule additions were preserved and later identified (n = 20). Grazers were initially added in the single and multiple frequency mesocosms in volumes determined by assigned level of supply magnitude. Grazers were experimentally added to mesocosms in multiple treatments weekly. Throughout the experiment, the ratio between supply magnitude treatments remained the same, although the total volume varied with availability. Some grazer propagules also colonized all mesocosms naturally via the sea water supply, thus providing a continual source of food for grazers and allowing grazer communities to develop in no addition controls. At the end of 6 weeks (2–3 generations for most grazer species) all grazers were collected and preserved. Algae that had settled and grown in the mesocosms were collected and wet mass was measured after excess water was removed via spinning (50). Grazers were identified to the lowest possible taxonomic group; some common species were lumped by genera because of the large number of juvenile individuals. The number of gravid females was also recorded. Samples were dried to constant mass at 60 °C, ashed at 450 °C, and massed again to obtain ash-free dry weights.
Statistical Analyses.
The effects of supply magnitude, frequency, and magnitude × frequency interaction on grazer abundance, richness, evenness, Shannon–Weiner diversity, ash-free dry weight, and algal biomass were analyzed via separate, fully crossed 2-factor ANOVA (n = 6). Effect size (ω2) was calculated for all significant treatments (51). Response variables were transformed as necessary to meet the assumptions of ANOVA. For species present in sufficient abundance, separate 2-factor ANOVAs tested the effects of supply magnitude and frequency on log-transformed total abundance and percentage gravid females.
To compare species composition between experimental communities, we conducted multivariate analyses on a matrix of Bray–Curtis similarities generated from fourth root-transformed abundances (52). The effects of supply magnitude and frequency on compositional similarity were investigated by ANOSIM, and protected pairwise tests were performed to test for differences between levels (n = 6). A hierarchical agglomerative cluster analysis with group average linking was performed on similarities to delineate samples with >80% similarity in species composition. To visualize differences in composition among treatment levels, a nonmetric MDS algorithm was performed on similarities with 50 iterations and the 2D configuration that best preserved similarity rankings (i.e., had the lowest stress value) was used to generate a MDS ordination plot.
We tested the effect of our supply treatments on the body-size distributions of the 4 most abundant species (n = 3). Body-size distributions were obtained by counting the number of individuals retained by each of a nested series of sieves (2.8, 2.0, 1.4, 1.0, 0.71, and 0.50 mm). Statistical comparison of body-size distributions by a 2-factor ANOSIM (factors = magnitude, frequency) performed on untransformed Euclidean distances.
Although the identity of individuals in our random propagule additions is unknown, we used preserved samples to estimate the richness of experimental treatments. Using EstimateS 8 (http://purl.oclc.org/estimates), we generated sample-based rarefaction curves to estimate species richness as a function of accumulated samples and thus the number of species added in each propagule addition treatment (SI Text and Fig. S1).
Supplementary Material
Acknowledgments.
We thank J. Kertesz, M. Kintzing, L. Ladwig, and J. Wall, the staff of the University of North Carolina's Institute of Marine Science (especially C. Lewis and A. Whichard), and J. Wells and R. Luettich for supporting our work at the Institute of Marine Sciences. Advice from M. O'Connor, E. Selig, and K. France greatly improved this manuscript. This work was supported by a National Science Foundation Predoctoroal Fellowship (to S.C.L.), an Environmental Protection Agency Science to Achieve Results Fellowship (to S.C.L), National Science Foundation Grant OCE0327191 (to J.F.B.), and the University of North Carolina. The University of North Carolina at Chapel Hill's office of the Vice Chancellor for Research and Economic Development provided support for open access publication.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809284106/DCSupplemental.
References
- 1.Elton C. The Ecology of Animals. London: Methuen; 1950. [Google Scholar]
- 2.Macarthur RH. Patterns of species diversity. Biol Rev Camb Philos Soc. 1965;40:510–533. [Google Scholar]
- 3.Cornell HV, Lawton JH. Species interactions, local and regional processes, and limits to the richness of ecological communities: A theoretical perspective. J Anim Ecol. 1992;61:1–12. [Google Scholar]
- 4.Loreau M. Are communities saturated? On the relationship between alpha, beta and gamma diversity. Ecol Lett. 2000;3:73–76. [Google Scholar]
- 5.Terborgh JW, Faaborg J. Saturation of bird communities in the West Indies. Am Nat. 1980;116:178–195. [Google Scholar]
- 6.Stachowicz JJ, Tilman D. In: Species Invasions: Insights into Ecology, Evolution, and Biogeography. Sax DF, Stachowicz JJ, Gaines SD, editors. Sunderland, MA: Sinauer; 2005. pp. 41–64. [Google Scholar]
- 7.Caswell H, Cohen JE. In: Species Diversity in Ecological Communities: Historical and Geographical Perspectives. Ricklefs RE, Schluter D, editors. Chicago: Univ of Chicago Press; 1993. pp. 99–107. [Google Scholar]
- 8.MacArthur RH, Wilson EO. The Theory of Island Biogeography. Princeton: Princeton Univ Press; 1967. [Google Scholar]
- 9.Chesson P. Recruitment limitation: A theoretical perspective. Aust J Ecol. 1998;23:234–240. [Google Scholar]
- 10.Tilman D. Community invasibility, recruitment limitation, and grassland biodiversity. Ecology. 1997;78:81–92. [Google Scholar]
- 11.Foster BL, Dickson TL, Murphy CA, Karel IS, Smith VH. Propagule pools mediate community assembly and diversity-ecosystem regulation along a grassland productivity gradient. J Ecol. 2004;92:435–449. [Google Scholar]
- 12.Gross KL, Mittelbach GG, Reynolds HL. Grassland invasibility and diversity: Responses to nutrients, seed input, and disturbance. Ecology. 2005;86:476–486. [Google Scholar]
- 13.Mouquet N, Leadley P, Meriguet J, Loreau M. Immigration and local competition in herbaceous plant communities: A three-year seed-sowing experiment. Oikos. 2004;104:77–90. [Google Scholar]
- 14.Clark CJ, Poulsen JR, Levey DJ, Osenberg CW. Are plant populations seed limited? A critique and meta-analysis of seed addition experiments. Am Nat. 2007;170:128–142. doi: 10.1086/518565. [DOI] [PubMed] [Google Scholar]
- 15.Turnbull LA, Crawley MJ, Rees M. Are plant populations seed-limited? A review of seed sowing experiments. Oikos. 2000;88:225–238. [Google Scholar]
- 16.Foster BL, Tilman D. Seed limitation and the regulation of community structure in oak savanna grassland. J Ecol. 2003;91:999–1007. [Google Scholar]
- 17.Tilman D, et al. The influence of functional diversity and composition on ecosystem processes. Science. 1997;277:1300–1302. [Google Scholar]
- 18.Shurin JB. Dispersal limitation, invasion resistance, and the structure of pond zooplankton communities. Ecology. 2000;81:3074–3086. [Google Scholar]
- 19.Caley MJ, et al. Recruitment and the local dynamics of open marine populations. Annu Rev Ecol Syst. 1996;27:477–500. [Google Scholar]
- 20.Doherty P, Fowler T. An empirical test of recruitment limitation in a coral reef fish. Science. 1994;263:935–939. doi: 10.1126/science.263.5149.935. [DOI] [PubMed] [Google Scholar]
- 21.Gaines S, Roughgarden J. Larval settlement rate: A leading determinant of structure in an ecological community of the marine intertidal zone. Proc Natl Acad Sci USA. 1985;82:3707–3711. doi: 10.1073/pnas.82.11.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaines SD, Bertness MD. Dispersal of juveniles and variable recruitment in sessile marine species. Nature. 1992;360:579–580. [Google Scholar]
- 23.Hughes TP, Tanner JE. Recruitment failure, life histories, and long-term decline of caribbean corals. Ecology. 2000;81:2250–2263. [Google Scholar]
- 24.Sale PF. In: The Ecology of Fishes on Coral Reefs. Sale PF, editor. San Diego: Academic; 1991. pp. 564–598. [Google Scholar]
- 25.Connolly SR, Menge BA, Roughgarden J. A latitudinal gradient in recruitment of intertidal invertebrates in the northeast pacific ocean. Ecology. 2001;82:1799–1813. [Google Scholar]
- 26.Menge BA, et al. Coastal oceanography sets the pace of rocky intertidal community dynamics. Proc Natl Acad Sci USA. 2003;100:12229–12234. doi: 10.1073/pnas.1534875100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navarrete SA, Wieters EA, Broitman BR, Castilla JC. Scales of benthic-pelagic and the intensity of species interactions: From recruitment limitation to top-down control. Proc Natl Acad Sci USA. 2005;102:18046–18051. doi: 10.1073/pnas.0509119102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chave J, Muller-Landau HC, Levin SA. Comparing classical community models: Theoretical consequences for patterns of diversity. Am Nat. 2002;159:1–23. doi: 10.1086/324112. [DOI] [PubMed] [Google Scholar]
- 29.Warner RR, Chesson PL. Coexistence mediated by recruitment fluctuations: A field guide to the storage effect. Am Nat. 1985;125:769–787. [Google Scholar]
- 30.Cardinale BJ, et al. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature. 2006;443:989–992. doi: 10.1038/nature05202. [DOI] [PubMed] [Google Scholar]
- 31.Zeiter M, Stampfli A, Newbery DM. Recruitment limitation constrains local species richness and productivity in dry grassland. Ecology. 2006;87:942–951. doi: 10.1890/0012-9658(2006)87[942:rlclsr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Loreau M, Holt RD. Spatial flows and the regulation of ecosystems. Am Nat. 2004;163:606–615. doi: 10.1086/382600. [DOI] [PubMed] [Google Scholar]
- 33.Bruno JF, Boyer KE, Duffy JE, Lee SC. Relative and interactive effects of plant and grazer richness in a benthic marine community. Ecology. 2008;89:2518–2528. doi: 10.1890/07-1345.1. [DOI] [PubMed] [Google Scholar]
- 34.Dethier MN, Duggins DO. An “indirect commensalism” between marine herbivores and the importance of competitive hierarchies. Am Nat. 1984;124:205–219. [Google Scholar]
- 35.Duffy JE. Marine Sciences. Chapel Hill: University of North Carolina; 1989. p. 148. PhD dissertation. [Google Scholar]
- 36.Cruz-Rivera E, Hay ME. The effects of diet mixing on consumer fitness: Macroalgae, epiphytes, and animal matter as food for marine amphipods. Oecologia. 2000;123:252–264. doi: 10.1007/s004420051012. [DOI] [PubMed] [Google Scholar]
- 37.Holt RD. Food webs in space: On the interplay of dynamic instability and spatial processes. Ecol Res. 2002;17:261–273. [Google Scholar]
- 38.McCann KS, Rasmussen JB, Umbanhowar J. The dynamics of spatially coupled food webs. Ecol Lett. 2005;8:513–523. doi: 10.1111/j.1461-0248.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 39.Bruno JF, O'Connor MI. Cascading effects of predator diversity and omnivory in a marine food web. Ecol Lett. 2005;8:1048–1056. [Google Scholar]
- 40.Duffy JE, et al. The functional role of biodiversity in ecosystems: Incorporating trophic complexity. Ecol Lett. 2007;10:522–538. doi: 10.1111/j.1461-0248.2007.01037.x. [DOI] [PubMed] [Google Scholar]
- 41.Edgar GJ. Patterns of colonization of mobile epifauna in a western Australian seagrass bed. J Exp Mar Biol Ecol. 1992;157:225–246. [Google Scholar]
- 42.Duffy JE, Harvilicz AM. Species-specific impacts of grazing, amphipods in an eelgrass-bed community. Marine Ecol Progress Ser. 2001;223:201–211. [Google Scholar]
- 43.Kohler SL. Identification of stream drift mechanisms: An experimental and observational approach. Ecology. 1985;66:1749–1761. [Google Scholar]
- 44.Weiner J. Asymmetric competition in plant populations. Trends Ecol Evol. 1990;5:360–364. doi: 10.1016/0169-5347(90)90095-U. [DOI] [PubMed] [Google Scholar]
- 45.Franz DR, Mohamed Y. Short-distance dispersal in a fouling community amphipod crustacean, Jassa marmorata. Holmes J Exp Marine Biol Ecol. 1989;133:1–13. [Google Scholar]
- 46.France KE, Duffy JE. Diversity and dispersal interactively affect predictability of ecosystem function. Nature. 2006;441:1139–1143. doi: 10.1038/nature04729. [DOI] [PubMed] [Google Scholar]
- 47.Shurin JB, Srivastava DS. In: Metacommunities: Spatial Dynamics and Ecological Communities. Holyoak M, Leibold MA, Holt RD, editors. Chicago: University of Chicago Press; 2005. pp. 399–417. [Google Scholar]
- 48.Tilman D. Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition, invasion, and community assembly. Proc Natl Acad Sci USA. 2004;101:10854–10861. doi: 10.1073/pnas.0403458101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edgar GJ. Artificial algae as habitats for mobile epifauna: Factors affecting colonization in a Japanese sargassum bed. Hydrobiologia. 1991;226:111–118. [Google Scholar]
- 50.Bruno JF, Boyer KE, Duffy JE, Lee SC, Kertesz JS. Effects of macroalgal species identity and richness on primary production in benthic marine communities. Ecol Lett. 2005;8:1165–1174. doi: 10.1111/j.1461-0248.2005.00823.x. [DOI] [PubMed] [Google Scholar]
- 51.Graham MH, Edwards MS. Statistical significance versus fit: Estimating the importance of individual factors in ecological analysis of variance. Oikos. 2001;93:505–513. [Google Scholar]
- 52.Clarke KR. Nonparametric multivariate analyses of changes in community structure. Aus J Ecol. 1993;18:117–143. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.