Abstract
The emergence of drug-resistant bacteria has compromised the use of many conventional antibiotics, leading to heightened interest in a variety of antimicrobial peptides. Although these peptides have attractive potential as antibiotics, their size, stability, tissue distribution, and toxicity have hampered attempts to harness these capabilities. To address such issues, we have developed small (molecular mass <1,000 Da) arylamide foldamers that mimic antimicrobial peptides. Hydrogen-bonded restraints in the arylamide template rigidify the conformation via hydrogen bond formation and increase activity toward Staphylococcus aureus and Escherichia coli. The designed foldamers are highly active against S. aureus in an animal model. These results demonstrate the application of foldamer templates as therapeutics.
Keywords: antibiotic, host defense peptide
Resistance to conventional antibiotics is rapidly increasing, posing a substantial threat to global public health (1). Recently, methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant Staphylococcus epidermidis, vancomycin-resistant enterococci (VRE), and ampicillin-resistant Escherichia coli have emerged as common nosocomial (hospital-acquired) infections (2–4). The escalating resistance to conventional agents has inspired a substantial research effort directed toward investigating the potential of antimicrobial peptides (AMPs). Although no mechanism of pharmaceutical intervention is devoid of problems associated with resistance, AMPs use a physical mechanism that targets the bacterial cell membranes, possibly decreasing the risk of resistance (5–7).
AMPs are found in a wide range of species, including: plants, frogs, worms, and humans (8, 9). These host-defense peptides are typically 20–50 residues in length and span several structural classes. Despite this diversity, they all tend to adopt highly amphiphilic topologies in which the hydrophilic and hydrophobic side chains segregate onto distinctly opposing regions or faces of the molecule. Electrostatic interactions between the positively charged AMPs and the negatively charged bacterial phospholipids provide an initial mode of interaction, whereas hydrophobic interactions allow the peptides to penetrate the cell membrane (10, 11), in some cases leading to depolarization of the bacterial membrane and cell death. AMPs with appropriate distributions of charged and hydrophobic residues are remarkably selective for killing bacterial cells relative to host cells (12, 13). This selectivity is a result of a fundamental difference between the lipid composition of bacterial membranes and that of eukaryotic membranes. Bacterial membranes are composed of ≈30% negatively charged phospholipids (phosphatidylglycerol), with phosphatidylcholine as their zwitterionic lipid (14), whereas the surface of a eukaryotic cell membrane is composed mainly of zwitterionic phospholipids (such as phosphatidylcholine, sphingomyelin phospholipids, and cholesterol).
Over the last decade, new classes of peptidic and nonpeptidic antimicrobial compounds with structures similar to those of cationic and facially amphiphilic host defense antimicrobial peptides have been extensively investigated as therapeutic agents. A number of studies reported antibiotics designed to follow the mechanism of natural AMPs, for example, peptides composed of α-amino acids (15–17), β-amino acids (18, 19), peptoids (20), aromatic oligomers (21–24), and synthetic polymers (25–27). Previously, we designed a series of arylamide foldamers that showed potential for both activity and selectivity (21). However, the compounds were not active and exhibited significant toxicity in animal models. Therefore, we altered the structural and physicochemical properties of the arylamide foldamers to improve their antimicrobial activity and selectivity against S. aureus and E. coli. Compared with magainin analogues (e.g., MSI-78) (28) these compounds have significantly enhanced selectivity and reduced toxicity.
Results
Design and Structure of an Arylamide Framework.
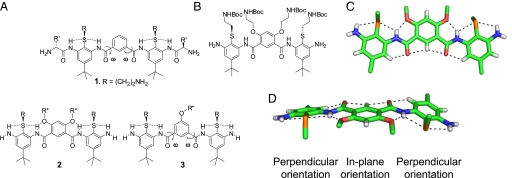
In previous studies (21), we prepared compounds related to generic structure 1, consisting of arylamides containing 2 1,3phenylene diamine units connected by a single isophthalic acid (Fig. 1A). A key feature in the design of these arylamides was a thioether moiety that provided a convenient point of attachment to the basic groups. The thioether also forms intramolecular hydrogen bonds (21, 22, 29–31) to neighboring amides, thereby restricting rotation about the N–C torsional angle between the amide nitrogen and the phenyl ring. However, the molecule retained significant torsional flexibility associated with ω, the torsional angle connecting the amide carbon and the phenyl group of the isophthalic acid ring.
Fig. 1.
Structures of arylamide foldamers. (A) Generic structures of conformationally restrained antimicrobial arylamide foldamers 1–3. (B–D) Chemical structure (B) and X-ray crystal structure (C and D) showing the network of hydrogen bonds through arylamide foldamers and the projection of the side chains from the plane of the ring [the side chains have been truncated; C (green), O (red), N (blue), polar H (white), and S (yellow)].
Herein, we increase the rigidity of the arylamide scaffold using a 4,6-dialkoxy-substituted isophthalic acid linker to form intramolecular O···H–N hydrogen bonds (generic structure 2), thereby restricting rotation around the aryl–CO bond. The ether substituents also provide convenient points from which to attach side chains (R″). To evaluate the conformational properties of this scaffold, we synthesized and determined the crystal structure of an arylamide foldamer with this substitution pattern (Fig. 1 B–D). In agreement with previous calculations (29–31), the tandem thioether and ether groups serve as conformational directing elements that restrict rotation around the aromatic ring–N bond and aromatic ring–C( O) bond, respectively. The thioether groups form intramolecular hydrogen bonds to adjacent amide and terminal amine protons (N···S distances = 3.02, 2.99, 2.99, and 2.97 Å); the 2 ether groups of the isophthalic amide linker also hydrogen bond to adjacent amide protons (N···O distances = 2.66 and 2.65 Å), forming 3-center intramolecular hydrogen bonds that rigidify the entire arylamide trimer. On the opposite face of the foldamer, we observed close proximity, typical of CH hydrogen bonds, between aryl CH-protons and amide oxygens (H···O distances = 2.22, 2.37, 2.37, and 2.32 Å). Although the angles are not optimal for a C–H···O hydrogen-bonded interaction (32), this feature might be important for maintaining the overall planar conformation. Thus, the overall network of consecutive N–H···S, N–H···O, and C–H···O interactions stabilizes the desired conformation and provides a platform on which to design a molecular recognition system with adjustable specificity.
O) bond, respectively. The thioether groups form intramolecular hydrogen bonds to adjacent amide and terminal amine protons (N···S distances = 3.02, 2.99, 2.99, and 2.97 Å); the 2 ether groups of the isophthalic amide linker also hydrogen bond to adjacent amide protons (N···O distances = 2.66 and 2.65 Å), forming 3-center intramolecular hydrogen bonds that rigidify the entire arylamide trimer. On the opposite face of the foldamer, we observed close proximity, typical of CH hydrogen bonds, between aryl CH-protons and amide oxygens (H···O distances = 2.22, 2.37, 2.37, and 2.32 Å). Although the angles are not optimal for a C–H···O hydrogen-bonded interaction (32), this feature might be important for maintaining the overall planar conformation. Thus, the overall network of consecutive N–H···S, N–H···O, and C–H···O interactions stabilizes the desired conformation and provides a platform on which to design a molecular recognition system with adjustable specificity.
To provide some measure by which to gauge effectiveness of the conformational restraints introduced into 2, we also prepared a series of compounds (generic structure 3) in which a single alkoxy group was introduced at the 5-position of the isophthalic acid ring. Although a direct comparison is complicated by the presence of two R″ groups in 2, while there is only one in 3, distinct structure-activity relationships (SAR) were found for the two series of compounds (Fig. 1A).
Antimicrobial and Hemolytic Activity.
The charge and hydrophobicity of the pendant functional groups were systematically varied (Tables 1–4) to determine how these parameters relate to activity in these 2 series of compounds. Furthermore, the terminal aniline amino groups in 2 and 3 (Fig. 1A) are very weakly basic and unlikely to be charged at neutral pH when bound to a membrane. Thus, we investigated the effect of converting these groups to the corresponding guanidines. The antimicrobial activities of compound 4a–g, 5a–c, and 6a–d were determined as the minimal inhibitory concentration (MIC) required to fully inhibit the growth of S. aureus ATCC27660 (Gram-positive) and E. coli D31 (Gram-negative) (Tables 1–4), along with hemolytic activity (HC50) against human erythrocytes. The MIC values are reproducible to within a factor of 2, whereas there is on the order of 10% to 20% error in the HC50 values depending on the source of human erythrocytes.
Table 1.
The effect of conformational restriction and guanidinylation of 4,6-disubstituted arylamide foldamers
Table 2.
The effect of guanidine for amine replacements of 4,6-disubstituted arylamide foldamers
Table 3.
The effect of guanidinylation of 5-monosubstituted arylamide foldamers
Table 4.
Antimicrobial and cytotoxic activities of compounds 6a–d
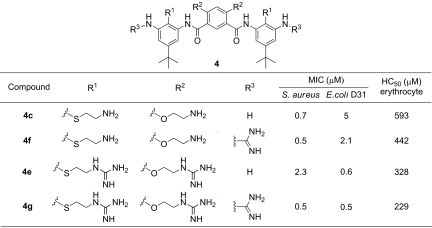
We observed an increase in both affinity and selectivity upon increasing the rigidity of the molecule (Table 1). As expected, the introduction of 2 methoxy groups in compound 4b increased the antimicrobial activity 17- and 8-fold against S. aureus and E. coli, respectively, when compared with 4a, which lacks these conformation-directing elements. Simultaneously, the hemolytic potency was decreased 10-fold (higher HC50) for an overall 170 and 80-fold gain in selectivity (HC50/MIC). These changes are attributed primarily to the more rigid structure of 4b induced by introduction of the ortho methoxy groups to the amides, although other factors including changes in hydrophobicity may also be important.
To determine the effect of the overall charge, we prepared compound 4c, in which 2 aminoethyl groups replace the methyl groups of 4b. This substitution resulted in retention in activity against S. aureus, although it decreased the potency ≈5-fold against E. coli. However, this modification also decreased the toxicity against red blood cells (HC50 = 0.6 mM), resulting in a very large (850-fold) selectivity for S. aureus versus human erythrocytes. Conversion of the alkyl-amino groups of 4c to guanidines led to successively greater loss in activity against S. aureus in 4d (with 2 guanidines) and 4e (with 4 guanidines). On the other hand, these modifications increased the activity against E. coli. Simultaneously, these changes tended to slightly decrease the HC50 values, indicating that the compounds had become less selective for S. aureus and more selective for E. coli.
The weakly basic aniline amino groups were guanidinylated to introduce a positive charge at these positions (Table 2). This modification to 4c did not significantly affect the antimicrobial or hemolytic activity (4f). On the other hand, guanidinylation of 4e gave rise to a 4-fold increase in antimicrobial activity (4g), without significantly affecting the activity against E. coli and the hemolytic potency. Thus, in contrast to data shown in Table 1, we observed that guanidinylation of the terminal amine (R3) resulted in a modest improvement in antimicrobial activity and selectivity against both bacteria.
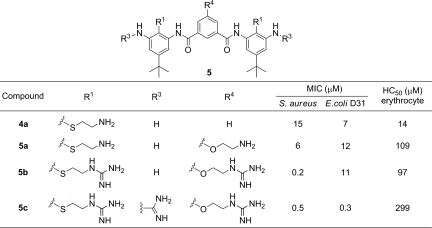
An entirely different SAR arises from consideration of the less conformationally restricted arylamides with a 5-monosubstituent at the isophthalic amide (Table 3). Similar to the effect on 4,6-disubstituted foldamer 4b, the introduction of a positively charged aminoethoxy group in 5a decreased toxicity toward erythrocytes without significantly affecting antimicrobial activity. Interestingly, although the guanidinylation of the 4,6-disubstituted foldamers tended to decrease their antimicrobial activity against S. aureus, the 5-monosubstituted compound 5b exhibited an unexpected 30-fold increase against S. aureus. This same substitution had no effect on the antimicrobial activity against E. coli, nor did it significantly change the hemolytic potency. Furthermore, in contrast to the results from 4e and 4g, guanidinylation of terminal aniline groups of 5b (to give 5c) led to a 3-fold decrease in the activity against S. aureus, a 40-fold increase against E. coli, and decreased toxicity toward erythrocytes. Thus, the effect of guanidinylation on the 2 series of compounds is strikingly different, presumably reflecting difference in overall charge as well as flexibility.
Finally, we explored additional substitutions (Table 4) into the conformationally constrained 2,4-dialkoxy isophthalic acid scaffold series of compounds. Based on previous work in which N-acyl groups had been shown to enhance activity of arylamides (21), we prepared compound 6a, in which a guanidino-pentanoyl side chain was appended to the terminal amines. The activity of this compound was improved further by decreasing its overall charge through the replacement of the 2 aminoalkyl side chains with methyl ethers. We also explored the introduction of fluoroalkyl subsituents (33) by replacing the t-butyl groups with less hydrophobic trifluoromethyl groups in compound 6c. Finally, in previous studies, a pyrimidine ring was used to control the conformation of antimicrobial arylamides in a manner similar to the dialkoxy substituents in 4c (24), prompting the synthesis of pyrimidine-containing compound 6d. In vitro, compounds 6c and 6d were highly activity against a variety of Gram-positive and Gram-negative bacteria (Table 4). They also exhibited minimal toxicity to mammalian cells as measured using erythrocytes (HC50), mouse 3T3, and human HepG2 cells. Comparison of the EC50 and HC50 values with the MIC for S. aureus indicated the high selectivity for bacteria over mammalian cells (250 to >20,000).
Cytoplasmic Membrane Permeabilization and Bacterial Killing.
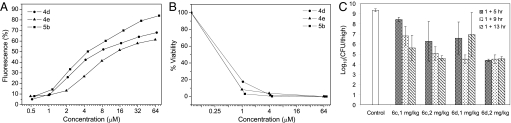
We examined the abilities of the arylamide foldamers, 4d, 4e, and 5b, to depolarize S. aureus membranes, using the membrane potential-sensitive dye DiSC3 (5). The distribution of this dye between the cell interior and the medium depends on the membrane potential gradient (34, 35), leading to an increase in fluorescence intensity in response to a loss of membrane potential. The potassium-selective ionophore valinomycin was used as a positive control. Maximum fluorescence was observed within 10 min after the addition of the arylamide compounds in a dose-dependent manner (Fig. 2A). We did not expect to see a perfect correlation between in vitro efficacy and ability to depolarize membranes, given the 2-fold error associated with determination of the MIC values and the limited spread of MIC values for this set of compounds. However, it is reassuring to note that the compound with the lowest MIC was most active in this assay, and the least active compound showed the least tendency to depolarize membranes.
Fig. 2.
In vitro and in vivo activities of arylamide foldamers. (A) Depolarization of the cytoplasmic membrane of S. aureus, assessed by the fluorescence intensity of membrane potential-sensitive dye DiSC3 (34, 35). (B) Survival of S. aureus in the presence of compounds 4d, 4e, and 5b. (C) In vivo efficacy of 6c and 6d in thigh burden infection model. Neutropenic mice (n = 4 per group) were inoculated in the posterior thigh muscles with S. aureus ATCC13709 at 1 × 106 CFU per thigh and then treated with 6c and 6d (1 or 2 mg/kg per dose) by i.v. bolus injection in the tail vein at 1 and 5, 1 and 9, or 1 and 13 h after infection.
For all compounds, the concentration required to cause membrane depolarization (after 10 min) was significantly higher than the MIC values, possibly because of differences in the assay conditions. The depolarization assay was conducted in 100 mM KCl with a bacterial density (OD600 = 0.02) 20-fold greater than the MIC assay. Thus, we evaluated the extent of cell death under the conditions of the fluorescence assay. After the fluorescence measurements, the cell suspension was diluted (30 min after addition of compound) and plated and the colony-forming units (CFUs) determined (Fig. 2B). The number of viable bacteria decreased in a dose-dependent manner over the same concentration range (≈1–20 μg/ml) that caused rapid depolarization of the membrane. Interestingly, although significant membrane depolarization occurred in this concentration range, full depolarization after 10 min did not appear to be necessary to elicit cell death after plating of the bacteria.
Resistance Study.
To investigate the potential for bacteria to develop resistance against the antimicrobial activity of the foldamers, S. aureus was serially passed in sublethal concentrations of 4e, and new MIC values were measured at 24-h intervals. Compound 4e was chosen for this study because it had intermediate activity and was available in sufficient quantities. As a positive control, parallel cultures were exposed to serial 2-fold dilutions of ciprofloxacin and norfloxacin, which are 2 broad-spectrum fluoroquinolones (36). No significant change in the MIC occurred for 4e over the entire 16 passages, whereas an increase in the MIC is readily observed by passage 6 for both ciprofloxacin and norfloxacin (supporting information (SI) Appendix). These results demonstrate that it is not particularly easy for S. aureus to evolve resistance to this arylamide. We are currently conducting more extensive studies at lower and higher drug concentrations to assess more fully the ability of various bacteria to evolve resistance the arylamides in vitro.
In Vivo Mouse Thigh Burden Infection Model.
The development of membrane-active antimicrobial peptides as systemic agents for treatment of bacterial infections has been beset with difficulties associated with toxicity and tissue distribution, and few molecules have been shown to be active in vivo (37–40). A widely used animal model for evaluating antimicrobial activity of preclinical compounds (41) is the thigh burden model, in which the thigh muscle of neutropenic mice is inoculated with bacteria, followed by i.v. administration of the compound. Although complete pharmacokinetic and efficacy studies are beyond the scope of this manuscript, we report our initial findings here.
Compounds 4c and 4e provided partial protection against infection with S. aureus (SI Appendix), although the degree of protection at the highest tolerated dose was less than that conferred by successful antibiotics. Much greater activity was observed for compound 6c and 6d, which were tolerated at doses as high as 20 mg/kg (single i.v. bolus injection). Compound 6c showed significant in vivo activity in the thigh burden model at doses of 10 mg/kg, 2 mg/kg, or 1 mg/kg when administered twice with a 6-hour interval between injections. At the highest dose, a 4-log10 decrease in CFU was observed (SI Appendix). For comparison, vancomycin typically produces a similar 104 to 105 reduction in viable CFU of S. aureus ATCC 13709 at its maximally efficacious i.v. dose of 30 mg/kg in this model. The efficacy of compound 6b could be enhanced by increasing the time between doses to 13 h; 2 doses of 1 mg/kg or 2 mg/kg gave rise to a 104- to 105-fold reduction in CFU (Fig. 2C). Compound 6d also showed excellent activity in this assay, although the degree of inhibition did not show the same time dependence observed for 6c. At 2 mg/kg, compound 6d showed excellent activity (105 reduction in viable CFU of S. aureus ATCC 13709) irrespective of the time between doses. At 1 mg/kg, the compound was only partially active, and considerable variability was observed.
Discussion
We have used arylamide foldamers to mimic the activities of antimicrobial peptides. These compounds are not only of potential use as systemic antibiotics but might also help elucidate the features required for antimicrobial activity. Like antimicrobial peptides, the arylamides cause rapid depolarization of the membrane in a concentration-dependent manner at concentrations close to those required to the MIC. Interestingly, only partial depolarization is required to lead to cell death, which occurs in a slower process that can take up to several hours at concentrations near the MIC (SI Appendix). Similar behavior has been observed for some AMPs, which show little membrane depolarization at concentrations that stopped growth (34, 35, 42). This behavior suggests that many AMPs and AMP mimics act by mixed mechanisms; at high concentrations they disrupt membranes sufficiently to lead to cell death, whereas at lower concentrations, other slower mechanisms become important. We are currently investigating the mechanisms of the antimicrobial arylamides, particularly the extent to which the rapid partial disruption of bacterial membranes is related to the slower processes that ultimately cause cell death.
The structure/activity relationships in this series of compounds illustrate the balance of forces required for activity. The arylamides are too short to span the hydrophobic length of the bilayer, and coarse-grained molecular dynamics simulations (43) suggest they work in part by a mechanism resembling the “carpet” mechanism of AMP activity (12). To reach their membrane targets, they must penetrate various physical barriers in Gram-positive and Gram-negative bacteria. Their relatively small size and conformationally constrained structures might aid in this process. Once they gain access to the cytoplasmic membrane, they must then bind with a sufficiently favorable free energy of association to allow disruption of the bilayer. Hydrophobic and electrostatic interactions appear to drive these interactions, but the overall hydrophobicity and charge must be carefully optimized to provide a sufficient driving force for binding while minimizing toxicity, aggregation, and nonspecific binding. Increasing the hydrophobicity beyond a certain threshold can lead to compounds with poor water solubility and toxicity toward mammalian cells. For example, we previously found that inclusion of a hydrophobic t-butyl substituent on the arylamide ring gave rise to good activity in vitro. Here, we find that substitution of this large hydrophobic substituent with a smaller trifluoromethyl group was tolerated without loss of antibacterial potency, while minimizing toxicity in vivo.
Many studies have explored the relationship between conformational flexibility and activity. Flexible compounds can be highly potent, possibly because membranes induce an amphiphilic conformation in the bound state (25, 40, 44). In such cases, additional electrostatic and hydrophobic interactions might be necessary to compensate for the loss in conformational entropy of binding in such compounds. Thus, the conformationally rigid compound 4b was highly active, although it had only 2 strongly basic side chains, whereas the less rigid compounds 5b and 5c required 3 and 5 positively charged groups, respectively, to provide comparable activity. It is also interesting to note that quite different SARs emerged from the 2 different series of compounds, suggesting that they might have subtly different conformations or modes of binding. Also, although good activity in vitro could be obtained for each of the scaffolds investigated here, only the scaffolds the largest number of potential hydrogen-bonding conformational restraints gave good activity in vivo. Possibly, the toxicities seen in the more flexible molecules are associated with conformations that are distinct from those required for antimicrobial activity.
It is particularly encouraging that the compounds show good in vivo activity at doses significantly lower than the maximal tolerated dose. Unexpectedly, compound 6c showed enhanced activity with an increasing delay time between doses. This effect might be related to its tissue distribution and pharmacokinetics, which have not yet been investigated. Alternatively it might have a mechanism similar to some AMPs, which can act indirectly through modulation of the host immune system (45). Thus, the increasing efficacy of 6c with longer delays between doses might be associated with the priming of an immune response, which is then augmented by the second dose. This explanation appears less likely, because the animals are neutropenic, and the time frame covers only 24 h; conditions that are suboptimal for immune priming to a level that could account for the magnitude of efficacy. Furthermore, 6d failed to show any statistically significant differences in activity with different dosing intervals when administered at either 1 mg/kg or 2 mg/kg. Pharmacokinetic analysis also showed that after i.v. injection of 6d in mice, the serum levels of drug reached concentrations greater than the MIC (SI Appendix).
Finally, this work illustrates the potential of foldamers for the development of pharmaceuticals. Beginning with AMPs of molecular masses in the range of 2,000–5,000 Da, it has been possible to design highly active arylamides with molecular masses in the range of 600–1,000 Da. These compounds might help address an urgent need for mechanistically novel drugs to combat the increasing threat posed by antibiotic-resistant bacteria. The in vivo activities seen for 6c and 6d are comparable with that of vancomycin at its maximum tolerated dose. Indeed, the good efficacy/safety ratios and broad-spectrum antibiotic activity seen in this study suggest mimics of AMPs might be excellent candidates for i.v. antibiotics
Methods
Method for determination of antimicrobial activity, toxicity, lipid depolarization, resistance, and in vivo antimicrobial activity can be found in SI Appendix. Synthetic details and crystallographic structure determination can be also found in SI Appendix.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grants AI74866 and GM54616.
Footnotes
Conflict of interest statement: W.F.D. is a member of the scientific advisory board of PolyMedix, a company that is conducting clinical trials of molecules related to the compounds described in this manuscript.
This article is a PNAS Direct Submission. A.S. is a guest editor invited by the Editorial Board.
Data deposition: The atomic coordinates have been deposited in the Cambridge Structural Database, Cambridge Crystallographic Data Centre, Cambridge CB2 1EZ, United Kingdom (CCDC reference no. 721880).
This article contains supporting information online at www.pnas.org/cgi/content/full/0811818106/DCSupplemental.
References
- 1.Levy SB, Marshall B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat Med. 2004;10:S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 2.Tomasz A. Multiple-antibiotic-resistant pathogenic bacteria. A report on the Rockefeller University Workshop. N Engl J Med. 1994;330:1247–1251. doi: 10.1056/NEJM199404283301725. [DOI] [PubMed] [Google Scholar]
- 3.Murray BE. Vancomycin-resistant enterococcal infections. N Engl J Med. 2000;342:710–721. doi: 10.1056/NEJM200003093421007. [DOI] [PubMed] [Google Scholar]
- 4.Aires de Sousa M, de Lencastre H. Evolution of sporadic isolates of methicillin-resistant Staphylococcus aureus (MRSA) in hospitals and their similarities to isolates of community-acquired MRSA. J Clin Microbiol. 2003;41:3806–3815. doi: 10.1128/JCM.41.8.3806-3815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 6.Perron GG, Zasloff M, Bell G. Experimental evolution of resistance to an antimicrobial peptide. Proc Biol Sci. 2006;273:251–256. doi: 10.1098/rspb.2005.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bishop JL, Finlay BB. Friend or foe? Antimicrobial peptides trigger pathogen virulence. Trends Mol Med. 2006;12:3–6. doi: 10.1016/j.molmed.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Boman HG. Innate immunity and the normal microflora. Immunol Rev. 2000;173:5–16. doi: 10.1034/j.1600-065x.2000.917301.x. [DOI] [PubMed] [Google Scholar]
- 9.Hancock RE, Lehrer R. Cationic peptides: A new source of antibiotics. Trends Biotechnol. 1998;16:82–88. doi: 10.1016/s0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 10.Boman HG. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 11.Hancock RE. Peptide antibiotics. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 12.Shai Y. Mode of action of membrane active antimicrobial peptides. Biopolymers. 2002;66:236–248. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- 13.Brogden KA. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 14.Mcphee JB, Hancock REW. Function and therapeutic potential of host defence peptides. J Pept Sci. 2005;11:677–687. doi: 10.1002/psc.704. [DOI] [PubMed] [Google Scholar]
- 15.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: Isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci USA. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, et al. Rational design of alpha-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index. J Biol Chem. 2005;280:12316–12329. doi: 10.1074/jbc.M413406200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Won HS, Jung SJ, Kim HE, Seo MD, Lee BJ. Systematic peptide engineering and structural characterization to search for the shortest antimicrobial peptide analogue of gaegurin 5. J Biol Chem. 2004;279:14784–14791. doi: 10.1074/jbc.M309822200. [DOI] [PubMed] [Google Scholar]
- 18.Hamuro Y, Schneider JP, DeGrado WF. De novo design of antibacterial β-peptides. J Am Chem Soc. 1999;121:12200–12201. [Google Scholar]
- 19.Porter EA, Wang X, Lee HS, Weisblum B, Gellman SH. Non-haemolytic beta-amino-acid oligomers. Nature. 2000;404:565. doi: 10.1038/35007145. [DOI] [PubMed] [Google Scholar]
- 20.Chongsiriwatana NP, et al. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proc Natl Acad Sci USA. 2008;105:2794–2799. doi: 10.1073/pnas.0708254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu D, et al. Nontoxic membrane-active antimicrobial arylamide oligomers. Angew Chem Int Ed Engl. 2004;43:1158–1162. doi: 10.1002/anie.200352791. [DOI] [PubMed] [Google Scholar]
- 22.Tew GN, et al. De novo design of biomimetic antimicrobial polymers. Proc Natl Acad Sci USA. 2002;99:5110–5114. doi: 10.1073/pnas.082046199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang H, Doerksen R, Tew GN. Synthesis of urea oligomers and their antibacterial activity. Chem Commun. 2005:1537–9. doi: 10.1039/b413679a. [DOI] [PubMed] [Google Scholar]
- 24.Tang H, Doerksen RJ, Jones TV, Klein ML, Tew GN. Biomimetic facially amphiphilic antibacterial oligomers with conformationally stiff backbones. Chem Biol. 2006;13:427–435. doi: 10.1016/j.chembiol.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Kuroda K, DeGrado WF. Amphiphilic polymethacrylate derivatives as antimicrobial agents. J Am Chem Soc. 2005;127:4128–4129. doi: 10.1021/ja044205+. [DOI] [PubMed] [Google Scholar]
- 26.Ilker MF, Nusslein K, Tew GN, Coughlin EB. Tuning the hemolytic and antibacterial activities of amphiphilic polynorbornene derivatives. J Am Chem Soc. 2004;126:15870–15875. doi: 10.1021/ja045664d. [DOI] [PubMed] [Google Scholar]
- 27.Gelman MA, Weisblum B, Lynn DM, Gellman SH. Biocidal activity of polystyrenes that are cationic by virtue of protonation. Org Lett. 2004;6:557–560. doi: 10.1021/ol036341+. [DOI] [PubMed] [Google Scholar]
- 28.Maloy WL, Kari UP. Structure-activity studies on magainins and other host defense peptides. Biopolymers. 1995;37:105–122. doi: 10.1002/bip.360370206. [DOI] [PubMed] [Google Scholar]
- 29.Doerksen RJ, et al. Controlling the conformation of arylamides: Computational studies of intramolecular hydrogen bonds between amides and ethers or thioethers. Chem Eur J. 2004;10:5008–5016. doi: 10.1002/chem.200400176. [DOI] [PubMed] [Google Scholar]
- 30.Vemparala S, Ivanov I, Pophristic V, Spiegel K, Klein ML. Ab initio calculations of intramolecular parameters for a class of arylamide polymers. J Comput Chem. 2006;27:693–700. doi: 10.1002/jcc.20382. [DOI] [PubMed] [Google Scholar]
- 31.Pophristic V, et al. Controlling the shape and flexibility of arylamides: A combined ab initio, molecular dynamics, and classical molecular dynamics study. J Phys Chem B. 2006;110:3517–3526. doi: 10.1021/jp054306+. [DOI] [PubMed] [Google Scholar]
- 32.Steiner T. The hydrogen bond in the solid state. Angew Chem Int Ed. 2002;41:48–76. doi: 10.1002/1521-3773(20020104)41:1<48::aid-anie48>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 33.Gottler LM, Bea Rdl S, Shelburne CE, Ramamoorthy A, Marsh ENG. Using fluorous amino acids to probe the effects of changing hydrophobicity on the physical and biological properties of the β-hairpin antimicrobial peptide Protegrin-1. Biochemistry. 2008;47:9243–9250. doi: 10.1021/bi801045n. [DOI] [PubMed] [Google Scholar]
- 34.Wu M, Maier E, Benz R, Hancock RE. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry. 1999;38:7235–7242. doi: 10.1021/bi9826299. [DOI] [PubMed] [Google Scholar]
- 35.Friedrich CL, Moyles D, Beveridge TJ, Hancock RE. Antibacterial action of structurally diverse cationic peptides on gram-positive bacteria. Antimicrob Agents Chemother. 2000;44:2086–2092. doi: 10.1128/aac.44.8.2086-2092.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Entenza JM, Vouillamoz J, Glauser MP, Moreillon P. Levofloxacin versus ciprofloxacin, flucloxacillin, or vancomycin for treatment of experimental endocarditis due to methicillin-susceptible or -resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:1662–1667. doi: 10.1128/aac.41.8.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez-Lopez S, et al. Antibacterial agents based on the cyclic D,L-alpha-peptide architecture. Nature. 2001;412:452–455. doi: 10.1038/35086601. [DOI] [PubMed] [Google Scholar]
- 38.Shai Y, Makovitzky A, Avrahami D. Host defense peptides and lipopeptides: Modes of action and potential candidates for the treatment of bacterial and fungal infections. Curr Protein Pept Sci. 2006;7:479–486. doi: 10.2174/138920306779025620. [DOI] [PubMed] [Google Scholar]
- 39.Vallon-Eberhard A, et al. Efficient clearance of Aspergillus fumigatus in murine lungs by an ultrashort antimicrobial lipopeptide, palmitoyl-lys-ala-DAla-lys. Antimicrob Agents Chemother. 2008;52:3118–3126. doi: 10.1128/AAC.00526-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radzishevsky IS, et al. Improved antimicrobial peptides based on acyl-lysine oligomers. Nat Biotechnol. 2007;25:657–659. doi: 10.1038/nbt1309. [DOI] [PubMed] [Google Scholar]
- 41.Andes D, van Ogtrop ML, Peng J, Craig WA. In vivo pharmacodynamics of a new oxazolidinone (linezolid) Antimicrob Agents Chemother. 2002;46:3484–3489. doi: 10.1128/AAC.46.11.3484-3489.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Dhillon P, Yan H, Farmer S, Hancock RE. Interactions of bacterial cationic peptide antibiotics with outer and cytoplasmic membranes of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2000;44:3317–3321. doi: 10.1128/aac.44.12.3317-3321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez CF, Nielsen SO, Srinivas G, Degrado WF, Klein ML. Probing membrane insertion activity of antimicrobial polymers via coarse-grain molecular dynamics. J Chem Theory Comput. 2006;2:649–655. doi: 10.1021/ct050298p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ivanov I, et al. Characterization of nonbiological antimicrobial polymers in aqueous solution and at water–lipid interfaces from all-atom molecular dynamics. J Am Chem Soc. 2006;128:1778–1779. doi: 10.1021/ja0564665. [DOI] [PubMed] [Google Scholar]
- 45.Scott MG, et al. An anti-infective peptide that selectively modulates the innate immune response. Nat Biotechol. 2007;25:465–472. doi: 10.1038/nbt1288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.