Abstract
Nonclassical estrogen receptor α (ERα) signaling can mediate E2 negative feedback actions in the reproductive axis; however, downstream pathways conveying these effects remain unclear. These studies tested the hypothesis that p21-activated kinase 1 (PAK1), a serine/threonine kinase rapidly activated by E2 in nonneural cells, functions as a downstream node for E2 signaling pathways in cells of the preoptic area, and it may thereby mediate E2 negative feedback effects. Treatment of ovariectomized (OVX) rats with estradiol benzoate (EB) caused rapid and transient induction of phosphorylated PAK1 immunoreactivity in the medial preoptic nucleus (MPN) but not the arcuate nucleus. To determine whether rapid induction of PAK phosphorylation by E2 is mediated by nonclassical [estrogen response element (ERE)-independent] ERα signaling, we used female ERα null (ERα−/−) mice possessing an ER knock-in mutation (E207A/G208A; AA), in which the mutant ERα is incapable of binding DNA and can signal only through membrane-initiated or ERE-independent genotropic pathways (ERα−/AA mice). After 1-h EB treatment, the number of pPAK1-immunoreactive cells in the MPN was increased in both wild-type (ERα+/+) and ERα−/AA mice but was unchanged in ERα−/− mice. Serum luteinizing hormone (LH) was likewise suppressed within 1 h after EB treatment in ERα+/+ and ERα−/AA but not ERα−/ − mice. In OVX rats, 5-min intracerebroventricular infusion of a PAK inhibitor peptide but not control peptide blocked rapid EB suppression of LH secretion. Taken together, our findings implicate PAK1 activation subsequent to nonclassical ERα signaling as an important component of the negative feedback actions of E2 in the brain.
Keywords: GnRH, LH, estrogen receptor α
Ovarian estradiol-17β (E2) conveys negative feedback actions within the reproductive axis that include inhibition of gonadotropin-releasing hormone (GnRH) neurosecretion and suppression of gonadotrope responsiveness to GnRH stimulation. Both actions can be sustained by E2 treatment regimens that maintain serum E2 levels in low physiological ranges (1) and they can also be manifested rapidly, within minutes after E2 injection (2). Studies of estrogen receptor α (ERα), ERβ, and ERα/β null mutant mice have clearly implicated ERα as the isoform essential for E2 negative feedback regulation in vivo (3, 4).
Cell signaling pathways that transduce ERα-mediated negative feedback are not well understood. In classical ERα signaling mechanisms, E2 binds nuclear ERs and recruits coactivators to consensus palindromic estrogen response elements (EREs). Direct binding of ERs to EREs thereby mediates alterations in transcription of target genes. Nonclassical ERα signaling mechanisms operate independently of ERα binding directly to EREs and include protein–protein interactions with transcription factors, such as AP1, SP1, and NF-κB (5), which in turn mediate transcriptional regulation at their cognate response elements. Nonclassical ERα signaling also includes membrane-associated receptor activation coupled to stimulation of cytoplasmic signaling pathways. To distinguish relative contributions of classical versus nonclassical ERα signaling to E2 actions in vivo, Jakacka et al. (6) developed a gene knockin mouse that expresses a mutant (E207A/G208A; AA) form of ERα with disrupted classical (ERE-dependent) but intact nonclassical (ERE-independent) ERα-signaling capacities. By using animals in which the AA mutant allele was introduced onto the ERα-null (ERα−/−) mutant background (ERα−/AA mice), we determined that nonclassical ERα signaling can rescue the majority of E2 negative feedback effects that are present in wild-type (ERα+/+) mice and completely absent in ERα−/− mice (7).
Here, we attempt to identify a downstream mediator of nonclassical ERα signaling mechanisms conveying E2 negative feedback in the brain. E2 can modulate dendrite morphogenesis and induce synaptogenesis in hypothalamic (8–11) and extrahypothalamic neuronal populations (12, 13), and such structural plasticity may mediate E2 feedback effects (14). That the ERα isoform appears to mediate many of these effects rapidly (15) is consistent with the idea that membrane-initiated, nonclassical ERα signaling can rapidly induce the actin-cytoskeletal reorganization required for synaptic remodeling (16). In nonneural cells, ERα signaling produces rapid alterations in cell shape, polarity, and motility by activating p21-activated kinase 1 (PAK1) (17), the best-characterized member of a family of conserved mammalian serine/threonine kinases that function as downstream effectors of activated Rho GTPases, Rac1 and Cdc42, as well as phosphatidylinositol 3-kinase (PI3K) (18). Because nonclassical ERα signaling and activated PAK1 share common effects on neuronal morphology (19), and because E2 can activate PAK1 through nonclassical mechanisms (20), we tested the hypothesis that the nonclassical negative feedback actions of E2 are conveyed in part via ERα-mediated activation of PAK1.
Results
E2 Rapidly Induces PAK1 Phosphorylation In Vivo.
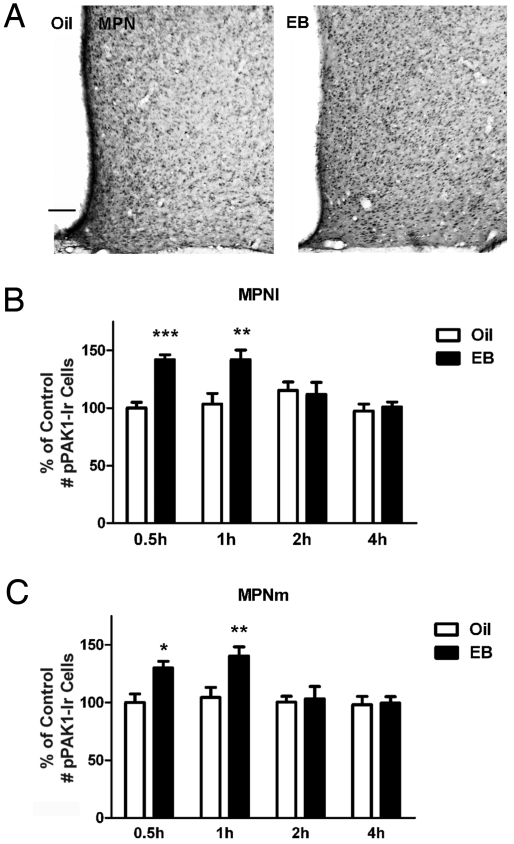
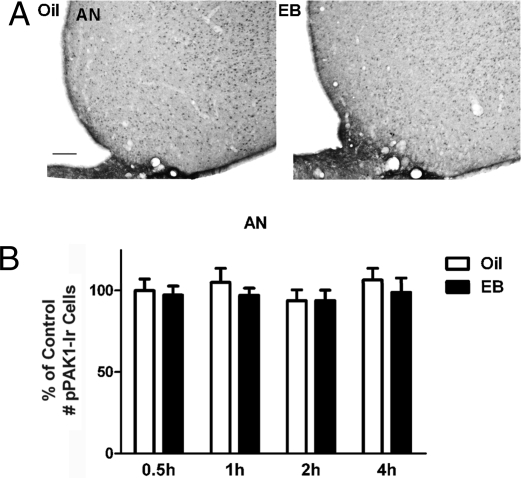
The effects of EB on the expression of pPAK1 were assessed by peroxidase immunohistochemical analyses of the lateral and medial subdivisions of medial preoptic nucleus (MPNl and MPNm, respectively) and the arcuate nucleus (AN). Treatment with EB but not oil vehicle produced a rapid and transient increase in the number of phosphorylated PAK1 immunoreactivity (pPAK1-Ir) cells in both subdivisions of the MPN. Micrographs of representative sections from 1 oil-treated and 1 EB-treated ovariectomized (OVX) rat 0.5 h after injections are given in Fig. 1A, demonstrating a greater number of pPAK1-Ir cells in the MPNl and MPNm of an EB-treated versus an oil-treated rat. Fig. 1 B and C summarizes pPAK1-Ir cell counts in the MPNl and MPNm for the 2 treatment groups at all time points. EB significantly increased the number of pPAK1-Ir cells as early as 0.5 h in the MPNl (P < 0.001; Fig. 1B) and MPNm (P < 0.05; Fig. 1C). This increase was maintained at 1 h after EB treatment (P < 0.01; Fig. 1 B and C). The number of pPAK1-Ir cells at 2 and 4 h was not significantly different from corresponding values in oil-treated controls. In the same OVX animals, the oil and EB treatments were without effect on the number of pPAK1-Ir cells in the AN. Representative tissue sections containing the AN from an oil-treated and an EB-treated rat are provided in Fig. 2A, along with the summary data of pPAK1-Ir cell counts for the 2 treatments at each time point (Fig. 2B). No significant differences between the treatment groups were observed for the pPAK1-Ir cell number in the AN at any time point.
Fig. 1.
Time dependence of E2-induced pPAK1-Ir in the MPN of female rats. OVX females rats treated with oil or EB were killed at 0.5, 1, 2, and 4 h after injection. (A) Representative photomicrographs of pPAK1-Ir in the MPN and adjacent regions of OVX rats 0.5 h after oil or EB injection. (Scale bar: 100 μm.) The number of pPAK1-Ir cells in the MPNl (B) and MPNm (C) was significantly greater after 0.5 and 1 h of EB treatment compared with corresponding oil treatment (n = 6–9). Data are represented as the mean ± SEM (***, P < 0.001; **, P < 0.01; *, P < 0.05).
Fig. 2.
E2-independent pPAK1-Ir in the AN of female rats. Animals were treated as described in Fig. 1. (A) Representative photomicrographs of pPAK1-Ir in the AN and adjacent regions of OVX rats 1 h after oil or EB injection. (Scale bar: 100 μm.) The number of pPAK1-Ir neurons in the AN was not significantly different after EB treatment compared with corresponding oil treatment (B). Data are represented as the mean ± SEM (n = 6–9).
Nonclassical ERα Signaling Mediates Rapid E2 Induction of pPAK1-Ir.
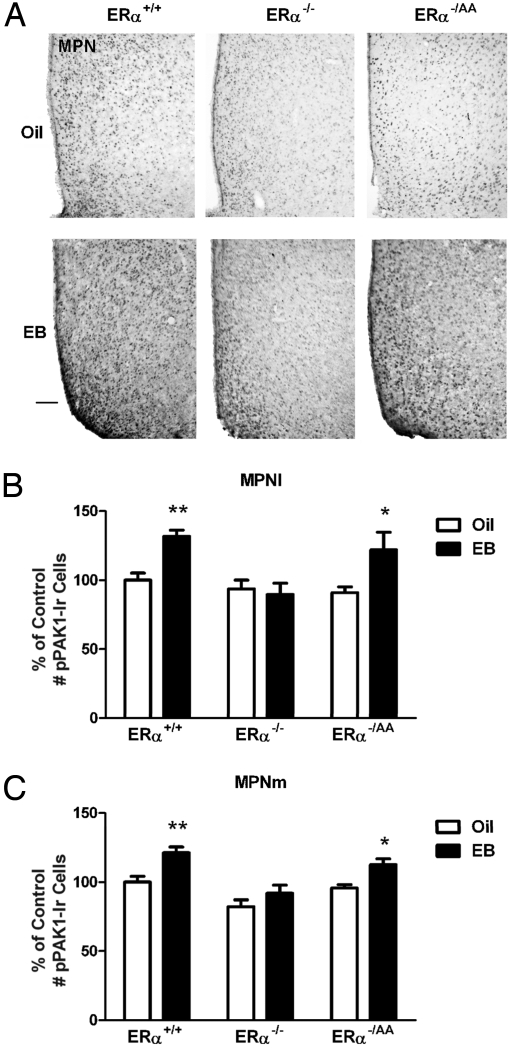
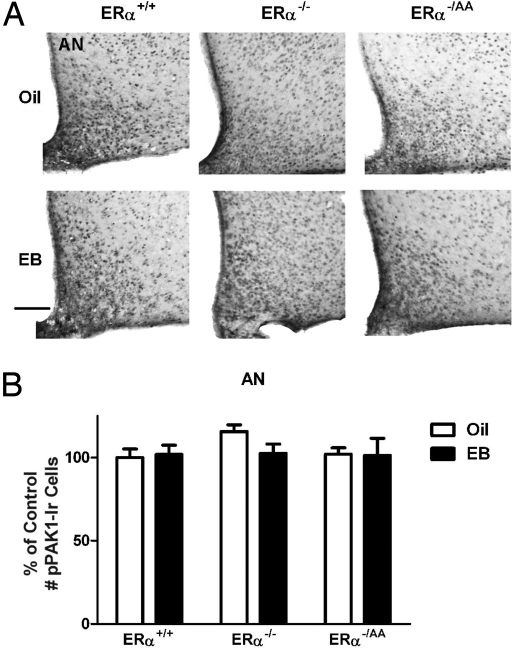
ERα can mediate negative feedback actions of E2 by nonclassical signaling mechanisms, and rapid, nonclassical ERα-mediated activation of PAK1 has been shown to occur in breast cancer cells (17). We therefore sought to determine whether nonclassical ERα signaling mediates PAK1 activation in preoptic cells. To assess the role of nonclassical ERα signaling, we compared the ability of 1 μg of EB to induce pPAK1-Ir in the preoptic areas of ERα+/+, ERα−/ −, and ERα−/AA mice within 1 h of treatment. Consistent with our findings in OVX rats, acute E2 treatment induced a significant increase in the number of pPAK1-Ir cells within 1 h in the MPNl and MPNm in OVX wild-type ERα+/+ mice (P < 0.01; Fig. 3 B and C). This effect was completely absent in the ERα−/− mice (Fig. 3 B and C), confirming the obligatory involvement of ERα in E2-mediated action. In the OVX ERα−/AA mice, the ability of E2 to induce PAK1 was restored to levels observed in the ERα+/+ mice (P < 0.05; Fig. 3 B and C), indicating that nonclassical ERα signaling is sufficient to mediate E2 effects on pPAK1 in the MPN. Representative photomicrographs of pPAK1-Ir in the MPN of the 3 genotypes are provided in Fig. 3A. The summary values for pPAK1-Ir in the 3 genotypes are depicted in Fig. 3 B and C. In contrast to the effects of E2 in the preoptic area, E2 was without any effect on pPAK1-Ir in the AN in any of the groups (Fig. 4).
Fig. 3.
E2-induced pPAK1-Ir in the MPN of female ERα+/+, ERα−/−, and ERα−/AA mice. OVX female mice treated with oil or EB were killed at 1 h after injection. (A) Representative photomicrographs of pPAK1-Ir in the MPN and adjacent regions of OVX mice 1 h after oil or EB injection. (Scale bar: 100 μm.) The number of pPAK1-Ir cells was significantly increased in the MPNl (B) and MPNm (C) in ERα+/+ and ERα−/AA but not ERα−/− mice (n = 5–9). Data are represented as the mean ± SEM (**, P < 0.01; *, P < 0.05).
Fig. 4.
E2-independent pPAK1-Ir in the AN of female ERα+/+, ERα−/−, and ERα−/AA mice. Animals were treated as described in Fig. 3. (A) Representative photomicrographs of pPAK1-Ir in the AN and adjacent regions of OVX mice 1 h after oil or EB injection. (Scale bar: 100 μm.) The number of pPAK1-Ir cells in the AN was not significantly different after EB treatment compared with values in animals receiving corresponding oil treatment (B). Data are represented as the mean ± SEM (n = 5–8).
Nonclassical ERα Signaling Mediates Rapid Negative Feedback Actions of E2.
If E2 can rapidly activate pPAK1 through nonclassical ERα signaling, and this mechanism is integral to E2 negative feedback, then it should be true that E2 can engage this mechanism to effect a rapid suppression of LH secretion. We therefore tested the ability of acute EB injections to rapidly suppress LH by nonclassical ERα signaling. To examine the rapid feedback actions of E2 on LH release, ERα+/+, ERα−/−, and ERα−/AA female mice were ovariectomized, and 7 days later (1,000–1,200 h) they received s.c. injections of 1 μg of EB or oil vehicle. Animals were killed 1 h after the injection. A total of 3 blood samples were collected from each mouse—one at the time of OVX, one just before the EB injection, and one at sacrifice 1 h later. LH RIA of the plasma samples revealed that LH levels before OVX were low in ERα+/+, slightly elevated in ERα−/AA, and greatly increased in ERα−/ − mice, as reported previously (7). The serum LH levels were significantly elevated in both ERα+/+ (P < 0.001; Fig. 5, a) and ERα−/AA (P < 0.05; Fig. 5, b) mice at 7 days after OVX compared with pre-OVX levels. In contrast, LH levels in ERα−/− mice were elevated before OVX and remained at these levels at 7 days after OVX. Treatment of OVX ERα+/+ mice with EB resulted in a suppression of LH within 1 h, whereas the same treatment was without effect in the OVX ERα−/− mice. In the OVX ERα−/AA mice, EB suppressed LH to the pre-OVX levels (P < 0.05; Fig. 5, d). The inhibitory action of EB in OVX ERα−/AA mice constituted ≈70% of the suppression seen in the OVX ERα+/+ mice (P < 0.001; Fig. 5, c).
Fig. 5.
The ERE-independent ERα signaling pathway is sufficient to convey rapid E2 negative feedback actions. Serum LH from intact, OVX, and OVX/EB-injected (1 h) females in the morning (n = 8–14). Data are represented as the mean ± SEM (***, P < 0.001; *, P < 0.05).
Inhibition of PAK Phosphorylation Blocks Acute EB Suppression of LH Secretions.
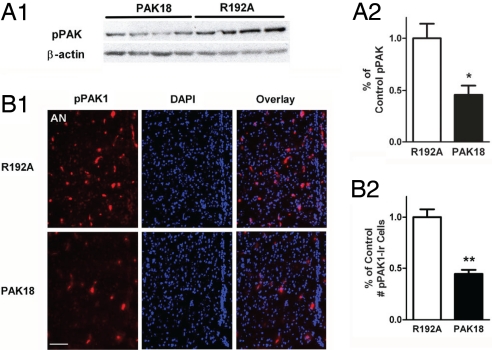
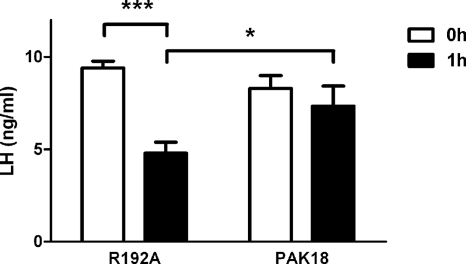
Within the PAK kinases, a conserved, proline-rich sequence of 18 aa called PAK18 binds tightly to the SH3 domain of PAK-interacting exchange factor (PIX). The PIX–PAK interaction was shown to be essential for PAK activation (21). The PAK18 peptide has been used to interfere selectively with the activation of PAKs 1–3 in cell cultures (22–26) and in vivo in the rat forebrain (22). PAK18 was conjugated to a cell-permeant HIV-1 TAT peptide sequence, which has been used to deliver functional biomolecules into cells (27). The TAT-facilitated cellular translocation has been shown to happen very rapidly (within 1–2 h) and results in neurofunctional effects in vitro and in vivo (28, 29). The PAK18 peptide but not the inactive peptide PAK18 R192A has been shown to reduce pPAK levels in the hippocampus by 80% after intracerebroventricular (icv) treatments, an effect that was accompanied by drebrin loss, cofilin pathology, and memory deficits (22). This suggests that PAK18 and PAK18 R192A are valid tools for inhibiting PAKs. We first verified the ability of PAK18 to block PAK phosphorylation by using hypothalamic GT1-7 cells in vitro. Western blot analysis of pPAK demonstrated that inhibition of PAK phosphorylation is rapid and significant when these cells are incubated with the peptide PAK18 (10 μM) for 1 h compared with R192A (P < 0.05; Fig. 6A). Subsequently, we infused peptide PAK18 (6 μg/μL, 1 μL/min in 5 min) or R192A in the lateral ventricle of OVX rats. The cerebrospinal fluid (CSF) volume was 250 μL per rat, with a physiological flow rate of 2.9 μL min−1 (30); therefore, the concentration of PAK18 and R192A in the CSF was ≈10 μM 1 h after the infusion, a dose found effective in vitro. Immediately after the peptide infusion, OVX animals were given an s.c. injection of EB (30 μg per rat). At 1 h after EB injection, animals were killed, and brains were removed rapidly to assess the ability of PAK18 to reduce pPAK1-Ir by immunohistofluorescence, indicating a suppression of PAK1 phosphorylation. As depicted in Fig. 6B, infusion of PAK18 peptide for 1 h resulted in a significant reduction in the number of pPAK1-Ir cells in the preoptic area compared with the control treatment (P < 0.01; Fig. 6B). To determine the effects of PAK18 infusions on responsiveness to the negative feedback actions of E2, blood samples were obtained just before infusions and at autopsy 1 h after the EB treatment. Analysis of serum LH levels by RIA revealed that in OVX rats, 5-min icv infusion of PAK18 but not control peptide R192A blocked rapid suppression of serum LH 1 h after EB administration (Fig. 7).
Fig. 6.
Inhibition of PAK phosphorylation. Inhibition of PAK phosphorylation by treatment with PAK18 inhibitory peptide to GT1-7 cells (A) and in the lateral ventricle of OVX rats (B) for 1 h (n = 4–6). (Scale bar: 100 μm.) Data are represented as the mean ± SEM (**, P < 0.01; *, P < 0.05).
Fig. 7.
Inhibition of PAK phosphorylation blocks acute E2 suppression of LH secretions. The LH level was significantly decreased at 1 h after R192A icv infusion and EB s.c. injection. In the contrast, the LH level was not significantly altered by treatment with PAK18 and EB (n = 4–6). Data are represented as the mean ± SEM (***, P < 0.001; *, P < 0.05).
Discussion
Our laboratories recently demonstrated that the majority of E2 negative feedback actions in the mouse can be exerted by a nonclassical ER α signaling mechanism that proceeds in the absence of direct binding of ERα to EREs in the promoters of target genes (7). In the present study, we have further determined that these inhibitory effects can occur within 1 h of E2 administration, and thus are likely exerted via nongenotropic signaling mechanisms. Our findings specifically implicate the PAKs as components of the nongenotropic ERα signaling pathways leading to the suppression of GnRH and LH, because they show that (i) E2 rapidly induces PAK1 phosphorylation in the MPN, (ii) nonclassical ERα signaling is sufficient to rescue rapid phosphorylation of PAK1 in preoptic cells, as well as suppression of LH secretion in ERα-null mutants, and (iii) acute inhibition of PAK phosphorylation in preoptic-hypothalamic areas blocks the acute negative feedback actions of E2.
The involvement of a nongenotropic mechanism in E2 negative feedback has long been suspected, given the ability of acute E2 treatments to suppress LH secretion in as little as 20 min (31). Although rapid steroid hormone effects on gene transcription are known, the rapid (<60 min) modulation of LH by E2 occurs within a temporal window that is generally held to be too short to additionally include RNA processing, translation, posttranslational enzymatic processing, intracellular transport, and neurosecretion. A variety of membrane-integrated or membrane-associated receptors and cell signaling mechanisms have instead been suggested to mediate at least some of the E2 effects on GnRH and LH. Classic work by Kelly et al. (62) revealed rapid effects of E2 on neuronal firing in preoptic neurons that were best explained by activation of membrane receptors for E2. Further work demonstrated that GnRH neurons themselves are rapidly hyperpolarized by E2, even during tetrodotoxin-induced blockade of synaptic inputs (32), suggesting direct electrophysiological suppression through membrane-associated receptors in GnRH neurons. The absence of ERα in GnRH neurons has led some to suggest that rapid, direct effects of E2 on GnRH neurons may be mediated by ERβ (33) and/or an unidentified G protein-coupled receptor (34). It has remained unclear, however, to what degree the rapid electrophysiological effects of E2 on GnRH neuronal activity in vitro may reflect the operation of negative feedback loop governing GnRH neurosecretion in vivo. Our studies clearly implicate the ERα isoform, which is not expressed in GnRH neurons, in the rapid feedback actions of E2 in vivo, and thus alternatively suggest an indirect mechanism conveyed via ERα-expressing afferents to GnRH neurons. The direct ERα-independent effects of E2 on GnRH neurons may provide additional components of negative feedback control of GnRH and LH release, or they may function to regulate other aspects of GnRH neuronal function. We also cannot exclude the possibility that ERα-dependent mechanisms may up-regulate ERα-independent E2 signaling through other receptors, which may in turn mediate E2 feedback effects.
Rapid inhibition of LH secretion can also occur via direct suppression of gonadotrope responsiveness to GnRH (35). It does not appear to be the case, however, that the nonclassical ERα signaling mechanisms described in the present studies are mediated by any such direct actions on the gonadotrope; pituitary responsiveness to GnRH is not enhanced in ERα-null mutants (3), nor is it reduced by E2 in ERα−/AA mice (7). We have also determined that nonclassical ERα signaling fails to rescue E2 effects on LH mRNA and pituitary LH content (36), even as it does effectively restore the majority of E2 suppression of LH secretion (7). Taken together, the weight of the foregoing evidence suggests that the majority of the rapid, nonclassical ERα-mediated suppression of LH secretion occurs through inhibitory actions on afferents to GnRH neurons. These effects are also complemented by classical, ERE-mediated transcriptional effects, because a residual 30% of E2 negative feedback actions are not rescued by nonclassical ERα signaling. As reported by Christian et al. (63), the portion of preopticohypothalamic negative feedback that is conveyed by classical ERα signaling may be mediated by suppression of GnRH neuronal firing.
We have observed that E2 induces rapid phosphorylation of PAK1 in MPN cells through a nonclassical ERα signaling mechanism, and that the inhibition of PAK1 in the basal forebrain blocks the inhibition of LH secretion, presumably by preventing E2 suppression of GnRH neurosecretion. Our findings are therefore consistent with the hypothesis that PAK activation in preoptic neurons mediates the negative feedback actions of E2 on GnRH release. Although these results do not reveal the cellular signaling pathways that mediate E2 activation of PAK1, the rapidity of this process effectively limits the possibilities to those that are initiated at the plasma membrane or within the cytoplasm and that are independent of transcriptional modulation. The actions of E2 in MPN cells parallel those observed previously in breast cancer cells, where E2 was found to activate PAK1 through a rapid, nontranscriptional mechanism.
A number of kinases have been reported to phosphorylate PAK1 and regulate its activity (37), including the cyclin B-bound Cdc2, which phosphorylates PAK1 at Thr-212, a site also targeted by the p35-bound form of Cdk5, a neuron-specific protein kinase (38, 39). Moreover, extracellular signal-regulated kinase 2 (ERK2) mediates phosphorylation of PAK1 at Thr-212 (40). PAK1 is also known to function as an effector protein of PI3K (18, 41). In the present study, ERα signaling was found to induce rapid phosphorylation of PAK1 at Thr-212 in MPN cells. Because E2 can rapidly influence MAPK/ERK and PI3K/Akt signaling pathways in a variety of cell types (42, 43) and in a variety of brain regions, including the MPN (44), it is possible that ERα signaling induces phosphorylation of PAK1 by ERα-mediated activation of either or both intermediate signaling kinases.
Consistent with the hypothesis that PAK1 mediates E2 feedback effects, recent studies have revealed that many of the cellular actions of E2 in the CNS are shared by those of activated PAK1. Both activated PAK1 (23) and ERα signaling (15, 45, 46) can induce rapid alterations in the dendritic cytoskeleton that are integral to morphological plasticity in central neurons. In cortical neurons, PAK1 activity promotes formation and maintenance of dendritic spines (47). A recent study showed that E2 also rapidly increases the number of nascent dendritic spines in cortical neurons (46), and thereby acutely increases neuronal connectivity, although these effects appear to be exerted in an ERα-independent manner. Estradiol also stimulates dendritic spinogenesis in hippocampal CA1 neurons, an effect that may occur rapidly via activation of ERα and MAPK signaling (45). Previous studies have also documented that E2 induces synaptic remodeling in the ventromedial nucleus, the anteroventral periventricular nucleus, the MPN, and the AN of the hypothalamus (10, 14).
Because ERα signaling and PAKs can both mediate rapid actin cytoskeletal organization and morphological plasticity (11, 23, 48–51), it is thus possible that these downstream cellular events comprise a major route by which E2-activated PAKs may mediate negative feedback control over GnRH and, hence, LH secretion. It remains to be determined whether such a rapid, PAK-mediated alteration in cell connectivity functions in this manner and, if so, which of the many known PAK substrates (e.g., actin-related protein 2/3 complex, filamin, and/or cofilin) (19) may mediate these actions. Further studies will also be necessary to identify the ERα-expressing cell populations in which PAKs mediate these E2 effects. The absence of ERα expression in GnRH neurons (52) makes it unlikely that the ERα-mediated negative feedback mechanism that we have characterized in these studies operates within GnRH neurons themselves. Expression of ERα does occur in GABAergic neurons, including those that appear to be afferents to GnRH neurons (53), and GABA release is modulated by E2 in a variety of brain regions. Moreover, ERα has been shown recently to be localized to axon terminals in hippocampal GABAergic interneurons (54, 55), suggesting their involvement in nongenotropic modulation of synaptic function. The localization of ERs to dendrites and axon terminals has been shown previously in hypothalamic neurons as well (56). Herbison and colleagues (57) have also recently characterized a nonclassical ERα signaling mechanism that mediates E2 modulation of GABAergic transmission on GnRH neurons.
Taken together, our findings support a model for the homeostatic negative feedback actions of E2 wherein the activation of extranuclear ERα is coupled via one or more cytoplasmic kinase signaling cascades to the activation of PAKs. Activated PAKs, which would presumably include the brain-enriched PAK1, thereafter mediate alterations in cell function and/or connectivity in neural circuitries that govern GnRH neurosecretion, and thereby suppress the release of the GnRH decapeptide into the hypophysial portal vasculature. Although several of the specific features of this model await testing, the present results provide initial evidence in a physiological context that nonclassical ERα signaling, leading to the activation of one or more of the PAKs, is a signaling pathway that is integral to the manifestation of physiological E2 negative feedback control in the reproductive axis.
Materials and Methods
Animals.
The ERα−/ − and ERα−/AA mutant mice were generated as described (6, 58, 59). Further details appear in SI Materials and Methods.
Effects of EB Treatment on PAK1 Phosphorylation.
Rats and mice were anesthetized and bilaterally OVX. On the morning of day 7 after OVX (0800–1000 hours), animals were given an s.c. injection of sesame oil vehicle or EB (10 μg per rat; 1 μg per mouse). Animals were anesthetized with 75 mg/kg i.p. ketamine (Fort Dodge Laboratories) and 5 mg/kg i.p. xylazine (Burns Veterinary Supply Inc.) and transcardially perfused with 4% paraformaldehyde (Sigma), pH 7.4, at the following time points after injection: 0.5, 1, 2, or 4 h for rats; 1 h for mice (60). Further details appear in SI Materials and Methods.
Effects of OVX and Acute EB Treatment on LH Release.
Female mice 2–4 months of age were anesthetized (0800–1000 hours), and blood samples were collected immediately before OVX. At 7 days after OVX, blood samples were obtained, and either 1 μg of EB or sesame oil vehicle injections was administered s.c. At 1 h after injections, blood samples were obtained by exsanguination following cardiac puncture. Further details appear in SI Materials and Methods.
Effects of PAK18 Inhibitory Peptide on EB Suppression of LH Release.
Details of stereotaxic surgery, PAK18 inhibitory peptide infusion, EB treatment, and blood sample collection can be found in SI Materials and Methods.
Cell Culture and Western Blot Analysis.
The effectiveness of the PAK18 peptide in suppressing PAK phosphorylation was tested in GT1-7 cells, an immortalized GnRH-producing cell line (61). Further details appear in SI Materials and Methods.
Immunohistochemistry and Analysis.
Brain sections were processed for immunohistochemistry following standard procedures. Details can be found in SI Materials and Methods.
Additional experimental procedures are presented in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We are grateful to Ms. Brigitte Mann for her technical expertise. These studies were supported by National Institutes of Health grants awarded through the National Institute of Child Health and Human Development and the Office of Research on Women's Health: R01 HD20677, P01 HD21921, and P50 HD44405.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812597106/DCSupplemental.
References
- 1.Leipheimer RE, Bona-Gallo A, Gallo RV. Influence of estradiol and progesterone on pulsatile LH secretion in 8-day ovariectomized rats. Neuroendocrinology. 1986;43:300–307. doi: 10.1159/000124544. [DOI] [PubMed] [Google Scholar]
- 2.Johnston CA, Tesone M, Negro-Vilar A. Cellular mechanisms of acute estrogen negative feedback on LH secretion: Norepinephrine, dopamine and 5-hydroxytryptamine metabolism in discrete regions of the rat brain. Brain Res Bull. 1984;13:363–369. doi: 10.1016/0361-9230(84)90086-8. [DOI] [PubMed] [Google Scholar]
- 3.Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- 4.Wintermantel TM, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman KM, Smith CL. Intracellular signaling pathways: Nongenomic actions of estrogens and ligand-independent activation of estrogen receptors. Front Biosci. 2001;6:D1379–D1391. doi: 10.2741/coleman. [DOI] [PubMed] [Google Scholar]
- 6.Jakacka M, et al. An estrogen receptor (ER)alpha deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol. 2002;16:2188–2201. doi: 10.1210/me.2001-0174. [DOI] [PubMed] [Google Scholar]
- 7.Glidewell-Kenney C, et al. Nonclassical estrogen receptor alpha signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci USA. 2007;104:8173–8177. doi: 10.1073/pnas.0611514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olmos G, et al. Synaptic remodeling in the rat arcuate nucleus during the estrous cycle. Neuroscience. 1989;32:663–667. doi: 10.1016/0306-4522(89)90288-1. [DOI] [PubMed] [Google Scholar]
- 9.Langub MC, Maley BE, Watson RE. Estrous cycle-associated axosomatic synaptic plasticity upon estrogen receptive neurons in the rat preoptic area. Brain Res. 1994;641:303–310. doi: 10.1016/0006-8993(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 10.Parducz A, et al. Synaptic remodeling induced by gonadal hormones: Neuronal plasticity as a mediator of neuroendocrine and behavioral responses to steroids. Neuroscience. 2006;138:977–985. doi: 10.1016/j.neuroscience.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Cohen RS, Pfaff DW. Ultrastructure of neurons in the ventromedial nucleus or the hypothalamus in ovariectomized rats with or without estrogen treatment. Cell Tissue Res. 1981;217:451–470. doi: 10.1007/BF00219357. [DOI] [PubMed] [Google Scholar]
- 12.Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naftolin F, et al. Estrogen-induced hypothalamic synaptic plasticity and pituitary sensitization in the control of the estrogen-induced gonadotrophin surge. Reprod Sci. 2007;14:101–116. doi: 10.1177/1933719107301059. [DOI] [PubMed] [Google Scholar]
- 15.Mukai H, et al. Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. J Neurochem. 2007;100:950–967. doi: 10.1111/j.1471-4159.2006.04264.x. [DOI] [PubMed] [Google Scholar]
- 16.Carlisle HJ, Kennedy MB. Spine architecture and synaptic plasticity. Trends Neurosci. 2005;28:182–187. doi: 10.1016/j.tins.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Mazumdar A, Kumar R. Estrogen regulation of Pak1 and FKHR pathways in breast cancer cells. FEBS Lett. 2003;535:6–10. doi: 10.1016/s0014-5793(02)03846-2. [DOI] [PubMed] [Google Scholar]
- 18.Tsakiridis T, Taha C, Grinstein S, Klip A. Insulin activates a p21-activated kinase in muscle cells via phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:19664–19667. doi: 10.1074/jbc.271.33.19664. [DOI] [PubMed] [Google Scholar]
- 19.Nikolic M. The pak1 kinase: An important regulator of neuronal morphology and function in the developing forebrain. Mol Neurobiol. 2008;37:187–202. doi: 10.1007/s12035-008-8032-1. [DOI] [PubMed] [Google Scholar]
- 20.Rayala SK, Kumar R. Sliding p21-activated kinase 1 to nucleus impacts tamoxifen sensitivity. Biomed Pharmacother. 2007;61:408–411. doi: 10.1016/j.biopha.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Manser E, et al. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- 22.Zhao L, et al. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9:234–242. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]
- 23.Penzes P, et al. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- 24.Maruta H, He H, Tikoo A, Nur-e-Kamal M. Cytoskeletal tumor suppressors that block oncogenic RAS signaling. Ann NY Acad Sci. 1999;886:48–57. doi: 10.1111/j.1749-6632.1999.tb09399.x. [DOI] [PubMed] [Google Scholar]
- 25.Obermeier A, et al. PAK promotes morphological changes by acting upstream of Rac. EMBO J. 1998;17:4328–4339. doi: 10.1093/emboj/17.15.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nheu T, et al. PAK is essential for RAS-induced upregulation of cyclin D1 during the G1 to S transition. Cell Cycle. 2004;3:71–74. [PubMed] [Google Scholar]
- 27.Wender PA, et al. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: Peptoid molecular transporters. Proc Natl Acad Sci USA. 2000;97:13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aarts M, et al. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- 29.Cao G, et al. In vivo delivery of a Bcl-xL fusion protein containing the TAT protein transduction domain protects against ischemic brain injury and neuronal apoptosis. J Neurosci. 2002;22:5423–5431. doi: 10.1523/JNEUROSCI.22-13-05423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusuhara H, Sugiyama Y. Efflux transport systems for drugs at the blood-brain barrier and blood-cerebrospinal fluid barrier (Part 2) Drug Discov Today. 2001;6:206–212. doi: 10.1016/s1359-6446(00)01643-3. [DOI] [PubMed] [Google Scholar]
- 31.Arreguin-Arevalo JA, Nett TM. A nongenomic action of estradiol as the mechanism underlying the acute suppression of secretion of luteinizing hormone in ovariectomized ewes. Biol Reprod. 2006;74:202–208. doi: 10.1095/biolreprod.105.044685. [DOI] [PubMed] [Google Scholar]
- 32.Kelly MJ, Ronnekleiv OK, Eskay RL. Identification of estrogen-responsive LHRH neurons in the guinea pig hypothalamus. Brain Res Bull. 1984;12:399–407. doi: 10.1016/0361-9230(84)90112-6. [DOI] [PubMed] [Google Scholar]
- 33.Abraham IM, et al. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu J, et al. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strobl FJ, Gilmore CA, Levine JE. Castration induces luteinizing hormone (LH) secretion in hypophysectomized pituitary-grafted rats receiving pulsatile LH-releasing hormone infusions. Endocrinology. 1989;124:1140–1144. doi: 10.1210/endo-124-3-1140. [DOI] [PubMed] [Google Scholar]
- 36.Glidewell-Kenney C, et al. Estrogen receptor alpha signaling pathways differentially regulate gonadotropin subunit gene expression and serum follicle-stimulating hormone in the female mouse. Endocrinology. 2008;149:4168–4176. doi: 10.1210/en.2007-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 38.Nikolic M, et al. The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature. 1998;395:194–198. doi: 10.1038/26034. [DOI] [PubMed] [Google Scholar]
- 39.Thiel DA, et al. Cell cycle-regulated phosphorylation of p21-activated kinase 1. Curr Biol. 2002;12:1227–1232. doi: 10.1016/s0960-9822(02)00931-4. [DOI] [PubMed] [Google Scholar]
- 40.Sundberg-Smith LJ, Doherty JT, Mack CP, Taylor JM. Adhesion stimulates direct PAK1/ERK2 association and leads to ERK-dependent PAK1 Thr212 phosphorylation. J Biol Chem. 2005;280:2055–2064. doi: 10.1074/jbc.M406013200. [DOI] [PubMed] [Google Scholar]
- 41.Adam L, et al. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J Biol Chem. 1998;273:28238–28246. doi: 10.1074/jbc.273.43.28238. [DOI] [PubMed] [Google Scholar]
- 42.Kato S, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 43.Sun M, et al. Phosphatidylinositol-3-OH Kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor alpha (ERalpha) via interaction between ERalpha and PI3K. Cancer Res. 2001;61:5985–5991. [PubMed] [Google Scholar]
- 44.Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- 45.Murakami G, et al. Comparison between basal and apical dendritic spines in estrogen-induced rapid spinogenesis of CA1 principal neurons in the adult hippocampus. Biochem Biophys Res Commun. 2006;351:553–558. doi: 10.1016/j.bbrc.2006.10.066. [DOI] [PubMed] [Google Scholar]
- 46.Srivastava DP, et al. Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc Natl Acad Sci USA. 2008;105:14650–14655. doi: 10.1073/pnas.0801581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi K, Ohshima T, Hashimoto M, Mikoshiba K. Pak1 regulates dendritic branching and spine formation. Dev Neurobiol. 2007;67:655–669. doi: 10.1002/dneu.20363. [DOI] [PubMed] [Google Scholar]
- 48.Meng Y, et al. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 2002;35:121–133. doi: 10.1016/s0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- 49.Boda B, et al. The mental retardation protein PAK3 contributes to synapse formation and plasticity in hippocampus. J Neurosci. 2004;24:10816–10825. doi: 10.1523/JNEUROSCI.2931-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tolias KF, et al. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron. 2005;45:525–538. doi: 10.1016/j.neuron.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 51.Zhao L, Brinton RD. Estrogen receptor alpha and beta differentially regulate intracellular Ca(2+) dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res. 2007;1172:48–59. doi: 10.1016/j.brainres.2007.06.092. [DOI] [PubMed] [Google Scholar]
- 52.Hrabovszky E, et al. Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–3264. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- 53.Christian CA, Moenter SM. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci. 2007;27:1913–1921. doi: 10.1523/JNEUROSCI.4738-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Milner TA, et al. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- 55.Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-alpha-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J Neurosci. 2007;27:2102–2111. doi: 10.1523/JNEUROSCI.5436-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blaustein JD, Lehman MN, Turcotte JC, Greene G. Estrogen receptors in dendrites and axon terminals in the guinea pig hypothalamus. Endocrinology. 1992;131:281–290. doi: 10.1210/endo.131.1.1612006. [DOI] [PubMed] [Google Scholar]
- 57.Romano N, et al. Nonclassical estrogen modulation of presynaptic GABA terminals modulates calcium dynamics in gonadotropin-releasing hormone neurons. Endocrinology. 2008;149:5335–5344. doi: 10.1210/en.2008-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McDevitt MA, et al. Estrogen response element-independent estrogen receptor (ER)-alpha signaling does not rescue sexual behavior but restores normal testosterone secretion in male ERalpha knockout mice. Endocrinology. 2007;148:5288–5294. doi: 10.1210/en.2007-0673. [DOI] [PubMed] [Google Scholar]
- 59.Dupont S, et al. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 60.Gu G, et al. Hormonal regulation of CREB phosphorylation in the anteroventral periventricular nucleus. J Neurosci. 1996;16:3035–3044. doi: 10.1523/JNEUROSCI.16-09-03035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mellon PL, et al. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron. 1990;5:1–10. doi: 10.1016/0896-6273(90)90028-e. [DOI] [PubMed] [Google Scholar]
- 62.Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114:152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- 63.Christian CA, Glidewell-Kenney C, Jameson JL, Moenter SM. Classical estrogen receptor alpha signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology. 2008;149:5328–5334. doi: 10.1210/en.2008-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.