SUMMARY
We describe a new approach for the identification and characterization by mass spectrometry of proteins that have been electroblotted onto nitrocellulose. Using this method (Blotting And Removal of Nitrocellulose, or BARN), proteins can be analyzed either as intact proteins for molecular weight determination or as peptides generated by on-membrane proteolysis. Acetone is used to dissolve the nitrocellulose and to precipitate the adsorbed proteins/peptides, thus removing the nitrocellulose which can interfere with mass spectrometry analysis. This method offers improved protein coverage, especially for membrane proteins such as uroplakins, since the extraction step after in-gel digestion is avoided. Moreover, removal of nitrocellulose from the sample solution allows sample analysis by both MALDI- and (LC) ESI-based mass spectrometers. Finally, we demonstrate the utility of BARN for the direct identification of soluble and membrane proteins after Western blotting, obtaining comparable or better results than with in-gel digestion.
INTRODUCTION
In-gel enzymatic digestion of gel-separated proteins followed by mass spectrometry (MS) analysis is a powerful tool for protein identification and characterization of posttranslational modifications (1). However, this method has several major shortcomings including low accessibility of proteases and other digestive reagents to the gel-entrapped proteins as well as a low recovery of large and/or hydrophobic peptides from the gels (2). These shortcomings can be especially problematic for the study of membrane proteins that typically contain hydrophobic peptides, and for the location and determination of posttranslational modifications, since modified residues and protein segments may escape detection (3). Several alternatives have therefore been developed to overcome these shortcomings, including the transfer of the gel-resolved proteins by electroblotting onto nitrocellulose (NC) (4–6) or poly(vinyl difluoride) (PVDF) membranes (7–9). An additional advantage of using electroblotted proteins is the possibility of analyzing intact proteins (3,5) by avoiding inefficient and time consuming gel extraction methods such as electroelution (10) and passive diffusion (11,12).
After electroblotting, proteins usually are either digested on the membrane followed by extraction of the peptides from the polymer bands (4) and subsequent MS analysis, or the NC bands are dissolved directly in MALDI matrix solution after on-membrane digestion for MS analysis (5,13).. The first procedure suffers, however, from a similar limitation as in-gel digestion because the recovery of large and/or hydrophobic peptides can be low. The second procedure solves this problem since the peptides adsorbed onto the membrane are dissolved together with the NC. Acetone was originally used to dissolve the NC membrane but the yield is low due to partial precipitation of proteins/peptides in acetone (5). We demonstrated in a previous paper (3) that this problem can be solved simply by replacing acetone with a mixture of 70:30 acetonitrile:methanol as the MALDI matrix solution. Compared to in-gel digestion, this improved method provided a doubling of the amino acid sequence coverage and better digestion efficiencies for integral membrane proteins such as uroplakins (3).
Although small amounts of NC have been added to the MALDI matrix solution to obtain homogeneous matrix crystallization and to enhance peptide detection (14,15), high concentrations of nitrocellulose can interfere with MALDI-MS performance (3,5). In addition, the presence of nitrocellulose in a sample mixture prevents the use of ESI-MS for analysis. In this article, we present a novel approach for the analysis of electroblotted proteins by both MALDI- and ESI-based mass spectrometers. This approach is based on the removal of the NC from the solution before MS analysis (Fig. 1), thus increasing the sensitivity and allowing the use of ESI-MS by direct infusion or after liquid chromatography (LC). This method (Blotting And Removal of Nitrocellulose, or BARN) also allows the identification of electroblotted proteins after Western blotting on the same NC band, thus significantly reducing the time and sample size needed per analysis and eliminating the need for the difficult correlation of protein bands or spots detected by Western blot on the NC to another stained gel or membrane (16).
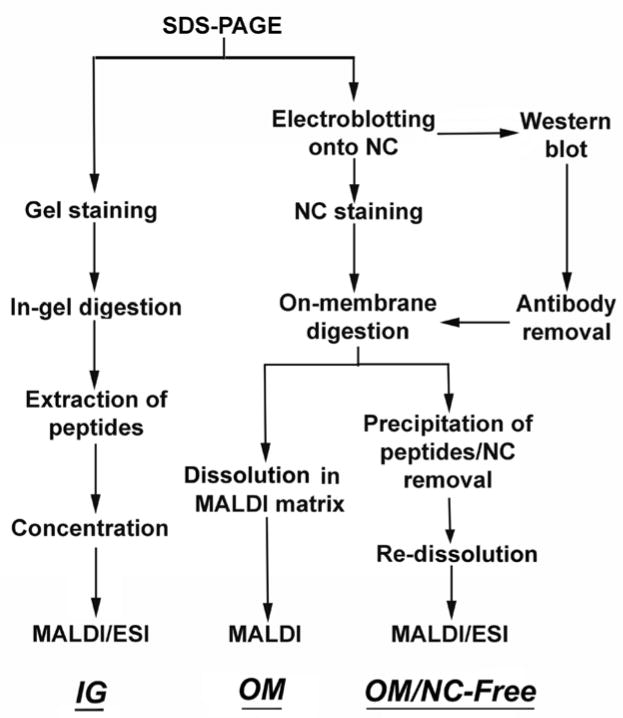
Fig. 1.
Steps required for in-gel, and on-membrane digestion with (OM) or without (OM/NC-Free) removal of the nitrocellulose.
EXPERIMENTAL PROCEDURES
SDS-PAGE and electroblotting
Proteins were separated by SDS-PAGE on 10% polyacrylamide gels as described by Laemmli.(17) After electrophoresis, proteins were electroblotted to a 100% Triton-free nitrocellulose membrane (pore size, 0.2 μm) (Bio-Rad) in a transfer buffer of 25 mM Tris, 192 mM glycine, 0.1% SDS, and 20% methanol (15ºC, 400 mA, and 1 h). MemCode (Pierce) staining and destaining were carried out according to the manufacturer’s instructions.
Molecular weight determination of intact proteins
NC bands containing electroblotted intact proteins were dissolved in acetone (90 μl acetone/4 mm2 NC), mixed by vortexing, and incubated for 30 min at room temperature to allow complete dissolution of the NC and precipitation of the protein/s. The supernatant fluid containing the acetone was removed and the precipitate was air-dried. The precipitated proteins were re-suspended in either 10 mg/ml α-CHCA (Sigma) prepared in 25:75 acetonitrile-methanol and 1% TFA (Sigma) for MALDI-MS analysis or in 2% acetonitrile, 3% FA (Sigma) for ESI-MS by direct infusion. The method used for validation based on direct dissolution of the NC bands in the MALDI matrix solution was performed as previously described (3).
On-membrane digestion
After electroblotting the proteins onto a NC membrane and staining with MemCode as described above, the bands containing the protein/s of interest were excised and destained. Nonspecific protein binding sites on the nitrocellulose were blocked by adding 0.5 ml of 0.5% (w/v) poly(vinylpyrrolidone) (PVP-40) (Sigma) in 100 mM acetic acid at 37ºC for 30 min before on-membrane digestion (4). After washing the NC bands at least 6 times with Milli-Q water to remove excess PVP-40, trypsin (Promega) at 12.5 ng/μl prepared in 50 mM NH4HCO3 buffer (pH = 8) was added to the NC bands and incubated at 37ºC overnight. After digestion, the samples were dried under vacuum, dissolved in acetone (90 μl acetone/4 mm2 NC), vortexed and incubated for 30 min at room temperature. The acetone containing the dissolved NC was carefully removed and the precipitated peptides were air-dried. Re-suspension of the peptides was carried out depending on the nature of the protein/s and the mass spectrometer used for further analysis. For proteins analyzed by MALDI-MS, re-suspension was carried out by adding 20 μl of 10 mg/ml of α-CHCA in either 1% TFA in 50:50 acetonitrile-water for soluble proteins or 1% TFA in 25:75 acetonitrile-methanol for membrane proteins. For soluble and membrane proteins analyzed by LC-ESI-MS, the peptides were resuspended in 20 μl of 2% acetonitrile in 0.1% FA. All solutions were sonicated for 10 min before MS analysis.
In-gel digestion and the alternative on-membrane digestion method used to validate the NC-free approach were carried out according to previously published protocols (1,3).
Western blotting and antibody removal
0.1–1 μg of purified bovine asymmetric unit membranes (AUM) (18–20) containing four major uroplakins (Ia, Ib, II and III) were dissolved in SDS loading buffer. Proteins were resolved by SDS PAGE and transferred onto a nitrocellulose membrane. Uroplakin II (UPII) was detected by a mono-specific polyclonal antibody against UPII and uroplakin III (UPIII) by a mouse monoclonal antibody against UPIII. HRP-conjugated goat anti-rabbit or goat anti-mouse sera were used as secondary antibodies (Sigma), respectively, and visualized by ECL reagent (Pierce). Clean new dishes were used to avoid contamination by BSA or casein from previous Western blots.
The developed film was superimposed onto the original nitrocellulose membrane and the proteins of interest were excised according to the Western blot signal. Antibody removal was carried out by three washes of the NC bands with 1.5 ml of 20 mM sodium bicarbonate buffer (pH 7.4) for 5 min each at room temperature, followed by three additional washes with 1.5 ml 100 mM glycine (pH 2.4) for 10 min each at room temperature. Finally, the bands were washed again with 1.5 ml of 20 mM bicarbonate buffer (pH 7.4) for 5 min. Subsequent on-membrane digestion was carried out as described above.
Mass spectrometry
Linear and reflectron mode MALDI-TOF mass spectra were acquired using a Micromass Tof Spec-2E mass spectrometer using standard parameters: a nitrogen laser (λ = 337 nm), laser pulse 39 ns, and accelerating voltage 20 Kv. External calibration was carried out using angiotensin I (average mass, 1296.5 Da), corticotrophin-like intermediate lobe peptide (ACTH clip 18–39, average mass, 2465.7) for peptides mass measurements, or cytochrome c (average mass, 12230 Da) and bovine serum albumin (average mass, 66430 Da) for analyses of intact proteins. Typically, 100–200 laser shots were summed into each mass spectrum. The spectra obtained were processed using MassLynx MaxEnt 3 (Micromass) software.
Nanoflow LC-MS/MS was also used for the analysis of peptide mixtures from tryptic on-membrane and in-gel digestions. The peptides were loaded onto a 0.3 x 1-mm C18 nano-precolumn (LC Packings), and then washed 5 min with 2% acetonitrile in 0.1% FA at a flow rate of 20 μl/min. After washing, flow was reversed through the precolumn and the peptides eluted with a gradient 2–90% acetonitrile in 0.1% FA. The gradient was delivered over 120 min by a CapLC (waters) HPLC system at a flow rate of 200 nl/min, obtained by a 15:1 precolumn flow split, through a 75 μm x 15 cm fused silica capillary C18 HPLC column (LC Packings PepMap) to a fused silica distal end-coated tip nano-electrospray needle (New Objective). The Q-TOF micro (Micromass) data acquisition involved MS survey scans and automatic data-dependent MS/MS acquisition, which were invoked after selected ions met preset parameters of minimum signal intensity of 12 counts per second, ion charge state +2, +3 or +4, and appropriate retention time. Survey scans of 1 s were followed by CID of the three most intense ions for up to 6 s each, or until 5000 total MS/MS ion counts per precursor peptide were achieved. The raw MS data were subsequently processed using manufacturer-supplied ProteinLynx 3.5 software, with the following settings: Background subtraction of polynomial order 10 below a 10% curve, 1 smooth with a window of two channels in Savitzky Golay mode, followed by centroid calculation of the top 80% of peaks based on a minimum peak width of 4 channels at half-height. On the basis of these parameters, pkl files incorporating parent ion mass and retention time as well as peak lists for each corresponding MS/MS spectrum were generated. In-house Mascot software (version 2.0.00, Matrix Science) was used for database searching and protein identification using the mammalian database from NCBI (downloaded 03/11/2005) with a minimum parent-ion and fragment-ion mass accuracy of 0.3 Da.
RESULTS
Optimization of the variables
We first wanted to optimize the conditions such as the composition and volume of the organic solvent used for dissolving the NC membrane and the precipitation of the proteins/peptides, the time and temperature of the precipitation step, and the solution used for re-dissolving the precipitated proteins/peptides before MS analysis.
One of the key features of our NC-free method is that we dissolve the NC membrane using an organic solvent which simultaneously precipitates the electroblotted proteins/peptides, thus separating them in a single step. We tested acetone, acetonitrile, methanol and ethanol for this purpose, and we found that the use of acetone provided the best MALDI-MS results for intact myoglobin and especially for intact BSA (Supplemental Fig. 1a). These data are consistent with the fact that acetone is one of the most widely used solvent for protein precipitation (21).
We tested precipitation times between 10 and 180 min. The MS signal increased after 10 to 30 min of precipitation, but started to decrease with longer times, possibly due to partial redissolution of the proteins/peptides after longer times. Thus, 30 min was selected as optimum precipitation time for subsequent experiments (Supplemental Fig. 1b).
We compared the use of acetone at room temperature (21°C) and −20°C. Even though most protocols use cold acetone to precipitate proteins, we found that acetone at room temperature actually gave better MS signals for both myoglobin (Supplemental Fig. 1c) and BSA (Supplemental Fig. 1d) (21). It is possible that although cold acetone is more efficient for precipitating proteins, it might be less effective in dissolving the NC, leading to the partial suppression of the MS signal.
Different volumes of acetone to dissolved the NC and precipitate the proteins/peptides were also tested. Using larger volumes of acetone (30 μl/mm2 NC) facilitated the dissolution of the NC membrane but probably decreased the recovery of the proteins thus leading to reduced MS signals. We found 22.5 μl of acetone per mm2 of NC to give the highest MS signals (Supplemental Fig. 1c,d).
We also tested various solutions to be used for re-dissolving the acetone-precipitated proteins/peptides. The optimal solution depended on whether intact proteins or peptides were analyzed and on what kind of mass spectrometer was to be used for subsequent analysis. For the analysis of intact proteins using MALDI-MS, we tested the ability of binary mixtures of acetonitrile/methanol at different ratios to re-dissolve soluble (myoglobin and BSA) and membrane (uroplakins II and III) proteins. All the solutions were prepared in 10 mg/ml α-cyano-4-hydrixycinnamic acid (α-CHCA) and 1% TFA (3). In all cases (Supplemental Fig. 2a), we obtained the best MS signal with the 25:75 acetonitrile/methanol mixture so this condition was used for all subsequent studies. For the analysis of digested proteins by MALDI-MS, we tested various mixtures of acetonitrile, methanol and water (Supplemental Fig. 2c,d). We obtained different results depending on the nature of the proteins analyzed. For soluble proteins, we obtained the best MS signal (Supplemental Fig. 2c) as well as the best protein coverage (Supplemental Fig. 2d) when using a binary mixture of 50:50 acetonitrile/water, which we therefore used for further experiments. For membrane proteins that contained many hydrophobic peptides, although mixtures acetonitrile/water gave the best MS signal (Supplemental Fig. 2c), acetonitrile/methanol gave a better protein coverage most likely due to the fact that hydrophobic peptides were better re-dissolved, and thus detected by MALDI-MS, in the absence of water (Supplemental Fig. 2d). For this reason, we used 75:25 methanol/acetonitrile to analyze digested membrane proteins in subsequent experiments. When using direct infusion ESI-MS, the solution used must be able to re-dissolve the precipitated proteins/peptides and to provide a good and stable ion spray. It is well-known that solutions with a moderate to high percentage of water provide a more stable spray. When we tested different mixtures of acetonitrile, methanol and water to re-dissolve intact proteins before ESI-MS analysis by direct infusion, we obtained the best results using 2% acetonitrile while solutions with a high organic solvent content could not be used since the spray was too unstable. Another key variable that highly increased the signal-to-noise ratio was the formic acid (FA) content. Different concentrations of FA were compared and the best results were obtained with solutions containing 3% FA (Supplemental Fig. 3). For LC-MS analysis, we re-suspended the samples in 2% acetonitrile, 0.1% FA to maximize binding of the proteins/peptides to the reverse phase column.
Evaluation of different protein digestion strategies
After electroblotting the proteins onto the NC membrane, the intact proteins can be enzymatically digested on-membrane before removal of the NC or digested in-solution after removal of the NC prior to mass spectrometry. For the former strategy, we digested electroblotted myoglobin and BSA on-membrane with or without Rapigest®. For the latter, we digested the same proteins in-solution in the presence or absence of 10% acetonitrile, urea, Rapigest® and the combination of 10% acetonitrile and urea. We obtained better coverage for both proteins using the on-membrane digestion strategy with no significant differences observed between using Rapigest® or not (Supplemental Fig. 4).
MALDI-MS analysis after removal of NC
To evaluate the suitability of the NC-free method for the determination of the molecular weight of intact protein by MALDI-MS, we compared this approach with a previously published method (3) that was based on the direct dissolution of the protein together with the NC band in the MALDI matrix solution. Myoglobin, BSA, uroplakin II (UPII) and uroplakin III (UPIII) were used as models of soluble and integral membrane proteins. Although both methods worked quite efficiently and could detect as little as 200 fmol of myoglobin, the NC-free approach gave better signal intensity providing almost twice the ion counts for the same protein concentration as compared to the previous method without removing the NC prior to MS (Supplemental Fig. 5).
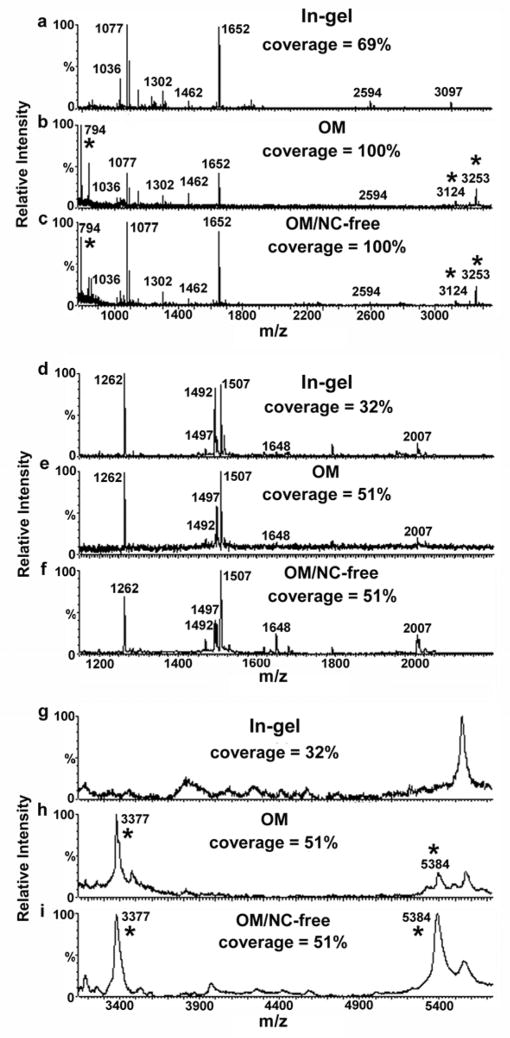
We also compared MALDI-TOF MS of digested membrane proteins prepared using the on-membrane digestion followed by NC-removal, in-gel digestion, or the previous on-membrane digestion method without NC removal (3). The NC-free on-membrane digestion method provided the same protein coverage as the previous on-membrane digestion method (almost double when compared with in-gel digestion for UPII and UPIII) but with a better signal-to-noise ratio, similar to that obtained after in-gel digestion (Fig. 2). We conclude that the NC-free approach is as sensitive as in-gel digestion but with the advantage of allowing the identification of large and/or hydrophobic peptides by MALDI-MS that can be missed after in-gel digestion.
Fig. 2.
Comparison of MALDI MS spectra obtained from (a–c) 10 pmol of UPII and from (d–i) 3 pmol of UPIII after (a, d, g) in-gel digestion, (b, e, h) on-membrane digestion followed by direct dissolution in the MALDI matrix and (c, f, i) on-membrane digestion followed by removal of the NC before MS analysis. Spectra a–f were collected in reflectron mode and spectra g–i in linear mode. Asterisks denote peaks corresponding to peptides found only after on-membrane but not in-gel digestion. "Coverage" refers to percentage of UPII or UPIII amino acid sequence accounted for by observed peptides.
LC-ESI-MS/MS analysis after on-membrane digestion followed by removal of NC
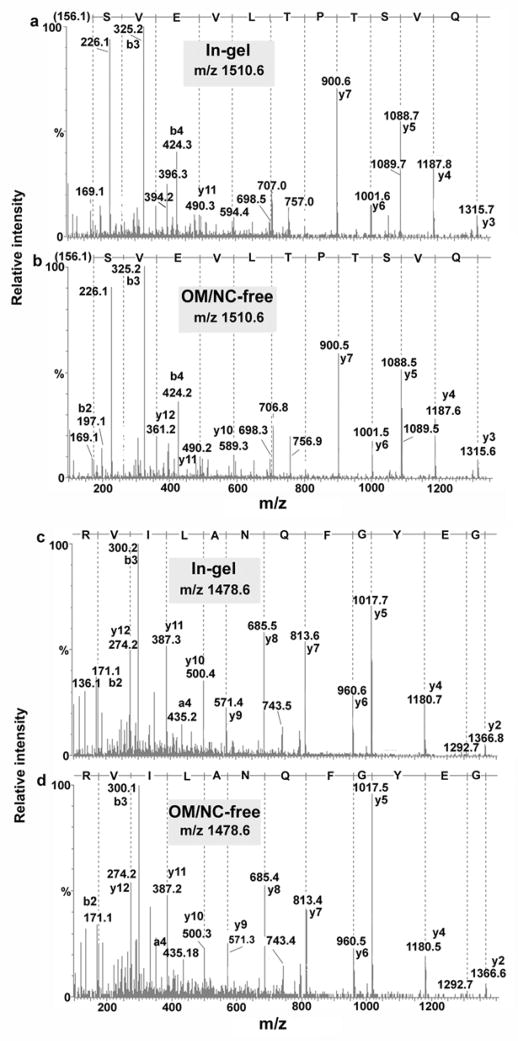
The main advantage of the NC-free on-membrane digestion method is that the nitrocellulose is removed from the solution before MS analysis, thus allowing the analysis of digested proteins by LC-ESI-MS for protein identification. When we compared this method with in-gel digestion, similar results were obtained in terms of protein sequence coverage, signal-to-noise ratio, and Mascot scores obtained by LC-MS/MS analysis. The number of peptides identified and the quality of the MS/MS spectra obtained for in-gel and on-membrane digested BSA and myoglobin were very similar (Fig. 3).
Fig. 3.
Comparison of LC-Q-TOF-MS/MS spectra obtained from 1 pmol BSA after (a, c) in-gel digestion and (b, d) on-membrane digestion (NC-free method).
Application of the NC-free/on-membrane digestion method to protein identification after Western blotting
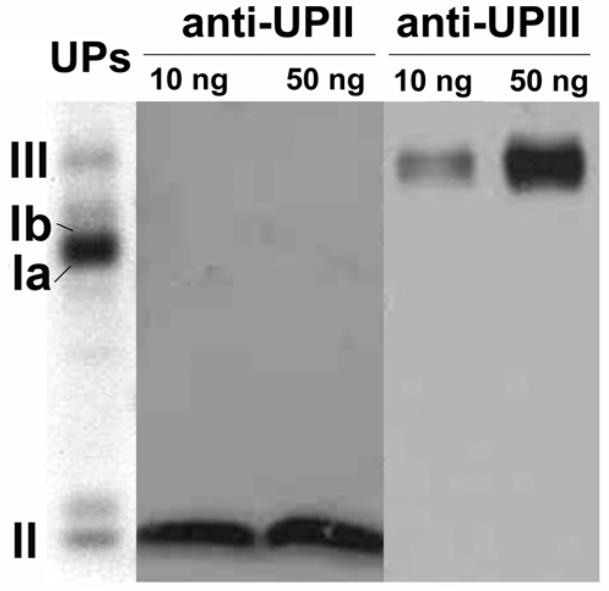
One of the most important advantages of this new method is the ability to identify proteins by MS after Western blotting, using the same NC membrane band for the identification and the Western blot. For this purpose, it is mandatory that we use a protein-free blocking agent during the Western blot procedure to avoid interfering with the subsequent mass spectrometric analysis. For this purpose, we tested a commercially available protein-free blocking buffer for the identification of uroplakins II (UPII) and III (UPIII) by Western blot (Fig. 4). After Western blotting, we excised the NC bands containing UPII and UPIII, removed bound antibodies with several glycine washes, and digested the proteins on membrane with trypsin (see the “methods” section for details). The same proteins at the same concentrations were also processed in parallel using conventional in-gel digestion for validation. Similar Mascot probability scores and protein coverage were obtained for the identification by LC-MS/MS of UPII (Table I). In the case of UPIII, 3 peptides were identified after in-gel digestion providing a Mascot score of 227 (Table I), while on-membrane digestion identified five peptides leading to a Mascot score of 394 (Table I). The two additional peptides detected after Western blotting and on-membrane digestion were identified with high quality MS/MS spectra and high Mascot scores (81 and 96) (Fig. 5). As previously demonstrated by MALDI-MS, we identified by LC-MS/MS an equal or larger number of peptides after on-membrane digestion of electroblotted proteins than after in-gel digestion, even after carrying out a previous Western blot on the same protein bands.
Fig. 4.
Western blot of UPII and UPIII using a commercial protein-free blocking agent. Left lane (UPs): Coomassie blue stain of uroplakin preparation blotted to the membrane.
TABLE I.
Comparison of the peptides detected by ESI-MS from uroplakins II and III after conventional in-gel digestion or on-membrane digestion after Western blot analysis
| m/z | Sequence | Scorea | |
|---|---|---|---|
| IGb | OM/NC-freec | ||
| Uroplakin II | |||
| 1033 | AESIGLAMAR + Ox(M)d | 31 | 13 |
| 1092 | EIPMSTFPR + Ox(M) | 30 | 23 |
| 1145 | KAESIGLAMAR + Ox(M) | 34 | 75 |
| 1148 | YYSYLVTK | 39 | 31 |
| 1462 | LSAYQVTNLAPGTK | 92 | 91 |
| 1651 | ELVSVVDSGSGFTVTR | 79 | 96 |
| Uroplakin III | |||
| 1261 | ASEILNAYLIR | 60 | 81 |
| 1491 | LTLYSAIDTWPGR | N.D.e | 81 |
| 1496 | SLGTSEPSYTSVNR | 78 | 32 |
| 1506 | TPLSSTFQQTQGGR | 89 | 104 |
| 2025 | GDADGATSHDSQITQEAVPK | N.D. | 96 |
Mascot score.
Conventional in-gel digestion.
On-membrane digestion carried out after Western blot analysis.
Oxidation of Methionine.
ND, not detected.
Fig. 5.
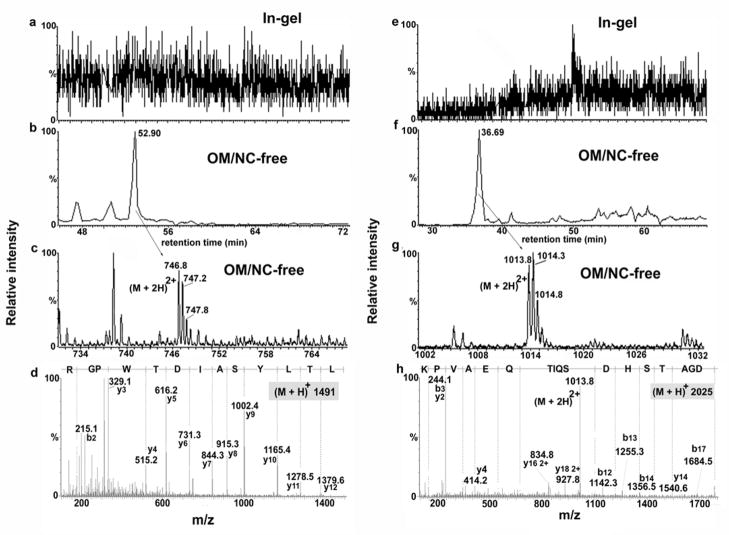
Chromatograms, MS and MS/MS spectra obtained by LC-MS/MS (Q-TOF) of peptides from UPIII identified after Western blotting followed by on-membrane digestion (NC-free method) but not by conventional in-gel digestion. (a, e) extracted ion chromatogram (m/z 747 and 1014, respectively) after in-gel digestion, (b, f) extracted ion chromatogram (m/z 747 and 1014, respectively) after on-membrane digestion, (c, g) MS spectra after on-membrane digestion and (d, h) MS/MS spectra after on-membrane digestion.
DISCUSSION
Here we describe a novel approach for mass spectrometric analysis of proteins that have been electroblotted onto NC membranes. Addition of acetone at room temperature to the blotted membranes dissolves the NC and at the same time precipitates the proteins and peptides. The precipitated proteins/peptides are then analyzed by mass spectrometry after the dissolved nitrocellulose is removed and the proteins/peptides redissolved. This method has several advantages over conventional in-gel digestion, especially when dealing with membrane proteins that usually contain large and/or hydrophobic tryptic peptides. The method has been designed to minimize the amount of NC in the final sample solution to avoid the suppression of the MS signal and to allow the use of ESI-based mass spectrometric techniques. Moreover, the method avoids the need for peptide extraction required by in-gel digestion and by some of the previously published methods for on-membrane digestion of electroblotted proteins. We demonstrated the utility of the method for both MALDI-MS and LC-MS/MS.
An important feature of this novel approach is that it enables the analysis of proteins by MS after Western blotting of the same NC band that contains the immunoreactive proteins. This can be extremely useful for determining antibody specificity, for the identification of cross-reactive as well as modified or degraded proteins, for the detection of a group of proteins with a common posttranslational modification using an antibody specific for that particular modification, and for protein crosslinking studies. Methods for analyzing proteins detected by Western blotting on NC (16,22) or PVDF (23) membranes have been described based on peptide extraction from the NC or PVDF after on-membrane digestion. However, it is known that proteins can bind strongly to NC or PVDF (5,24), leading to inefficient extraction from the membranes, especially for large and/or hydrophobic peptides. Our method circumvents this problem by avoiding any extraction step.
We validated our NC-free method using two membrane proteins, uroplakins II and III, and the results showed that successful on-membrane digestion of proteins after Western blotting follow by MS analysis is not only possible, but it can even provide better protein sequence coverage and thus, more confident protein identification, than conventional in-gel digestion.
Supplementary Material
Acknowledgments
We acknowledge support from NIH grants P30 NS050276 and S10 RR14662 to T.A.N., NIH grants DK39753 and DK52206 to T.T.S., and the Consejería de Educación y Ciencia (Junta de Andalucía, Spain) for support to J.L.L.G.
ABBREVIATIONS
- α-CHCA
α-cyano-4-hydrixycinnamic acid
- BARN
Blotting And Removal of Nitrocellulose
- FA
Formic Acid
- HRP
Horseradish Peroxidase
- NC
nitrocellulose
- NCBI
National Center for Biotechnology Information
- PVP-40
Poly (vinylpyrrolidone)
- UPII
Uroplakin II
- UPIII
Uroplakin III
- OM
on-membrane digestion
- OM/NC-free
on-membrane digestion, nitrocellulose-free
References
- 1.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 2.Jonsson AP, Aissouni Y, Palmberg C, Percipalle P, Nordling E, Daneholt B, Jornvall H, Bergman T. Recovery of gel-separated proteins for in-solution digestion and mass spectrometry. Anal Chem. 2001;73:5370–5377. doi: 10.1021/ac010486h. [DOI] [PubMed] [Google Scholar]
- 3.Luque-Garcia JL, Zhou G, Sun TT, Neubert TA. Use of nitrocellulose membranes for protein characterization by matrix-assisted laser desorption/ionization mass spectrometry. Anal Chem. 2006;78:5102–5108. doi: 10.1021/ac060344t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Oostveen I, Ducret A, Aebersold R. Colloidal silver staining of electroblotted proteins for high sensitivity peptide mapping by liquid chromatography-electrospray ionization tandem mass spectrometry. Anal Biochem. 1997;247:310–318. doi: 10.1006/abio.1997.2052. [DOI] [PubMed] [Google Scholar]
- 5.Liang X, Bai J, Liu YH, Lubman DM. Characterization of SDS--PAGE-separated proteins by matrix-assisted laser desorption/ionization mass spectrometry. Anal Chem. 1996;68:1012–1018. doi: 10.1021/ac950685z. [DOI] [PubMed] [Google Scholar]
- 6.Bai J, Qian MG, Liu Y, Liang XDML. Peptide mapping by CNBr degradation on a nitrocellulose membrane with analysis by matrix-assisted laser desorption/ionization mass spectrometry. Anal Chem. 1995;67:1705–1710. [Google Scholar]
- 7.Bunai K, Nozaki M, Hamano M, Ogane S, Inoue T, Nemoto T, Nakanishi H, Yamane K. Proteomic analysis of acrylamide gel separated proteins immobilized on polyvinylidene difluoride membranes following proteolytic digestion in the presence of 80% acetonitrile. Proteomics. 2003;3:1738–1749. doi: 10.1002/pmic.200300529. [DOI] [PubMed] [Google Scholar]
- 8.Vestling MM, Fenselau C. Poly(vinylidene difluoride) membranes as the interface between laser desorption mass spectrometry, gel electrophoresis, and in situ proteolysis. Anal Chem. 1994;66:471–477. [Google Scholar]
- 9.Strupat K, Karas M, Hillenkamp F. Matrix-assisted laser desorption ionization mass spectrometry of proteins electroblotted after polyacrylamide gel electrophoresis. Anal Chem. 1994;66:464–470. [Google Scholar]
- 10.Haebel S, Jensen C, Andersen SO, Roepstorff P. Isoforms of a cuticular protein from larvae of the meal beetle, Tenebrio molitor, studied by mass spectrometry in combination with Edman degradation and two-dimensional polyacrylamide gel electrophoresis. Protein Sci. 1995;4:394–404. doi: 10.1002/pro.5560040306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen SL, Chait BT. Mass spectrometry of whole proteins eluted from sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. Anal Biochem. 1997;247:257–267. doi: 10.1006/abio.1997.2072. [DOI] [PubMed] [Google Scholar]
- 12.Jorgensen CS, Jagd M, Sorensen BK, McGuire J, Barkholt V, Hojrup P, Houen G. Efficacy and compatibility with mass spectrometry of methods for elution of proteins from sodium dodecyl sulfate-polyacrylamide gels and polyvinyldifluoride membranes. Anal Biochem. 2004;330:87–97. doi: 10.1016/j.ab.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Dukan S, Turlin E, Biville F, Bolbach G, Touati D, Tabet JC, Blais JC. Coupling 2D SDS-PAGE with CNBr cleavage and MALDI-TOFMS: a strategy applied to the identification of proteins induced by a hypochlorous acid stress in Escherichia coli. Anal Chem. 1998;70:4433–4440. doi: 10.1021/ac980132z. [DOI] [PubMed] [Google Scholar]
- 14.Landry F, Lombardo CR, Smith JW. A method for application of samples to matrix-assisted laser desorption ionization time-of-flight targets that enhances peptide detection. Anal Biochem. 2000;279:1–8. doi: 10.1006/abio.1999.4468. [DOI] [PubMed] [Google Scholar]
- 15.Preston LM, Murray KK, Russell DH. Reproducibility and quantitation of matrix-assisted laser desorption ionization mass spectrometry: effects of nitrocellulose on peptide ion yields. Biol Mass Spectrom. 1993;22:544–550. doi: 10.1002/bms.1200220908. [DOI] [PubMed] [Google Scholar]
- 16.Dufresne-Martin G, Lemay JF, Lavigne P, Klarskov K. Peptide mass fingerprinting by matrix-assisted laser desorption ionization mass spectrometry of proteins detected by immunostaining on nitrocellulose. Proteomics. 2005;5:55–66. doi: 10.1002/pmic.200400902. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Wu XR, Manabe M, Yu J, Sun TT. Large scale purification and immunolocalization of bovine uroplakins I, II, and III. Molecular markers of urothelial differentiation. J Biol Chem. 1990;265:19170–19179. [PubMed] [Google Scholar]
- 19.Wu XR, Lin JH, Walz T, Haner M, Yu J, Aebi U, Sun TT. Mammalian uroplakins. A group of highly conserved urothelial differentiation-related membrane proteins. J Biol Chem. 1994;269:13716–13724. [PubMed] [Google Scholar]
- 20.Liang F, Kachar B, Ding M, Zhai Z, Wu XR, Sun TT. Urothelial hinge as a highly specialized membrane: detergent-insolubility, urohingin association, and in vitro formation. Differentiation. 1999;65:59–69. doi: 10.1046/j.1432-0436.1999.6510059.x. [DOI] [PubMed] [Google Scholar]
- 21.Gorg A, Weiss W, Dunn MJ. Current two-dimensional electrophoresis technology for proteomics. Proteomics. 2004;4:3665–3685. doi: 10.1002/pmic.200401031. [DOI] [PubMed] [Google Scholar]
- 22.Klarskov K, Naylor S. India ink staining after sodium dodecyl sulfate polyacrylamide gel electrophoresis and in conjunction with Western blots for peptide mapping by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2002;16:35–42. doi: 10.1002/rcm.522. [DOI] [PubMed] [Google Scholar]
- 23.Methogo RM, Dufresne-Martin G, Leclerc P, Leduc R, Klarskov K. Mass spectrometric peptide fingerprinting of proteins after Western blotting on polyvinylidene fluoride and enhanced chemiluminescence detection. J Proteome Res. 2005;4:2216–2224. doi: 10.1021/pr050014+. [DOI] [PubMed] [Google Scholar]
- 24.Gershoni JM, Palade GE. Protein blotting: principles and applications. Anal Biochem. 1983;131:1–15. doi: 10.1016/0003-2697(83)90128-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.