Abstract
Chronic restraint stress for 6h/21d causes hippocampal CA3 apical dendritic retraction, which parallels spatial memory impairments in male rats. Recent research suggests that chronic immobilization stress for 2h/10d induces CA3 dendritic retraction (Vyas et al., 2002) and questions whether CA3 dendritic retraction and spatial memory deficits can be produced sooner than found following 6h/21d of restraint stress. Therefore, this study investigated the effects of four different durations of chronic restraint stress (varied by hours/day and total number of days) and the subsequent effects on hippocampal CA3 morphology and spatial memory in the same male Sprague-Dawley rats. The results showed that only rats exposed to the 6h/21d restraint paradigm exhibited CA3 apical dendritic retraction, consistent spatial memory deficits, and decreased body weight gain compared to experimental counterparts and controls. While chronically stressing a rat with wire mesh restraint has a physical component, it acts primarily as a psychological stressor, and these findings support the interpretation that chronic psychological stress produces hippocampal-dependent cognitive deficits that are consistent with hippocampal structural changes. Differences in stress effects observed across different studies may be due to rat strain, type of stressor, and housing conditions; however, the current findings support the use of chronic restraint stress, with wire mesh, for 6h/21d as a reliable and efficient method to produce psychological stress and to cause CA3 dendritic retraction and spatial memory deficits in male Sprague-Dawley rats.
Keywords: Chronic restraint stress, hippocampus, spatial memory, Sprague-Dawley
1. Introduction
The hippocampus plays a critical role in spatial memory ability because damage to the hippocampus corresponds with spatial memory impairments in both lab animals (Conrad et al., 1996; Fortin et al., 2002; Kleen et al., 2006; Luine et al., 1996) and humans (Astur et al., 2002; Kessels et al., 2001; King et al., 2002). Chronic stress may affect hippocampal function through such mechanisms as CA3 neuronal remodeling (for review, see Conrad, 2006), suppression of synaptic activity (Kim and Diamond, 2002; Stewart et al., 2005), and altered neurogenesis (for review, see Leuner et al., 2006; Shors, 2006). In male rats, stress-induced alterations in CA3 apical dendritic arborization parallel deficits in hippocampal function, such as spatial memory impairments in the radial arm maze (Luine et al., 1994) and Y-maze (Conrad et al., 1996; Conrad et al., 2003; Wright and Conrad, 2005), and spatial learning deficits in the water maze (Sandi et al., 2003; Venero et al., 2002). Therefore, stress-induced changes in hippocampal CA3 neurons are consistent with deficits in hippocampal function, including spatial memory.
Hippocampal CA3 dendritic retraction, a remodeling of CA3 dendritic properties, is characterized by decreases in branch points, branch length, and synaptic suppression (Sousa et al., 2000). A variety of experimental conditions produce CA3 dendritic retraction, including 21 days of predator stress combined with high-fat diet (Baran et al., 2005), six days of activity stress combined with food restriction (Lambert et al., 1998), one month of chronic, unpredictable stress (Sousa et al., 2000) and chronic social stress paradigms, including 14 days of social defeat stress (McKittrick et al., 2000) and 11 sessions of social defeat stress (Kole et al., 2004). In rodents, a common stress paradigm used to elicit CA3 dendritic retraction is chronic restraint stress (Conrad et al., 1999; Magarinos and McEwen, 1995; Stewart et al., 2005; Watanabe et al., 1992a; Watanabe et al., 1992b). Experimental preference for this paradigm includes such factors as the reversal of stress-induced dendritic retraction within 4-10 days of restraint termination (no permanent cell death) and its ease and cost-effectiveness for the researcher. In the chronic restraint paradigm, rats are placed in ventilated, but snug wire mesh restraints for 6h/21d. This type of restraint involves a physical component (immobilization), but acts primarily as a psychological stressor through awareness of the inability to escape (Glavin et al., 1994; Servatius et al., 2000). In addition to inducing hippocampal dendritic retraction, chronic restraint stress has also been widely used to assess other hippocampal properties including molecular expression and synaptic activity (Donahue et al., 2006; Ejchel-Cohen et al., 2006; Gao et al., 2006; McEwen, 1999; Stewart et al., 2005; Venero et al., 2002), and hippocampal-dependent behaviors such as spatial memory (Bowman et al., 2003; Conrad et al., 1996; Kleen et al., 2006; Luine et al., 1996; McLaughlin et al., 2005; Sandi et al., 2003; Srikumar et al., 2006). Earlier work with chronic restraint has emphasized that 6h/13d of restraint does not alter hippocampal dendritic complexity, and males tested under this paradigm actually show a slight enhancement in spatial memory on the radial arm maze (Luine et al., 1996). However, a recent report suggests that CA3 dendritic retraction can be produced with a shorter duration of restraint (2h/10d, Vyas et al., 2002). This finding questions whether researchers should adapt to a new chronic stress paradigm.
A concern when comparing across studies is that different rat strains and restraint mechanisms are used. Rat strains can perceive stressors differently, which can subsequently influence hormonal profiles in response to stress. Therefore, the purpose of the current experiment was to compare different durations of chronic stress, using wire mesh restraint, on CA3 dendritic morphology and spatial memory within the same male Sprague-Dawley rats. The comparison of these different restraint paradigms will be useful in determining the most efficient and effective form of restraint stress needed to induce CA3 dendritic retraction and spatial memory impairments. Moreover, this study will measure brain and behavioral outcomes within the same group of rats to directly demonstrate whether changes in CA3 dendritic complexity are consistent with spatial ability.
2. Results
2.1 CA3 dendritic retraction
Rats exposed to restraint for 6h/21d exhibited apical CA3 dendritic retraction (Fig 1 & 2). Two separate one-way ANOVA’s revealed a significant main effect of treatment on apical branch points (F(4, 24) = 3.03, p < .05) and apical branch length (F(4,24) = 3.07, p < .05), respectively. Rats in the 6h/21d group had significantly fewer apical branch points compared to controls and had a significant reduction in overall apical branch length compared to control and all other restrained groups (2h/10d, 2h/21d, 6h/10d, Fig. 2). There were no statistically significant differences for basal dendritic properties across the different conditions.
Figure 1. CA3 Neuronal Tracings.

Camera Lucida drawings (320x) taken from Golgi-stained CA3 cells represent dendritic properties of each experimental group. Note the decrease in apical dendritic arbors in the 6h/21d group compared to the control group. In addition, the 6h/21d group shows a reduction in overall apical branch length compared to controls and all other experimental groups.
Figure 2. CA3 Dendritic Properties.

Rats exposed to restraint for 6h/21d had a statistically significant reduction in the number of apical branch points and in overall branch length. There were no significant differences in basal dendritic properties among the groups.
* p < .05 Comparison between 6h/21d restraint and control group
▲ p< .05 Comparison between 6h/21d restraint and all other stress conditions
2.2 Spatial Memory: Y-maze
Four of the five groups (control, 2h/10d, 2h/21d, 6h/10d) entered the novel arm significantly more than the other arm across the five minutes of testing according to a Wilcoxon matched-pairs test (p < .05; Fig 3). However, the 6h/21d rats entered the novel and other arms a similar number of times. A two-way ANOVA (treatment × minute) comparing total entries into all arms showed a significant effect of minute (F(4, 160) = 9.96, p < .001), with all groups making significantly more entries during min 1 compared to any other minute. Therefore, additional analyses were run on arm entries during min 1 to avoid the potential confound of habituation to the maze. A Wilcoxon matched pairs test revealed a within group effect of novel arm preference over the other arm. Rats restrained for 2h/21d, 6h/10d, or 6h/21d made a statistically similar number of entries in the novel arm compared to the other arm during min 1. A one-way ANOVA comparing group entry into all the arms did not detect any significant differences in arm preference (n.s).
Figure 3. Entries into the Y-maze.

Across the 5 minutes of testing, rats in the control, 2h/10d, 2h/21d, and 6h/10d entered the novel arm more than the other arm. When entries were compared during minute 1, rats in the control or 2h/10d groups entered the novel arm more than the other arm. The 6h/21d group failed to enter the novel arm more than the other arm at both time points (5 min and 1 min).
*p< .05 Comparison between entries into the novel vs other arm.
2.2 Body Weight
2.3.1. 10 day stress paradigms
Chronic restraint significantly altered body weight gain (Fig 4A). A 3×3 mixed-factor ANOVA (treatment × day) for body weight gain in rats over 10 days of restraint revealed a significant main effect of day (F(2, 42) = 312.35, p < .01) and a significant interaction between treatment and day, (F(4,42) = 23.09, p < .01), with no main effect of treatment on body weight (n.s.). All groups had similar weights at the beginning of restraint; however, at day 10 of restraint, both stress groups gained less weight than controls, with the 6h/10d rats weighing significantly less than the 2h/10d rats.
Figure 4. Body Weight.
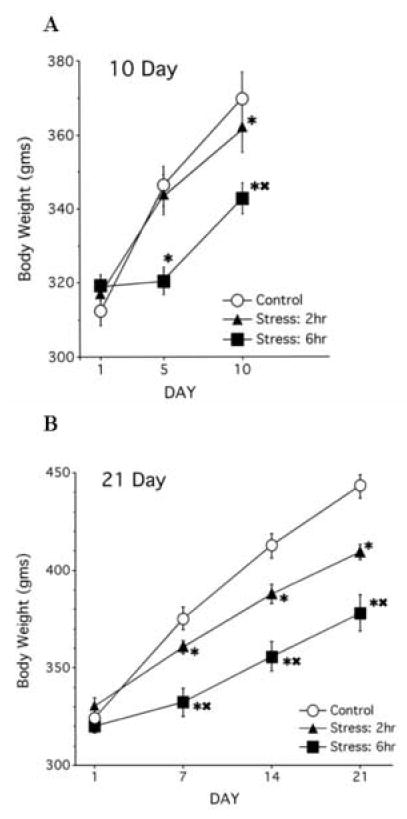
(A) Chronic restraint stress over 10 days significantly reduced weight gain in both the 2h/10d and 6h/10 groups compared to controls. In addition, the 6h/10d rats gained significantly less weight compared to the 2hr/d rats at both day 5 and 10. (B) Chronic restraint stress over 21 days significantly reduced weight gain in both the 2h/21d and 6h/21d groups compared to controls, with differences in body weight emerging at day 7.
* p < .05 Comparison between stress groups and control group
▲ p< .05 Comparison between 6h stress groups to 2h stress conditions
2.3.2 21 day stress paradigms
A 3×4 mixed-factor ANOVA (treatment × day) for body weight gain in rats over 21 days of restraint showed significant main effects of treatment (F(2,21) = 15.78, p < .01), day (F(3,63) = 505.14, p < .01) and a significant interaction between treatment and day (F(6,63) = 23.40, p < .01). While all groups showed similar weights at the start of the study, chronic stress reduced body weight gain by day 21, with rats in the 6h/d/21d group gaining the least amount of weight compared to the other groups (Fig 4B).
3. Discussion
Our findings support the continued use of chronic restraint stress using wire mesh for 6h/21d as a valid procedure to produce CA3 dendritic retraction, which corresponds with spatial memory deficits in male Sprague-Dawley rats. The 6h/21d restraint group was the only group to display CA3 apical dendritic retraction in both branch points and overall branch length and to show consistent impairments on the Y-maze. Moreover, the 6h/21d group gained significantly less weight compared to the 2h/21d and control groups, demonstrating that 6h/21d restraint was perceived as being a successful stressor.
While the 6h/21d group may have had less access to food compared to the other groups, we have previously shown that chronically stressed rats that are food restricted actually consume more food to maintain their weights than control rats (Kleen et al., 2006). Additional evidence illustrating that stress-induced weight loss is not dependent upon food access is shown in studies using the stress hormone, corticosterone. Rats administered corticosterone (via drinking water or daily injections) over weeks show decreased weight gain compared to control counterparts (Coburn-Litvak et al., 2003; Conrad et al., 2004; Sousa et al., 1998; Watanabe et al., 1992a). In another example, rats exposed to daily tone-footshock pairings showed reduced weight gain over 21 days compared to controls given tones without footshock (Trentani et al., 2002). These studies demonstrate that even when rats have similar access to food, chronic stress or corticosterone-treated rats gain weight more slowly than their control counterparts. Therefore, food availability is not likely to contribute to differences in weight gain between the groups in the current study.
Total entries were analyzed to rule out the possibility that non-mnemonic factors, such as motivation and changes in motor ability, may have contributed to behavioral differences among the groups. While some reports demonstrate that chronic stress impairs motivation (Mizoguchi et al., 2002), including studies using chronic restraint for 6h/21d (Kleen et al., 2006), most chronically stressed rats tested on the Y-maze show motivation to explore (Conrad et al., 1996; Wright and Conrad, 2005; Wright et al., 2006). One of the advantages of using the Y-maze is that motivation is dependent on a rat’s innate interest in novelty seeking, and food restriction is not needed to motivate rats to perform (for review see Conrad, 2006). Indeed, when restraint for 6h/21d is combined with food restriction, rats show decreases in motivation for food reward, but continue to show motivation to explore the Y-maze (Kleen et al., 2006). Moreover, both Mizoguchi et al (2002) and Kleen et al (2006) show that while chronic stress may affect some aspects of motivation, motor ability remains functional. The current results also support these findings, as there were no group differences in total entries within the maze. Another interpretation of the data may be that stress influenced the preference for novelty, specifically. This seems unlikely considering that chronically stressed male rats successfully seek out the novel arm when intrinsic cues are present in the maze or when the inter-trial interval is decreased between training and testing (Bellani et al., 2006; Kleen et al., 2006; Wright and Conrad, 2005). The construction of dark and narrow walls within the Y-maze may also allow rats to enter the arms and maintain novelty exploration without interfering with thigmotaxic behavior (Wallace and Whishaw, 2005).
Interestingly, the 6h/10d group showed large variability in min 1 novel arm entries, accompanied by elevated novel arm entries, which may reflect a transitional period. Kleen et. al (2006) looked at daily operant response rates in rats exposed to 6h/21d restraint and found that motivation for appetitive responses began to decrease during the second week of restraint. This finding, combined with the slight improvement in performance associated with 6h/13d restraint stress (Luine et al., 1996), suggests that chronic stress-related changes in behavior may occur between days 7-13. Perhaps the large variability in performance in the 6h/10d group reflects this transition.
The current findings demonstrate that restraint procedures used to produce hippocampal CA3 dendritic retraction and spatial memory deficits are not equivalent and emphasize several important variables. First, individual rat strains have unique profiles in response to stress (Herman et al., 1999) and extrapolating findings from one strain in order to apply them to another should be performed with caution. In the current study, we used Sprague-Dawley rats, which are commonly used with 6h/21d restraint (Beck and Luine, 2002; Conrad et al., 1996; Pham et al., 2003; Wood et al., 2003; Wright et al., 2006). In contrast, Vyas et al. (2002) used Wistar rats, which are commonly used in unpredictable stress protocols and shorter restraint/immobilization paradigms (Gerrits et al., 2003; Marin et al., 2007; Mitra et al., 2005; Mizoguchi et al., 2001; Sousa et al., 2000; Vyas et al., 2002; Vyas and Chattarji, 2004). Rat strain becomes important in stress research because of differences in stress sensitivity. For example, Wistar-Kyoto, Fisher, and Long Evans strains are more likely to express stress-induced ulcers than Wistar and Sprague-Dawley strains (Pare, 1990). Wistar-Kyoto are considered to be hyperreactive to stressors compared to most other strains, including Fisher and Wistar rats (Redei et al., 1994), and they have different behavioral profiles compared to Sprague-Dawley rats (Zafar et al., 1997). Moreover, stress effects on neurotransmitter systems such as serotonin and norepinephrine also differ between Wistar-Kyoto and Sprague-Dawley strains (Pare and Tejani-Butt, 1996; Tejani-Butt et al., 1994). Therefore, it is unlikely that chronic stress produces similar responses across strains and the duration of restraint should be carefully considered according to rat strain.
Second, the type of restrainer used to immobilize the rats may play a critical role in how rats perceive the stress procedure because different stressors have varying effects on behavior and stress profiles (for review see Glavin et al., 1994; Marin et al., 2007). Vyas et al., (2002) restrained rats in “immobilization bags” which were not clearly described. Perhaps using immobilization bags for 2h/10d produced CA3 dendritic retraction because rats perceived this paradigm differently compared to wire mesh restraints. Using immobilization bags may increase the risk for contact with urine and feces and may also affect thermoregulation due to the restrictive nature and materials of the bag. Maintaining thermoregulatory control is important because body temperature increases in response to a variety of stressors, causing a stress-induced hyperthermia (Marazziti et al., 1992; Olivier et al., 2003). Moreover, the duration that body temperature remains elevated after termination of stress depends on the severity of the stressor (Bhatnagar et al., 2006). For example, chronic mild stress models which use 4 weeks of combination stressors including food deprivation, light cycle changes, and soiled bedding exposure (excessive contact with urine and feces), terminate the normal rhythmic pattern in rats. Furthermore, temperature in these rats is significantly higher than in controls. Interestingly, changes in body temperature are commonly observed in depressed patients, and both antidepressant (Souetre et al., 1988) and electroconvulsive therapies (Szuba et al., 1997) can restore circadian temperature rhythms. These potential outcomes suggest that immobilization bags may have a greater physical stress component than that associated with wire mesh restraints. Therefore, perception of restraint as more of a physical stressor compared to a psychological stressor may affect the brain regions activated during immobilization stress, which can contribute to differences among studies.
Third, housing conditions may contribute to differences between studies. Sprague-Dawley rats were pair housed in the current research, whereas Wistar rats were housed 3 to a cage in the Vyas et al., (2002) research. Levels of sociability also differ between strains and cagemates and may relieve stress for some rats while acting as additional stressors for others. There is a dichotomy in response to stress within the Sprague-Dawley strain itself, with acute restraint stress decreasing aggressive attacks to cagemates compared to controls and chronic restraint stress increasing aggressive attacks directed to cagemates compared to controls (Wood et al., 2003). In addition, aggressive behaviors seem to emerge around day 14 in rats subjected to 6h/21d restraint, which supports the idea that there is a shift in the stress response during the second week of restraint, as described earlier (Kleen et al., 2006). Interestingly, housing also affects hippocampal-dependent behavior in male rats. For example, object location memory is impaired in single-housed Sprague-Dawley rats exposed to 6h/21d restraint stress but not in pair-housed rats (Beck and Luine, 2002). These findings suggest that housing manipulations can interact with stress manipulations to influence cognitive abilities.
The current study focused specifically on the relationship between CA3 morphology and spatial memory for important reasons. Stress-induced reductions in dendritic arbors are expressed within the CA3 region first before other hippocampal areas show changes. For instance, chronic restraint (6h/21d) or corticosterone treatment (21d) in rats, and psychosocial stress (28d) in tree shrews produces CA3 dendritic retraction without altering the dendritic tree in the dentate gyrus, CA1, or CA2 regions (Magarinos and McEwen, 1995; Magarinos et al., 1996; McLaughlin et al., 2005; Woolley et al., 1990). Prolonged and fatal stress in vervet monkeys reduces CA3 and CA4 pyramidal cells, without significantly altering CA1 and dentate gyrus neuronal numbers (Uno et al., 1989), a finding that is consistent with another study using rats and water immersion for 30d (Mizoguchi et al., 1992). Chronic stress even alters CA3 neoplasm, a measure of nuclear activity and vulnerability, after 20d and similar changes occur after in CA1 after 28d (Fuchs et al., 1995). While chronic stress can certainly influence other hippocampal regions (Lambert et al., 1998; Sousa et al., 2000) and brain areas (Liston et al., 2006; Radley et al., 2005; Vyas et al., 2002), these studies show that the CA3 region is highly sensitive to chronic stress and appears to be one of the first hippocampal regions to respond to chronic stress. Since damage to the CA3 region can impair hippocampal function (Conrad et al., 1996), using a chronic stress paradigm under a time frame that influences hippocampal CA3 dendritic arbors should also influence spatial ability, as we have illustrated in this study.
In conclusion, while chronic restraint with wire mesh has a physical component, it acts primarily as a psychological stressor and these findings support the interpretation that chronic psychological stress produces hippocampal-dependent cognitive deficits that are consistent with hippocampal structural changes. Specifically, our findings support the use of the 6h/21d chronic restraint paradigm as a reliable and efficient method to induce CA3 dendritic retraction and spatial memory deficits in male Sprague-Dawley rats. This study emphasizes using caution when extrapolating data between studies and demonstrates the importance of rat strain, stress paradigm, housing conditions, and other factors which may influence experimental outcomes.
4. Experimental Procedure
Federal and institutional guidelines set forth by the Institutional Animal Care and Use Committee for animals in research at Arizona State University approved all procedures used for this research. Adequate measures were taken to minimize the number of rats used and to minimize pain or discomfort.
4.1 Subjects
Male Sprague-Dawley rats (CD strain, n = 48) were purchased from Charles River Labs (Hartford, CT) and were pair housed with access to food and water ad libitum. All rats were randomly assigned to either a control or stress condition: 2 hours daily restraint or 6 hours daily restraint which was repeated for 10 or 21 days to produce 4 experimental groups: 2h/10d, 2h/21d, 6h/10d, 6h/21d. Control, 2h and 6h, and stressed rats, 2hr and 6h, were housed in separate, but identical chambers with a temperature of 21-22 °C and a reversed 12/12 light cycle (lights off at 6 AM).
4.2 Restraint and handling procedure
The 6h/21d restraint and handling procedure was the same as that described in previous studies (Conrad et al., 2004; Kleen et al., 2006; McLaughlin et al., 2005; Wright and Conrad, 2005). Modifications of this paradigm, including the amount of time in restraint each day and the number of days of restraint, served as other experimental conditions. All restraint occurred between 9AM-3PM. Rats were restrained in their home cages in wire mesh restrainers (18 cm circumference × 24 cm long; wire mesh from Flynn and Enslow Inc, San Francisco, CA) with wire ends sealed with grip guard sealer (ACE hardware) and were transferred to larger restrainers (23 cm circumference × 28 cm long) if they outgrew the smaller restrainers. Control rats remained undisturbed during the time of restraint stress. Body weights were taken on days 1, 5, 10 of restraint in the 10 day groups and on days 1, 7, 14, 21 of restraint in the 21 day groups.
4.3 Y-Maze paradigm
The Y-maze has been validated as a spatial memory task that depends on an intact hippocampus for successful performance (Conrad et al., 1996) and the training and testing procedure was the same as that previously described (Kleen et al., 2006; McLaughlin et al., 2005; Wright and Conrad, 2005; Wright et al., 2006).
4.4 Golgi Procedure and Histology
Unperfused brains were rapidly removed and processed according to FD Rapid Golgistain™ Kits (FD NeuroTechnologies, Baltimore, MD). The staining procedure followed previously established methods that successfully stain hippocampal pyramidal cells (Kleen et al., 2006; McLaughlin et al., 2005). For Golgi analyses, cells were chosen based on the following criteria: 1) the cell body and dendrites were fully impregnated, 2) the cell was relatively isolated from surrounding neurons, and 3) the cell was located in the CA3 region of the hippocampus. Histological quantification included Camera Lucida neuronal tracings and assessment of CA3 dendritic branch points and branch length (Kleen et al., 2006; McLaughlin et al., 2005). A total of 30 rats were chosen in which sufficient numbers of Golgi-impregnated cells could be identified (control n = 7); 2h/10d (n = 5); 2h/21d (n = 6); 6h/10d (n = 6); 6h/21d (n = 5)). Dendritic length and branch points were measured separately for the apical and basal sections of the neuron. For each rat, analyses for branch points and length were obtained by averaging 2-5 SS and LS neurons separately and the means of these cells were combined for one value to represent apical and basal dendritic properties, respectively.
4.5 Statistical analyses
There was no statistical difference for either behavior or morphology for the control conditions (2hr or 6hr), so control groups were combined for analyses. In addition, three rats were removed from behavioral analyses because they failed to explore the Y-maze (n = 2, one each from 6h/10d and 6h/21d) or for technical reasons (n = 1, from 2h/21d). Thus, the final statistical analyses for behavior included: control (n = 16), 2h/10d (n = 8), 2h/21d (n = 7), 6h/10d (n = 7), 6h/21d (n = 7).
Parametric data were analyzed by ANOVA and Newman-Keuls post hocs were computed when statistical significance reached p = .05. Non-parametric data were analyzed by Wilcoxon matched-pairs tests. Data are represented by means ± S.E.M. The start arm was not included in Wilcoxon analyses because rats were initially placed there by the experimenter, potentially creating a bias against re-entering that arm.
Acknowledgments
This research was funded by MH64747 (Conrad) and the Howard Hughes Medical Institute through the Undergraduate Biology Enrichment Program (Gomez). The contributions of the following individuals are gratefully appreciated: Mariam Ashmawy, Rudi Bellani, Shelly Dubbs, Roda Hajo, Gillian Hamilton, James Harman, Jocelyn Janni, and Ryan Wright.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behavioral Brain Research. 2002;132:77–84. doi: 10.1016/s0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- Baran SE, Campbell AM, Kleen JK, Foltz CH, Wright RL, Diamond DM, Conrad CD. Combination of high fat diet and chronic stress retracts hippocampal dendrites. Neuroreport. 2005;16:39–43. doi: 10.1097/00001756-200501190-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiol Behav. 2002;75:661–73. doi: 10.1016/s0031-9384(02)00670-4. [DOI] [PubMed] [Google Scholar]
- Bellani R, Luecken LJ, Conrad CD. Peripubertal anxiety profile can predict predisposition to spatial memory impairments following chronic stress. Behav Brain Res. 2006;166:263–70. doi: 10.1016/j.bbr.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol. 2006;18:13–24. doi: 10.1111/j.1365-2826.2005.01375.x. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Coburn-Litvak PS, Pothakos K, Tata DA, McCloskey DP, Anderson BJ. Chronic administration of corticosterone impairs spatial reference memory before spatial working memory in rats. Neurobiol Learn Mem. 2003;80:11–23. doi: 10.1016/s1074-7427(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–34. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–13. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Grote KA, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiol Learn Mem. 2003;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wise LS. Chronic stress enhances ibotenic acid-induced damage selectively within the hippocampal CA3 region of male, but not female rats. Neuroscience. 2004;125:759–67. doi: 10.1016/j.neuroscience.2004.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav Cogn Neurosci Rev. 2006;5:41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue CP, Kosik KS, Shors TJ. Growth hormone is produced within the hippocampus where it responds to age, sex, and stress. Proc Natl Acad Sci U S A. 2006;103:6031–6. doi: 10.1073/pnas.0507776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejchel-Cohen TF, Wood GE, Wang JF, Barlow K, Nobrega JN, SM B, Trevor Young L. Chronic restraint stress decreases the expression of glutathione S-transferase pi2 in the mouse hippocampus. Brain Res. 2006;1090:156–62. doi: 10.1016/j.brainres.2006.03.062. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nature Neuroscience. 2002;5:458–62. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Uno H, Flugge G. Chronic psychosocial stress induces morphological alterations in hippocampal pyramidal neurons of the tree shrew. Brain Res. 1995;673:275–82. doi: 10.1016/0006-8993(94)01424-g. [DOI] [PubMed] [Google Scholar]
- Gao Y, Bezchlibnyk YB, Sun X, Wang JF, McEwen BS, Young LT. Effects of restraint stress on the expression of proteins involved in synaptic vesicle exocytosis in the hippocampus. Neuroscience. 2006;141:1139–48. doi: 10.1016/j.neuroscience.2006.04.066. [DOI] [PubMed] [Google Scholar]
- Gerrits M, Westenbroek C, Fokkema DS, Jongsma ME, Den Boer JA, Ter Horst GJ. Increased stress vulnerability after a prefrontal cortex lesion in female rats. Brain Res Bull. 2003;61:627–35. doi: 10.1016/j.brainresbull.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Glavin GB, Pare WP, Sandbak T, Bakke HK, Murison R. Restraint stress in biomedical research: an update. Neurosci Biobehav Rev. 1994;18:223–49. doi: 10.1016/0149-7634(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Watson SJ, Spencer RL. Defense of adrenocorticosteroid receptor expression in rat hippocampus: effects of stress and strain. Endocrinology. 1999;140:3981–91. doi: 10.1210/endo.140.9.6962. [DOI] [PubMed] [Google Scholar]
- Kessels RP, de Haan EH, Kappelle LJ, Postma A. Varieties of human spatial memory: a meta-analysis on the effects of hippocampal lesions. Brain Research Brain Research Review. 2001;35:295–303. doi: 10.1016/s0165-0173(01)00058-3. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–62. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- King JA, Burgess N, Hartley T, Vargha-Khadem F, O’Keefe J. Human hippocampus and viewpoint dependence in spatial memory. Hippocampus. 2002;12:811–20. doi: 10.1002/hipo.10070. [DOI] [PubMed] [Google Scholar]
- Kleen JK, Sitomer MT, Killeen PR, Conrad CD. Chronic stress impairs spatial memory and motivation for reward without disrupting motor ability and motivation to explore. Behav Neurosci. 2006;120:842–51. doi: 10.1037/0735-7044.120.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH, Costoli T, Koolhaas JM, Fuchs E. Bidirectional shift in the cornu ammonis 3 pyramidal dendritic organization following brief stress. Neuroscience. 2004;125:337–47. doi: 10.1016/j.neuroscience.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Lambert KG, Buckelew SK, Staffiso-Sandoz G, Gaffga S, Carpenter W, Fisher J, Kinsley CH. Activity-stress induces atrophy of apical dendrites of hippocampal pyramidal neurons in male rats. Physiol Behav. 1998;65:43–9. doi: 10.1016/s0031-9384(98)00114-0. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–24. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–4. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–70. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Luine V, Martinez C, Villegas M, Magarinos AM, McEwen BS. Restraint stress reversibly enhances spatial memory performance. Physiology and Behavior. 1996;59:27–32. doi: 10.1016/0031-9384(95)02016-0. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995;69:83–8. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–40. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, Di Muro A, Castrogiovanni P. Psychological stress and body temperature changes in humans. Physiol Behav. 1992;52:393–5. doi: 10.1016/0031-9384(92)90290-i. [DOI] [PubMed] [Google Scholar]
- Marin MT, Cruz FC, Planeta CS. Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiol Behav. 2007;90:29–35. doi: 10.1016/j.physbeh.2006.08.021. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–22. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Magarinos AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36:85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Wright RL, Conrad CD. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: possible involvement of CA1 neurons. Neuroscience. 2005;135:1045–1054. doi: 10.1016/j.neuroscience.2005.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Vyas A, Chatterjee G, Chattarji S. Chronic-stress induced modulation of different states of anxiety-like behavior in female rats. Neurosci Lett. 2005;383:278–83. doi: 10.1016/j.neulet.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress differentially regulates glucocorticoid negative feedback response in rats. Psychoneuroendocrinology. 2001;26:443–59. doi: 10.1016/s0306-4530(01)00004-x. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Tabira T. Chronic stress impairs rotarod performance in rats: implications for depressive state. Pharmacol Biochem Behav. 2002;71:79–84. doi: 10.1016/s0091-3057(01)00636-0. [DOI] [PubMed] [Google Scholar]
- Olivier B, Zethof T, Pattij T, van Boogaert M, van Oorschot R, Leahy C, Oosting R, Bouwknecht A, Veening J, van der Gugten J, Groenink L. Stress-induced hyperthermia and anxiety: pharmacological validation. Eur J Pharmacol. 2003;463:117–32. doi: 10.1016/s0014-2999(03)01326-8. [DOI] [PubMed] [Google Scholar]
- Pare WP. Technique and strain comparisons in stress ulcer. Ann N Y Acad Sci. 1990;597:223–30. doi: 10.1111/j.1749-6632.1990.tb16170.x. [DOI] [PubMed] [Google Scholar]
- Pare WP, Tejani-Butt SM. Effect of stress on the behavior and 5-HT system in Sprague-Dawley and Wistar Kyoto rat strains. Integr Physiol Behav Sci. 1996;31:112–21. doi: 10.1007/BF02699783. [DOI] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. European Journal of Neuroscience. 2003;17:879–86. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005 doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Redei E, Pare WP, Aird F, Kluczynski J. Strain differences in hypothalamic-pituitary-adrenal activity and stress ulcer. Am J Physiol. 1994;266:R53–60. doi: 10.1152/ajpregu.1994.266.2.R353. [DOI] [PubMed] [Google Scholar]
- Sandi C, Davies HA, Cordero MI, Rodriguez JJ, Popov VI, Stewart MG. Rapid reversal of stress induced loss of synapses in CA3 of rat hippocampus following water maze training. Eur J Neurosci. 2003;17:2447–56. doi: 10.1046/j.1460-9568.2003.02675.x. [DOI] [PubMed] [Google Scholar]
- Servatius RJ, Salameh G, Coyle KM, Pare WP. Restraint Stress. In: Fink G, editor. Encyclopedia of Stress. Vol. 3. Academic Press; New York: 2000. pp. 376–377. [Google Scholar]
- Shors TJ. Significant life events and the shape of memories to come: a hypothesis. Neurobiol Learn Mem. 2006;85:103–15. doi: 10.1016/j.nlm.2005.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souetre E, Salvati E, Wehr TA, Sack DA, Krebs B, Darcourt G. Twenty-four-hour profiles of body temperature and plasma TSH in bipolar patients during depression and during remission and in normal control subjects. Am J Psychiatry. 1988;145:1133–7. doi: 10.1176/ajp.145.9.1133. [DOI] [PubMed] [Google Scholar]
- Sousa N, Madeira MD, Paula-Barbosa MM. Effects of corticosterone treatment and rehabilitation on the hippocampal formation of neonatal and adult rats. An unbiased stereological study. Brain Res. 1998;794:199–210. doi: 10.1016/s0006-8993(98)00218-2. [DOI] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–66. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Srikumar BN, Raju TR, Shankaranarayana Rao BS. The involvement of cholinergic and noradrenergic systems in behavioral recovery following oxotremorine treatment to chronically stressed rats. Neuroscience. 2006;143:679–88. doi: 10.1016/j.neuroscience.2006.08.041. [DOI] [PubMed] [Google Scholar]
- Stewart MG, Davies HA, Sandi C, Kraev IV, Rogachevsky VV, Peddie CJ, Rodriguez JJ, Cordero MI, Donohue HS, Gabbott PL, Popov VI. Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: a three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience. 2005;131:43–54. doi: 10.1016/j.neuroscience.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Szuba MP, Guze BH, Baxter LR., Jr Electroconvulsive therapy increases circadian amplitude and lowers core body temperature in depressed subjects. Biol Psychiatry. 1997;42:1130–7. doi: 10.1016/s0006-3223(97)00046-2. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt SM, Pare WP, Yang J. Effect of repeated novel stressors on depressive behavior and brain norepinephrine receptor system in Sprague-Dawley and Wistar Kyoto (WKY) rats. Brain Res. 1994;649:27–35. doi: 10.1016/0006-8993(94)91045-6. [DOI] [PubMed] [Google Scholar]
- Trentani A, Kuipers SD, Ter Horst GJ, Den Boer JA. Selective chronic stress-induced in vivo ERK1/2 hyperphosphorylation in medial prefrontocortical dendrites: implications for stress-related cortical pathology? Eur J Neurosci. 2002;15:1681–91. doi: 10.1046/j.1460-9568.2002.02000.x. [DOI] [PubMed] [Google Scholar]
- Venero C, Tilling T, Hermans-Borgmeyer I, Schmidt R, Schachner M, Sandi C. Chronic stress induces opposite changes in the mRNA expression of the cell adhesion molecules NCAM and L1. Neuroscience. 2002;115:1211–9. doi: 10.1016/s0306-4522(02)00543-2. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Chattarji S. Modulation of different states of anxiety-like behavior by chronic stress. Behav Neurosci. 2004;118:1450–4. doi: 10.1037/0735-7044.118.6.1450. [DOI] [PubMed] [Google Scholar]
- Wallace DG, Whishaw IQ. Dead Reckoning. In: Whishaw IQ, Kolb B, editors. The behavior of the laboratory rat. Vol. Oxford University Press; New York: 2005. pp. 401–409. [Google Scholar]
- Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS. Phenytoin prevents stress-and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992a;2:431–5. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Daniels DC, Cameron H, McEwen BS. Tianeptine attenuates stress-induced morphological changes in the hippocampus. Eur J Pharmacol. 1992b;222:157–62. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- Wood GE, Young LT, Reagan LP, McEwen BS. Acute and chronic restraint stress alter the incidence of social conflict in male rats. Horm Behav. 2003;43:205–13. doi: 10.1016/s0018-506x(02)00026-0. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–31. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Chronic stress leaves novelty-seeking behavior intact while impairing spatial recognition memory in the Y-maze. Stress. 2005;8:151–4. doi: 10.1080/10253890500156663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RL, Lightner EN, Harman JS, Meijer OC, Conrad CD. Attenuating corticosterone levels on the day of memory assessment prevents chronic stress-induced impairments in spatial memory. Eur J Neurosci. 2006;24:595–605. doi: 10.1111/j.1460-9568.2006.04948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar HM, Pare WP, Tejani-Butt SM. Effect of acute or repeated stress on behavior and brain norepinephrine system in Wistar-Kyoto (WKY) rats. Brain Res Bull. 1997;44:289–95. doi: 10.1016/s0361-9230(97)00140-8. [DOI] [PubMed] [Google Scholar]


