Abstract
The Nef protein is an important virulence factor of primate lentiviruses, yet the mechanisms by which it exerts this influence are imperfectly understood. Here, using an inducible system, we demonstrate that Nef increases IL-2 secretion from T cells stimulated via CD3 or CD28. This effect requires the conservation of the Nef myristoylation signal and SH3-binding proline-based motif. Together with several proteins involved in the initiation and propagation of T cell signaling, Nef associates with membrane microdomains known as rafts. The Nef-mediated superinduction of IL-2 reflects the activation of both NFAT and NFκB. Accordingly, Nef also enhances HIV-1 transcription in response to CD3 or CD28 stimulation. Nef-induced IL-2 hyperresponsiveness is also observed in primary CD4 lymphocytes. Overall, these data suggest that Nef acts at the level of rafts to prime T cells for activation. Likely consequences of this effect are the promotion of HIV-1 replication and the facilitation of virus spread.
Nef is an early gene of primate lentiviruses that encodes a factor important for the virulence of both human and simian immunodeficiency viruses (1–4). To account for this influence, several biological effects of Nef have been identified, including the down-regulation of CD4 and MHC-1, the stimulation of virion infectivity, and the alteration of T cell activation pathways.
Recent experimental evidence suggests that Nef might promote T cell activation. Transgenic mice (Tg) expressing HIV-1 nef in CD4+ T cells and in cells of the macrophage/dendritic lineages developed several AIDS-like pathologies (5). Furthermore, the thymocytes of such mice exhibited a state of hyperactivation and of anti-CD3 hyperresponsiveness (5, 6). In Jurkat human T lymphoid cells, the surface expression of a CD8-Nef chimera resulted in activation and death by apoptosis whereas its intracytoplasmic accumulation led to a state of apparent anergy (7). In an IL-2-dependent rhesus-monkey T lymphoid cell line infected with herpesvirus saimiri, nef-positive simian immunodeficiency virus could induce IL-2 production (8). Finally, simian immunodeficiency virus strains with Nef variants harboring amino acid sequences that resemble immunoreceptor tyrosine-based activation motifs could replicate to high levels in peripheral blood mononuclear cells without a need for exogenous stimulation (9). Contrasting with these results, other reports have suggested that Nef inhibits lymphocyte activation (10–19): for instance, in Jurkat cell clones stably expressing HIV-1 nef (10–14) or electroporated with recombinant nonmyristoylated Nef (15, 16). However, interpretation is complicated by the possible selection of cells tolerized for Nef (10, 14) or the improper subcellular localization of nonmyristoylated Nef (15,16). Two other studies relied on an inducible system, but the inducing agent was phorbol myristate acetate (PMA), itself a major modulator of T cell activation (17, 18).
Several molecular interactions constitute putative links between Nef and signal transduction pathways. A conserved proline-rich repeat in Nef can capture the SH3 domain of the Hck and Lyn nonreceptor protein tyrosine kinases (20, 21). Nef can also bind the T cell-specific Lck (17, 22) and frees Lck from CD4 upon triggering CD4 endocytosis (23, 24). Finally, HIV-1 Nef can recruit a member of the p-21 activated kinase family (25, 26) as well as another yet unidentified serine/threonine kinase (22, 27), and can associate with the θ isoform of protein kinase C (28) and with Vav (29).
The present study aimed at further investigating the potential impact of Nef on T cell activation pathways. To avoid possible artifacts linked to the use of chimeric molecules and to circumvent the potential toxicity of Nef in T cells, a conditional expression system was used. Our results indicate that Nef associates with membrane microdomains critically involved in the initiation and propagation of T cell signaling and that it primes T cells for activation, thereby promoting IL-2 secretion and viral transcription.
Materials and Methods
Antibodies and Reagents.
Anti-CD3 (HIT3A), R-phycoerythrin-conjugated anti-CD4 or -CD28, and fluorescein isothiocyanate (FITC)-conjugated anti-CD3 or -MHC-1 monoclonal antibodies (mAbs) were from PharMingen, CD28-specific mAb (Leu-28) was from Becton Dickinson, LAT-, Lck-, PLC-γ1-, and phosphotyrosine-specific antibodies were from Upstate Biotechnology (Lake Placid, NY), and anti-Fyn, -Src, and -Vav antibodies were from Transduction Laboratories (Lexington, KY). The rabbit anti-Nef serum was previously described (30). Phorbol myristate acetate (PMA) and A23187 were from Calbiochem, tetracycline was from Sigma, and recombinant human tumor necrosis factor α was from R & D Systems.
Establishment of Nef-Inducible Cell Lines.
cDNAs encoding wild-type R7 Nef, NefG2A, and Nef(PXX)4− (30) were inserted into pTet-Splice (D. Schatz, Yale University) to generate pTet-Nef, pTet-NefG2A, or pTet-Nef(PXX)4− in which nef is placed under the control of the tetracycline-controlled transactivator-dependent promoter. The resulting plasmids were electroporated into D4, a tetracycline-controlled transactivator-expressing Jurkat derivative (J. Sodroski, Harvard Medical School) (31), together with a vector expressing the hygromycin B resistance marker. Clones selected by limiting dilution were maintained in the presence of 200 μg of G418, 150 μg of hygromycin B, and 2 μg of tetracycline per milliliter. To induce nef expression, cells were cultured without tetracycline for 5 days. On average, 20% of the clones exhibited very low levels of Nef in tetracycline and high nef expression without the drug.
Cell Stimulation and IL-2 Quantification.
Aliquots (2.5 × 106) of cells were treated with 3 μg/ml Leu-28 or HIT3A, 50 ng/ml PMA, 1 μg/ml A23187, and 10 ng/ml tumor necrosis factor α, either alone or in combinations. Where indicated, anti-CD3 antibodies were crosslinked by first incubating cells for 30 min at 4°C with 3 μg/ml of HIT3A mAb and then transferring them to wells precoated at 4°C overnight with 10 μg/ml goat anti-mouse antibodies in 35 mM bicarbonate/15 mM carbonate (pH 9.6) buffer. Sixteen to eighteen hours later, aliquots of supernatants were assayed for IL-2 by ELISA (R & D Systems).
Detergent-Insoluble Glycolipid-Enriched Microdomain (DIG) Isolation and Analyses.
DIGs were isolated essentially as described (32, 33). Cells were washed with ice-cold PBS and were lysed by 10 strokes of Dounce homogenizer in 1.3 ml of hypotonic buffer (10 mM Tris⋅hydrochloride, pH 7.5/5 mM EDTA/500 μM Na3VO4/10 mM NaF/5 μg/ml aprotinin/1 mM phenylmethylsulfonyl fluoride). After centrifugation at 4°C for 10 min, the 1-ml postnuclear supernatant was incubated with 1% Triton X-100/150 mM NaCl for 1 h at 4°C, was adjusted to 42.5% wt/vol sucrose by the addition of 1 ml of 85% sucrose in buffer A (10 mM Tris⋅hydrochloride, pH 7.5/5 mM EDTA/150 mM NaCl/500 μM Na3VO4/10 mM NaF), and was overlaid with 6 ml of 30% wt/vol and 3.5 ml 5% wt/vol sucrose in buffer A. After centrifugation for 16–18 h at 34,000 rpm in an SW41 rotor (Beckman Coulter) at 4°C, 1-ml fractions were harvested from the top. DIGs were recovered from low density fractions 2–5.
Luciferase Assays.
pNFAT-Luc (G. Crabtree, Stanford University), pAP-1-Luc, pNFκB-Luc, pHIV-1-Luc, and pHIV-1-κB-mut.-Luc (K. Jones, Salk Institute) were electroporated into JNef and control cells grown with or without tetracycline. All experiments were performed in duplicate transfections, and inductions/activations were carried out on aliquots of a single pool of transfected cells. Cells were processed by using the Luciferase Assay System (Promega) 14 h after activation. For transient Nef expression, D4 cells were electroporated with 5 μg of luciferase reporter plasmids, 20 μg of Tet-Nef, and 2 μg of pRL-TK (Promega). Twenty-four hours later, aliquots either were left unstimulated or were treated with 3 μg/ml anti-CD3 and/or CD28 mAb. Cells were processed for luciferase assays 14 h later, normalizing for transfection efficiency by using the cotransfected Renilla Luciferase activity.
CD4 T Cell Transduction and Stimulation.
CD4+ T lymphocytes (>95% purity) were obtained from peripheral blood as described (34), were stimulated with 5 μg/ml phytohemagglutinin for 24 h, and were maintained in RPMI containing 10 units/ml rIL-2 and 10% FCS. One week later, cells were infected with the nef-expressing HR-EF1α-Nef (in which Nef is expressed from the elongation factor-1 α promoter) or a control, VSV G-pseudotyped, HIV-based vector as described (34, 35). Three days later, cells were stimulated with anti-CD3/CD28 antibodies, and IL-2 production in the supernatant was measured by ELISA. An aliquot of the cells was stained with phycoerythrin-conjugated CD4-specific antibody to monitor CD4 down-regulation.
Results
Inducible Expression of HIV-1 Nef in Jurkat Human T Lymphoid Cells.
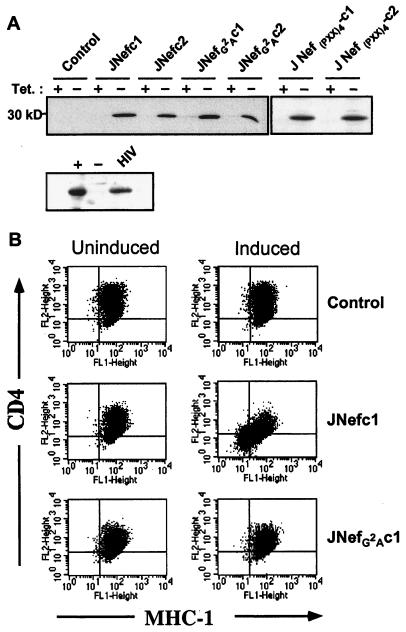
Human T lymphoid Jurkat cells expressing Nef in a controllable manner were created by using the tetracycline-repressible system. JNefc1 and JNefc2 are two clones that express high levels of HIV-1 Nef only in the absence of tetracycline, at levels comparable to those observed in Jurkat cells chronically infected with HIV-1, whereas JNefG2Ac1 and JNefG2Ac2 similarly produce a nonmyristoylated version of Nef (Fig. 1A). Control clones were obtained in parallel by transfection with empty vectors. All clones exhibited comparable cell surface levels of CD3, CD28, and CD45 (data not shown). Wild-type but not nonmyristoylated Nef triggered CD4 and MHC-1 down-regulation (Fig. 1B).
Figure 1.
Characterization of Nef-inducible Jurkat T lymphoid cell lines. (A) Nef-specific Western blot analysis of indicated cells grown in the presence or absence of tetracycline for 5 days. Nef levels in nef-induced (+), control (−), and HIV-1-infected (HIV) Jurkat cells are compared underneath. (B) Cell surface levels of CD4 and MHC-I measured by fluorescence-activated cell sorter analysis. Result is representative of the two clones tested in each case.
Nef Enhances IL-2 Production in Response to CD3 or CD28 Stimulation.
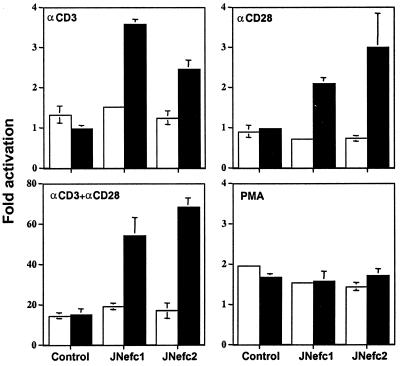
IL-2 production was measured in the supernatant of nef-expressing and control clones after treatment with various activating agents under either uninduced (with tetracycline) or induced (without tetracycline) conditions (Fig. 2). Without stimulation, no IL-2 was detected, whether Nef was expressed or not (data not shown), indicating that the viral protein cannot alone fully trigger the T cell activation cascade. However, wild-type Nef increased IL-2 production in response to stimulation with CD3- (≈5 fold), CD28- (≈10 fold), or CD3+CD28-specific (≈3 fold) antibodies (Fig. 2 A and B). The CD25, CD69, and CTLA-4 receptors were present at very low levels in the absence of stimulation. Upon CD3 and CD28 ligation, the cell surface levels of all three markers increased; although Nef significantly enhanced CTLA-4 induction, it did not affect CD25 or CD69 (data not shown). Finally, Nef did not enhance IL-2 production when cells were treated with the protein kinase C activator PMA and the calcium ionophore A23187, either separately or in combination (data not shown).
Figure 2.
Nef enhances IL-2 production in response to CD3 or CD28 stimulation. (A and B) Uninduced (open bars) or induced (solid bars) cells were stimulated with the indicated antibodies, with crosslinking for CD3. IL-2 concentration in the supernatant was measured by ELISA 16–18 h later. Without stimulation, no IL-2 was detected (not shown). In C, results are expressed as the ratio of IL-2 values measured in the supernatants of induced vs. uninduced cells. Data are averages from representative duplicate experiments.
Nef Myristoylation and Proline-Rich Repeat Are Necessary for IL-2 Superinduction.
It was previously suggested that Nef can either activate or inhibit early T cell signaling events, depending on whether it is localized at the plasma membrane or in the cytosol (7). In partial agreement with this proposal, no superinduction of IL-2 secretion was observed in the supernatant of the JNefG2A clones (Fig. 2). Nevertheless, these cells responded well to CD3 and CD28 ligation (data not shown), indicating that T cell activation is not blocked by the cytosolic accumulation of Nef. As previously described (30), the Nef(PXX)4− variant was about half as active as the wild type at down-regulating CD4 (not illustrated). However, it completely failed to induce IL-2 hyperresponsiveness (Fig. 2). The positive effect of Nef on T cell activation might therefore require the recruitment of SH3-containing proteins.
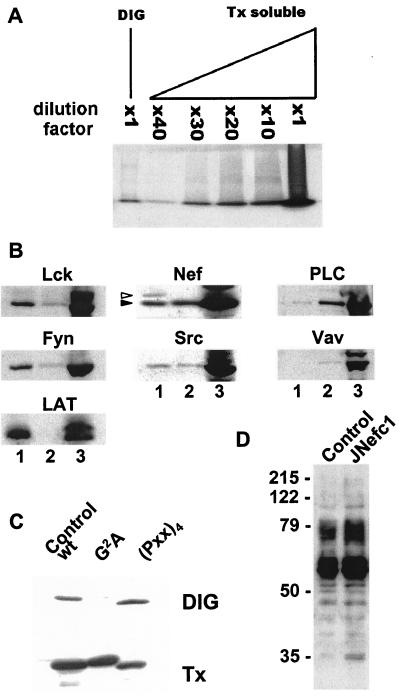
Nef Associates with DIGs.
Many factors that are key to initiating and propagating T cell receptor (TCR) signal transduction, such as Lck and LAT, are concentrated in detergent-insoluble glycolipid-enriched microdomains (DIGs or GEMs) of the plasma membrane, also called rafts (36). To ask whether the viral protein is contained in such structures, nef-expressing cells were lysed in a buffer containing a nonionic detergent, and detergent-resistant membrane constituents were fractionated by isopycnic sucrose gradient centrifugation. DIG fractions contained ≈30× less total protein than detergent-soluble fractions (Fig. 3A). Fraction samples normalized for protein content were subjected to Western blotting with various antibodies (Fig. 3B). Lck, Fyn, and LAT were markedly enriched in DIGs. In contrast, PLC-γ1 and Vav were significantly more abundant in detergent-soluble fractions. Nef was found at about equal concentrations in both compartments, indicating that it was associated with, yet not restricted to, DIGs. The protein tyrosine kinase Src exhibited a similar distribution. A Nef species with a higher apparent molecular weight, perhaps corresponding to a phosphorylated form of the protein, was detected only in DIGs. This demonstrates the validity of our fractionation technique, in addition to strongly supporting the specificity and functional relevance of the Nef-DIG association. The latter required the myristoylation but not the proline-rich motif of Nef (Fig. 3C) and did not change upon CD3/CD28 stimulation (not shown). Finally, even in the absence of stimulation, Nef expression was associated with the accumulation of tyrosine-phosphorylated proteins in DIGs (Fig. 3D).
Figure 3.
Myristoylation-dependent association of Nef with DIG microdomains. (A) Equal volumes from the DIG- and Triton X-100 (Tx)-soluble fractions, or diluates from the latter, were subjected to SDS/PAGE. Electrophoresis was stopped just after the dye front entered the separating gel, and proteins were revealed by Coomassie blue staining. (B) Equal volumes from DIG- (lane 1) and Tx-soluble (lane 3) fractions, or a 1:30 dilution of the latter (lane 2), were analyzed by Western blotting with indicated antibodies. Two distinct species of Nef (arrows) are detected in DIG- but not Tx-soluble fractions. (C) Nef-specific Western blot analysis of DIG- and Tx-soluble fractions from control and nef-expressing cells, loading equal amounts of protein in all cases. The two species of Nef (visible in B) are not clearly separated on this low resolution gel. (D) Phosphotyrosine-specific Western blot analysis of DIG fractions of control (−) and nef-expressing (+) cells.
Nef Hyperactivates Transcription from the IL-2 Promoter.
To examine whether the Nef effect on IL-2 production was exerted at a transcriptional level, an IL-2 promoter-luciferase construct was transfected into JNef or control clones. After stimulation with CD3-, CD28-, or CD3+CD28-specific antibodies, the IL-2 promoter activity was enhanced 2- to 4-fold in Nef-producing cells, compared with the control (Fig. 4). Corroborating the observations made on IL-2 secretion, this hyperactivation was not seen without stimulation or in cells treated with PMA (data not shown).
Figure 4.
Nef potentiates IL-2 promoter activation. Cells grown 4 days in tetracycline-free medium were electroporated with an IL-2 promoter luciferase reporter plasmid and were kept either with (open bars) or without (dark bars) tetracycline. Forty-eight hours later, aliquots of cells from each group either were left untreated or were stimulated as indicated (with crosslinking for α-CD3) for fourteen hours before measuring luciferase activity. Fold activation represents the ratio of values in test sample vs. its uninduced/unstimulated control. Data are averages from representative duplicate experiment, with variability as error bar. Without stimulation, Nef had no effect (not shown).
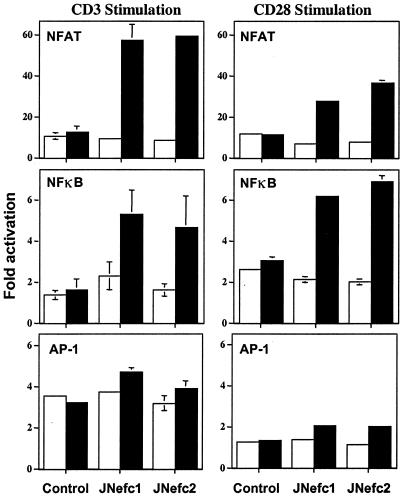
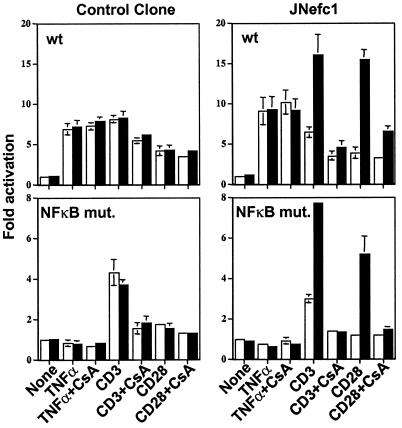
NFAT and NFκB Relay Nef-Associated T Cell Hyperactivation.
IL-2 promoter activation requires the induction of several transcription factors, including NFκB, NFAT, and AP-1 (37). To investigate which transcription factor(s) was (were) mediating the positive effect of Nef on IL-2 transcription, reporter plasmids in which luciferase was expressed from minimal promoters bearing the NFAT, NFκB, or AP-1 elements were transfected into nef-inducible or control Jurkat cells. Cells were then stimulated with CD3- and/or CD28-specific antibodies under uninduced or induced conditions. Nef production enhanced NFAT and NFκB transcriptional activities severalfold within the context of CD3 or CD28 stimulation (Fig. 5 Top and Middle). In contrast, it exerted only a marginal effect on AP-1-driven transcription (Fig. 5 Bottom), suggesting a relatively lesser impact on the Ras-Raf-MAPK and c-Jun N-terminal kinase activation pathways. Consistent with the IL-2 analyses, Nef did not change the basal activities of the various promoters in the absence of stimulation, indicating that additional signaling events must occur to unveil its influence (data not shown).
Figure 5.
Nef superinduces NFAT and NFκB activities in response to CD3 or CD28 stimulation. NFAT- (Top), NFκB- (Middle), and AP-1- (Bottom) luciferase plasmids were transfected into indicated cells, using a procedure similar to that described for Fig. 4 (without crosslinking). Without stimulation, Nef had no effect (not shown). Values are averages of two independent transfections. Open bars, uninduced cells; solid bar, induced cells.
Nef Enhances HIV Promoter Response to Stimulation.
The HIV-1 enhancer contains two tandem repeats of the NFκB regulatory element, which overlap with a binding site for NFATc, a member of the family of Rel-related components of NFAT (38, 39). Correspondingly, viral transcription is greatly enhanced by T cell activation. As predicted from this premise and from the observed effect of Nef on NFκB and NFAT responsiveness, the viral protein enhanced transcription from an HIV-1 long terminal repeat (LTR)-reporter construct by 2- to 4-fold after CD3 and/or CD28 stimulation (Fig. 6). To ask whether Nef exerted this influence primarily through NFATc or through NFκB, advantage was taken of a point mutant of the HIV-1 LTR that is defective in NFκB binding but can still recruit NFATc (38, 39). Tumor necrosis factor α induced expression from the wild-type HIV-1 LTR 6- to 10-fold. This activation was insensitive to cyclosporin A, a blocker of NFATc activation (40), but abrogated by the NFκB binding site mutation. Nef failed to synergize significantly with tumor necrosis factor α to promote transcription from the HIV-1 LTR. Although the NFκB LTR mutant was less responsive than its wild-type counterpart to CD3 or CD28 stimulation, it was still hyperactivated by nef expression (2- to 4-fold). Presumably, this reflected an increase in NFAT activity, as it was cyclosporin A-sensitive. Conversely, the wild-type HIV-1 LTR still showed some degree of Nef-associated hyperactivation in the presence of cyclosporin A, particularly in cells stimulated via CD28, pointing to the participation of NFκB. Taken together, these results indicate that Nef can positively regulate the transcriptional activity of the HIV-1 LTR by potentiating the dual activation of NFAT and NFκB.
Figure 6.
Nef enhances HIV-1 promoter responsiveness to CD3 or CD28 stimulation. Wild-type or NF-κB-mutated HIV-1 LTR luciferase plasmids were transfected into control or Nef-producing Jurkat cells. Other experimental conditions were the same as for Fig. 4 (without crosslinking). Values are averages of two independent transfections. Open bars, uninduced cells; solid bar, induced cells.
Nef-Induced Hyperactivation of NFAT- and HIV LTR-Driven Transcription in Transiently Transfected Cells.
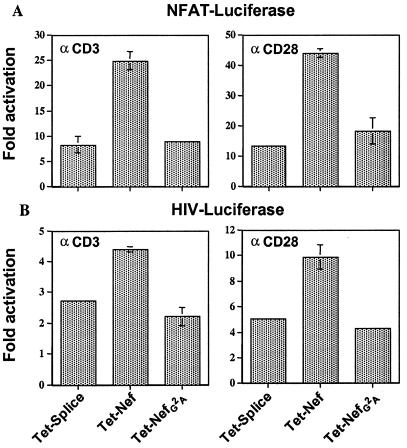
To exclude the possibility of clonal artifacts, D4 Jurkat cells were transiently cotransfected with NFAT or HIV-1 LTR luciferase reporter plasmids, together with vectors expressing wild-type or nonmyristoylated Nef (Fig. 7). Confirming the data obtained in stable clones, Nef expression was associated with the hyperresponsiveness of both promoters to CD3 or CD28 stimulation, in a myristoylation-dependent manner.
Figure 7.
Nef increases NFAT and HIV-1 LTR transcriptional responsiveness in transiently transfected Jurkat T cells. D4 Jurkat T cells were cotransfected with NFAT (A) or HIV-1 LTR (B) luciferase plasmids and either an empty vector (pTet-Splice) or the indicated Nef expression plasmids, plus pRL-TK as an internal control. Twenty-four hours later, cells either were left unstimulated or were treated with 3 μg/ml anti-CD3 or CD28 antibodies for fourteen hours and were analyzed for luciferase activity. Ratios of values obtained with vs. without stimulation are shown, averaging two independent transfections.
IL-2 Superinduction in nef-Expressing Primary CD4 T Cells.
To validate our results in primary cells, CD4+ T lymphocytes purified from the peripheral blood were transduced with a control or a nef-expressing lentiviral vector as described (34, 35). Three days posttransduction, the cells were stimulated with anti-CD3/CD28 antibodies, and IL-2 production was measured in the supernatant (Table 1). Even though only 30% of the cells exposed to the Nef vector expressed significant levels of the viral protein, as assessed from the proportion exhibiting CD4 down-regulation (not illustrated), IL-2 production in the population's supernatant was 300% of that released by control cells. Nef therefore induces IL-2 hyperresponsiveness in primary CD4 T cells, the main targets of HIV in vivo.
Table 1.
Nef enhances IL-2 induction in primary CD4 lymphocytes
| Control cells | Nef-transduced cells | |
|---|---|---|
| Experiment 1 | 4,908 pg/ml | 16,606 pg/ml |
| Experiment 2 | 5,904 ± 173 pg/ml* | 16,347 ± 962 pg/ml* |
Primary human CD4 T lymphocytes were transduced with the nef-expressing HR-EF1α-Nef or with a control lentiviral vector. Three days later, cells were stimulated with 5 μg/ml CD3/CD28-specific antibodies, and IL-2 production in the supernatant was measured at 22 h by ELISA.
*Quadruplicate measurements.
Discussion
The present work reveals that the Nef protein of HIV-1 primes human T lymphocytes for signaling through the CD3 and CD28 receptors, thereby promoting the activation of transcription factors such as NFAT and NFκB. Enhanced IL-2 secretion and a stimulation of HIV-1 transcriptional activity ensue, both of which could partly account for the positive influence exerted by Nef on viral replication in vivo.
The functional outcome of T cell receptor (TCR) engagement is normally conditioned by the absence or presence of costimulatory signals delivered via accessory molecules such as CD28 (41). Ligation of either the TCR or CD28 alone usually induces minimal levels of T cell activation or, for the TCR, can even lead to a state of anergy (42). T cells polarize toward the point of contact with the antigen-presenting cell, thereby forming a highly structured synapse (43–45). CD28 costimulation potentiates TCR signaling by recruiting rafts into the immune synapse (46). Rafts, also known as detergent-insoluble glycolipid-enriched microdomains (DIGs or GEMs), represent discrete subdomains of the plasma membrane that concentrate glycophosphatidylinositol-linked proteins, glycosphingolipids, and mediators of T cell activation, including Lck, Fyn, and LAT (32, 36, 46–48). Correspondingly, DIG integrity is a prerequisite for efficient TCR signal transduction (49). The association of Nef with rafts is therefore most likely central to its ability to prime the T cell for activation and alleviate at least partly the need for the coordinated ligation of CD3 and CD28.
A proline-based motif governing the SH3-mediated recruitment of some members of the Src family of tyrosine kinases was essential for Nef-induced IL-2 superinduction. Although it suggests that the binding of Nef to such proteins is key to its action, one cannot exclude that interactions with other SH3-containing proteins, for instance, playing the role of adapters, might be involved. In that respect, it was recently demonstrated that the Nef PxxP motif binds Vav and that this interaction can lead to the activation of Vav and its downstream effectors, such as the c-Jun N-terminal kinase (29). However, our results with an AP-1 reporter construct suggest that the c-Jun N-terminal kinase pathway may not be the primary target of Nef in human lymphocytes. Of note, the proline-rich motif of Nef is not essential for its association with DIG.
Several models, not mutually exclusive, can be proposed to explain the mechanisms of Nef action. For instance, Nef could gather the signaling molecules-containing rafts to the vicinity of the TCR even in the absence of stimulation. Alternatively, Nef might establish connections between internal components of the rafts, or recruit additional proteins into rafts, increasing the chance that these effectors will interact with and perhaps activate each other. Finally, Nef could directly preactivate some of the early mediators of T cell activation: for instance, p56lck or the θ isoform of protein kinase C. The accumulation of tyrosine-phosphorylated proteins in DIGs of nef-expressing cells is compatible with all three hypotheses. Our data concur with recent reports showing that Nef increases T cell activation in a stimulus-dependent manner (50) and that thymocytes of nef transgenic mice exhibit a state of hyperactivation and of α-CD3 hyperresponsiveness (5). Our study further indicates that the viral protein promotes the response of T cells to suboptimal modes of stimulation and provides leads for understanding the molecular mechanisms of this phenomenon.
Nef is the most abundant viral protein during the early phase of HIV gene expression (1, 2), and in certain forms of latency it may be the only viral gene product made to significant levels (51). The following model can thus be proposed to integrate our results within the context of HIV biology. Lowering the threshold necessary for triggering T cell activation will augment the chance that an infected cell presents an environment suitable for high level viral gene expression. In addition, an increased production of IL-2 will further boost viral transcription through autocrine mechanisms. Finally, the diffusion of IL-2 in the surrounding milieu will promote viral spread by augmenting the permissiveness of neighboring T lymphocytes for the early steps of viral replication. Indeed, resting T lymphocytes do not support a productive HIV-1 infection in part because reverse transcription and nuclear import are inefficient in the absence of activation (52, 53). Remarkably, IL-2 can alleviate this block even in the absence of cell proliferation (54). Together with the stimulation of viral gene expression, this effect of Nef could thus partly account for the positive influence exerted by this viral protein in vivo.
Acknowledgments
We thank K. Jones, G. Crabtree, D. Schatz, and J. Sodroski for the gift of reagents, R. Zufferey for help with the construction of lentiviral vectors, and M. Loche for the artwork. J.-K.W. and E.K. were the recipients of fellowships from the University of California Universitywide AIDS Research Program and from the Japanese Foundation for AIDS Prevention, respectively. This work was supported by grants from the National Institutes of Health (R37 AI 34306), the Swiss National Science Foundation, and the Gabriella Giorgi-Cavaglieri Foundation.
Abbreviations
- PMA
phorbol myristate acetate
- DIG
detergent-insoluble glycolipid-enriched microdomain
- TCR
T cell receptor
- LTR
long terminal repeat
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kim S, Byrn R, Groopman J, Baltimore D. J Virol. 1989;63:3708–3713. doi: 10.1128/jvi.63.9.3708-3713.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klotman M E, Kim S, Buchbinder A, De Rossi A, Baltimore D, Wong-Staal F. Proc Natl Acad Sci USA. 1991;88:5011–5015. doi: 10.1073/pnas.88.11.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kestler H W, Ringler D J, Mori K, Panicali D L, Desrosiers R C. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 4.Deacon N J, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker D J, McPhee D A, Greenway A L, Ellett A, Chatfield C, et al. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 5.Hanna Z, Kay D G, Rebai N, Guimond A, Jothy S, Jolicoeur P. Cell. 1998;95:163–175. doi: 10.1016/s0092-8674(00)81748-1. [DOI] [PubMed] [Google Scholar]
- 6.Skowronski J, Parks D, Mariani R. EMBO J. 1993;12:703–713. doi: 10.1002/j.1460-2075.1993.tb05704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baur A S, Sawai E T, Dazin P, Fantl W J, Cheng-Mayer C, Peterlin B M. Immunity. 1994;1:373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 8.Alexander L, Du Z, Rosenzweig M, Jung J U, Desrosiers R C. J Virol. 1997;71:6094–6099. doi: 10.1128/jvi.71.8.6094-6099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Z, Lang S M, Sasseville V G, Lackner A A, Ilyinskii P O, Daniel M D, Jung J U, Desrosiers R C. Cell. 1995;82:665–674. doi: 10.1016/0092-8674(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 10.Bandres J, Ratner L. J Virol. 1994;68:3243–3249. doi: 10.1128/jvi.68.5.3243-3249.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collette Y, Chang H L, Cerdan C, Chambot H, Algarte M, Mawas C, Imbert J, Burny A, Olive D. J Imunol. 1996;156:360–370. [PubMed] [Google Scholar]
- 12.Luria S, Chambers I, Berg P. Proc Natl Acad Sci USA. 1991;88:5326–5330. doi: 10.1073/pnas.88.12.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niederman T M J, Garcia V J, Hastings W R, Luria S, Ratner L. J Virol. 1992;66:6213–6219. doi: 10.1128/jvi.66.10.6213-6219.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niederman T M J, Hastings W R, Luria S, Bandres J, Ratner L. Virology. 1993;194:338–344. doi: 10.1006/viro.1993.1264. [DOI] [PubMed] [Google Scholar]
- 15.Greenway A, Azad A, McPhee D. J Virol. 1995;69:1842–1850. doi: 10.1128/jvi.69.3.1842-1850.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenway A, Azad A, Mills J, McPhee D. J Virol. 1996;70:6701–6708. doi: 10.1128/jvi.70.10.6701-6708.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collette Y, Dutartre H, Benziane A, Ramos-Morales F, Benarous R, Harris M, Olive D. J Biol Chem. 1996;271:6333–6341. doi: 10.1074/jbc.271.11.6333. [DOI] [PubMed] [Google Scholar]
- 18.Collette Y, Mawas C, Olive D. Eur J Immunol. 1996;26:1788–1793. doi: 10.1002/eji.1830260819. [DOI] [PubMed] [Google Scholar]
- 19.Iafrate A J, Bronson S, Skowronski J. EMBO J. 1997;16:673–684. doi: 10.1093/emboj/16.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saksela K, Cheng G, Baltimore D. EMBO J. 1995;14:484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee C H, Saksela K, Mirza U A, Chait B T, Kuriyan J. Cell. 1996;85:931–942. doi: 10.1016/s0092-8674(00)81276-3. [DOI] [PubMed] [Google Scholar]
- 22.Baur A S, Sass G, Laffert B, Willbold D, Cheng-Mayer C, Peterlin B M. Immunity. 1997;6:283–291. doi: 10.1016/s1074-7613(00)80331-3. [DOI] [PubMed] [Google Scholar]
- 23.Aiken C, Konner J, Landau N R, Lenburg M C, Trono D. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 24.Rhee S S, Marsh J W. J Virol. 1994;68:5156–5163. doi: 10.1128/jvi.68.8.5156-5163.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawai E T, Baur A, Struble H, Peterlin B M, Levy J A, Cheng-Mayer C. Proc Natl Acad Sci USA. 1994;91:1539–1543. doi: 10.1073/pnas.91.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nunn M F, Marsh J W. J Virol. 1996;70:6157–6161. doi: 10.1128/jvi.70.9.6157-6161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bodeus M, Cardine A M, Bougeret C, Ramos-Morales F, Benarous R. J Gen Viol. 1995;76:1337–1344. doi: 10.1099/0022-1317-76-6-1337. [DOI] [PubMed] [Google Scholar]
- 28.Smith B L, Krusherlnycky B W, Mochly-Rosen D, Berg P. J Biol Chem. 1996;271:16753–16757. doi: 10.1074/jbc.271.28.16753. [DOI] [PubMed] [Google Scholar]
- 29.Fackler O T, Luo W, Geyer M, Alberts A S, Peterlin B M. Mol Cell. 1999;3:729–739. doi: 10.1016/s1097-2765(01)80005-8. [DOI] [PubMed] [Google Scholar]
- 30.Aiken C, Krause L, Chen Y-L, Trono D. Virology. 1996;217:293–300. doi: 10.1006/viro.1996.0116. [DOI] [PubMed] [Google Scholar]
- 31.Cao J, Park I-W, Cooper A, Sodroski J. J Virol. 1996;70:1340–1354. doi: 10.1128/jvi.70.3.1340-1354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodgers W, Rose J K. J Cell Biol. 1996;135:1515–1523. doi: 10.1083/jcb.135.6.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montixi C, Langlet C, Bernard A M, Thimonier J, Dubois C, Wurbel M A, Chauvin J-P, Pierres M, He H-T. EMBO J. 1998;17:5334–5348. doi: 10.1093/emboj/17.18.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unutmaz D, KewalRamani V N, Marmon S, Littman D R. J Exp Med. 1999;189:1735–1746. doi: 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zufferey R, Nagy D, Mandel R J, Naldini L, Trono D. Nat Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 36.Lanzavecchia A, Iezzi G, Viola A. Cell. 1999;96:1–4. doi: 10.1016/s0092-8674(00)80952-6. [DOI] [PubMed] [Google Scholar]
- 37.Serfling E, Avots A, Neumann M. Biochem Biophys Acta. 1995;1263:181–200. doi: 10.1016/0167-4781(95)00112-t. [DOI] [PubMed] [Google Scholar]
- 38.Nabel G, Baltimore D. Nature (London) 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 39.Kinoshita S, Su L, Amano M, Timmerman L A, Kaneshima H, Nolan G P. Immunity. 1997;6:235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- 40.Crabtree G R. Cell. 1999;96:611–614. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- 41.June C H, Ledbetter J A, Linsley P J, Thompson C B. Immunol Today. 1990;11:211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- 42.Harding F A, McArthur J G, Gross J A, Rauler D H, Allison J P. Nature (London) 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 43.Dustin M L, Olszowy M W, Holdorf A D, Li J, Bromley S, Desai N, Widder P, Rosenberger F, van der Merwe P A, Allen P M, Shaw A S. Cell. 1998;94:667–677. doi: 10.1016/s0092-8674(00)81608-6. [DOI] [PubMed] [Google Scholar]
- 44.Wülfing C, Davis M. Science. 1998;282:2266–2269. doi: 10.1126/science.282.5397.2266. [DOI] [PubMed] [Google Scholar]
- 45.Monks C R, Freiberg B A, Kupfer H, Sciaki N, Kupfer A. Nature (London) 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 46.Viola A, Schroeder S, Sakabira Y, Lanzavecchia A. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 47.Simons K, Ikonen E. Nature (London) 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 48.Zhang W, Trible R P, Samelson L. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- 49.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 50.Schrager J A, Marsh J W. Proc Natl Acad Sci USA. 1999;96:8167–8172. doi: 10.1073/pnas.96.14.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pomerantz R J, Trono D, Feinberg M B, Baltimore D. Cell. 1990;61:1271–1276. doi: 10.1016/0092-8674(90)90691-7. [DOI] [PubMed] [Google Scholar]
- 52.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S Y. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 53.Stevenson M, Stanwick T L, Dempsey M P, Lamonica C A. EMBO J. 1990;9:1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kinter A L, Poli G, Fox L, Hardy E, Fauci A S. J Immunol. 1995;154:2448–2459. [PubMed] [Google Scholar]