Abstract
To examine the significance of chemokine activation of CXCR2 in wound healing after chemical burn, cutaneous injury was created by topical application of nitrogen mustard on CXCR2 wild type (+/+), heterozygous (+/−), and knockout (−/−) mice. Wounds were analyzed histologically for neutrophil and monocyte infiltration and for reepithelialization at postwound days 4, 7, and 10. Neutrophil recruitment to the wound site was reduced through postwound day 7 in CXCR2 −/− mice as indicated by myeloperoxidase assay and by visual quantitation. Because there is always concern that mice with targeted deletion of a specific receptor may undergo developmental adaptations to offset the loss of the receptor, we also accessed chemical wound repair in the presence of a small molecule antagonist of CXCR2. Dietary supplementation with a CXCR2 antagonist (SB-265610) during the wound repair process also markedly delayed healing parameters in CXCR2 +/+ mice, even greater than treatment with glucocorticoids. These parallel studies further establish that mice deficient in CXCR2 function exhibit delayed cutaneous wound healing that may be primarily linked to impaired neutrophil recruitment after chemical burn with nitrogen mustard. Thus, there may be a potential therapeutic benefit of treating nitrogen mustard-induced skin lesions with agonists of CXCR2 to facilitate the wound repair process.
Chemokine/chemokine receptor interactions have a well-established relevance for many processes that occur (1 for review). Expression of the cysteine-X-cysteine chemokine ligand (CXCL) 1, 5, 7, 8, 9, and 10 as well as the cysteine-cysteine chemokine ligand (CCL)-2 are temporally modulated during cutaneous wound repair.1 Moreover, the display of chemokine receptors is also modulated in keratinocytes, endothelial cells, and leukocytes.1 Previously we reported that loss of CXC receptor-2 (CXCR2) is associated with delayed wound closure, delayed neutrophil recruitment, and delayed angiogenic responses during repair of excisional wounds in murine skin.2 The present study was designed to investigate how the loss of CXCR2 might impact wound healing parameters when nitrogen mustard (NM), a reagent frequently used for topical treatment in patients with cutaneous T-cell lymphoma and Hodgkin’s disease,3–6 was applied to skin. If CXCR2 is also involved in this process, then delivery of an agonist to CXCR2 might potentially reduce some of the cutaneous problems incurred with use of NM in treatment of these diseases.
While NM initially affects exposed epithelial tissues of the skin, the eye, and the respiratory tract, higher doses can produce systemic toxicity.3,7 The severity of NM toxicity is highly variable, resulting in severe irritation, edema, necrosis, and ulceration, depending upon dose, temperature, and site of application. NM was selected in the present study because it is an excellent model for evaluation of a pathogen-free inflammatory response in skin lesions. Among its multiple targets, NM can reportedly diffuse across cellular membranes.3 Microscopic changes become visible by 3–6 hours after exposure as evidenced by pyknotic nuclei in basal keratinocytes. During progression of injury, one typically notes the dissolution of the basal cell layer, loss of immunoreactivity for laminin, and the subsequent separation of dermis from epidermis (blistering), leading to significant fluid losses and the creation of an easy portal for pathogenic agents.3 A substantial number of patients with cutaneous T-cell lymphoma undergoing treatment with NM are reported to develop cutaneous intolerance to this therapy, often resulting in spongiotic dermatitis.4,5
Previous histological and biochemical studies have shown that neutrophils infiltrate mustard lesions with a concurrent elevation of the neutrophil-associated enzyme myeloperoxidase (MPO) as early as 3 hours after exposure.3 The purpose of the current study was twofold: to evaluate the role of CXCR2 in injury due to NM and to thoroughly characterize the implications of a chemical wound model where neutrophilic recruitment is severely impaired by either a CXCR2 −/− background or a CXCR2 blockade.
MATERIALS AND METHODS
BALB/C mice heterozygous (+/−) for CXCR2 were obtained through Genentech, Inc. (San Francisco, CA), bred, and maintained by Charles River (Wilmington, MA) in a gnotobiotic facility. CXCR2 knockout (−/−) and wild (+/+) type mice were obtained from breeding CXCR2 (+/−) mice at Vanderbilt Medical Center. Mice were maintained under a 12-hour light/dark cycle and the protocols utilized were with the approval of the Institutional Animal Care and Use Committee. All experiments were performed using the guidelines established by the NIH for humane care as outlined in the Guide for the Care and Use of Laboratory Animals. In our experiments with CXCR2 antagonist (SB-265610) and glucocorticosteroid (prednisolone), we used C57 black wild type (+/+) mice. Loss of CXCR2 is associated with a compromised ability to fight infection. Therefore, these mice were bred in a gnotobiotic facility at the Charles River laboratory and maintained under pathogen-free conditions, using sterile/autoclaved food and water, autoclaved wood chips within the cage, a HEPA-filtered cage, and a HEPA-filtered room. This protocol reduces the infection rate of the mice and enhances disease-free survival.
Genotyping by polymerase chain reaction
Tail clippings were performed on anesthetized 4-week-old mice. Tail tissues were digested at 60°C overnight in lysis buffer containing 1 mg/ml Proteinase K (Gibco BRL, Gaithersburg, MD), 0.05% sodium dodecyl sulfate, 100 mM EDTA, and 50 mM Tris pH 8.0. The genomic DNA was extracted in three sequential phenol-chloroform solutions, ethanol precipitated, and resuspended in sterile water. Using a 32-cycle polymerase chain reaction (PCR; each cycle is: 94°C-5minutes; 94°C-30 seconds; 56°C-30 seconds; 72°C-1 minute, followed by 72°C-10 minutes and 4°C for 12 hours), the genomic DNA was amplified with specific primers in an automatic temperature cycler (PTC-100, MJ Research Inc., Watertown, MA). For PCR, the primers were as follows: CXCR2A (5′ to 3′): GGC AAG GTC AGG GCA AAG AA, and CXCR2 S, sequence (5′ to 3′): GGC GGG GTA AGA CAA GAA TC; Neo 1 (5′ to 3′): CGG TTC TTT TTG TCA AGA C; Neo 2 (5′ to 3′) ATC CTC GCC GTC GGG CAT GC. Each reaction mixture contained 12.5 pmol of each primer, 0.5 U Tag polymerase (Gibco BRL), 0.2 mM of dNTP, and 1μg of DNA. PCR amplification products were electrophoresed through a 1% agarose gel containing ethidium bromide.
Chemical wounding
NM or bis {2-Chloroethyl}methylamine, [(ClCH2CH2) 2NCH3 × HCl (purity 98%) was obtained from Aldrich Chemical Company, Inc. (Milwaukee, WI). This chemical was used following safety precautions described by our Vanderbilt chemical safety guidelines. The application of this reagent was performed under a chemical fume hood with the use of double gloves and with skin and eye covering to protect the research assistant performing the protocols. In the preparation for wounding, mice were anesthetized with an intraperitoneal injection of ketamine (20 mg/kg) and xylazine (0.32 mg/kg) from Bayer Corporation (West Haven, CT). The dorsal fur was removed with clippers and NM (10 μg of a 2.0% solution in phosphate buffered saline solution) was applied by micropipette over an area approximately 0.8 cm in diameter. Wounds were harvested at 4, 7, and 10 days after chemical exposure.
Histologic analysis of inflammatory infiltrates
Wound beds surrounded by a margin of nonwounded skin were collected 4, 7, and 10 days postwounding after mice had been euthanized. Skin samples were fixed for 18 hours in 4% paraformaldehyde at 4°C, embedded in paraffin, sectioned, stained with hematoxylin and eosin (H & E), and photographed. Sections were viewed on an Olympus AH light microscope (Melville, NY) and morphometric quantification of neutrophils and monocytes in the wound bed was performed by two individuals (SM and LBN) with LBN blinded to the mouse genotype. The temporal analysis of inflammatory infiltrates for each time point was performed on a minimum of six wounds (two wounds per mouse on three different mice per time point).
Based on well-established features of polymorphonuclear leukocytes,8 the density of these cells was determined by manually counting neutrophils within a standardized microscopic area. The cellular density of monocyte/macrophage infiltration was assessed in a similar fashion.
Myeloperoxidase assay
MPO activity was estimated in the wounded skin according to a method previously described,9 with slight modification. MPO was assessed 16 hours after application of the test agent. Wounded tissue from postwound days 1, 2, 3, 4, 7, and 10 was collected, snap frozen in liquid nitrogen, and later homogenized in 1 ml of phosphate buffer containing 0.5% hexadecyl trimethyl ammonium bromide (HTAB) in 50 mM phosphate buffer, pH 6.0, to extract MPO. HTAB is a detergent that releases MPO from primary granules of neutrophils. To ensure complete extraction of MPO from PMN granules, tissues were sonicated on ice for 15 seconds (Sonic 300 Model 300 (Fisher Scientific)). After one or two freeze-thaw cycles, samples were centrifuged 20 minutes at 15,000 × g at 4°C. Protein concentrations were determined using the Bio-Rad protein analysis method (Bio-Rad Laboratories, Hercules, CA). Aliquots of lysate containing equivalent amounts of protein (40 μg) were assayed for MPO. MPO was assayed by mixing 0.1 ml of the supernatant with 0.5 ml of potassium phosphate buffer (50 mM), pH 6.0, containing 0.167 mg O-dianosidine dihydrochloride (Sigma Chemical Co., St. Louis, MO) and 0.0005% H2O2. The reaction was started by the addition of hydrogen peroxide. The change in absorbance at 490 nm was measured spectrophotometrically, using the BECK-MAN-DU 7000 (Beckman Coulter, Inc., Fullerton, CA). One- and 2-minute time points were compared for each aliquot of wound bed lysate from CXCR2 (+/+), (+/−), and (−/−) mice.
Pretreatment with glucocorticoid, prednisolone, and CXCR2 antagonist
Wild type mice (C57-Black) were fed for 5 days with food containing either 10 mg/kg/day glucocorticoid (prednisolone; Sigma) or with 100 mg/kg/day of the CXCR2 antagonist, SB-265610 (GSK Pharmaceuticals, King of Prussia, PA). Serum levels of SB-265610 were assayed by Glaxo-SmithKline from samples collected immediately prior to euthanasia and were found to be in the range of ~700 ng/ml.
Statistical analyses
Data were analyzed for statistically significant differences using the Student’s paired t-test. p-values less than 0.05 were taken as evidence for statistically significant differences between data sets.
RESULTS
When chemical burns were created by NM application to the skin, the responses differed in accordance with the CXCR2 genotype of the mice. For those injuries inflicted on either CXCR2 +/+ (control) mice or CXCR2 +/− (heterozygotes), the recruitment of neutrophils was early and maximal at day 4 (Table 1) This was in marked contrast to the significantly diminished neutrophilic influx observed on the fourth day after injury in CXCR2 −/− mice (Table 1). These data indicate that NM serves as a robust inflammatory stimulus in normal animals. Interestingly, mice heterozygous for CXCR2 were still capable of mounting an immune response that appeared morphologically similar to that observed in normal mice. By contrast, at 4 days after injury, the NM lesions were essentially nonhealing in CXCR2 knockout mice. By postwound day 7, the distinct morphological differences between the NM-treated group and the control group continued to be evident (Figure 1A). Wounds on CXCR2 +/+ mice showed normal reparative responses. As is typical in the absence of acute infection, the neutrophilic infiltration was diminishing, but the monocyte infiltration was still at its peak. The resurfacing of these chemical wounds was complete or nearly complete (Figure 1B). No appreciable difference was noted in the morphological appearance of the wounds in the CXCR2 +/+ mice as compared to the CXCR2 +/−mice (data not shown). These findings suggest that even the lower levels of CXCR2 present on the CXCR2 +/− mice are adequate for recruitment of neutrophils. Eventually, the wounds in CXCR2 −/− mice did exhibit neutrophilic recruitment, but this delay was accompanied by a severely delayed response in the animals (Figure 1A).
Table 1.
Delayed neutrophil recruitment in CXCR2 knockout mice after chemical wounding with nitrogen mustard
| CXCR2 Genotype |
|||
|---|---|---|---|
| Time post-wounding (d) | +/+ | +/− | −/− |
| 4 | 102.00 ± 10.50 | 88.00 ± 9.01 | 36.33 ± 4.33* |
| 7 | 90.60 ± 3.84 | 79.33 ± 4.66 | 47.00 ± 6.02* |
| 10 | 77.66 ± 1.76 | 56.66 ± 2.33 | 63.66 ± 2.40 |
Neutrophil recruitment to the wound bed at days 4, 7, and 10 after cutanous wounding. Total numbers of neutrophils in CXCR2 knockout, wild type, and heterozygous mice were manually counted within standardized areas of H&E stain sections of the wound bed at 100×. Data are expressed as the mean ± SEM for the total number of neutrophils along the wounded skin based on three different mice at each time point after wounding, based upon Student’s two-tailed t-test. The level of statistical significance of the comparison between wild type and CXCR2 (−/−) mice is indicated by the * to denote p values < 0.05.
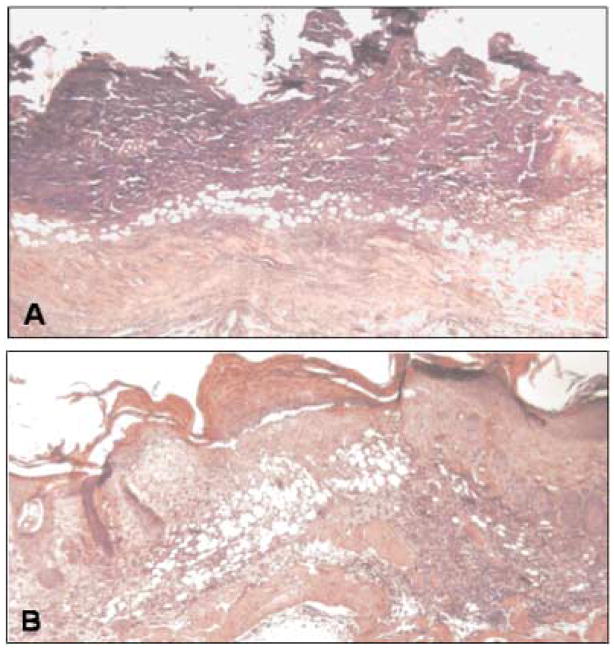
FIGURE 1.
Histological effects of CXCR2 status on NM injury. (A) shows the lack of resurfacing, the extent of the inflammatory response, and the immaturity within the wound bed 7 days after injury in the CXCR (−/−) mouse. (B) By contrast, in a CXCR2 (+/+) mouse at 4 days after injury, the resurfacing is complete, the epidermis is stratifying, and a neodermis has been formed.
By day 10 after injury, the slowness in the wound repair in the CXCR2 −/− mice as compared to the normal wild type was still highly evident (data not shown). The epidermis over the control mice was largely restored and mature as evidenced by the differentiation of the outer epidermal layers, whereas the epidermis over the NM sites was still largely undifferentiated and immature. The characteristics of the residual inflammatory population within the maturing neo-dermis were essentially identical among all groups during this maturational phase of healing. The temporal analysis following chemical injury with NM suggests that CXCR2 status is an early modifier of wound repair, yet receptor status for this particular form of the receptor does not appear to be an essential requirement, because all wounds eventually undergo a total repair of the defect.
CXCR2 status influences monocyte/macrophage influx following injury with nitrogen mustard
The role of inflammation in the pathogenesis of NM skin lesions appears to involve complex dynamic interactions from a variety of discrete cell populations. Although neutrophils are the initial cells that are recruited during the acute inflammatory period, monocytes/macrophages typically become predominant during the intermediate stages of inflammation. Not surprisingly, the monocyte/macrophage density peaked in the CXCR2 +/+ mice at 4 days and diminished over 7 and 10 days (Table 2). By contrast the monocyte/macrophage recruitment appeared identical between the CXCR2 −/− and +/− mice. The monocyte infiltrate remained high at 7 days postwounding and had begun to decline by day 10 (for CXCR2 −/− and +/−mice). The difference in monocyte infiltrate in the wound bed between CXCR2 −/− and CXCR2 +/+ mice was significant at 7 days.
Table 2.
Monocyte recruitment in CXCR2 +/+, +/−, and −/− mice after nitrogen mustard wounding
| CXCR2 Genotype |
|||
|---|---|---|---|
| Time post-wounding (d) | +/+ | +/− | −/− |
| 4 | 38.33 ± 0.88 | 47.00 ± 2.00 | 39.67 ± 1.20 |
| 7 | 22.33 ± 0.88 | 40.00 ± 0.57 | 38.00 ± 1.15* |
| 10 | 19.66 ± 0.66 | 17.33 ± 0.57 | 25.33 ± 1.45* |
Monocyte infiltration to the wound bed on days 4, 7, and 10 after chemical wounding of the skin with NM. Total numbers of monocyte in CXCR2 wild type, CXCR2 heterozygous, and CXCR2 knockout mice were manually determined and counted along the wounded areas at 100×. Data are expressed as the mean ± SEM. Statistical significance of the comparison between CXCR2 wild type, CXCR2 knockout, and CXCR2 heterozygous mice, was based upon the Student’s two-tailed t-test. The level of statistical significance of the comparison between wild type and CXCR2 (−/−) mice is indicated by the * denote p values < 0.05, showing statistical difference between these two genotypes at postwound day 7 and 10.
CXCR2 status influences MPO levels following injury with nitrogen mustard
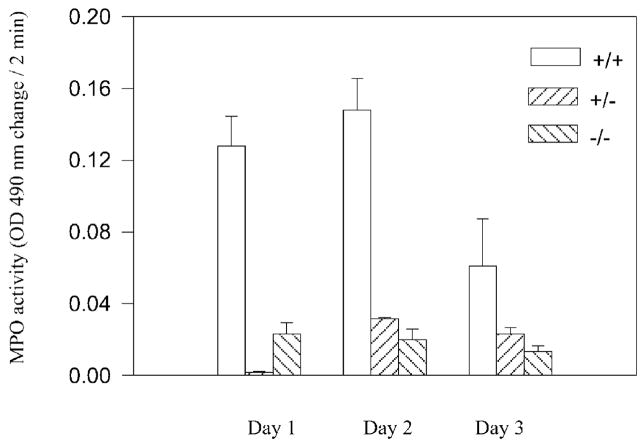
In our study, the accumulation of MPO, an enzyme found at high concentration in neutrophils, was used as a biomarker to assess the inflammatory response to this skin irritant. Prominent erythema, a hallmark of acute inflammation, was observed in the period immediately after application of NM. To confirm the extent of CXCR2-mediated neutrophil recruitment, wounded skin was collected from three differing genotypes (CXCR2 +/+, +/−, and −/−), and additionally examined by quantification of neutrophils from histological sections (Table 1). As expected, the peak MPO activity after treatment with nitrogen mustard was attained on days 1 and 2 in the CXCR2 +/+ mice (Figure 2). By contrast, the MPO level in CXCR2 −/− mice was significantly lower than in wild type mice or heterozygous mice on postwound days 2 and 3 (Figure 2). MPO activity in CXCR2 +/− mice was not significantly different than in CXCR2 −/− mice on postwound days 2–3. Neutrophilic influx was blunted as well as severely delayed in CXCR2 −/− mice as compared to CXCR2 +/+ mice during the wounding period from days 4–10 (Table 1).
FIGURE 2.
Reduced MPO activity following NM treatment. MPO activity was measured using 40 μg protein from lysate (postwound days 1, 2, and 3), as described. Data are graphed as change in absorbance at 490 nm over a 2-minute time period normalized to protein. Bars indicate the mean ± SEM statistical significance of the comparison between CXCR2 (+/+) wild type, CXCR2 (−/−) knockout, and CXCR2 (+/−) heterozygous mice. In the CXCR2 (−/−) knockout mice, MPO activity is significantly lower than in wild type (+/+) mice at postwound days 1, 2, and 3 based upon the Student’s two-tailed t-test (p < 0.05).
Chemical antagonists of CXCR2 produce similar disruptions to neutrophilic influx following injury
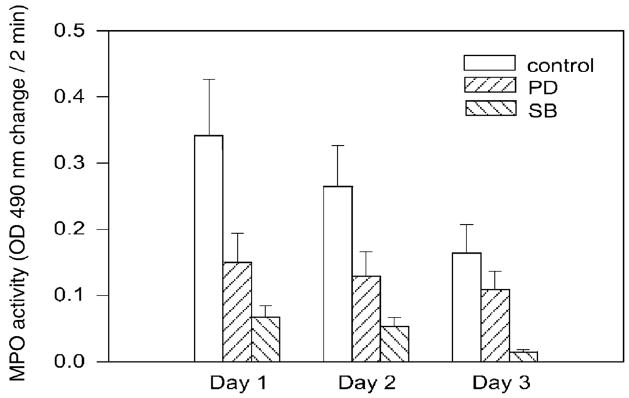
Although our model comparing three genotypically different mouse lines seemed to indicate that CXCR2 status was pivotal to neutrophilic influence and ultimately to wound repair, we sought to confirm these findings using a pharmacological antagonist to CXCR2. A competitive CXCR2 antagonist known as SB-265610 was employed. In addition, a more global inhibitor of inflammatory processes, glucocorticoid (prednisolone), was also used as a model system because it exhibits less selective anti-inflammatory and immunosuppressive properties.10 Mice pretreated with the CXCR2 antagonist exhibited significantly lower MPO activity in the acute period from days 1, 2, and 3 as compared with nontreated CXCR2 wild type (+/+) mice (Figure 3). Mice pretreated with the CXCR2 antagonist also exhibited significantly lower MPO activity than those provided with the nonselective inflammatory inhibitor (Figure 3). Our previous work with this competitive CXCR2 antagonist (SB-265610) in a differing model of injury likewise led to a reduction in neutrophil sequestration.11 In the present chemical burn model, we observed that the CXCR2 antagonist, SB-265610, markedly impaired the wound healing process (Figure 3). For some reason the baseline MPO value was higher in these experiments than in the experiments described in Figure 2, where NM treatment was compared among CXCR2 +/+, +/−, and −/−mice. It is not clear why this variation in baseline MPO was observed in the different series of experiments, but it likely was related to differences in the ambient temperature at the time of treatment for each experiment.12 Previous work has shown that skin toxicity increases as ambient temperatures rise.12 The level of SB-265610 in the serum of mice at the time of euthanasia was in the range of 700 ng/ml. This serum level of SB-265610 indicates that there was adequate delivery of this selective CXCR2 antagonist during the experimental protocol.
FIGURE 3.
Reduced MPO activity in CXCR2 (+/+) mice treated with the CXCR2 antagonist, SB-265610 or with prednisolone. MPO activity was measured using 40 μg protein from wound lysates, prepared from homogenates of postwound days 1, 2, and 3 in mice treated with SB-265610 or prednisolone delivered in food chow. Data are graphed as change in absorbance at 490 nm over a period of 2 minutes, normalized to the protein concentration. The bars indicate the mean ± SEM statistical significance between CXCR2 (+/+) mice treated with CXCR2 antagonist SB-265610 or prednisolone. MPO activity is significantly lower in mice treated with CXCR2 antagonist, SB-265610 at days 1, 2, and 3 based upon the Student’s two-tailed t-test (p < 0.05).
DISCUSSION
The present study provides additional support that the involvement of CXCR2 is a prime determinate of the inflammatory response following chemical injury. Our findings were consistent whether the CXCR2 status was manipulated by altering the genotype of the mouse or by using a selective inhibitor of CXCR2. No matter which strategy was employed, wounds that were deficient in CXCR2 showed impaired neutrophilic recruitment that appeared to lead to a general impairment in cutaneous wound repair.
A unique feature of the present study was the chemical burn model that was selected. The chemical agent NM was selected for use in the present study because it produces an acute inflammatory response without an immediate breach in the skin. Although blistering eventually occurs between 12 and 24 hours after injury, this interval allows for the initiation of the neutrophilic response in the absence of other stimulators of the inflammatory response, such as the entrance of pathogens and the release of lipopolysaccharide. This global finding is consistent with our earlier findings where we showed using a murine excisional wound model that loss of CXCR2 results in a delay in wound closure as well as a delay in neutrophilic recruitment and a delay in angiogenesis.2 That study, combined with the present mechanistic studies, provides an explanation for our earlier descriptive studies with human burn wounds where we were able to detect a modulation in expression of CXCR2 in keratinocytes of the epidermis and in the endothelial cells undergoing neovascularization.13
In the present study we noted that recruitment of neutrophils to the wound site was significantly reduced in CXCR2 −/− mice through postwound day 10. Normally, recruitment of monocytes and lymphocytes sequentially follows neutrophilic recruitment, then rapidly subsides. The present study indicates that whereas monocyte recruitment diminished after postwound day 4 in CXCR2 +/+ mice, the monocyte infiltrate was still elevated in CXCR2 −/− mice on postwound days 7 and day 10 (Table 2). CXCR2 is expressed to some extent on monocytes in addition to its robust expression on neutrophils. However, monocyte migration into the wound bed is not compromised in the CXCR2 −/− mice, suggesting that other chemokine/chemokine receptor interactions are the major modulators of monocyte infiltration into the wound bed. Together these data suggest that loss of CXCR2 delays the timing of recruitment of monocytes into the wound bed and significantly delays the wound healing process.
A more practical way to examine the role of CXCR2 in chemical burn wound healing responses is to utilize a small molecule antagonist of CXCR2. We delivered the SB-265610 CXCR2 antagonist to CXCR2 +/+ mice through the oral route and then examined the wound healing response. We observed that as with CXCR2 −/− mice, administration of the CXCR2 antagonist, SB-265610, markedly impaired the wound healing of CXCR2 +/+ mice, and also impaired the inflammatory response at the wound site based upon MPO assay. The data presented in this study also show reduced MPO activity in glucocorticoid-treated mice, probably due to reduced invasion of cytokine-producing inflammatory cells into the wound.
Other chemokines/chemokine receptor interactions have also been shown to facilitate the wound healing process. In fact, many chemokines and their receptors have been implicated in chronic inflammatory wounds of the skin, including psoriasis and atopic dermatitis. CXCL1 and CXCL8, as well as their receptor, CXCR2, are among the chemokine receptor interactions reported to be up-regulated in these disease processes. Indeed, thermal wounds undergoing delay in wound healing often exhibit persistently higher levels of CXCL8.14 In cutaneous T-cell lymphoma, high serum levels of interleukin-8 (IL-8) are associated with poor outcome.15 In the chicken, another CXC chemokine, cCAF, may facilitate the differentiation of wound bed fibroblasts into myofibroblasts during the wound repair process to facilitate wound closure.16 Although macrophage inflammatory protein-α (MIP-1α) is reported to be a critical macrophage chemoattractant in murine models of wound repair,17 another report indicates that monocyte chemoattractant protein-1 (MCP-1) −/−mice, but not MIP-1α −/− mice, exhibit delayed wound healing. The most significant difference in wound healing in MCP-1 −/− mice was observed at postwound day 3. At postwound day 5 there was also a significant delay in wound angiogenesis and collagen synthesis,18 however, there was no change in the number of wound macrophages recruited into the wounds, suggesting that other factors can compensate for loss of MCP-1 to recruit macrophages during cutaneous wound repair.
The recruitment of monocytes and mast cells into the wound bed is accompanied by release of IL-4 (1 for review). IL-4 has the ability to down-regulate the expression of pro-inflammatory chemokines such as CCL2 and CXCL8. This is thought to be an important aspect of shutting down the early inflammatory stage of wound healing. Moreover, by postwound day 4, a shift in the cytokine profile from tumor necrosis factor-α and IL-1 to one of interferon-γ may explain the induction of the interferon-γ–inducible chemokines, CXCL10 (IP-10) and CXCL9 (mig), after day 4 of cutaneous wound healing.1,19 Overexpression of CXCL10 in keratinocytes is accompanied by a delay in cutaneous wound healing.20 Thus, the temporal pattern of cellular induction and response to chemokines and cytokines must be carefully orchestrated to optimally bring about wound healing.
Understanding of the mechanisms by which harsh chemicals such as high concentrations of NM inflict serious cutaneous injury is important. We have previously demonstrated that mice deficient in CXCR2 exhibit delayed wound healing, impaired neutrophil recruitment, and reduced angiogenesis during excisional wound repair.2 The results presented here show that this is also true for highly inflammatory chemical burns induced with NM. Future work in this area may increase our knowledge of the acute cutaneous responses, delayed and impaired cutaneous healing, and long-term effects of NM exposure including carcinogenic effects. Moreover, our finding here that the chemokine receptor antagonists may be useful mediators of the wound healing process take our initial findings a step further to show that acute loss of CXCR2 function results in impaired wound healing. These findings suggest that there may be a potential therapeutic effect of treatment of skin lesions in cutaneous T-cell lymphoma patients undergoing NM treatment with ointments containing agonist to CXCR2 to facilitate the wound repair process.
Acknowledgments
This work was supported in part by funds from a Senior Career Scientist award from the Department of Veterans Affairs (AR), from a grant from the Defense and Department of Veterans Affairs, and from NIH grants GM40437 (LBN), and P30 AR41943 (AR,LBN).
- CCL
Cysteine-cysteine chemokine ligand
- CXCL
Cysteine-X-cysteine chemokine ligand
- CXCR2
CXC chemokine receptor-2
- H & E
Hematoxylin and eosin
- IL
Interleukin
- MPO
Myeloperoxidase
- NM
Nitrogen mustard
- PCR
Polymerase chain reaction
References
- 1.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leuk Biol. 2001;69:513–21. [PubMed] [Google Scholar]
- 2.Devalaraja RM, Nanney LB, Qian Q, Du J, Yu Y, Devalaraja MN, Richmond A. Delayed wound healing in CXCR2 knockout mice. J Invest Dermatol. 2000;115:234–44. doi: 10.1046/j.1523-1747.2000.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith KJ, Smith WJ, Hamilton T, Skelton HG, Graham JS, Okerberg C, Moeller R. Histopathologic and immunohistochemical features in human skin after exposure to nitrogen and sulfur mustard. Am J Dermatopathol. 1998:22–8. doi: 10.1097/00000372-199802000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Esteve E, Bagot M, Joly P, Souteyrand P, Beylot-Barry M, Vaillant L, Delaunay M, Avril MF, Laroche L, Grange F, Thomine E, Wechsler J. A prospective study of cutaneous intolerance to topical mechlorethamine therapy in patients with cutaneous T-cell lymphomas. French study group of cutaneous lymphomas. Arch Dermatol. 1999;135:1349–53. doi: 10.1001/archderm.135.11.1349. [DOI] [PubMed] [Google Scholar]
- 5.Foulc P, Evrard V, Dalac S, Guillot B, Delaunay M, Verret J-L, Dreno B. Contact dermatitis allergy: evaluation of a 1-h exposure time to mechlorethamine in patients undergoing topical treatment. Br J Dermatol. 2002;147:926–30. doi: 10.1046/j.1365-2133.2002.04802.x. [DOI] [PubMed] [Google Scholar]
- 6.Reddy VB, Ramsay D, Garcia JA, Kamino H. Atypical cutaneous changes after topical treatment with nitrogen mustard in patients with mycosis fungoides. Am J Dermatopathol. 1996;18:19–23. doi: 10.1097/00000372-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Petrali JP, Ogelsby SB, Mills KR, Hamilton TA. Comparative morphology of the effects of mustard gas in the hairless guinea pig and human skin equivalent. J Submicro Cyto Pathol. 1993;25:113–8. [PubMed] [Google Scholar]
- 8.Leeson TS, Leeson CR, Paparo AA. General histological principles and primary tissues text/atlas of histology. Philadelphia: Saunders; 1988. pp. 206–7. [Google Scholar]
- 9.Trush MA, Egner PA, Kensler TW. Myeloperoxidase as a biomarker of skin irritation and inflamation. Food Chem Toxic. 1994;32:143–7. doi: 10.1016/0278-6915(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 10.Hubner G, Brauchle M, Smola H, Madlener M, Fassler R, Werner S. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine. 1996;8:548–56. doi: 10.1006/cyto.1996.0074. [DOI] [PubMed] [Google Scholar]
- 11.White RJ, Lee JM, Young PR, Hertzberg RP, Jurewicz AJ, Chaikin MA, Widdowson K, Foley JJ, Martin LD, Grinswold DE, Sarau HM. Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J Biol Chem. 1998;273:10095–8. doi: 10.1074/jbc.273.17.10095. [DOI] [PubMed] [Google Scholar]
- 12.Borak J, Sidell FR. Agents of chemical warfare: sulfur mustard. Ann Emerg Med. 1992;21:303–8. doi: 10.1016/s0196-0644(05)80892-3. [DOI] [PubMed] [Google Scholar]
- 13.Nanney L, Mueller S, Bueno R, Peiper S, Richmond A. Distributions of MGSA/GRO and the IL-8 receptor B in wound repair. Am J Path. 1995;147:1248–60. [PMC free article] [PubMed] [Google Scholar]
- 14.Iocono JA, Colleran KR, Remick DG, Gillespie BW, Ehrlich HP, Garner WL. Interleukin-8 levels and activity in delayed-healing human thermal wounds. Wound Rep Reg. 2000;8:216–25. doi: 10.1046/j.1524-475x.2000.00216.x. [DOI] [PubMed] [Google Scholar]
- 15.Poszepczynska E, Martinvalet D, Bouloc A, Echchakir H, Wechsler J, Becherel PA, Boumsell L, Bensussan A, Bagot M. Erythrodermic cutaneous T-cell lymphoma with disseminated pustulosis. Production of high levels of interleukin-8 by tumour cells. Br J Dermatol. 2001;144:1073–9. doi: 10.1046/j.1365-2133.2001.04203.x. [DOI] [PubMed] [Google Scholar]
- 16.Feugate JE, Li Q, Wong L, Martins-Green M. The CXC: chemokine cCAF stimulates differentiation of fibroblasts into myofibroblasts and accelerates wound closure. J Cell Biol. 2002;156:161–72. doi: 10.1083/jcb.200103062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiPietro LA, Burdick M, Low QE, Kunkel SL, Strieter RJ. MIP 1-alpha as critical macrophage chemoattractant in murine wound repair. J Clin Invest. 1995;101:1693–8. doi: 10.1172/JCI1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Low QE, Drugea IA, Duffner LA, Quinn DG, Cook DN, Rollins BJ, Kovacs EJ, DiPietro LA. Wound healing in MIP-1 alpha (−/−) and MCP-1 (−/−) mice. Am J Pathol. 2001;159:457–63. doi: 10.1016/s0002-9440(10)61717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Opdenakker G, Van Damme J. Cytokine-regulated proteases in auto-immune disease. Immunol Today. 1994;15:103–7. doi: 10.1016/0167-5699(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 20.Luster AD, Cardiff RD, MacLean JA, Crowe K, Granstein RD. Delayed wound healing and disorganized neovascularization in transgenic mice expressing the IP-10 chemokine. Proc Assoc Am Physicians. 1998;110:183–96. [PubMed] [Google Scholar]