Abstract
The MGSA/GRO protein is endogenously expressed in almost 70% of the melanoma cell lines and tumors, but not in normal melanocytes. We have previously demonstrated that over-expression of human MGSA/GROα, β or γ in immortalized murine melanocytes (melan-a cells) enables these cells to form tumors in SCID and nude mice. To examine the possibility that the MGSA/GRO effect on melanocyte transformation requires expression of other genes, differential display was performed. One of the mRNA’s identified in the screen as overexpressed in MGSA/GRO transformed melan-a clones was the newly described M-Ras or R-Ras3 gene, a member of the Ras gene superfamily. Over-expression of MGSA/GRO upregulates M-Ras expression at both the mRNA and protein levels, and this induction requires an intact glutamine-leucine-arginine (ELR)-motif in the MGSA/GRO protein. Western blot examination of Ras expression revealed that K- and N-Ras proteins are also elevated in MGSA/GRO-expressing melan-a clones, leading to an overall increase in the amount of activated Ras. MGSA/GRO-expressing melan-a clones exhibited enhanced AP-1 activity. The effects of MGSA/GRO on AP-1 activation could be mimicked by over-expression of wild-type M-Ras or a constitutively activated M-Ras mutant in control melan-a cells as monitored by an AP-1-luciferase reporter, while expression of a dominant negative M-Ras blocked AP-1-luciferase activity in MGSA/GRO-transformed melan-a clones. In the in vitro transformation assay, over-expression of M-Ras mimicked the effects of MGSA/GRO by inducing cellular transformation in control melan-a cells, while over-expression of dominant negative M-Ras in MGSA/GROα-expressing melan-a-6 cells blocked transformation. These data suggest that MGSA/GRO-mediated transformation requires Ras activation in melanocytes.
Keywords: chemokine, MGSA/GRO, Ras, AP-1, melanocytes, transformation
Introduction
Melanoma growth stimulatory activity/growth regulated protein (MGSA/GRO) is a member of the CXC chemokine family. MGSA/GRO plays a fundamental role in recruitment and activation of neutrophils, lymphocytes and monocytes in host defense. There are four MGSA/GRO genes. Three of them, MGSA/GROα, β and γ, encode closely related proteins (Haskill et al., 1990; Iida and Grotendorst 1990; Kurdowska et al., 1994), and the other, MGSA/GROδ, appears to be a pseudogene (Shattuck-Brandt et al., 1997). All three MGSA/GRO proteins bind to the CXC chemokine receptor designated CXCR2, a member of G-protein coupled receptor family. The order of potency for these three isoforms regarding neutrophil and basophil chemotactic activity, Ca2+ flux, respiratory burst, exocytosis, shape change, and CXCR2 receptor binding is MGSA/GROα>γ>β (Geiser et al., 1993). An ELR (glutamic acid, leucine and arginine)-motif is present near the N-terminus of all the neutrophil-activating CXC chemokines that bind to CXCR1 and CXCR2. Muta-genesis studies on MGSA/GROα have revealed that the ELR motif is a critical domain for receptor binding and biological activity (Hesselgesser et al., 1995; Arenberg et al., 1997). The order of loss of ligand sensitivity upon mutation of this motif is R>E>L (Hébert et al., 1991).
MGSA/GROα expression is correlated with melanocyte tumor progression (Richmond and Thomas, 1986; 1988; Balentien et al., 1991; Mattei et al., 1994; Shattuck et al., 1994). Our laboratory has demonstrated that mouse immortalized melanocytes (melan-a cells) stably transfected with MGSA/GROα, β, or γ exhibit enhanced ability to form large colonies in soft agar and tumors in nude mice (Balentien et al., 1991; Owen et al., 1997). In contrast, parental melan-a cells (immortalized murine melanocytes), melan-a cells expressing empty vector (V1 controls), or melan-a cells expressing ELR motif mutant forms of MGSA/GROα form few, if any, tumors in nude mice (Bennett et al., 1987; Haghnegahdar et al., 2000). Moreover, neutralizing antibodies to MGSA/GROα or γ proteins slowed or inhibited the growth of these MGSA/GRO-induced melan-a tumors in SCID mice (Haghnegahdar et al., 2000).
The signaling pathways leading to MGSA/GRO-induced melanocyte transformation are unclear. Activation of the PI3-kinase/Ras/Raf/Soc/MEK/ERK pathway is common for G protein-coupled receptors (Hawes et al., 1996; Della Rocca et al., 1997; Lopez-llasaca et al., 1997). Stimulation of CXCR2 with IL-8 revealed that activation of the PI3-kinase/Ras/Raf pathway is required for human neutrophil migration. However, the regulation of cell migration by IL-8 is independent of ERK activation (Knall et al., 1997), but is dependent upon Gβγ activation (Neptune and Bourne, 1997). For some cell types, MGSA/GRO activation of ERK can also be through a Ras/Raf1 independent pathway (Shyamala and Khoja, 1998; Yang et al., 1999). In some cell systems, activation of Ras is accompanied by enhanced expression of c-Fos and enhanced formation of the activation protein-1 (AP-1) complex, and these are crucial in cellular transformation (Johnson et al., 1996; van Darn et al., 1998; Saez et al., 1995). Blocking of AP-1 activity in Ras-transformed cells has been shown to suppress transformation (Lloyd et al., 1991). Enhanced AP-1 activity is frequently associated with in vivo and in vitro models of skin cancer (Rutberg et al., 1997; Domann et al., 1994).
We postulated that continuous expression of MGSA/GRO in melanocytes would be accompanied by an altered mRNA expression profile. It is known that IL-8 induces α4β7 αLβ2 and αvβ2-integrin expression in leukocytes (Sadhu et al., 1998), and that MGSA/GRO induces α6 integrin expression in keratinocytes (Rennekampff et al., 1997). In an attempt to further explore the mechanism by which MGSA/GRO induces melanocyte transformation, we performed differential display to identify mRNAs over-represented in MGSA/GROα- or γ-expressing melan-a cells, as compared to vector control cells. One gene identified in this screen is a member of the Ras family, called M-Ras or R-Ras3 (Matsumoto et al., 1997; Kimmelman et al., 1997). Additional experiments revealed that expression of N- and K-Ras was also induced, with a resultant marked increase in the presence of activated Ras. To further characterize the M-Ras function in melanocytes, we examined the activation of the transcription factor AP-1, which is a downstream target of Ras. Over-expression of wild-type M-Ras or a constitutively activated M-Ras mutant in control melan-a cells (V1) increased activation of AP-1. Over-expression of a dominant negative M-Ras mutant inhibited MGSA/GRO-induced AP-1 activation. In the in vitro transformation assay, wild-type M-Ras over-expression induced cellular transformation, while dominant negative M-Ras blocked the MGSA/GROα-induced transformation in melanocytes. Our results suggest that MGSA/GRO-induced melanocyte transformation is through a Ras dependent mechanism which leads to activation of specific transcription complexes, and altered gene expression.
Results
Identification of M-Ras by differential display
The over-expression of MGSA/GRO plays a key role in melanocyte transformation (Balentien et al., 1991; Owen et al., 1997). However, changes in gene expression that occur as a result of continuous expression of MGSA/GRO in melanocytes have not been characterized. To determine whether constitutive MGSA/GRO altered the expression of genes whose protein products may be involved in melanocyte transformation, differential display was performed. Total RNA preparations extracted from the transformed MGSA/GROα-expressing Mel-a-6 cells, the MGSA/GROγ-expressing γ3-14 cells, and the non-transformed melan-a cells expressing the neomycin selective vector alone (V1 cells) (Table 1) were used to perform differential display using 24 different primer combinations. The 16 reamplified differentially expressed cDNA fragments originally identified by the differential display analysis (see Materials and methods) were sub-cloned into the PCR-TRAP vector and sequenced. Sequence homology searches on the obtained cDNA sequences indicated that three of the fragments match known sequences: M-Ras; homeodomain protein Meis (a transcription factor which regulates cell differentiation); and extracellular matrix associated protein (sc1). Thirteen cDNA fragments did not match any genes in the database, as shown in Table 2. We focus here on M-Ras, which was expressed at a higher level in Mel-a-6 and γ3-14 cells than in the V1 cells in the differential display assay (Table 2). The amino acid sequence of murine M-Ras shares 49 and 46% identity to mouse H-Ras and R-Ras, respectively. M-Ras contains a conserved domain including the GTP-binding site and the consensus prenylation signal CAAX known to play an important role in the plasma membrane localization of Ras (Matsumoto et al., 1997; Kimmelman et al., 1997).
Table 1.
Name of melan-a clones
| Clone name | Vector | Expressing gene | Cells transfected | Cellular transformation |
|---|---|---|---|---|
| V1 | pRC/CMV | Parental melan-a cells | No | |
| V4 | ||||
| V6 | ||||
| γ3-14 | pRC/CMV | MGSA/GROγ (ELR) | Parental melan-a cells | Yes |
| γ3-12 | ||||
| γ3-37 | ||||
| Mel-a-6 | pRC/CMV | MGSA/GROα (ELR) | Parental melan-a cells | Yes |
| Mel-a-4 | ||||
| Mel-a-9 | ||||
| E6A | pRC/CMV | MGSA/GROα (ALR) | Parental melan-a cells | No |
| L7A | pRC/CMV | MGSA/GROα (EAR) | Parental melan-a cells | No |
| R8A | pRC/CMV | MGSA/GROα (ELA) | Parental melan-a cells | No |
Table 2.
Differential display clones with altered expression in transfectant cells
| Differential display clone | Expression in transfectant cell | Proposed identity |
|---|---|---|
| 1 | Mel-a-6 | No match |
| 2 | γ3-14 | No match |
| 3 | γ3-14, Mel-a-6 | No match |
| 4 | γ3-14, Mel-a-6 | M-Ras |
| 5 | Mel-a-6 | No match |
| 6 | γ3-14, Mel-a-6 | No match |
| 7 | β2-19 | No match |
| 8 | V1, γ3-14 | No match |
| 9 | V1, β2-19 | Meis 2 |
| 10 | V1, β2-19 | SC1 |
| 11 | V1, γ3-14, Mel-a-6 | No match |
| 12 | Mel-a-6 | No match |
| 13 | β2-19 | No match |
| 14 | V1 | No match |
| 15 | β2-19 | No match |
| 16 | β2-19 | No match |
Differential display assay − 250 ng of total RNA from V1, β2-19, γ3-14 and Mel-a-6 were subjected to the differential display assay to examine M-Ras expression using the primers (H-T11G and H-AP4) and conditions described in Materials and methods. The M-Ras identified by DNA sequence analysis was differentially detected
Over-expression of MGSA/GRO up-regulates M-Ras mRNA expression
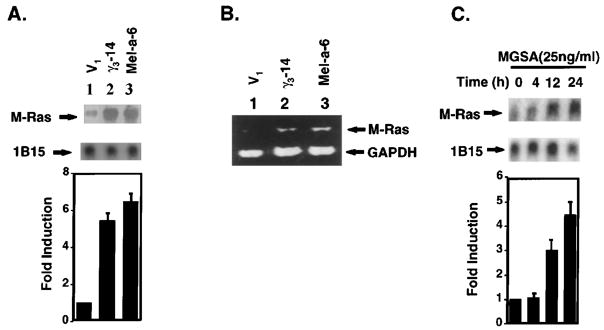
M-Ras differential expression was verified by Northern blot using the cDNA fragments of M-Ras (Figure 1a, upper panel) and by RT–PCR (Figure 1b). The probe for M-Ras is a specific 200 bp-cDNA fragment that only hybridizes M-Ras mRNA, not other members of the Ras super-family. A cyclophilin (1B15) probe was used as a loading control (Figure 1a, middle panel). The bar graph data (lower panel) presented in Figure 1a shows the band intensity corresponding to M-Ras after normalization to cyclophilin (1B15) mRNA. The quantitation of the Northern blot reveals that Mel-a-6 cells have a 6.5-fold higher level of expression of the 1.0 kb M-Ras transcript, whereas the γ3-14 cells show a 4.5-fold higher level of expression as compared to the V1 cells (Figure 1a, lower panel). The RT–PCR results are consistent with the Northern blot results (Figure 1b). There is a similar level of receptor expression on the surface of melan-a clones, based on the observation that V1, γ3-14 and Mel-a-6 cells exhibit equivalent 125I-MGSA/GROα specific binding activity (1477, 1717, 1369 c.p.m.s per 105 cells, respectively). These data suggest that the differences in the expression of M-Ras exhibited by V1, γ3-14 and Mel-a-6 cells are not due to clonal differences in receptor expression.
Figure 1.
MGSA/GRO up-regulates M-Ras expression in mRNA level in melanocytes (a) Northern blot analysis of M-Ras: Total cellular RNA (20 μg) from V1 (lane 1), γ3-14 (lane 2) or Mel-a-6 (lane 3) cells was extracted, separated on a formaldehyde-agarose gel, and transferred to a Nytran Plus filter. The filter was hybridized with the 32P-labeled mouse M-Ras differential display fragment. Hybridizing bands were identified at 1.0 kb. The filter was re-probed with 32P-labeled cyclophilin (1B15). The relative M-Ras band density is M-Ras band density normalized to cyclophilin (1B15) band density quantitated by a densitometer. The bar graph presents a mean (±s.e.m.) of fold induction of relative M-Ras band intensity from three independent experiments (lower panel). (b) RT– PCR: Total cellular RNA (20 μg) from V1 (lane 1), γ3-14 (lane 2) or Mel-a-6 (lane 3) cells was extracted and RT–PCR amplification was performed as described in Materials and methods, using specific oligonucleotides (5′ primer: ACCAGCGCTGTTCCAAGTGAA; 3′ primer: TCACAAGATGACACA CTGCAG). The post-PCR products were analysed in an ethidium bromide-stained 1.5% agarose gel. (c) Northern blot analysis of M-Ras induced by exogenous MGSA/GROα: Total cellular RNA (20 μg) from parental melan-a cells treated with recombinant human MGSA/GROα at 25 ng/ml for the indicated period was used in Northern blot analysis as described above. The relative M-Ras band density is M-Ras band density normalized to cyclophilin (1B15) band density quantitated by a densitometer. The bar graph presents a mean (±s.e.m.) of fold-induction of relative M-Ras band intensity from three independent experiments (lower panel)
In parallel, to determine whether the addition of exogenous MGSA/GROα increases M-Ras gene expression in parental melan-a cells, Northern blot analyses were performed on total RNA from the parental melan-a cells treated with recombinant human MGSA/GROα (25 ng/ml) for 0, 4, 12 or 24 h (Figure 1c, upper panel). A cyclophilin (1B15) probe was used as an internal control to monitor RNA loading (Figure 1c, middle panel). Confirming the above results, a 3.0- or 4.5-fold increase in the M-Ras mRNA transcript was noticed after treatment of parental melan-a cells with MGSA/GROα for 12 or 24 h, respectively (Figure 1c, lower panel). These results demonstrate that MGSA/GRO up-regulates the 1.0 kb M-Ras transcript in melan-a cells.
Over-expression of MGSA/GRO up-regulates M-Ras protein expression
To confirm whether this MGSA/GRO-enhanced M-Ras mRNA expression results in an increase in M-Ras protein, we examined M-Ras expression in V1, γ3-14 and Mel-a-6 cells by Western blot using an anti-M-Ras antibody. The 24 kDa M-Ras band migrated with the control recombinant M-Ras protein expressed in transfected HEK293 cells (Figure 2a, lane 6). M-Ras protein levels were significantly higher in γ3-14 and Mel-a-6 cells, compared with the V1 cells (Figure 2a lanes 2, 3 versus lane 1). In order to determine whether the higher expression of M-Ras in MGSA/GRO-expressing cells is a result of MGSA/GRO over-expression, stable melan-a clones over-expressing the ELR-motif mutant forms of MGSA/GROα (AAA/ELR) and MGSA/GROα (EAR/ELR) (E6A) were analysed for M-Ras protein expression. The level of M-Ras protein expression in melan-a clones either expressing mutant MGSA/GROα (ELR/AAA) or mutant MGSA/GROα (ELR/ALR) protein was similar to the level in V1 cells (Figure 2a lanes 4 and 5 versus lane 1) and was much lower than the level in Mel-a-6 and γ3-14 cells (Figure 2a lanes 4 and 5 versus lanes 2 and 3). ELISA data for MGSA/GROα revealed equal levels of MGSA/GROα secretion in cells expressing wild type and mutant forms of this chemokine (data not shown). A ligand-binding assay revealed equal levels of 125I-MGSA/GROα binding to melanocyte clones expressing wild type and mutant MGSA/GROα (data not shown). The failure of the MGSA/GROα ELR-mutant to increase M-Ras protein expression demonstrates that the elevated expression of M-Ras in Mel-a-6 or γ3-14 cells is a result of biological responses induced by MGSA/GRO over-expression and does not result from non-specific clonal differences in gene expression, since the MGSA/GROα ELR-mutant has reduced affinity for CXCR2 and little biological activity.
Figure 2.
Over-expressing MGSA/GRO in melanocytes up-regulates M-Ras protein expression. (a) Western blot analyses of M-Ras protein expression: Whole cell extracts (50 μg) from melan-a clones V1 (lane 1), γ3-14 (lane 2), Mel-a-6 (lane 3), AAA-MGSA/GROα (lane 4), or E6A-MGSA/GROα mutant (lane 5) were resolved by 12% SDS-polyacrylamide gel electrophoresis, blotted onto nitrocellulose membranes, and probed with anti-M-Ras antibody as described in Materials and methods. Lane 6 represents the whole cell extract (20 μg) from HEK293 cells over-expressing M-Ras protein as a positive control. The band density was quantitated by a densitometer, and the results are reported as a mean of fold-induction (±s.e.m.) from three independent experiments (low panel). (b) Western blot analyses of Ras protein expression: Whole cell extracts (30 μg) from V1 (lane 1), γ3-14 (lane 2), or Mel-a-6 (lane 3) were resolved by 12% SDS-polyacrylamide gel electrophoresis, blotted onto nitrocellulose membranes, and probed with anti-pan-ras, anti-N-Ras, anti-K-Ras, anti-H-Ras or anti-Bcl2 antibody as described in Materials and methods. The bar graph represents a mean of fold induction (±s.e.m.) of the total Ras band density quantitated by a densitometer from three independent experiments. (c) Western blot analyses of Ras protein expression in ELR-mutant clones: Western blots were performed by using whole cell extract (30 μg) from V1 (lane 1), Mel-a-6 (lane 2), E6A (lane 3), R8A (lane 4) or L7A (lane 5) and probed with anti-pan-Ras as described above
To examine the overall expression of Ras (H, N and K-Ras), Western blots were performed using a pan-Ras antibody (AB-3) (Oncogene Research Products, Cambridge, MA, USA) which reacts with mammalian H, N and K-Ras proteins. Overall Ras protein levels were significantly higher in γ3-14 and Mel-a-6 cells, as compared to the V1 cells (Figure 2b, top panel). Ras expression from three different clones from each vector, MGSA/GROα- and MGSA/GROγ-expressing melan-a cells was examined and similar results were obtained (data not shown). The higher Ras expression in Mel-a-6 cells is mostly contributed by K-Ras and N-Ras, while the higher Ras expression in γ3-14 is contributed by N-Ras. There is no significant difference in H-Ras expression between these cells (Figure 2b). To confirm that equivalent amounts of protein were loaded on the gel, the Western blot was probed with antibody to Bcl-2. All the extracts from these clones exhibited the same level of Bcl-2 protein expression (Figure 2b, lower panel). In addition, overall Ras protein expression levels in melan-a clones expressing ELR-mutant forms of MGSA/GROα were determined by Western blot using a pan-ras antibody (AB-3). All three melan-a clones expressing ELR-motif mutants failed to show an increased level of Ras protein expression, as compared to the V1 cells (Figure 2c). However, the L7A mutant form of MGSA/GROα exhibited a higher level of Ras protein expression than E6A and R8A mutant forms of MGSA/GROα, a result which is consistent with the higher affinity of this ligand for CXCR2 (Hebert et al., 1991). These results indicate that the ELR-motif is required for MGSA/GRO-enhanced Ras expression.
Enhanced Ras expression is correlated with Ras activity
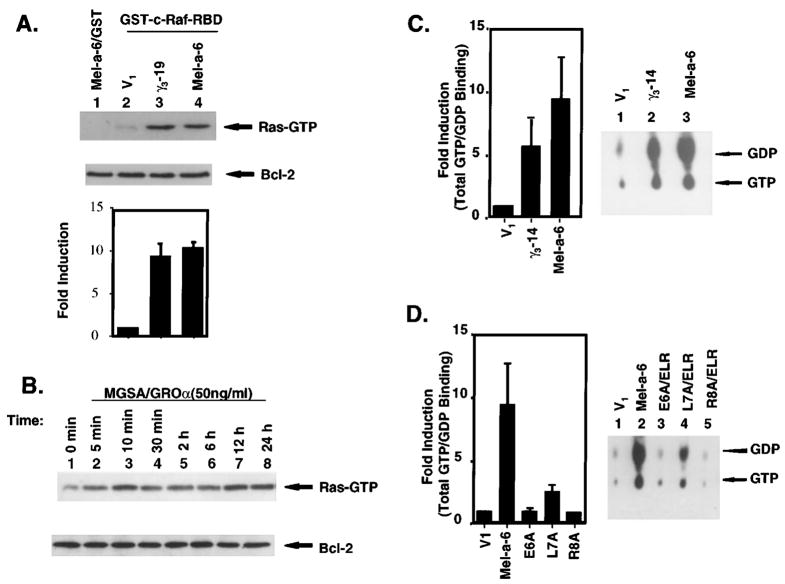
Based on the current knowledge of Ras effector molecules, activation-specific probes for endogenous activated Ras have recently been developed based upon the ability of activated Ras to bind Raf through a specific activated Ras binding domain on Raf (Raf RBD) (Taylor and Shalloway, 1996). The Raf RBD has a significantly lower affinity for Ras-GDP than for Ras-GTP, such that only Ras-GTP is detected under the conditions of this assay (Taylor and Shalloway, 1996). This construct preferentially recognizes and precipitates GTP-loaded forms of Ras and allows determination of endogenous Ras activation in cells. To test whether over-expression of MGSA/GRO increases endogenous Ras activation in melanocytes, the level of endogenous Ras activation in MGSA/GRO-expressing clones was examined by determining the amount of Ras-GTP (activated) that would bind to a GST-cRaf-1 RBD through the Ras binding domain. The level of Ras-GTP was 10-fold higher in γ3-14 and Mel-a-6 cells, compared with the V1 cells (Figure 3a, lanes 3 and 4 versus lane 2). These results demonstrate that over-expression of MGSA/GRO constitutively increases a total amount of activated Ras. Furthermore, we examined whether addition of exogenous MGSA/GROα increased the amount of endogenous GTP-bound Ras in the parental melan-a cells treated with recombinant human MGSA/GROα (50 ng/ml) for the indicated time (Figure 3b). MGSA/GROα increases Ras activation in 5 min and lasts 24 h. In an early response (from 5 – 120 min), MGSA/GROα enhanced Ras activation without changing Ras expression (data not shown). In a late response (from 6 – 24 h), MGSA/GROα-enhanced Ras activity is contributed by increasing the level of Ras proteins (data not shown). To further determine whether over-expression of MGSA/GRO alters the Ras exchange ratio, Ras-GTP/GDP binding and GTPase assays were performed. Mel-a-6 and γ3-14 cells exhibit greater Ras-GTP/GDP-binding, as compared to V1 (9.5 and 5.7-fold, respectively) (Figure 3c, left panel). The level of Ras-GTP-binding is in close agreement with the level of Ras-GTP measured by precipitating the Ras-GTP using the Ras binding domain of GST-c-Raf-1 RBD (Figure 3a). The GTP/GDP ratio is not changed in any of the clones (Figure 3c, right panel). To examine the possibility that failure to see a change in the GTP/GDP ratio in these clones was due to the long reaction time resulting in saturation of the GTP/GDP ratio, the GTP/GDP ratio over a time course was measured to follow the GTPase activity over time. No difference in the Ras-GTP/GDP ratio was observed in any of these three clones (data not shown). These results demonstrate that overexpression of MGSA/GRO results in an increase in the total amount of GTP and GDP bound to Ras, but does not greatly alter the GTP/GDP exchange ratio. These data suggest that over-expression of MGSA/GRO does not change the Ras-GTPase activity per molecule, but MGSA/GRO-enhanced Ras expression results in an increase in total constitutive Ras activation. Consistent with the results of Ras protein expression, continuous expression of mutant ELR-motif forms of MGSA/GROα fails to enhance Ras activation. Mutant MGSA/GROα (E6A and R8A) expressing clones have the same levels of Ras-GTP and Ras GDP as the V1 control. The mutant L7A cells slightly increased Ras-GTP and Ras GDP, as compared to V1 (2.5-fold) (Figure 3d, left panel). The Ras-GTP/GDP ratio is not greatly changed in any clones (Figure 3d, right panel). These data further confirm that the high level of total constitutive Ras activity in γ3-14 and Mel-a-6 clones is a specific response to over-expression of MGSA/GROγ and α in these cells.
Figure 3.
Ras activation. (a) Activation of endogenous Ras in MGSA/GRO-expressing clones: GTP-bound Ras was affinity-precipitated from 400 μg of whole cell extract of V1, γ3-14 or Mel-a-6 using a GST-c-Raf-1/Ras binding domain (RBD) fusion protein. Ras proteins were detected with a pan-Ras antibody. Lane 1 is a negative control of GST alone to precipitate Ras proteins from extract of Mel-a-6 cells. The amount of Bcl2 protein is shown in the lower panel for control of total proteins in extracts from different clones. The bar graph represents the mean of fold-induction (±s.e.m.) of the band density of activated Ras quantitated by a densitometer from three independent experiments. (b) Activation of endogenous Ras in parental melan-a cells treated with MGSA/GROα: GTP-bound Ras was affinity-precipitated from 400 μg of whole cell extract of parental melan-a cells treated with MGSA/GROα for the indicated times using the GST-c-Raf-1 RBD fusion protein. Ras protein bands were detected with a pan-Ras antibody. The expression of Bcl2 protein is shown in the lower panel to control for protein content in extracts. (c) Ras-GTP/GDP-binding and GTPase activity in MGSA/GRO-expressing clones: Left-panel: Ras proteins were immunoprecipitated from 400 μg of whole cell extract of V1, γ3-14 or Mel-a-6 with 2 μg of anti-pan-ras antibody (AB-3). Ras immune complexes were incubated with 1 μl of [α-32P]GTP (3000 Ci/mmol, Amersham) in 100 μl GTP/GDP-binding buffer and processed as described in Materials and methods. The results are reported as a mean of fold-induction (±s.e.m.) from three independent experiments. The fold induction was calculated as the radioactivity (c.p.m.) of 32P-labeled nucleotides from γ3-14 or Mel-a-6 divided by the radioactivity of 32P-labeled nucleotides from V1. Right-panel: The released 32P-labeled GTP and GDP were loaded on PEI-cellulose plates, resolved by 0.75 M KH2PO4 and then visualized by autoradiography. (d) Ras-GTP/GDP-binding and GTPase activity in ELR-mutant clones: GTP/GDP-binding and GTPase activity assays were performed using 400 μg of whole cell extract of V1, Mel-a-6, E6A/ELR, L7A/ELR and R8A/ELR as described above
MGSA/GROα increases the activation of AP-1
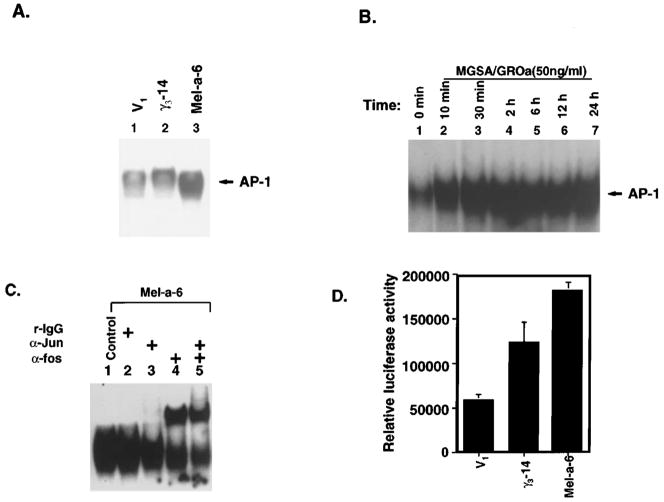
We hypothesized that MGSA/GRO uses a Ras pathway to activate AP-1, an event that results in the modulation of expression of genes whose encoded proteins are crucial for this transformation event. To evaluate whether melanocytes continuously expressing MGSA/GRO exhibit enhanced basal AP-1 activation, two different approaches were used: electrophoretic mobility shift assay (EMSA) and luciferase reporter transactivation assay. EMSA was performed to evaluate the DNA binding activity of AP-1 in V1, γ3-14 and Mel-a-6 cells using the consensus AP-1 DNA binding sequence as the radiolabeled probe. The results showed that the level of basal AP-1 DNA binding activity was higher in γ3-14 cells and Mel-a-6 cells than V1 cells (Figure 4a). To exclude the possibility that these observations were the result of clonal differences, AP-1 EMSAs were performed on nuclear extracts from MGSA/GROα-expressing Mel-a-4 and Mel-a-9 clones; from MGSA/GROγ-expressing γ3-12 and γ1-37 clones; and from two additional vector control clones, V4 and V6 (Table 1). These results confirmed that the level of AP-1 DNA binding activity was elevated in the MGSA/GROγ-expressing clones (γ3-14, γ3-12 and γ1-37) and MGSA/GROα-expressing clones (Mel-a-6, Mel-a-4 and Mel-a-9) (data not shown). To determine whether addition of exogenous MGSA/GROα to parental melan-a cells also induces AP-1 DNA binding activity, the AP-1 DNA binding activity in nuclear extracts from parental melan-a cells treated with MGSA/GROα was analysed over a time course of 10 min to 24 h. The increased AP-1 binding activity was observed after 10 min and lasted to 24 h (Figure 4b). The results show that addition of exogenous MGSA/GROα induced the AP-1 DNA binding activity in parental melan-a cells. Super-shift EMSA using antibodies which recognize most forms of Jun (pan-Jun Ab) or Fos (pan-Fos Ab) identified the subunit composition of the MGSA/GRO-induced AP-1 complex observed by EMSA. As shown in Figure 4c, the pan-Fos antibody super-shifted the AP-1 complex. In contrast, the pan-Jun antibody had no effect on the AP-1 complex in the EMSA. These results indicate that MGSA/GROα induces AP-1 DNA binding activity through increased nuclear localization and activation of Fos in melan-a cells. To examine the effect of MGSA/GRO on AP-1 transactivation, cells expressing MGSA/GROα or γ were cotransfected with a luciferase reporter gene driven by the SV40 promoter containing two copies of the consensus AP-1 binding site and a pSV-β-Gal expression construct. AP-1 luciferase and β-Gal activities were measured 48 h after transfection. The mean of the relative activity of the AP-1 luciferase after normalization for transfection efficiency based upon β-gal activity was threefold higher in the Mel-a-6 clone (P<0.0003) and 2.1-fold higher in the γ3-14 clone (P<0.03), as compared with the V1 control (Figure 4d). These data demonstrate that over-expression of MGSA/GROα enhances AP-1 transactivation.
Figure 4.
MGSA/GRO stimulates AP-1 activity. (a) AP-1 binding activity in MGSA/GRO expressing clones: EMSA was performed on nuclear extracts (5 μg) from V1 (lane 1), γ3-14 (lane 2), and Mel-a-6 (lane 3), using radiolabeled double-stranded AP-1 consensus oligonucleotide probe (CGCTTGATGAGTCAGCCGGAA) as described in Materials and methods. (b) AP-1 binding activity in parental melan-a cells treated with MGSA/GROα: EMSA was performed on nuclear extracts (5 μg) from parental melan-a cells treated with 50 ng/ml MGSA/GROα for the indicated times (lanes 2 – 7) or with carrier buffer alone (lane 1). (c) Identification of protein composition of AP-1 complex by EMSA super-shift analyses: The EMSA experiment was performed as in Figure 3a after incubation of nuclear extracts (5 μg) from Mel-a-6 clone with 1 μg of antibody to pan-Jun (lane 3), pan-Fos (lane 4) or both (lane 5) at room temperature for 30 min. (d) AP-1 luciferase transactivation analyses: Transient co-transfections were performed by the calcium phosphate method, using 2.5 μg of the AP-1 luciferase reporter gene and the pSV-β-Gal expression plasmid in V1, γ3-14 and Mel-a-6 cells. The luciferase activity and β-Gal activity were measured 48 h after transfection. The graph shows the average of relative luciferase activities (absolute activity of sample was normalized by β-gal activity) from four independent transfections. The error bars indicate the s.e.m
M-Ras mediates AP-1 activation
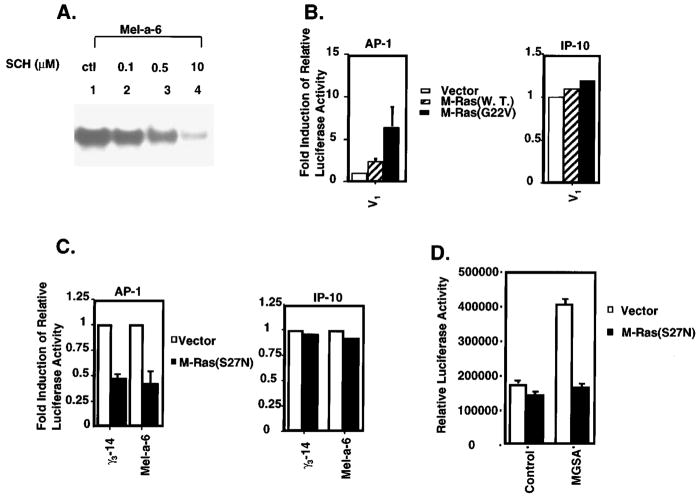
In order to determine whether the Ras farnesyl-transferase inhibitor blocked AP-1 activation in MGSA/GROα-expressing cells, nuclear extracts from the Mel-a-6 cells treated with solvent alone or with Ras farnesyl-transferase inhibitor (SCH44342) were analysed by EMSA with the consensus AP-1 DNA binding sequence as the probe (Figure 5a). The SCH44342 inhibitor blocked about 50% of the AP-1 binding activity at the 0.5 μM concentration (lane 3) and 90% of the AP-1 binding activity at the 10 μM concentration (lane 4). This result suggests that the MGSA/GROα-induced AP-1 activation is through a Ras-dependent pathway. To determine whether Ras mediates MGSA/GRO-induced AP-1 activation, we examined the effect of M-Ras (w.t.), dominant active M-Ras (G22V) or dominant negative M-Ras (S27N) on MGSA/GRO-induced AP-1 transactivation. V1 cells were cotransfected with the AP-1 luciferase reporter construct and an M-Ras expression plasmid (either w.t., constitutively active, or control pBK-CMV vector). Over-expression of wild-type M-Ras or a constitutively active form of M-Ras (G22V) increased basal AP-1 activity in V1 cells by 2.3- and 6.4-fold, respectively (Figure 5b, left panel). Since over-expression of wild-type M-Ras or dominant active M-Ras in melanocytes did not affect transactivation of the IP-10 CAT reporter gene (Figure 5b, right panel), the effects of Ras on AP-1 appear to be specific. The induction of AP-1 transactivation by M-Ras in the absence of any additional stimulus indicates that the M-Ras protein can activate AP-1 similarly to other Ras proteins including H-Ras, K-Ras or N-Ras (Johnson et al., 1996; Vojtek and Der 1998).
Figure 5.
Ras mediated AP-1 activation. (a) Ras inhibitor (SCH44342) blocks AP-1 binding activity: Mel-a-6 cells were pre-treated with the indicated concentrations of SCH44342 (lanes 2 – 4) or the solvent control (lane 1) overnight. Nuclear proteins were extracted and EMSA’s were performed by using 5 μg of protein and 0.4 ng of 32P-labeled AP-1 consensus oligonucleotide in EMSA. (b) M-Ras increases basal activation of AP-1: Left panel: V1 cells were cotransfected with 2.5 μg AP-1 luciferase reporter and either 5 μg pBK-CMV vector (empty bar), M-Ras (striped bar) or the dominant active form of M-Ras (G22V) (solid bar), together with 2.5 μg pBL-CAT3 plasmid. Luciferase and CAT activity were measured 48 h later. The relative luciferase activity represents the luciferase activity normalized by CAT activity. The results are reported as a mean (±s.e.m.) of fold-induction (the relative luciferase activity of dominant active form of M-Ras divided by the relative luciferase activity of pBK-CMV vector) from three independent experiments. Right panel: V1 cells were cotransfected with 2.5 μg IP-10 CAT reporter and 5 μg pBK-CMV vector (empty bar), M-Ras (striped bar) or the dominant active form of M-Ras (G22V) (solid bar), together with 2.5 μg pSV-β-Gal expression plasmid. CAT and Luciferase activity were measured 48 h later. (c) Dominant negative M-Ras blocks MGSA/GRO-increased basal AP-1 activation: Left panel: γ3-14 or Mel-a-6 cells were cotransfected with 2.5 μg AP-1 luciferase reporter and 5 μg pBK-CMV vector (empty bar) or the dominant negative M-Ras (solid bar), together with 2.5 μg pBL-CAT3 plasmid. Luciferase and CAT activity were measured 48 h later. The relative luciferase activity represents the luciferase activity normalized by CAT activity. The results are reported as a mean (±s.e.m.) of relative inhibition (the relative luciferase activity of dominant negative form of M-Ras divided by the relative luciferase activity of pBK-CMV vector) from three independent experiments. Right panel: γ3-14 or Mel-a-6 cells were cotransfected with 2.5 μg IP-10 CAT reporter and 5 μg pBK-CMV vector (empty bar) or the dominant negative M-Ras (solid bar), together with 2.5 μg pSV-β-Gal expression plasmid. CAT and Luciferase activity were measured 48 h later. (d) Dominant negative M-Ras blocks MGSA/GRO-induced AP-1 activation in V1 cells: V1 cells were cotransfected with 2.5 μg AP-1 luciferase reporter and 5 μg pBK-CMV vector (empty bar) or the dominant negative M-Ras (solid bar), together with 2.5 μg pBL-CAT3 plasmid. After transfection, cells were treated with carrier buffer alone or with 50 ng/ml MGSA/GROα for 48 h. The relative luciferase activity represents the luciferase activity normalized by CAT activity. The results are reported as a mean (±s.e.m.) of relative luciferase activity from three independent experiments
In a second series of experiments, dominant negative M-Ras (S27N) expression and AP-1 reporter constructs were cotransfected into γ3-14 and Mel-a-6 cells. The dominant negative M-Ras (S27N) blocked the basal AP-1 activity in γ3-14 or Mel-a-6 cells by 53 and 60%, respectively (Figure 5c, left panel). Since over-expression of dominant negative M-Ras in γ3-14 or Mel-a-6 cells did not affect transactivation of the IP-10 CAT reporter gene (Figure 5c, right panel), the effects of dominant negative M-Ras on AP-1 are not non-specific. In addition, we characterized the effect of the Ras inhibitor on the basal transcription of a minimal MGSA/GROα-350 bp promoter where the NF-kB and IUR elements have been mutated, but the Sp-1 element is left intact (Wood and Richmond 1995). We saw no effect of co-transfection with the dominant negative Ras on the basal transcription of this promoter (data not shown). To further confirm whether dominant negative M-Ras can directly block MGSA/GROα-induced AP-1 activation, dominant negative M-Ras (S27N) expression constructs and AP-1 reporter constructs were cotransfected into V1 cells, where the AP-1 reporter is stimulated by MGSA/GROα treatment after the transfection. The dominant negative M-Ras (S27N) completely inhibited MGSA/GROα-induced AP-1 activation, but did not affect the basal AP-1 activation (Figure 5d). Taken together, these data demonstrate that Ras mediates MGSA/GRO-enhanced AP-1 activation. Since it is likely that the S27N mutant of M-Ras also blocks events downstream of H-, N- and K-Ras, our results demonstrate that a generalized increase in Ras activity (not limited to M-Ras) is essential for the enhanced AP-1 activity observed in MGSA/GROα- and γ-expressing melan-a cells.
M-Ras induces melanocyte transformation
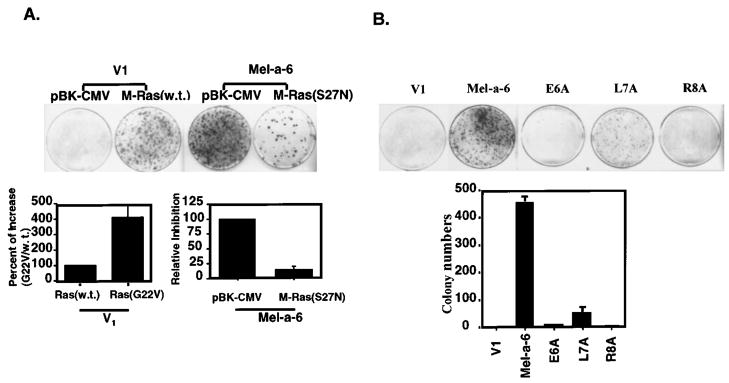
To test the ability of M-Ras to induce melanocyte transformation, melan-a clones were transfected with wild-type, dominant active, or dominant negative forms of M-Ras in an in vitro transformation assay. V1 cells transfected with M-Ras (w.t.) formed transformed foci, while V1 cells transfected with the vector control alone (pBK-CMV) did not form foci (Figure 6a). This result indicates that over-expression of M-Ras can induce melanocyte transformation. V1 cells transfected with dominant active form of M-Ras (G22V) formed four-times more transformed foci than those transfected with wild-type Ras (Figure 6, lower-left panel). MGSA/GROα-expressing Mel-a-6 cells transfected with the dominant negative form of M-Ras (S27N) showed 85% inhibition of focus formation (Figure 6, lower-right panel). Since the dominant negative M-Ras would potentially inhibit all forms of Ras activity, we conclude that Ras is involved in MGSA/GROα-induced melanocyte transformation.
Figure 6.
M-Ras induces focus formation and dominant negative M-Ras inhibits MGSA/GROα-induced transformation. (a) V1 or Mel-a-6 cells were cotransfected with a puromycin resistant vector (pBABE) and either a pBK/CMV, wild-type M-Ras, dominant active M-Ras (G22V) or dominant negative M-Ras (S27N). The cells were cultured in 10% FBS DMEM with G418 and 1 μg/ml puromycin. Foci of transformed cells were stained with crystal violet and counted 18 days after transfection. Each data point in the bar graph represents the mean (±s.e.m.) of fold induction from a total of six different platings in three independent experiments. (b) The V1, Mel-a-6, E6A, L7A or R8A cells were cultured in 10% FBS DMEM and the foci were stained with crystal violet and counted 18 days after transfection. The data shown represents one of three independent experiments with similar results each time (top panel). Each data point represents the mean (±s.e.m.) of colonies of six different platings in three independent experiments (lower panel)
To examine the effect of loss of each residue in the ELR-motif of MGSA/GROα on cellular transformation, an in vitro transformation assay was performed. Mel-a-6 cells expressing MGSA/GROα exhibited greater formation of transformed foci, as compared to V1 cells. In contrast, ELR-motif mutants (E6A/ELR and R8A/ELR) failed to exhibit focus formation and L7A/ELR mutant clones exhibited fewer transformed foci (Figure 6b). This result is consistent with the observation that the mutant forms of MGSA/GROα fail to enhance Ras expression, further confirming that high Ras expression in Mel-a-6 is functionally correlated to MGSA/GRO-induced transformation.
Discussion
In an attempt to identify MGSA/GRO-regulated genes which may be involved in MGSA/GRO-induced melanocyte transformation, using differential display we found that continuous expression of MGSA/GRO up-regulates the expression of M-Ras at both the mRNA and protein levels, as well as K-Ras and N-Ras expression. MGSA/GRO induction of Ras is through the CXCR2 receptor, since melan-a clones expressing the ELR-motif mutant forms of MGSA/GROα failed to exhibit increased Ras protein expression. Our results show that over-expression of MGSA/GRO does not affect the specific activity of the Ras-GTPase (activity per molecule of Ras). Rather, over-expression of MGSA/GRO increased the total amount of the activated Ras by increasing the level of Ras protein expression. These data suggest a mechanism by which Ras activity can be regulated at both the mRNA and protein levels, in addition to the post-translational level of GTP binding and GTPase activity.
It is not clear how MGSA/GRO regulates the expression of M-Ras and other Ras family members. Recently, the Renauld group reported that Interleukin-9 induces mRNA expression of M-Ras within 6 h after addition of IL-9, with induction maximal at 24 h. This induction was postulated to be regulated at the transcriptional level (Louahed et al., 1999). UV-B induces 5 – 10-fold higher cHa-Ras gene expression in both papillomas and carcinomas (Husain et al., 1990). Moreover, estrogen induces c-Ha-Ras expression in uterine endometrial fibroblasts and cancer cells (Fujimoto et al., 1995). Some of these reagents that induce Ras expression also cause induction of P38 stress-associated kinase, an event which enhances mRNA stability (Winzen et al., 1999). The increase in M-Ras mRNA in the MGSA/GRO-expressing melan-a clones could be due to the alteration in the transcription and/ or enhanced stability of M-Ras mRNA. Since transcription of Ras is thought to be regulated largely by Sp1 and is therefore not inducible, we postulate that MGSA/GRO induces Ras expression by increasing the stability of Ras mRNA, consequent to alterations in signals such as p38 or ERK1, 2.
Human melanoma appears to belong to the group of tumors whose growth and tumorigenicity depend on autocrine loops (Rodeck et al., 1991). MGSA/GRO has been shown to act through both autocrine and paracrine modes to enhance tumor progression (Owen et al., 1997; Luan et al., 1997). The deregulation of MGSA/GRO may also mimic effects of aberrant Ras function even in the absence of a Ras gene mutation. Thus, the influence of normal and aberrant Ras function on the biology of human melanoma may be even greater than expected from the frequency of Ras gene mutations. Indeed, overexpression of receptor tyrosine kinases such as ErbB2 or epidermal growth factor receptor leads to cell transformation that involves the participation of Ras protein expression (Smith et al., 1987; Huang et al., 1997; Kolibaba and Druker 1997).
M-Ras is closely related to R-Ras, Tc21, H-Ras, K-Ras and N-Ras. Ras family members play very important roles in cell growth, differentiation, transformation and apoptosis (Vojtek and Der, 1998). Over-expression of activated Ras in melanocytes null for p16 induces melanoma tumors (Chin et al., 1999) and over-expression of mutant activated M-Ras induces transforming foci in NIH3T3 cells, although the ability of M-Ras to induce transforming foci is weaker than that of Ha-Ras (Quilliam et al., 1999). It has been reported that over-expression of any of three normal Ras genes, N-Ras (McKay et al., 1986), H-Ras (Westaway et al., 1986; Zhang et al., 1997) or K-Ras (George et al., 1986) leads to in vitro transformation. In vivo, over-expression of normal N-Ras is associated with development of hyperplasia and tumors in transgenic mice (Mangues et al., 1992). Here, we show for the first time that over-expression of normal M-Ras induces melanocyte transformation in vitro. The foci that formed grew in the absence of serum or exogenous growth factors and without attachment to the substrate. These results indicate that over-expression of normal M-Ras protein can induce melanocyte transformation, similarly to H-Ras, K-Ras or N-Ras in other cell types. Moreover, it is conceivable that the upregulation of Ras expression is a consequence of the MGSA/GROα- and γ-induced transformation event, based upon experiments showing that expression of dominant negative M-Ras, which probably inhibits other forms of Ras in addition to M-Ras, inhibited MGSA/GRO-induced cellular transformation. These data suggest that Ras expression enhanced by over-expression of MGSA/GRO is required for melanocyte transformation. However, it is possible that MGSA/GRO induction of Ras would be essential for both the MGSA/GRO induction of cell growth and the transformation process. Then dominant negative M-Ras may block cell transformation by directly interfering with the transformation process, by indirectly inhibiting cell proliferation or by both. Since it is not possible to monitor transformation in the absence of cell proliferation, we cannot discern between these three possible mechanisms. Taken together, these data indicate that M-Ras can play a role in MGSA/GRO-induced melanocyte transformation as well as can other members of Ras. It is highly probable that over-expression of wild-type M-Ras will also contribute to the development of malignancies in vivo.
Interpretation of our data is complicated by the observation that melan-a clones expressing MGSA/GROβ only weakly induce Ras expression and activity (M-Ras plus pan-Ras) (data not shown). There are now several reports that MGSA/GROβ binds the receptor differently and has different biological activities from the α and γ forms of this chemokine (Geiser et al., 1993; Cao et al., 1995; Rosenkilde and Schwartz 2000). A recent report demonstrated that MGSA/GROβ activates the sphingomyelinase/ceramide pathway (Limatola et al., 1999). Thus, the mechanism by which MGSA/GROβ affects progression of immortalized melanocytes to melanoma may be different from that for MGSA/GROα and γ. We postulate that other pathways may be activated in MGSA/GROβ-expressing melan-a clones to effect transformation. For example, we found that MGSA/GROβ-expressing melan-a clones exhibit enhanced VEGF expression, while MGSA/GROα or γ clones do not (data not shown). Since V-EGF induces endothelial cell proliferation, migration, and angiogenesis, the additive effect of V-EGF secretion must supplement the weak induction of Ras in MGSA/GROβ-expressing melan-a clones to facilitate the transformation process.
To our knowledge, this is the first report that chemokines can up-regulate the expression of Ras and enhance AP-1 activation. Over-expression of M-Ras in melanocytes induces cellular transformation, and continuous expression of MGSA/GRO-induced transformation can be blocked by expression of a dominant negative form of M-Ras.
Materials and methods
Cell culture
The non-transformed mouse immortalized melanocyte cell line, parental melan-a (gift of Dr Dorthea Bennett), was cultured in DMEM supplemented with 50 units/ml penicillin, 50 μg/ml streptomycin, 3 mM glutamine, 10% heat-inactivated fetal bovine serum (GIBCO – BRL, Rockville, MD, USA) and 200 nM TPA (Sigma, St. Louis, MO, USA). The MGSA/GRO expression plasmids were constructed by inserting human MGSA/GROα, γ or mutant MGSA/GROα into the pRC/CMV vector and stably transfecting into parental melan-a cells (Table 1). These clones were cultured in the same media supplemented with 800 μg/ml G418 (Sigma, St. Louis, MO, USA) as previously described (Owen et al., 1997). The level of chemokine expression in these clones has been previously characterized (Owen et al., 1997). 125I-MGSA/GROα was purchased from DuPont NEN (Boston, MA, USA) at a specific activity of 272 mCi/mg.
125I-MGSA/GROα binding assay
The binding assay was conducted 48 h after the initial plating as previously described (Yang et al., 1999). Non-specific binding was monitored by determining the binding of 125I-MGSA/GROα in the presence of a 100-fold excess of cold MGSA/GROα. The specific binding activity was calculated by subtracting non-specific binding from total 125I-MGSA/GROα binding activity.
Differential display
Differential display was performed using the RNA image kit (GenHunter, Nashville, TN, USA) following manufacturer’s instruction. 250 ng of total RNA from V1, γ3-14 or Mel-a-6 clones was reverse transcribed by using 1 μl MMLV reverse transcriptase and one of three different one-base anchored oligo-dT primers (H-T11G, H-T11A and H-T11C). The resulting cDNA was amplified by PCR using 0.2 μl Taq DNA polymerase (Qiagen, Valencia, CA, USA) and one of three oligo-dT primers and one of eight arbitrary 13-mers (H-AP1, H-AP2, H-AP3, H-AP4, H-AP5, H-AP6, H-AP7 and H-AP8) in the presence of [α33P]dATP (DuPont-NEN, Boston, MA, USA). The resulting products were fractionated on a 6% polyacrylamide sequence gel that was dried and exposed to film. Sixteen differentially expressed bands were recovered from sequencing gels and reamplified using the same primer set and PCR conditions, except the dNTP concentration was at 20 μM. PCR products were run on a 1.5% agarose gel and stained with ethidium bromide. The bands were recovered from the agarose gel. The cDNA fragments were either used as probes for Northern blots or cloned into the PCR-TRAP vector using the PCR-TRAP Cloning System Kit (GenHunter, Nashville, TN, USA) for sequencing.
DNA sequencing and DNA sequence homology search
DNA sequencing of cDNA fragments in the PCR-TRAP vector was performed by the dideoxychain termination method using the T7 sequenase 2.0 polymerase kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA) with Lgh or Rgh primers that flank the cloning site of the PCR–TRAP vector. Gene database searches were performed at the National Center for Biotechnology Information by using the BLAST network service.
Northern blot
20 μg of total cellular RNA, isolated from cells using the Ultraspec RNA Isolation System (Biotecx Laboratories, Inc., Houston, TX, USA), was resolved under denaturing conditions on a 1.0% formaldehyde-agarose gel, and transferred to Nytran Plus filters (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The filter was hybridized at 42°C for 12 – 16 h with gel-purified 32P-labeled differential display cDNA fragments, which were labeled using the Multiprime DNA labeling system (Amersham Pharmacia Biotech). After hybridization, the filter was washed twice with 1× saline sodium citrate with 0.1% SDS for 15 min at 25°C, followed by two washes with 0.25× saline sodium citrate with 0.1% SDS for 20 min at 45°C, and then exposed to film.
Whole cell extracts and Western blot
Western blots were performed following protocols provided by Santa Cruz Biotechnology Inc. The cells were washed at 4°C with 1× PBS and lysed in 0.25 – 0.5 ml of RIPA buffer (1× PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) with protease inhibitor cocktail tablets (Boehringer Mannheim Corp., Indianapolis, IN, USA). 50 μg soluble protein was boiled, loaded and separated on a 12% SDS-polyacrylamide gel and then electrophoretically transferred to a 0.45 μm nitrocellulose membrane (BIO-RAD, Hercules, CA, USA). The membrane was blocked with 5% dry milk in TBS-T buffer (10 mM Tris-HCl, pH 8.0; 150 mM NaCl; 0.05% Tween-20) for 1 h and then incubated for 12 – 16 h at 4°C in a 1:200 dilution of the anti-M-Ras antibody (gift from Dr Endo), in a 1:3000 dilution of the anti-pan-Ras antibody (AB-3) (Oncogene Research Products, Cambridge, MA, USA), in a 1:200 dilution of anti-N-Ras, anti-K-Ras or anti-H-Ras (Santa Cruz Biotechnology Inc) or in a 1:1000 dilution of the anti-Bcl2 (Santa Cruz Biotechnology Inc). The membrane was then incubated in a 1:3000 dilution of the anti-mouse or anti-rabbit immunoglobulin conjugated with horseradish peroxidase (Boehringer Mannheim Corp.) in TBS-T buffer with 5% dry milk for 1 h at room temperature. The protein bands were detected with ECL Western blotting detection reagents (Amersham Pharmacia Biotech) according to the manufacturer’s instructions.
Activation assays for Ras
Activation assays for Ras were performed as described in detail (Taylor and Shalloway, 1996). Cells were washed twice with ice-cold HBS and lysed in Mg2+-containing lysis buffer (MLB: 25 mM HEPES, pH 7.5, 150 mM NaCl, 1% NP-40, 0.25% Na deoxycholate, 10% glycerol, 25 mM NaF, 10 mM MgCl2, 1mM EDTA, 1 mM, protease inhibitor cocktail tablets). After lysis for 15 min, lysates were spun at 16 000 g for 20 min, and supernatants collected. The protein concentrations of the lysates were then determined. Equal amounts of total protein of cell lysate (400 μg) were immediately affinity-precipitated for 1 h using 20 μg of recombinant GST-c-Raf-1 RBD (1 – 149) (a gift from Dr David Shalloway) fusion proteins, which had been freshly coupled to glutathione-Sepharose beads (Amersham Pharmacia Biotech). The precipitates were washed three times with MLB and eluted by boiling in SDS –PAGE sample buffer. The proteins were separated on a 12% SDS-polyacrylamind gel and then immunoblotted with pan-Ras antibody (AB-3) (Oncogene Research Products, Cambridge, MA, USA).
GTP/GDP-binding and GTPase assay
Immunoprecipitations were performed by using 400 μg of whole cell lysates of melan-a clones incubated overnight at 4°C with 2 μg of anti-pan-ras antibody (AB-3). Subsequently, 30 μl of protein-A/G-agarose (Sigma, St. Louis, MO, USA) was added and incubated at 4°C for 3 h. The Ras immune complexes were pelleted and washed six times with RIPA buffer and once with GTP/GDP-binding buffer (50 mM NaCl, 50 mM Tris-HCl [pH 7.5], 5 mM EDTA, 0.1 mM EGTA, 0.1 mM DTT and 10 μM ATP). The GTP/GDP-binding and GTPase assays were carried out as previously described (Matsumoto et al., 1997). Briefly, each Ras immune complex bound to protein-A/G-agarose was incubated with 1 μl of [α-32P]GTP (3000 Ci/mmol, Amersham Pharmacia Biotech) in 100 μl GTP/GDP-binding buffer at 37°C for 10 min. After the pellets were washed six times with wash buffer (20 nM MgCl2, 50 mM Tris-HCl [pH 7.5], 1 mM DTT and 1 mg/ml bovine serum albumin), the GTPase reaction was performed in GTPase reaction buffer (50 mM NaCl, 5 mM MgCl2, 50 mM Tris-HCl [pH 7.5], 5 mM DTT) at 37°C for 30 min. The pellets were washed with wash buffer twice, and bound nucleotides were eluted by incubating the pellets in 1% SDS and 20 mM EDTA at 68°C for 20 min. The released 32P-labeled nucleotides were counted directly by Cerenkov scintillation counting (Beckman LS 3801), and then GTP and GDP were loaded on PEI-cellulose plates (JT Baker Inc., Phillipsburg, NJ, USA), resolved by 0.75 M KH2PO4 and then visualized by autoradiography.
Nuclear extracts and mobility shift assay
Cells were lysed with buffer (10 mM HEPES, 10 mM sodium chloride, 1.5 mM magnesium chloride, 0.5 mM DTT, 5 mM β-mercaptoethanol, 100 μM PMSF) containing 0.5% Nonidet P-40. The nuclear proteins were extracted from the nuclear pellets using high salt extraction (25% glycerol, 0.2 mM EDTA, 20 mM HEPES, 1.5 mM magnesium chloride, 0.5 mM DTT, 10 mM potassium chloride, 240 mM sodium chloride) with protease inhibitors and phosphatase inhibitors, as described previously (Shattuck and Richmond, 1997). EMSA was performed by incubating 0.25 ng double stranded oligonucleotide end-labeled with 32P by polynucleotide kinase (New England Biolabs) and equal amounts of nuclear protein (5 μg) for 30 min at room temperature, as described previously (Shattuck and Richmond, 1997). The binding reaction mixtures were resolved on a 6% nondenaturing polyacrylamide gel. Antibody super-shift-EMSA was performed by incubating nuclear extract proteins with antibodies (1 μg) against pan-Fos, pan-Jun, or control antibody r-IgG (Santa Cruz Biotechnology Inc) at room temperature for 1 h before adding the labeled probe.
Transient transfection assay
M-Ras (w.t.), constitutively active M-Ras (G22V), dominant negative M-Ras (S27N) expression constructs and an empty expression vector (pBK-CMV) were generated as described previously (Matsumoto et al., 1997). An AP-1 luciferase reporter gene construct was constructed by inserting two copies of the AP-1 consensus DNA binding sequence into the pGL-3 promoter vector (Promega, Madison, WI, USA). The IP-10 CAT reporter gene construct is a gift from Dr Peng Liang. 5×105 cells (30 mm dishes) were transiently co-transfected with either 0.5 μg AP-1 luciferase reporter gene or 0.5 μg IP-10 CAT reporter gene with either 5 μg pBK-CMV vector or 5 μg M-Ras expression construct and 2.5 μg PBL-CAT by the calcium phosphate precipitation technique previously described (Shattuck et al., 1994). Three hours later, the cells were glycerol shocked for 3 min. The cells were washed twice with complete medium, and then incubated with complete medium at 37°C/5% CO2. Two days later, the cells were washed with cold PBS and lysed in 1× reporter lysis buffer (Promega, Madison, WI, USA) for 15 min at room temperature and the lysate was cleared by centrifugation. The luciferase or CAT activity was measured according to standard protocols (Promega, Madison, WI, USA) using a Monolight 2010 luminometer (Analytical Luminescence Laboratory, San Diego, CA, USA) or Beckman LS 3801.
Transformation assays
Focus formation assays were performed in duplicate in three independent experiments. V1, Mel-a-6, E6A/ELR, L7A/ELR or R8A/ELR cells (Table 2) were cultured in DMEM supplemented with 10% FBS, and the foci of transformed cells were counted 18 days after plating the cells. For M-Ras transformation experiments, V1 or Mel-a-6 cells were cotransfected with 0.2 μg of a puromycin resistant vector (pBABE) and either 2 μg of a pBK/CMV, M-Ras (w.t.), dominant active M-Ras (G22V) or dominant negative M-Ras (S27N) by LIPOFECTAMINE PLUS reagent following manufacturer’s protocols (GIBCO – BRL, Rockville, MD, USA). The cells were cultured in 10% FBS DMEM with 0.8 mg/ml G418 and 0.5 mg/ml puromycin, and the foci of transformed cells were counted 18 days after transfection.
Acknowledgments
We are indebted to the NIH for support: CA56704 (A Richmond); Vanderbilt Cancer Center CA 68485, Department of Veterans Affairs Merit Award and Career Scientist Award (A Richmond), and to James Owen and Eddy Balentien for developing the MGSA/GRO expressing melan-a clones. We are also indebted to Ben Johnston and Amy Pruitt for excellent technical assistance.
Abbreviations
- CXC chemokine
chemokine with the first two conserved cysteine residues separated by an intervening amino acid
- CMV
cytomegalovirus
- DMEM
Dulbecco’s modified Eagle’s medium
- IL-8
interleukin-8
- MGSA/GRO
melanoma growth-stimulatory activity/growth-regulated protein
- AP-1
activation protein-1
- RT-PCR
reverse transcription-polymerase chain reaction
- SV-40
simian virus-40
- EMSA
electrophoresis mobility shift assay
- MMLV
moloney murine virus
- ELR motif
glutamine-leucine-arginine tripeptide sequence near the N-terminus of MGSA/GRO proteins
References
- Arenberg DA, Polverini PJ, Kunkel S, Shanafelt A, Hesselgesser J, Horuk R, Strieter M. J Leukocyte Biol. 1997;62:554 – 562. doi: 10.1002/jlb.62.5.554. [DOI] [PubMed] [Google Scholar]
- Balentien E, Mufson BE, Shattuck RL, Derynck R, Richmond A. Oncogene. 1991;6:1115 – 1124. [PubMed] [Google Scholar]
- Bennett DC, Cooper PJ, Hart IR. Int J Cancer. 1987;39:414 – 418. doi: 10.1002/ijc.2910390324. [DOI] [PubMed] [Google Scholar]
- Cao Y, Chen C, Weatherbee JA, Tsang M, Folkman J. J Exp Med. 1995;182:2069 – 2077. doi: 10.1084/jem.182.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L, Tam A, Pomerantz J, Wong M, Holash J, Bardeesy N, Shen Q, O’Hagan R, Pantginis J, Zhou H, Horner JW, Cordon-Cardo C, Yancopoulos GD, DePinho RA. Nature. 1999;400:468 – 472. doi: 10.1038/22788. [DOI] [PubMed] [Google Scholar]
- Della Rocca GJ, van Biesen T, Daaka Y, Luttrell DK, Luttrell LM, Lefkowitz RJ. J Biol Chem. 1997;272:19125 – 19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- Domann FE, Jr, Levy JP, Finch JS, Bowden GT. Mol Carcinog. 1994;9:61 – 66. doi: 10.1002/mc.2940090202. [DOI] [PubMed] [Google Scholar]
- Fujimoto J, Ichigo S, Hori M, Morishita S, Tamaya T. Mol Biol. 1995;55:25 – 33. doi: 10.1016/0960-0760(95)00145-p. [DOI] [PubMed] [Google Scholar]
- Geiser T, Dewald B, Ehrengruber MU, Clark-Lewis I, Baggiolini M. J Biol Chem. 1993;268:15419 – 15424. [PubMed] [Google Scholar]
- George DL, Glick B, Trusko S, Freeman N. Proc Natl Acad Sci USA. 1986;83:1651 – 1655. doi: 10.1073/pnas.83.6.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghnegahdar H, Du J, Wang D, Strieter RM, Burdick MD, Nanney LB, Cardwell N, Luan J, Shattuck-Brandt R, Richmond A. J Leuk Biol. 2000;67:53 – 62. doi: 10.1002/jlb.67.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskill S, Peace A, Morris J, Sporn SA, Anisowicz A, Lee SW, Smith T, Martin G, Ralph P, Sager R. Proc Natl Acad Sci USA. 1990;87:7732 – 7736. doi: 10.1073/pnas.87.19.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes BE, Luttrell LM, van Biesen T, Lefkowitz RJ. J Biol Chem. 1996;271:12133 – 12136. doi: 10.1074/jbc.271.21.12133. [DOI] [PubMed] [Google Scholar]
- Hébert CA, Vitangcol RV, Baker JB. J Biol Chem. 1991;266:18989 – 18994. [PubMed] [Google Scholar]
- Hesselgesser J, Chitnis CE, Miller LH, Yansura DG, Simmons LC, Fairbrother WJ, Kotts C, Wirth C, Gillece-Castro BL, Horuk R. J Biol Chem. 1995;270:11472 – 11476. doi: 10.1074/jbc.270.19.11472. [DOI] [PubMed] [Google Scholar]
- Huang HS, Nagane M, Klingbeil CK, Lin H, Nishikawa R, Ji XD, Huang CM, Gill GN, Wiley HS, Cavenee WK. J Biol Chem. 1997;272:2927 – 2935. doi: 10.1074/jbc.272.5.2927. [DOI] [PubMed] [Google Scholar]
- Husain Z, Yang QM, Biswas D. Arch Dermatol. 1990;126:324 – 330. doi: 10.1001/archderm.126.3.324. [DOI] [PubMed] [Google Scholar]
- Iida N, Grotendorst GR. Mol Cell Biol. 1990;10:5596 – 5599. doi: 10.1128/mcb.10.10.5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R, Spiegelman BM, Hanahan D, Wisdom R. Mol Cell Biol. 1996;18:4504 – 4511. doi: 10.1128/mcb.16.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmelman A, Tolkacheva T, Lorenzi MV, Osada M, Chan AM. Oncogene. 1997;15:2675 – 2685. doi: 10.1038/sj.onc.1201674. [DOI] [PubMed] [Google Scholar]
- Knall C, Worthen GS, Johnson GL. Proc Natl Acad Sci USA. 1997;94:3052 – 3057. doi: 10.1073/pnas.94.7.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolibaba KS, Druker BJ. Biochim Biophys Acta. 1997;1333:F217 – F248. doi: 10.1016/s0304-419x(97)00022-x. [DOI] [PubMed] [Google Scholar]
- Kurdowska A, Cohen AB, Carr FK, Stevens MD, Miller EJ, Mullenbach G, Tekamp-Olson P. Cytokine. 1994;6:124 – 134. doi: 10.1016/1043-4666(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Limatola C, Mileo AM, Giovannelli A, Vacca F, Ciotti MT, Mercanti D, Santoni A, Eusebi F. J Biol Chem. 1999;274:36537 – 36543. doi: 10.1074/jbc.274.51.36537. [DOI] [PubMed] [Google Scholar]
- Lloyd AC, Yancheva N, Wasylyk B. Nature. 1991;352:635 – 638. doi: 10.1038/352635a0. [DOI] [PubMed] [Google Scholar]
- Lopez-Ilasaca M, Crespo P, Pellici PG, Gutkind JS, Wetzker R. Science. 1997;275:394 – 397. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- Louahed J, Grasso L, De Smet C, Van Roost E, Wildmann C, Nicolaides NC, Levitt RC, Renauld JC. Blood. 1999;94:1701 – 1710. [PubMed] [Google Scholar]
- Luan J, Shattuck-Brandt R, Haghnegahdar H, Owen JD, Strieter R, Burdick M, Nirodi C, Beauchamp D, Johnson KN, Richmond A. J Leuk Biol. 1997;62:588 – 597. doi: 10.1002/jlb.62.5.588. [DOI] [PubMed] [Google Scholar]
- Mangues R, Seidman I, Gordon JW, Pellicer A. Oncogene. 1992;7:2073 – 2076. [PubMed] [Google Scholar]
- Matsumoto K, Asano T, Endo T. Oncogene. 1997;15:2409 – 2417. doi: 10.1038/sj.onc.1201416. [DOI] [PubMed] [Google Scholar]
- Mattei S, Colombo MP, Melani C, Silvani A, Parmiani G, Herlyn M. Int J Cancer. 1994;56:853 – 857. doi: 10.1002/ijc.2910560617. [DOI] [PubMed] [Google Scholar]
- McKay IA, Marshall CJ, Cales C, Hall A. EMBO J. 1986;5:2617 – 2621. doi: 10.1002/j.1460-2075.1986.tb04542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune ER, Bourne HR. Proc Natl Acad Sci USA. 1997;94:14489 – 14494. doi: 10.1073/pnas.94.26.14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen JD, Strieter R, Burdick M, Haghnegahdar H, Nanney L, Shattuck-Brandt R, Richmond A. Int J Cancer. 1997;73:94 – 103. doi: 10.1002/(sici)1097-0215(19970926)73:1<94::aid-ijc15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Quilliam LA, Castro AF, Rogers-Graham KS, Martin CB, Der CJ, Bi C. J Biol Chem. 1999;274:23850 – 23857. doi: 10.1074/jbc.274.34.23850. [DOI] [PubMed] [Google Scholar]
- Rennekampff HO, Hansbrough JF, Woods V, Jr, Dore C, Kiessig V, Schroder JM. Arch Dermatol Res. 1997;289:204 – 212. doi: 10.1007/s004030050181. [DOI] [PubMed] [Google Scholar]
- Richmond A, Thomas HG. J Cell Physiol. 1986;129:375 – 384. doi: 10.1002/jcp.1041290316. [DOI] [PubMed] [Google Scholar]
- Richmond A, Thomas HG. J Cell Biochem. 1988;36:185 – 198. doi: 10.1002/jcb.240360209. [DOI] [PubMed] [Google Scholar]
- Rodeck U, Becker D, Herlyn M. Cancer Cells. 1991;3:308 – 311. [PubMed] [Google Scholar]
- Rosenkilde MM, Schwartz TW. Mol Pharmacol. 2000;57:602 – 609. doi: 10.1124/mol.57.3.602. [DOI] [PubMed] [Google Scholar]
- Rutberg SE, Lee EJ, Hansen LH, Glick AB, Yuspa SH. Mol Carcinog. 1997;20:88 – 98. [PubMed] [Google Scholar]
- Sadhu C, Masinovsky B, Staunton DE. J Immunol. 1998;160:5622 – 5628. [PubMed] [Google Scholar]
- Saez E, Rutberg SE, Mueller E. Cell. 1995;82:721 – 732. doi: 10.1016/0092-8674(95)90469-7. [DOI] [PubMed] [Google Scholar]
- Shattuck RL, Wood LD, Jaffe GJ, Richmond A. Mol Cell Biol. 1994;14:791 – 802. doi: 10.1128/mcb.14.1.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck-Brandt RL, Wood LD, Richmond A. DNA Sequence. 1997;7:379 – 386. doi: 10.3109/10425179709034060. [DOI] [PubMed] [Google Scholar]
- Shattuck-Brandt RL, Richmond A. Cancer Res. 1997;57:3032 – 3039. [PubMed] [Google Scholar]
- Shyamala V, Khoja H. Biochemistry. 1998;37:15918 – 15924. doi: 10.1021/bi9811415. [DOI] [PubMed] [Google Scholar]
- Smith JJ, Derynck R, Korc M. Proc Natl Acad Sci USA. 1987;84:7567 – 7570. doi: 10.1073/pnas.84.21.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SJ, Shalloway D. Curr Biol. 1996;6:1621 – 1627. doi: 10.1016/s0960-9822(02)70785-9. [DOI] [PubMed] [Google Scholar]
- van Dam H, Huguier S, Kooistra K, Baguet J, Vial E, Vandereb AJ, Herrlich P, Angel P, Castellazzi M. Genes Dev. 1998;12:1227 – 1239. doi: 10.1101/gad.12.8.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtek AB, Der CJ. J Biol Chem. 1998;273:19925 – 19928. doi: 10.1074/jbc.273.32.19925. [DOI] [PubMed] [Google Scholar]
- Westaway D, Papkoff J, Moscovici C, Varmus HE. EMBO J. 1986;5:301 – 309. doi: 10.1002/j.1460-2075.1986.tb04213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzen R, Kracht M, Ritter B, Wilhelm A, Chen C-YA, Shyu A-B, Muller M, Gaestel M, Resch K, Holtmann H. EMBO J. 1999;18:4969 – 4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LD, Richmond A. J Biol Chem. 1995;270:30619 – 30626. doi: 10.1074/jbc.270.51.30619. [DOI] [PubMed] [Google Scholar]
- Zhang B, Shih JW, Wear DJ, Tsai S, Lo SC. Proc Sco Exp Biol Med. 1997;214:359 – 366. doi: 10.3181/00379727-214-44104. [DOI] [PubMed] [Google Scholar]
- Yang W, Wang D, Richmond A. J Biol Chem. 1999;274:11328 – 11333. doi: 10.1074/jbc.274.16.11328. [DOI] [PubMed] [Google Scholar]