Abstract
Background: Secular trends over the past several decades suggest an environmental influence on body mass index (BMI). However, twin models that incorporate a gene-environment correlation and gene × environment interaction have not been applied to elucidate specific environmental factors that affect the heritability of BMI.
Objective: Our aim was to determine whether one putative environmental predictor of obesity, vigorous exercise, shows evidence of a gene-environment correlation or gene × environment interaction with BMI among twins.
Design: Twin structural equation modeling was used to examine a gene-environment correlation and a gene × environment interaction of vigorous exercise with BMI among 2710 monozygotic and 2327 dizygotic male-male twin pairs from the Vietnam Era Twin Registry—a national registry of twin pairs who served in the military during the Vietnam War era.
Results: Vigorous exercise significantly modified the additive genetic component of BMI, which indicated a gene × environment interaction (P < 0.001). BMI showed the greatest genetic influence among those who did not report vigorous exercise, with diminished genetic influence among those who did. Furthermore, vigorous exercise had a small but significant environmental effect on BMI (P = 0.006)—a finding confirmed among monozygotic co-twins discordant for vigorous exercise.
Conclusions: Genetic influences on BMI are lower among those who report vigorous exercise. Consistent with an emerging literature, this suggests that vigorous exercise may mitigate some of the genetic influence on obesity. Molecular genetic studies of obesity should consider incorporating measures of behavioral and demographic factors to maximize the identification of novel obesity genes.
INTRODUCTION
Although recent genome-wide association studies have, for the first time, produced confirmed genetic associations with body mass index (BMI; in kg/m2) (1–6), the variance attributable to these markers remains small (≈2%). Heritability is frequently cited as an estimate of the upper boundary for total genetic contributions to a trait. Because heritability estimates for BMI commonly exceed 0.60 (7), indicating that 60% of the variance in BMI is attributable to genetic factors, a substantial proportion of the heritability remains to be explained. However, estimation of the heritability coefficient is well known to rely on a number of key assumptions, including lack of both a gene-environment correlation and gene × environment interaction. Given the pronounced secular trends in obesity over the past several decades (8–10), it is plausible that both a gene-environment correlation and gene × environment interaction contribute to variability in BMI.
One likely contributor to the rise in BMI is a decrease in physical activity levels. As levels of BMI have increased (10, 11), overall physical activity levels have decreased (12). Several population-based studies found a cross-sectional association between higher levels of self-reported physical activity and lower BMI (13–16). Research further suggests that time spent performing vigorous aerobic activities may be especially important for reducing the likelihood of being overweight or obese (15, 17–20).
Studies of discordant twins identified the direct effects of physical activity on BMI, regardless of the genetic risk of obesity. For example, in studies of monozygotic (MZ) twins discordant for level of physical activity, twins who reported higher levels of physical activity had lower BMI than their less active co-twins (21, 22). Furthermore, active co-twins gained less weight over time relative to their less physically active co-twins (23, 24). These studies clearly support an environmental effect of physical activity on BMI.
Twin studies have further evaluated whether physical activity confers a differential effect on BMI by the level of the genetic risk of obesity (gene × environment interaction) and have reported mixed results (21, 25, 26). Only one study used state-of-the-art structural equation modeling methods to estimate the gene × environment interaction within the twin design, and it found reduced heritability of BMI among those who are physically active (27). This type of study has added significance in light of a recent candidate gene study suggesting that the effect of specific genetic markers on BMI may vary by vigorous activity. Andreasen et al (28) reported that the effect of the fat mass and obesity–associated (FTO) polymorphism on BMI varies by vigorous activity, such that a strong genetic association of FTO with BMI is present among those who are inactive, whereas no such association is seen among those who are active.
In this article, we used twin structural equation models of a gene-environment correlation and gene × environment interaction to test 1) whether common genetic or environmental factors predict both vigorous exercise (VE) and BMI and 2) whether VE modifies genetic influences on BMI.
SUBJECTS AND METHODS
Sample
The Vietnam Era Twins Registry (VETR) is a nationally distributed US cohort consisting of male-male twin pairs born between 1939 and 1957 in which both siblings served on active military duty during the Vietnam War era (29). Zygosity was determined using a questionnaire and blood group typing methodology that achieved 95% accuracy (29). Registry members are representative of all twins who served in the military during the Vietnam War on a variety of sociodemographic and other variables (30, 31). The data used in the present study were from a National Heart, Lung, and Blood Institute survey (1990). Exclusion criteria included unresolved zygosity (n = 302 individuals) and missing data for both BMI and VE (n = 3507 individuals). Subjects included in the analyses and those who were not were fairly comparable in terms of age [excluded: males (M) = 40.7 (SD = 3.2) y; retained: M = 40.1 (SD = 3.0) y], years of schooling [excluded: M = 13.9 (SD = 2.6); retained: M = 14.4 (SD = 2.5)], marital status (excluded: 86% married; retained: 90% married), and BMI by self-report 3 y earlier [excluded: M = 25.6 (SD = 3.5); retained: M = 25.4 (SD = 3.6)]. However, those retained were more likely to identify their racial category as white (95% compared with 87%) relative to those excluded from the present analyses and to report annual family income >$35,000 (46% compared with 35%). A total of 3590 [2024 MZ and 1566 dizygotic (DZ)] male-male Vietnam-era twin pairs were available for the estimation of cross-twin relationships. Individual twins from an additional 1447 pairs (686 MZ and 761 DZ) in which the other twin was missing both BMI and VE information were also included in the analyses; they contribute to the estimation of within-twin, but not cross-twin, relations. Therefore, the overall sample comprises 8627 individuals drawn from 5037 twin pairs.
All participants gave verbal informed consent at the time of the interviews. The current data analysis was approved by the Miriam Hospital Institutional Review Board, and procedures that were followed were in accordance with the Miriam Hospital Guidelines.
Measures
Height and weight were measured by self-report. Previous studies have shown that self-report of weight is a valid measure of actual weight with an average error of only 1–2 lb (32). Research also suggests that underreporting of weight does not appear to vary as a function of physical activity (33). VE was defined by self-reported regular participation in one or more of 5 common vigorous-intensity aerobic activities over the past 3 mo (34). The activities included the following 1) jog or run ≥10 miles/wk (1 mile = 1.6 km), 2) play strenuous racquet sports (eg, singles tennis, paddle ball) ≥5 h/wk, 3) ride a bicycle ≥50 miles/wk, 4) swim ≥2 miles/wk, or 5) play other strenuous sports (eg, basketball, soccer). Participants who endorsed one or more activities were designated as vigorous exercisers, whereas participants who did not endorse any of these activities were designated as nonvigorous exercisers.
Statistical analyses
The primary method of analysis was twin structural modeling, which aims to explain the observed total phenotypic variation and covariation between MZ and DZ twins in terms of latent causes due to additive (A) or nonadditive (D) genetic effects and shared (C) or unique (E) environmental effects. The primary goals of the analyses were 1) to determine whether common genetic factors contributed to both VE and BMI (gene-environment correlation) and 2) to determine whether VE moderates genetic influences on BMI (gene × environment interaction). As described in detail below, bivariate twin models estimate common genetic and environmental influences across VE and BMI and will be used to estimate the extent to which common genetic and/or environmental factors are correlated across VE and BMI. Next, gene × environment interaction models were fit. These models determine whether genetic or environmental factors differ as a function of a measured environmental factor, which here is VE. To the extent that the magnitude of genetic influence differs as a function of the environmental measure, this would suggest that gene × measured environment (eg, VE) interaction is present for the outcome of interest, which here is BMI. Gene × environment interaction models are described in detail below.
All models and maximum-likelihood parameter estimates were calculated by using the raw data capabilities of the Mx program (35), although Mplus provides an additional method for conducting such analyses (36). The significance of individual parameters was determined by comparing the fit of models that omitted the parameters of interest with the fit of a full model, with twice the difference in the log-likelihood ratio distributed asymptotically as a chi-square variable with df reflecting the difference in the number of parameters between the full and reduced models. However, for tests of the variance components, the corresponding P values were halved, because their value under the null hypothesis was on the boundary of the parameter space (37). As there was little evidence that correlations among MZ twins substantially exceeded twice those among DZ twins, we focused primarily on ACE (instead of ADE) models.
Bivariate models
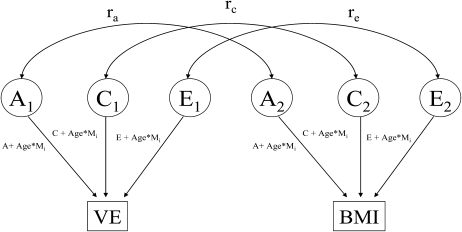
The polychoric correlation between the BMI and VE, as well as the extent to which it is attributable to common additive genetic, shared environmental, or unique environmental variance may be estimated using bivariate twin modeling (Figure 1, adapted from reference 38). Using this approach, the correlation between additive genetic, shared environmental, and nonshared environmental components of VE and BMI may be quantified, with the first specifically reflecting gene-environment correlation. Because the VE measure was binary, and Mx cannot currently model polyserial correlations between ordinal and continuous data, polychoric correlations instead were obtained by discretizing BMI into 7 categories chosen to retain information while also ensuring adequate cell counts. Polychoric correlations between pairs of ordinal variables can be estimated on the basis of a liability model (39), which presumes an underlying, normally distributed susceptibility for the expression of the phenotype of interest with zero mean and unit variance. Categories were defined by thresholds on this underlying curve, such that the area under the curve reflects the proportion of the population in each category. To ensure convergence, the thresholds in the liability scale were assumed to be fixed across the 2 VE levels. Furthermore, because we wanted to examine the bivariate association between BMI and VE adjusted for any potential confounding by age, we allowed age to moderate all genetic, shared, and nonshared environmental components of their variance and covariance matrix.
FIGURE 1.
Bivariate model adapted from reference 38. VE, vigorous exercise; A1 and A2, additive genetic effects for twins 1 and 2 within a twin pair, respectively; C1 and C2, shared environmental effects for twins 1 and 2 within a twin pair, respectively; Mi, age moderator values for the ith individual within each twin pair; ra, additive genetic correlation; rc, shared environmental correlation; re, nonshared environmental correlation.
Gene × environment interaction models
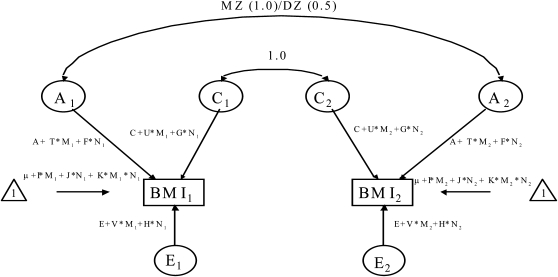
Gene × measured environment interaction may be detected within twin models by modeling the variance components attributable to latent genetic, shared, and nonshared environmental effects as a function of the putative environmental moderator (40). VE and age were evaluated as putative joint moderators of both BMI mean and all 3 of the BMI variance components (Figure 2). Modeling the effects of a moderator on the mean of the liability distribution avoids any potential confounding of a gene × environment interaction by gene-environment correlation by regressing out genetic and environmental effects common to both BMI and the moderator. To avoid possible model misspecification, we deliberately overparameterized the model for the mean structure of the multiple moderation model (eg, μ + I × Mi + J × Ni + K × Mi × Ni, where Mi, and Ni, reflect VE and age moderator values for the ith individuals within a twin pair; see Figure 2 for definitions of the variables), irrespective of the statistical significance of the individual regression coefficients I, J, and K. VE was coded as a 0/1 indicator of VE participation, whereas age was standardized to zero mean and unit variance using the entries of Table 1. Variability in residual susceptibility to BMI was described in terms of 3 latent variables—A, C, and E—with the path coefficients associated with each variable expressed as linear functions of the moderators (eg, A + T × Mi + F × Ni, C + U × Mi + G × Ni, E + V × Mi + H × Ni, with T, U, and V reflecting the effects of VE on additive genetic, shared environmental, and nonshared environmental variance in BMI and with F, G, and H reflecting the effects of age on additive genetic, shared environmental, and nonshared environmental variance in BMI). The full model (IJK-ACE-TUV-FGH) was first fit to the data. Next, an overall test of moderation of the variance components by VE (T, U, and V fixed to zero) was conducted in the presence of moderation by age. If significant, a backward stepwise elimination procedure was followed for the individual interaction variables, testing the extent to which the variable associated with the smallest change in log-likelihood ratio contributes significantly to the model. A similar series of models is evaluated for age moderation in the presence of moderation by VE.
FIGURE 2.
Gene × environment interaction model adapted from reference 40. VE, vigorous exercise; BMI1 and BMI2, BMI in twins 1 and 2 within a twin pair, respectively; A1 and A2, additive genetic effects for twins 1 and 2 within a twin pair, respectively; C1 and C2, shared environmental effects for twins 1 and 2 within a twin pair, respectively; E1 and E2, nonshared environmental effects for twins 1 and 2 within a twin pair, respectively; M, moderator 1 (VE); N, moderator 2 (age); T, VE-moderated component of A; U, VE-moderated component of C; V, VE-moderated component of E; F, age-moderated component of A; G, age-moderated component of C; H, age-moderated component of E; I, effect of VE on mean BMI; J, effect of age on mean BMI; K, effect of VE × age interaction on mean BMI.
TABLE 1.
Demographic characteristics and descriptive statistics for the Vietnam Era Twin Registry sample1
| Full sample (n = 8627) | MZ twins (n = 4734) | DZ twins (n = 3893) | |
| Age (y) | 41.07 ± 3.002 | 41.06 ± 3.15 | 41.07 ± 2.80 |
| Education (y) | 14.44 ± 2.52 | 41.52 ± 2.50 | 14.34 ± 2.53 |
| BMI (continuous) | 25.75 ± 3.69 | 25.73 ± 3.67 | 25.78 ± 3.71 |
| Race [n (%)] | |||
| White | 8149 (94.51) | 4472 (94.53) | 3677 (94.50) |
| African American | 442 (5.13) | 238 (5.03) | 204 (5.24) |
| Hispanic | 5 (0.06) | 3 (0.06) | 2 (0.05) |
| Other | 26 (0.30) | 18 (0.38) | 8 (0.21) |
| Annual family income [n (%)] | |||
| <$35,000 | 4466 (54.11) | 2454 (54.02) | 2012 (54.22) |
| ≥$35,000 | 3788 (45.89) | 2089 (45.98) | 1699 (45.78) |
| Married [n (%)] | |||
| Yes | 7550 (89.53) | 4132 (89.05) | 3418 (90.11) |
| No | 883 (10.47) | 508 (10.95) | 375 (9.89) |
| Vigorous exercise [n (%)] | |||
| Yes | 2190 (25.51) | 1224 (25.98) | 966 (24.94) |
| No | 6395 (74.49) | 3487 (74.02) | 2908 (75.06) |
| BMI, categorical [n (%)] | |||
| Normal | 4136 (48.20) | 2258 (48.00) | 1878 (48.43) |
| Overweight | 3506 (40.85) | 1952 (41.50) | 1554 (40.07) |
| Obese | 940 (10.95) | 494 (10.50) | 446 (11.50) |
MZ, monozygotic; DZ, dizygotic.
Mean ± SD (all such values).
Note that it would have been possible to formulate models for testing for gene × environment interaction in the presence of gene-environment correlation (40). However, we chose not to examine such models because the joint distribution of VE and BMI is no longer a bivariate normal once VE is allowed to moderate both common and specific additive genetic paths (41).
To obtain CIs for parameter estimates, thresholds, and genetic and environmental correlations, bootstrapping methods were used. Specifically, at each bootstrap iteration, pairs were drawn with replacement from the original sample of the same size. All runs in which Mx gave warning messages about a possible lack of convergence were dropped, and the process was repeated until 1000 bootstrap iterations had converged successfully. For each variable of interest, the estimates were ordered and endpoints of 95% bootstrap CIs were obtained from the 2.5 and 97.5 bootstrap sample percentiles.
In twin structural equation modeling, it is assumed that the rearing environment for the behaviors under study was similar for MZ and DZ twin pairs. Empirical tests of the equal environment assumption by zygosity suggest that it holds for many traits (42–44). In addition, a lack of assortative mating by phenotype is typically assumed. Although assortative mating effects have been shown for BMI (45), they appear to contribute only a small proportion to genetic variance in BMI (7), which suggests that this assumption is reasonable for the present analyses.
RESULTS
Sample and demographics
Demographic information and descriptive statistics for VE and BMI are presented in Table 1. In the full sample, participants were, on average, 41.1 y of age at the time of the National Heart, Lung and Blood Institute Survey and reported 14.4 y of schooling, 95% were white, 54% had an annual family income <$35,000, and 90% were currently married. Participants had, on average, a BMI of 25.8, which reflected a BMI in the overweight range with 41% in the overweight range and 11% classified as obese. Only 25.5% of the participants reported VE over the past 3 mo. Equality of variances and means for the natural logarithm of BMI was tested by fitting separate bivariate normal models to observations of MZ and DZ twin pairs and then testing equality across zygosity groups of the first 2 moments of the corresponding univariate normal margins. Neither equality of variances [χ2(1) = 0.382, P = 0.537] nor equality of means under the assumption of equal variances [χ2(1) = 0.209, P = 0.648] could be rejected given the data at hand. Similarly, separate bivariate liability distributions for MZ and DZ pairs were estimated for VE. Again, neither equality of variances [χ2(1) < 0.001, P = 0.992] nor equality of thresholds under the assumption of equal variances [χ2(1) = 1.193, P = 0.275] could be rejected for this sample. Results agree with the sample summaries shown in Table 1, which show negligible differences in mean BMIs and VE prevalences by zygosity group.
Univariate models
Before estimating models examining genetic and environmental factors common across VE and BMI, we first estimated genetic and environmental contributions to each at the mean of age. Additive genetic, shared environmental, and nonshared environmental contributions to VE and BMI for a typical 41-y-old study participant are listed in Table 2. Additive genetic effects did not contribute significantly to VE, whereas shared and nonshared environmental variance did. For BMI, additive genetic and nonshared environmental effects contributed significantly with little contribution of shared environment.
TABLE 2.
Additive genetic, shared environmental, and nonshared environmental effects for vigorous exercise (VE) and BMI and their correlation1
| a2 | c2 | e2 | ra | rc | re | |
| VE | 0.10 | 0.18 | 0.72 | — | — | — |
| (0.00, 0.31) | (0.01, 0.30) | (0.63, 0.80) | — | — | — | |
| BMI | 0.67 | 0.04 | 0.29 | — | — | — |
| (0.56, 0.072) | (0.00, 0.14) | (0.27, 0.32) | — | — | — | |
| VE-BMI | — | — | — | −0.03 | −0.05 | −0.09 |
| — | — | — | (−0.54, 0.36) | (−0.99, 0.99) | (−0.16, −0.02) |
n = 8627 study participants drawn from 2710 monozygotic and 2327 dizygotic twin pairs (results of gene × environment interaction twin structural equation modeling). 95% CIs are in parentheses. a2, additive genetic variance; c2, shared environmental variance; e2, nonshared environmental variance; ra, additive genetic correlation; rc, shared environmental correlation; re, nonshared environmental correlation.
Bivariate models
Because preliminary analyses revealed no evidence of age moderation of any component of the association between BMI and VE [χ2(3) = 2.039, P = 0.564], the primary analyses focused solely on the bivariate association between VE and BMI. Comparison of the ACE model to one in which all 3 of its components were set to zero suggested the existence of a significant overall association between BMI and VE [χ2(3) = 27.064, P < 0.001]. Bootstrap estimation confirmed these results (Table 2), which indicated the presence of a small, but significant, correlation between VE and BMI (r = −0.06; 95% CI: −0.09, −0.02), such that lower BMI levels were observed among subjects reporting VE. When the association was broken down into individual A, C, and E components, results indicated that nonshared environmental effects (E) contributed significantly to this inverse relation (re = −0.09; 95% CI: −0.16, −0.02), which suggests that environmental factors that differ across twins contribute to the correlation between VE and BMI. In contrast, additive genetic effects (ra = −0.03; 95% CI: −0.54, 0.36) did not contribute to the association between VE and BMI, which indicates a lack of gene-environment correlation. Shared environmental factors also did not contribute to the association between VE and BMI (rc = −0.05; 95% CI: −0.99, 0.99). Taken together, these results suggest that familial factors did not contribute to the association between VE and BMI in middle-aged men.
The nonshared environmental effects can be illustrated by examining differences among MZ co-twins discordant for VE. In this sample, 614 MZ twin pairs were discordant for VE (one reported VE and the other did not). The biserial correlation between VE and BMI (r = −0.16, P = 0.001) was statistically significant, but small, accounting for a mean difference in BMI of 1.3%, or 0.33 BMI units between MZ co-twins who were discordant for VE.
Gene × environment interaction models
Comparative model fits that test the extent to which VE serves as a moderator of the natural logarithm of BMI—a measure whose distribution is more symmetric than BMI itself—are presented in Table 3. The comparative model fitting showed moderation of BMI by both VE and age (models 2 and 3, respectively: P < 0.001). Subsequent evaluation of additive genetic, shared environmental, and nonshared environmental moderation indicated that VE moderated the additive genetic effects (model 2a: P < 0.001) and nonshared environmental effects (model 2c: P = 0.04) on BMI over and above any moderation of these components by age. Furthermore, age moderated both additive genetic (model 3a: P = 0.02) and shared environmental components (model 3b: P = 0.04) of BMI beyond any moderation of these components by VE.
TABLE 3.
Comparative model fits for participation in vigorous exercise (VE) and age as moderators of BMI1
| Model fit |
Comparative model fit |
|||||
| Model | −2LL | df | Δ−2LL | df | P value | Test |
| 1: Full model | 18,356.58 | 7060 | ||||
| 2: No VE moderation | 18,426.50 | 7063 | 69.92 | 3 | <0.001 | 2 vs 1 |
| 2a: No additive genetic moderation | 18,376.40 | 7061 | 19.82 | 1 | <0.001 | 2a vs 1 |
| 2b: No shared environmental moderation | 18,357.25 | 7061 | 0.67 | 1 | 0.42 | 2b vs 1 |
| 2c: No nonshared moderation | 18,360.89 | 7061 | 4.31 | 1 | 0.04 | 2c vs 1 |
| 3: No age moderation | 18,367.97 | 7063 | 11.39 | 3 | <0.001 | 3 vs 1 |
| 3a: No additive genetic moderation | 18,362.08 | 7061 | 5.50 | 1 | 0.02 | 3a vs 1 |
| 3b: No shared environmental moderation | 18,360.94 | 7061 | 4.36 | 1 | 0.04 | 3b vs 1 |
| 3c: No nonshared moderation | 18,358.24 | 7061 | 1.66 | 1 | 0.20 | 3c vs 1 |
n = 8627 study participants drawn from 2710 monozygotic and 2327 dizygotic twin pairs (results of gene × environment interaction twin structural equation modeling).
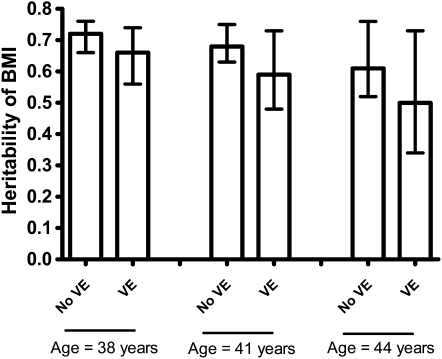
The range of heritability in BMI by the presence or absence of VE and age is presented in Figure 3. VE and older age were each individually associated with a reduction in the heritability of BMI. For subjects 1 SD below the mean (38 y), those who did report VE participation showed a 6-point decrease in BMI heritability compared with those who did not report VE participation, with heritability reduced from 0.72 (95% CI: 0.61, 0.76) to 0.66 (95% CI: 0.50, 0.74). Subjects at the mean age of 41 y recorded a corresponding 9-point decrease, with heritability reduced from 0.68 (95% CI: 0.59, 0.72) to 0.59 (95% CI: 0.44, 0.70). Finally, for subjects 1 SD above the mean (44 y), heritability decreased from 0.61 (95% CI: 0.47, 0.71) to 0.50 (95% CI: 0.29, 0.67), representing an 11-point reduction. As for age effects, even within a restricted age range of our sample, a 2-SD increase in age from 38 to 44 y, which included the majority of VETR participants, resulted in an 11-point reduction in heritability among those who did not report VE participation and a 16-point reduction among those who did.
FIGURE 3.
The heritability of BMI (point estimate and 95% CI) by self-report of vigorous exercise (VE) and age. Gene × environment interaction estimated by twin structural equation modeling. n = 8627 study participants drawn from 2710 monozygotic and 2327 dizygotic twin pairs.
DISCUSSION
The results of this study highlight the importance of VE when evaluating genetic influences on BMI. The heritability of BMI differed in the presence or absence of self-reported VE, such that the heritability of BMI was greater among those who did not report VE and blunted among those who reported VE (gene × environment interaction). Consistent with an emerging literature, these results suggest that participation in VE may buffer genetic influences on BMI. Furthermore, these results suggest that molecular genetic studies of BMI and obesity may benefit from the incorporation of relevant behavioral factors, such as VE, to maximize the identification of novel obesity genes.
In the gene × environment interaction models, age also moderated genetic influences on BMI and operated synergistically with VE in the heritability scale, with heritability effects attributable to VE participation increasing in magnitude from a 6- to an 11-point reduction in BMI heritability over a narrow 6-y age range (38–44 y). This suggests that the effect of VE on the heritability of BMI strengthens with increasing age. However, the clinical relevance of these results remains to be determined given the restricted age range of this sample.
We also observed a small but significant inverse relation between VE and BMI. Twin modeling indicated that the nonshared environment alone contributed significantly to this inverse relation. A nonshared environmental contribution suggests that environmental factors specific to twins, and not common across twins, account for the association between VE and BMI. This result is entirely consistent with prior reports of greater BMI in inactive MZ twins relative to their more active co-twins (21–23). However, we incorporated the full richness of our data set, including discordant and concordant MZ and DZ twins, to derive our estimates. To further illustrate these effects in our data set, we selected MZ twins discordant for VE and showed that inactive MZ twins weigh more than their active co-twins in direct replication of the prior research.
A direct environmental effect of VE on BMI that is independent of genetic effects could occur, for example, through reduction in appetite and sustaining of a negative energy balance (46) or through favorable changes in body composition that could elevate resting metabolic rate (47). Nonshared environmental correlation may also reflect correlated measurement error. This is a possibility for both our study and the study by Pietilainen et al (19), given that both used self-report measures to calculate BMI and physical activity. We believe that the possibility of correlated measurement error is diminished by direct replication of 2 other studies, which defined body mass using measured height and weight (21, 22). It is also possible that a nonshared environmental correlation reflects the effect of a third, unmeasured variable on both VE and BMI, such as chronic disability.
We did not identify any correlated genetic effects across VE and BMI. The finding that genetic factors that contributed to VE did not also contribute to BMI can be interpreted as a lack of gene-environment correlation. However, it may also have been attributable to the small contribution of genetic effects to VE observed in this sample (a2 = 0.10; 95% CI: 0.00, 0.31), relative to previous twins studies addressing this topic (48–50), because the covariance of 2 random variables is zero when one of them shows no variability. Given the low levels of shared environmental variance estimated for BMI (c2 = 0.04; 95% CI: 0.00, 0.14), a similar explanation may lie behind the absence of any correlated shared environmental effects across the 2 phenotypes of interest. In contrast, both phenotypes of interest displayed strong nonshared variability in the latent liability scale (BMI: e2 = 0.29, 95% CI: 0.27, 0.32; VE: e2 = 0.72, 95% CI: 0.63, 0.80).
Overall, these results are consistent with the general public health message that exercise, particularly that which is performed at a vigorous intensity, is essential for promoting and maintaining health (51). More specifically, these findings support research indicating that higher levels of VE participation may be important for weight control, particularly as one becomes older (19, 52–54). Indeed, VE could serve as a protective factor against obesity even in those individuals who are at high genetic risk of obesity.
These results also suggest that, in the search for genes related to BMI, accounting for behavioral factors such as VE and demographic factors such as age may improve the ability to detect genetic effects. In this study, VE contributed to environmental variation in BMI, as further documented by the association of VE with lower BMI among discordant MZ twins. This suggests that controlling for VE in genetic studies of BMI would reduce environmental effects on the outcome, permitting a greater chance of detecting genetic effects using the residual variance, given the same sample size. Second, VE moderated the heritability of BMI, which indicates that genetic markers may show differential effects at differing levels of VE. It is plausible that stratifying by VE may identify novel genetic predictors of BMI or elucidate the conditions under which identified genes show their strongest effects, although this is likely to require very large sample sizes to achieve sufficient statistical power. Nonetheless, at least one study now suggests that FTO (28) may show its strongest effects among inactive participants, which suggests, consistent with the present results, that VE may counteract some of the genetic effects on BMI and render detection of genetic effects more difficult if not considered.
It is important to note the limitations of the present study. First, the VETR is composed entirely of men who are predominantly white. The generalizability of these results to civilians, women, and ethnic minorities remains to be determined. In addition, we were unable to examine whether sex might moderate the heritability of BMI and had insufficient variability to identify racial and ethnic effects on the heritability of BMI. We used VE as one potential environmental predictor of BMI. It is possible that lower intensities and other types of structured exercise as well as non-exercise-related activity not examined in this study could also affect the heritability of BMI as well as dietary and other related health behaviors. Furthermore, our study was cross-sectional in nature, and we were not able to determine whether the chronicity of VE also affects the heritability of BMI or whether VE is relevant to change in weight over time.
Acknowledgments
The US Department of Veterans Affairs provided financial support for the development and maintenance of the VETR. Numerous organizations provided invaluable assistance in the conduct of this study, including the Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; the National Opinion Research Center; the National Research Council, National Academy of Sciences; and the Institute for Survey Research, Temple University. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VETR and their families. Without their contribution this research would not have been possible.
The authors' responsibilities were as follows—JMM and GDP: had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the conceptualization and the writing of the manuscript and provided critical feedback on the manuscript. None of the authors had any financial involvement or affiliation with any organization whose financial interests may be affected by material in the manuscript or that might potentially bias it.
REFERENCES
- 1.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007;316:889–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 2007;316:1341–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loos RJ, Lindgren CM, Li S, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet 2008;40:768–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willer CJ, Speliotes EK, Loos RJ, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 2009;41:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thorleifsson G, Walters GB, Gudbjartsson DF, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet 2009;41:18–24 [DOI] [PubMed] [Google Scholar]
- 6.Sabatti C, Service SK, Hartikainen AL, et al. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat Genet 2009;41:35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet 1997;27:325–51 [DOI] [PubMed] [Google Scholar]
- 8.Lewis CE, Jacobs DR, Jr, McCreath H, et al. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol 2000;151:1172–81 [DOI] [PubMed] [Google Scholar]
- 9.Sturm R. Increases in clinically severe obesity in the United States, 1986-2000. Arch Intern Med 2003;163:2146–8 [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev 2007;29:6–28 [DOI] [PubMed] [Google Scholar]
- 11.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology 2007;132:2087–102 [DOI] [PubMed] [Google Scholar]
- 12.Brownson RC, Boehmer TK, Luke DA. Declining rates of physical activity in the United States: what are the contributors? Annu Rev Public Health 2005;26:421–43 [DOI] [PubMed] [Google Scholar]
- 13.Ball K, Owen N, Salmon J, Bauman A, Gore CJ. Associations of physical activity with body weight and fat in men and women. Int J Obes Relat Metab Disord 2001;25:914–9 [DOI] [PubMed] [Google Scholar]
- 14.Chan CB, Spangler E, Valcour J, Tudor-Locke C. Cross-sectional relationship of pedometer-determined ambulatory activity to indicators of health. Obes Res 2003;11:1563–70 [DOI] [PubMed] [Google Scholar]
- 15.King GA, Fitzhugh EC, Bassett DR, Jr, et al. Relationship of leisure-time physical activity and occupational activity to the prevalence of obesity. Int J Obes Relat Metab Disord 2001;25:606–12 [DOI] [PubMed] [Google Scholar]
- 16.Wyatt HR, Peters JC, Reed GW, Barry M, Hill JO. A Colorado statewide survey of walking and its relation to excessive weight. Med Sci Sports Exerc 2005;37:724–30 [DOI] [PubMed] [Google Scholar]
- 17.Bernstein MS, Costanza MC, Morabia A. Association of physical activity intensity levels with overweight and obesity in a population-based sample of adults. Prev Med 2004;38:94–104 [DOI] [PubMed] [Google Scholar]
- 18.Coakley EH, Rimm EB, Colditz G, Kawachi I, Willett W. Predictors of weight change in men: results from the Health Professionals Follow-up Study. Int J Obes Relat Metab Disord 1998;22:89–96 [DOI] [PubMed] [Google Scholar]
- 19.Williams PT. Maintaining vigorous activity attenuates 7-yr weight gain in 8340 runners. Med Sci Sports Exerc 2007;39:801–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshioka M, Ayabe M, Yahiro T, et al. Long-period accelerometer monitoring shows the role of physical activity in overweight and obesity. Int J Obes (Lond) 2005;29:502–8 [DOI] [PubMed] [Google Scholar]
- 21.Samaras K, Kelly PJ, Chiano MN, Spector TD, Campbell LV. Genetic and environmental influences on total-body and central abdominal fat: the effect of physical activity in female twins. Ann Intern Med 1999;130:873–82 [DOI] [PubMed] [Google Scholar]
- 22.Williams PT, Blanche PJ, Krauss RM. Behavioral versus genetic correlates of lipoproteins and adiposity in identical twins discordant for exercise. Circulation 2005;112:350–6 [DOI] [PubMed] [Google Scholar]
- 23.Pietilainen KH, Kaprio J, Borg P, et al. Physical inactivity and obesity: a vicious circle. Obesity (Silver Spring) 2008;16:409–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waller K, Kaprio J, Kujala UM. Associations between long-term physical activity, waist circumference and weight gain: a 30-year longitudinal twin study. Int J Obes (Lond) 2008;32:353–61 [DOI] [PubMed] [Google Scholar]
- 25.Karnehed N, Tynelius P, Heitmann BL, Rasmussen F. Physical activity, diet and gene-environment interactions in relation to body mass index and waist circumference: the Swedish young male twins study. Public Health Nutr 2006;9:851–8 [DOI] [PubMed] [Google Scholar]
- 26.Heitmann BL, Kaprio J, Harris JR, Rissanen A, Korkeila M, Koskenvuo M. Are genetic determinants of weight gain modified by leisure-time physical activity? A prospective study of Finnish twins. Am J Clin Nutr 1997;66:672–8 [DOI] [PubMed] [Google Scholar]
- 27.Mustelin L, Silventoinen K, Pietilainen K, Rissanen A, Kaprio J. Physical activity reduces the influence of genetic effects on BMI and waist circumference: a study in young adult twins. Int J Obes (Lond) 2008;33:29–36 [DOI] [PubMed] [Google Scholar]
- 28.Andreasen CH, Stender-Petersen KL, Mogensen MS, et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes 2008;57:95–101 [DOI] [PubMed] [Google Scholar]
- 29.Eisen S, True W, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: method of construction. Acta Genet Med Gemellol (Roma) 1987;36:61–6 [DOI] [PubMed] [Google Scholar]
- 30.Goldberg J, True W, Eisen S, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: ascertainment bias. Acta Genet Med Gemellol (Roma) 1987;36:67–78 [DOI] [PubMed] [Google Scholar]
- 31.Henderson WG, Eisen S, Goldberg J, True WR, Barnes JE, Vitek ME. The Vietnam Era Twin Registry: a resource for medical research. Public Health Rep 1990;105:368–73 [PMC free article] [PubMed] [Google Scholar]
- 32.Villanueva EV. The validity of self-reported weight in US adults: a population based cross-sectional study. BMC Public Health 2001;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Field AE, Aneja P, Rosner B. The validity of self-reported weight change among adolescents and young adults. Obesity (Silver Spring) 2007;15:2357–64 [DOI] [PubMed] [Google Scholar]
- 34.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32:S498–504 [DOI] [PubMed] [Google Scholar]
- 35.Neale M, Boker S, Xie G, Maes H. Statistical modeling. 6th ed Richmond, VA: Department of Psychiatry, 2002 [Google Scholar]
- 36.Muthen LK, Muthen BO. Mplus user's guide Los Angeles, CA: Muthen & Muthen, 2007 [Google Scholar]
- 37.Self SG, Liang K-Y. Asymptotic properties of maximum likelihood estimators and likelihood ratio tests under nonstandard conditions. J Am Stat Assoc 1987;82:605–10 [Google Scholar]
- 38.Neale M, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht, Netherlands: Kluwer Academic Publishers, 1992 [Google Scholar]
- 39.Falconer DS. The inheritance of liability to certain diseases, estimated from the incidence among relatives. Ann Hum Genet 1967;31:1–20 [DOI] [PubMed] [Google Scholar]
- 40.Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Res 2002;5:554–71 [DOI] [PubMed] [Google Scholar]
- 41.Rathouz PJ, Van Hulle CA, Rodgers JL, Waldman ID, Lahey BB. Specification, testing, and interpretation of gene-by-measured-environment interaction models in the presence of gene-environment correlation. Behav Genet 2008;38:301–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A test of the equal-environment assumption in twin studies of psychiatric illness. Behav Genet 1993;23:21–7 [DOI] [PubMed] [Google Scholar]
- 43.Matheny AP., Jr Appraisal of parental bias in twin studies: ascribed zygosity and IQ differences in twins. Acta Genet Med Gemellol (Roma) 1979;28:155–60 [DOI] [PubMed] [Google Scholar]
- 44.Scarr S, Carter-Saltzman L. Twin method: defense of a critical assumption. Behav Genet 1979;9:527–42 [DOI] [PubMed] [Google Scholar]
- 45.Silventoinen K, Kaprio J, Lahelma E, Viken RJ, Rose RJ. Assortative mating by body height and BMI: Finnish twins and their spouses. Am J Hum Biol 2003;15:620–7 [DOI] [PubMed] [Google Scholar]
- 46.Blundell JE, Stubbs RJ, Hughes DA, Whybrow S, King NA. Cross talk between physical activity and appetite control: does physical activity stimulate appetite? Proc Nutr Soc 2003;62:651–61 [DOI] [PubMed] [Google Scholar]
- 47.Slentz CA, Duscha BD, Johnson JL, et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE—a randomized controlled study. Arch Intern Med 2004;164:31–9 [DOI] [PubMed] [Google Scholar]
- 48.Carlsson S, Andersson T, Lichtenstein P, Michaelsson K, Ahlbom A. Genetic effects on physical activity: results from the Swedish Twin Registry. Med Sci Sports Exerc 2006;38:1396–401 [DOI] [PubMed] [Google Scholar]
- 49.Frederiksen H, Christensen K. The influence of genetic factors on physical functioning and exercise in second half of life. Scand J Med Sci Sports 2003;13:9–18 [DOI] [PubMed] [Google Scholar]
- 50.Stubbe JH, Boomsma DI, Vink JM, et al. Genetic influences on exercise participation in 37,051 twin pairs from seven countries. PLoS One 2006;1:e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 2007;39:1423–34 [DOI] [PubMed] [Google Scholar]
- 52.Di Pietro L, Dziura J, Blair SN. Estimated change in physical activity level (PAL) and prediction of 5-year weight change in men: the Aerobics Center Longitudinal Study. Int J Obes Relat Metab Disord 2004;28:1541–7 [DOI] [PubMed] [Google Scholar]
- 53.Littman AJ, Kristal AR, White E. Effects of physical activity intensity, frequency, and activity type on 10-y weight change in middle-aged men and women. Int J Obes (Lond) 2005;29:524–33 [DOI] [PubMed] [Google Scholar]
- 54.Williams PT, Wood PD. The effects of changing exercise levels on weight and age-related weight gain. Int J Obes (Lond) 2006;30:543–51 [DOI] [PMC free article] [PubMed] [Google Scholar]