Abstract
Background: Exercise intensity may affect the selective loss of abdominal adipose tissue.
Objective: This study showed whether aerobic exercise intensity affects the loss of abdominal fat and improvement in cardiovascular disease risk factors under conditions of equal energy deficit in women with abdominal obesity.
Design: This was a randomized trial in 112 overweight and obese [body mass index (in kg/m2): 25–40; waist circumference >88 cm], postmenopausal women assigned to one of three 20-wk interventions of equal energy deficit: calorie restriction (CR only), CR plus moderate-intensity aerobic exercise (CR + moderate-intensity), or CR plus vigorous-intensity exercise (CR + vigorous-intensity). The diet was a controlled program of underfeeding during which meals were provided at individual calorie levels (≈400 kcal/d). Exercise (3 d/wk) involved treadmill walking at an intensity of 45–50% (moderate-intensity) or 70–75% (vigorous-intensity) of heart rate reserve. The primary outcome was abdominal visceral fat volume.
Results: Average weight loss for the 95 women who completed the study was 12.1 kg (±4.5 kg) and was not significantly different across groups. Maximal oxygen uptake ( O2max) increased more in the CR + vigorous-intensity group than in either of the other groups (P < 0.05). The CR-only group lost relatively more lean mass than did either exercise group (P < 0.05). All groups showed similar decreases in abdominal visceral fat (≈25%; P < 0.001 for all). However, changes in visceral fat were inversely related to increases in
O2max) increased more in the CR + vigorous-intensity group than in either of the other groups (P < 0.05). The CR-only group lost relatively more lean mass than did either exercise group (P < 0.05). All groups showed similar decreases in abdominal visceral fat (≈25%; P < 0.001 for all). However, changes in visceral fat were inversely related to increases in  O2max (P < 0.01). Changes in lipids, fasting glucose or insulin, and 2-h glucose and insulin areas during the oral-glucose-tolerance test were similar across treatment groups.
O2max (P < 0.01). Changes in lipids, fasting glucose or insulin, and 2-h glucose and insulin areas during the oral-glucose-tolerance test were similar across treatment groups.
Conclusion: With a similar amount of total weight loss, lean mass is preserved, but there is not a preferential loss of abdominal fat when either moderate- or vigorous-intensity aerobic exercise is performed during caloric restriction. This trial was registered at clinicaltrials.gov as NCT00664729.
INTRODUCTION
The prevalence of obesity increases with age and is highest among middle-aged and older women (1). In addition, redistribution of body fat from the gluteofemoral to the abdominal region often occurs after menopause in women (2, 3). This excess adipose tissue in the abdomen, especially around visceral organs, increases metabolic risk of cardiovascular disease (CVD) independent of the total amount of adipose tissue (4, 5). In fact, evidence-based guidelines on the management of obesity promote the use of waist circumference as a measure of abdominal obesity for predicting excess relative risk of disease in overweight and class I obese persons [body mass index (BMI; in kg/m2): 25.0–34.9] (6). Therefore, therapies that selectively target the loss of abdominal fat may be more effective at reducing the CVD risk attributed to obesity in postmenopausal women.
The current consensus is that a combination of a hypocaloric diet and regular aerobic exercise is the most effective treatment of abdominal obesity. Current practice guidelines advocate inclusion of physical activity for 30 min/d most days of the week, increasing, when appropriate, to 60 min/d as part of an overall obesity treatment program (7–9). However, although a greater total volume of exercise (in kcal expenditure as a function of exercise intensity, duration, and frequency) results in greater loss of total and abdominal fat and better metabolic profiles (10–15), the ideal intensity of exercise for a given level of caloric expenditure necessary to maximize these health benefits is not known. Observational studies indicate that, compared with reported low- to moderate-intensity physical activity, higher-intensity activity is associated with less total and abdominal obesity and reduced risk of CVD, independent of the total energy expenditure of exercise (16–20). In addition, some intervention trials show that, in normal-weight persons, vigorous-intensity exercise training, performed in the absence of caloric restriction, results in greater improvement in risk factors of CVD than low- or moderate-intensity training (21–24). Thus, the intensity of exercise may be an important factor affecting the selective loss of abdominal fat and improvement in CVD risk when combined with caloric restriction.
The primary purpose of this trial, the Diet, Exercise, and Metabolism for Older Women Study, was to determine whether intensity of aerobic exercise affects the loss of abdominal (both subcutaneous and visceral) adipose tissue and improvement in CVD risk factors (glucose tolerance and HDL-cholesterol and triglyceride concentrations) under controlled conditions of equal energy deficit in postmenopausal women with abdominal obesity.
SUBJECTS AND METHODS
Design overview
The Diet, Exercise, and Metabolism for Older Women Study (2003–2007) was a randomized trial comparing the effects of 1) caloric restriction alone (CR only), 2) caloric restriction plus moderate-intensity aerobic exercise (CR + moderate-intensity), and 3) caloric restriction plus vigorous-intensity exercise (CR + vigorous-intensity). The diet included a controlled program of underfeeding during which meals were prepared and provided at individual calorie levels. The degree of caloric restriction was adjusted so that total caloric deficit (≈400 kcal/d; 2800 kcal/wk) was similar for all groups. All exercise sessions (3 d/wk) were supervised. The study was statistically powered to detect group differences in the primary outcome of abdominal visceral fat volume and in the secondary outcomes of glucose tolerance and HDL-cholesterol and triglyceride concentrations. The study and protocol were approved by the Wake Forest University School of Medicine Institutional Review Board, and all participants provided written informed consent. A subset of the data collected during this trial was published previously (25–28).
Setting and participants
Women from Forsyth County, NC, and the surrounding areas were recruited through local advertisement. Women were enrolled based on the following criteria: 1) abdominal obesity (BMI: 25–40; and waist circumference > 88 cm), 2) age (50–70 y), 3) postmenopausal status (no menses for >1 y), 4) nonsmoking (for >1 y), 5) not on hormone replacement therapy, 6) sedentary (<15 min exercise 2 times/wk in the past 6 mo), and 7) weight stable (<5% weight change) for ≥6 mo before enrollment.
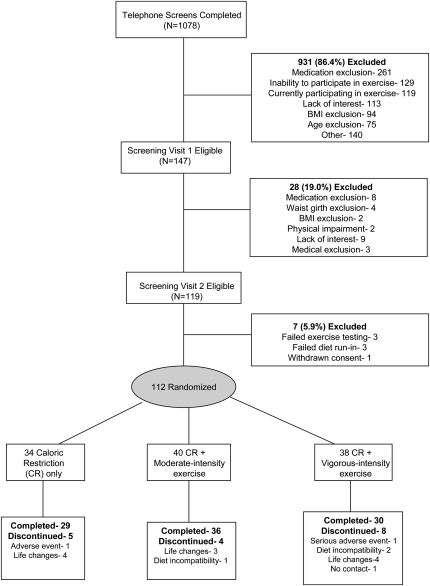
A total of 1078 women were initially screened by telephone (Figure 1). Of those women, 147 were further screened in the clinic and underwent a medical history review, physical examination, cognitive (Mini-Mental State Examination) and depression (Center for Epidemiologic Studies Depression Scale) screening, fasting blood draw, and 12-lead resting electrocardiogram. Those women with evidence of untreated hypertension (blood pressure >160/90 mm Hg) or depression (Center for Epidemiologic Studies Depression Scale >16); hypertriglyceridemia (triglycerides >400 mg/dL or 4.5 mmol/L); insulin-dependent or uncontrolled diabetes (fasting glucose >140 mg/dL or 7.7 mmol/L); active cancer, liver, renal, or hematologic disease; cognitive dysfunction (Mini-Mental State Examination <25); or other medical disorders that could affect the results or compliance were excluded. Medication use was also recorded, and women were excluded if they were currently taking medications known to affect body weight (except thyroid medication, statin therapy, or oral hypoglycemic medication). A total of 6 women were taking thyroid medication (evenly distributed among groups), 8 were taking statins (n = 2, 4, and 2 in the CR-only, CR + moderate-intensity, and CR + vigorous-intensity groups, respectively), and 7 were taking oral hypoglycemic medication (n = 2, 4, and 1 in the CR-only, CR + moderate-intensity, and CR + vigorous-intensity groups, respectively). No difference was observed in the mean amount of weight lost among these women and those not taking these medications (data not shown). On successful completion of the initial screening, 119 women attended another screening visit in which they underwent a graded exercise test to voluntary exhaustion to exclude those with exercise-induced ischemia (29). All women with abnormal test results were referred to their physician for evaluation.
FIGURE 1.
Participant recruitment, random assignment, and follow-up.
Participants who were medically eligible for the study were next interviewed by the General Clinical Research Center (GCRC) dietitian to discuss food preferences and to ascertain their willingness to comply with the dietary intervention. They also underwent a 7-d dietary run-in to assess their compatibility with the study menus and their compliance with picking up food 3 d/wk. Three women failed this run-in and were considered ineligible for the study.
Randomization and interventions
A total of 112 women met all study criteria and were randomly assigned (before baseline assessments) to 1 of the 3 interventions (Figure 1) by random number generation. We anticipated a greater loss to follow-up in women assigned to exercise; therefore, we enrolled 10% more women in these groups.
The goal of the dietary intervention was to elicit a similar energy deficit and amount of total weight loss between the 3 groups. Individual diets were developed by a registered dietitian (RD) and provided to each woman by the GCRC metabolic kitchen. On entry into the study, all women completed a 4-d food record, which was used as an initial measure of dietary habits. Individual energy needs for weight maintenance were calculated from each participants' resting metabolic rate (by using a MedGraphics CCM/D cart and BREEZE 6.2 software (MedGraphics, St Paul, MN) for indirect calorimetry after an overnight fast) and an activity factor based on self-reported daily activity (1.2–1.3 for sedentary lifestyle). The calorie deficits of all women were adjusted to ≈2800 kcal/wk (≈400 kcal/d), but no woman was provided with <1100 kcal/d. The deficits for the CR-only group resulted totally from reduction in dietary intake, whereas deficits for the CR + moderate- and vigorous-intensity exercise groups resulted from both reductions in dietary intake and exercise energy expenditure. Participants in the CR-only group were asked not to alter their sedentary lifestyle throughout the course of the study.
Throughout the course of the 20-wk intervention, all women were provided with daily lunch, dinner, and snacks. These meals were prepared according to each participant's choices from a menu designed by the RD. In consultation with the dietitian, women purchased and prepared their breakfast meal from a provided menu plan. They were asked to eat only the food that was given to them or that was approved from the breakfast menu. The macronutrient content was ≈25–30% fat, 15–20% protein, and 50–60% carbohydrate. Women were allowed to consume as many noncaloric, noncaffeinated beverages as they liked. They were also allowed 2 free days per month during which they were not provided food, but they were given guidelines for diet intake at their prescribed energy level and asked to report all food and beverage intake on these days. All women were provided with daily calcium supplements (500 mg, 2 times/d). They picked up their food 3 times/wk and were asked to keep a log of all foods consumed. The records were monitored weekly by the RD to verify compliance.
The goal of the exercise intervention was to test the specific effects of exercise intensity, while holding energy expenditure attributable to an exercise constant (at ≈700 kcal/wk which is consistent with the 8 kcal · kg body weight−1 · wk−1 reported as the dose for sedentary, obese postmenopausal women who follow the public health exercise recommendation) (12). This was accomplished by altering the duration of exercise between the exercise groups. Participants exercised 3 d/wk under the supervision of an exercise physiologist. Blood pressure and heart rate were measured before each exercise session, and participants warmed up by walking for 3–5 min at a slow pace. After flexibility exercise, women walked on a treadmill at an intensity of 45–50% (moderate-intensity) or 70–75% (vigorous-intensity) of heart rate reserve (HRR). Exercise progressed from 20–25 min the first week to 55 min by the end of the sixth week and thereafter for the moderate-intensity group. The duration of exercise for the vigorous-intensity group progressed from 10–15 min the first week to 30 min by the end of the sixth week and thereafter. Treadmill speed and grade were adjusted on an individual basis to ensure women exercised at their prescribed exercise intensity based on each woman's target heart rate calculated from the Karvonen equation [(HRR × intensity) + resting heart rate] (30), where HRR is the maximal heart rate, obtained from each subject's maximal oxygen uptake ( O2max) test, minus resting heart rate. At least 2 heart rate readings (measured by Polar heart rate monitors; Polar Electro Inc, Lake Success, NY) were taken during each exercise session and recorded in a log book to monitor compliance to the prescribed exercise intensity.
O2max) test, minus resting heart rate. At least 2 heart rate readings (measured by Polar heart rate monitors; Polar Electro Inc, Lake Success, NY) were taken during each exercise session and recorded in a log book to monitor compliance to the prescribed exercise intensity.
Outcomes and follow-up
Dietary intake, body composition, abdominal fat,  O2max, lipoprotein lipids, and glucose tolerance were measured at baseline and after the 20-wk interventions. All procedures were performed at the GCRC or the Geriatric Research Center in the J Paul Sticht Center on Aging. Staff that measured the primary (abdominal visceral fat volume) and secondary (CVD risk factors) outcomes were blinded to group assignment.
O2max, lipoprotein lipids, and glucose tolerance were measured at baseline and after the 20-wk interventions. All procedures were performed at the GCRC or the Geriatric Research Center in the J Paul Sticht Center on Aging. Staff that measured the primary (abdominal visceral fat volume) and secondary (CVD risk factors) outcomes were blinded to group assignment.
Body composition and fat distribution
Height and weight were measured with shoes and outer garments removed. Whole-body fat mass, lean mass, and percentage body fat were measured by dual-energy X-ray absorptiometry (Hologic Delphi QDR, Bedford, MA). Waist (minimal circumference) and hip (maximal gluteal protuberance) were measured in triplicate, and waist-to-hip ratio was calculated. Visceral and subcutaneous adipose tissue volumes around the abdomen were measured by multidetector computed tomography (GE Medical Systems, Milwaukee, WI). Women were positioned supine, with the arms above the head and legs positioned flat. Computed tomography slices within 15 mm centered at the L4–5 level were obtained. Quantitative measures of adipose tissue volume were obtained with the Advantage Windows 4.2 Volume Viewer (GE Healthcare, Waukesha, WI). Visceral fat was defined as fat enclosed by the inner aspect of the abdominal wall, and subcutaneous fat was defined as fat outside the outer aspects of the abdominal wall. The same technician analyzed all scans, and the intraclass correlation coefficient of the measurement of visceral fat volume in our laboratory is 0.99.
Maximal oxygen uptake
 O2max was measured on a treadmill (Medical Graphics Corporation, Minneapolis, MN) during a progressive exercise test to voluntary exhaustion. A ramp treadmill protocol was used—the speed was set at a constant rate according to individual ability, and the incline increased at small intervals continuously throughout the test (31). A valid
O2max was measured on a treadmill (Medical Graphics Corporation, Minneapolis, MN) during a progressive exercise test to voluntary exhaustion. A ramp treadmill protocol was used—the speed was set at a constant rate according to individual ability, and the incline increased at small intervals continuously throughout the test (31). A valid  O2max was obtained when at least 2 of these 3 criteria were met: 1) plateau in
O2max was obtained when at least 2 of these 3 criteria were met: 1) plateau in  O2 (<200 mL/min change) with increasing work rate, 2) maximal heart rate >90% of age-predicted maximal heart rate (220 beats/min for age), and 3) respiratory exchange ratio of ≥1.10. If the participant did not reach these criteria, the test was repeated.
O2 (<200 mL/min change) with increasing work rate, 2) maximal heart rate >90% of age-predicted maximal heart rate (220 beats/min for age), and 3) respiratory exchange ratio of ≥1.10. If the participant did not reach these criteria, the test was repeated.
Lipoprotein lipids
Blood samples were collected in EDTA-treated evacuated tubes by venipuncture in the early morning after a 12-h fast on duplicate testing days both before and after the interventions. The values reported are the average of these 2 d. Plasma triglycerides, total cholesterol, HDL cholesterol, and LDL cholesterol were measured by standardized hospital laboratory methods.
Oral-glucose-tolerance test
After an overnight fast, a 20-gauge polyethylene catheter was placed in an antecubital vein to facilitate blood sampling. Blood samples were drawn before (−10 and 0 min) and after (30, 60, 90, and 120 min) a 75-g glucose ingestion. Plasma glucose was measured with the glucose hexokinase method (Bayer Diagnostics, Tarrytown, NY). Plasma insulin was determined by a chemiluminescent immunoassay with the use of an IMMULITE analyzer (Diagnostics Products Corporation, Los Angeles, CA). Glucose and insulin areas were determined using Tai's model: 1/2 × 30 × (y0min + 2y30min + 2y60min + 2y90min + y120min), where y represents insulin or glucose at the different time points (32).
Statistical analysis
On the basis of prior data, we calculated that completion of 30 women per group would provide ≥85% power (α probability of 0.05) to detect a statistically significant overall group difference in our primary outcome of abdominal visceral fat volume, and ≥80% power to detect group differences in our secondary outcomes. All analyses were performed with the use of SAS software, version 9.1 (SAS Institute, Cary, NC), and an α of 0.05 was used as the type I error rate. All analyses were conducted under the intent-to-treat model with intervention assignment based on the assigned intervention at time of randomization. Baseline characteristics are reported as mean (±SD) or as frequency in percentage. Absolute changes in all outcomes were calculated as baseline value subtracted from the follow-up value (after intervention). Group differences for baseline and change values were analyzed with the use of one-way analysis of variance. Within-group differences between baseline and follow-up values were determined with a paired t test.
Simple bivariate correlation and regression analyses were performed to examine relations between mean changes in the primary and secondary outcomes with baseline values of these outcomes, changes in body weight, changes in  O2max, age, and race in all women combined. In addition, multiple stepwise linear regression analysis was used to model postintervention values of abdominal visceral fat volume, HDL-cholesterol and triglyceride concentrations, and glucose tolerance with baseline values, total amount of weight loss, change in visceral fat (for the metabolic variables), change in
O2max, age, and race in all women combined. In addition, multiple stepwise linear regression analysis was used to model postintervention values of abdominal visceral fat volume, HDL-cholesterol and triglyceride concentrations, and glucose tolerance with baseline values, total amount of weight loss, change in visceral fat (for the metabolic variables), change in  O2max, race, and treatment group as factors in the model.
O2max, race, and treatment group as factors in the model.
RESULTS
A total of 95 women completed the study and returned for follow-up testing (85% retention). Most (n = 11) of the 17 women who dropped out did so reportedly because of life changes unrelated to the study interventions, including unanticipated illness, change in work schedules, new time constraints, relocation, or family circumstances. Three women dropped out because of incompatibility with the dietary intervention, and 1 woman did not provide a reason. There were 2 adverse events (pneumonia and sinusitis with medication reaction) that led to dropout, and both were unrelated to study participation. The 17 women who dropped out of the study did not differ from women who completed the study for any baseline characteristic (data not shown). A total of 5 women assigned to exercise (4 in the CR + moderate-intensity group and 1 in the CR + vigorous-intensity group) completed the study and stayed on the prescribed diet throughout the 20-wk intervention but discontinued exercising within the first 2 mo because of time restraints (n = 2) or chronic, reoccurring injuries (n = 3). Data from all women were analyzed according to original group assignment.
Participant demographics and physical characteristics at baseline for all randomly assigned women are shown in Table 1. There were no differences among groups for any of these variables, and this was also true when only data from those women who completed the study (eg, n = 95) were compared. Overall, women were an average age of 58.4 y, 14.1 y past menopause, and mainly white (65%). Their upper-body obesity is reflected by a high waist circumference, waist-to-hip ratio, and abdominal visceral fat volume. The low maximal aerobic fitness level reflects their sedentary status. Of note, although there was large interindividual variability in baseline reported energy intakes (likely because of differences in energy needs as well as accuracy with self-reported dietary intake), there were no group differences.
TABLE 1.
Demographics and physical characteristics of randomly assigned women at baseline by group1
| CR only (n = 34) | CR + moderate-intensity (n = 40) | CR + vigorous-intensity (n = 38) | |
| Age (y) | 58.4 ± 6.02 | 57.7 ± 5.5 | 59.0 ± 5.0 |
| Years past menopause (y) | 14.7 ± 9.5 | 12.7 ± 10.6 | 12.3 ± 10.1 |
| Race-ethnicity [n (%)] | |||
| Non-Hispanic white | 19 (56) | 25 (62) | 29 (76) |
| African American | 14 (41) | 15 (38) | 9 (24) |
| Other | 1 (3) | 0 | 0 |
| Body composition | |||
| Body weight (kg) | 91.8 ± 10.4 | 90.4 ± 10.6 | 88.7 ± 12.7 |
| BMI (kg/m2) | 33.9 ± 4.0 | 33.7 ± 3.5 | 32.9 ± 3.7 |
| Total fat mass (kg) | 39.7 ± 7.2 | 39.3 ± 6.0 | 38.6 ± 7.7 |
| Total lean mass (kg) | 53.5 ± 4.5 | 52.7 ± 6.8 | 51.3 ± 6.0 |
| Body fat (%) | 42.4 ± 3.8 | 42.7 ± 3.3 | 42.7 ± 3.0 |
| Body fat distribution | |||
| Waist circumference (cm) | 98.5 ± 8.6 | 98.5 ± 8.6 | 97.5 ± 8.8 |
| Hip circumference (cm) | 118.8 ± 9.5 | 119.2 ± 8.5 | 116.6 ± 9.3 |
| Waist-hip ratio | 0.83 ± 0.07 | 0.83 ± 0.08 | 0.84 ± 0.06 |
| Thigh circumference (cm) | 60.5 ± 6.0 | 59.3 ± 5.8 | 58.1 ± 5.7 |
| Abdominal visceral fat (cm3) | 2369 ± 870 | 2252 ± 859 | 2509 ± 737 |
| Abdominal subcutaneous fat (cm3) | 5767 ± 1547 | 6212 ± 1519 | 5592 ± 1722 |
| Medication use [n (%)] | |||
| Antihypertensive | 11 (32.4) | 10 (25) | 8 (21.1) |
| Cholesterol-lowering | 6 (17.6) | 7 (17.5) | 7 (18.4) |
| Glucose control | 3 (8.8) | 4 (10) | 3 (7.9) |
| Thyroid | 4 (11.8) | 7 (17.5) | 6 (15.8) |
| Antidepressant or mood-altering | 0 | 1 (2.5) | 1 (2.6) |
| Reported energy intake (kcal/d) | 1844 ± 595 | 1736 ± 419 | 1692 ± 428 |
| Exercise variables | |||
Absolute  O2max (L/min) O2max (L/min) |
1.86 ± 2.71 | 1.89 ± 3.0 | 1.78 ± 3.94 |
Relative  O2max (mL · kg−1 · min−1) O2max (mL · kg−1 · min−1) |
20.6 ± 2.9 | 21.5 ± 3.2 | 20.3 ± 3.7 |
| Maximal heart rate (beats/min) | 157 ± 15 | 156 ± 15 | 156 ± 13 |
| Respiratory exchange ratio at max | 1.12 ± 0.10 | 1.12 ± 0.08 | 1.12 ± 0.07 |
CR, calorie restriction;  O2max, maximal oxygen uptake. No significant differences among groups were observed for any variable (one-factor ANOVA).
O2max, maximal oxygen uptake. No significant differences among groups were observed for any variable (one-factor ANOVA).
Mean ± SD (all such values).
The study intervention prescription and compliance data for women who completed the study are shown in Table 2. The average estimated weight maintenance energy level (determined from each woman's resting metabolic rate and activity factor) and the average reduction in energy intake did not differ among groups. The levels of calorie restriction comprised an ≈19–20% reduction (for the exercise groups) and a 23% reduction (for the CR-only group) in daily energy intake needed for weight maintenance. Self-reported compliance with the dietary intervention showed excellent compliance (Table 2); however, as noted below, interindividual variability in weight loss suggests that self-report measures of compliance may not be accurate for all women. Also of note, the actual exercise intensity (recorded heart rates during exercise) and duration matched the prescribed intensity and duration for each of the exercise groups (Table 2).
TABLE 2.
Study intervention data for women who completed the study1
| CR only (n = 29) | CR + moderate-intensity (n = 36) | CR + vigorous-intensity (n = 30) | |
| Dietary intervention | |||
| Estimated weight maintenance calorie level (kcal/d)2 | 1673 ± 2463 | 1616 ± 223 | 1618 ± 209 |
| Prescribed calorie level (kcal/d)2 | 1283 ± 163 | 1276 ± 156 | 1316 ± 176 |
| Absolute caloric reduction (kcal/d) | 390 ± 110 | 340 ± 88 | 302 ± 76 |
| Relative caloric reduction (% of reduction from maintenance) | 23.0 ± 4.4 | 20.8 ± 3.9 | 18.6 ± 3.8 |
| Recorded dietary compliance (% of deviation from provided kcal level)2 | 99.8 ± 1.4 | 100.3 ± 1.8 | 100.4 ± 1.7 |
| Exercise intervention4 | |||
| Prescribed intensity level (HR) | — | 110 ± 9 | 129 ± 11 |
| Prescribed duration (min/d) | — | 55 | 30 |
| Prescribed frequency (d/wk) | — | 3 | 3 |
| Actual intensity (HR) | — | 111.3 ± 8.9 | 125.6 ± 13.8 |
| Actual duration (min/d) | — | 54.1 ± 3.4 | 29.7 ± 1.9 |
| Actual frequency (d/wk) | — | 2.57 ± 0.1 | 2.49 ± 0.22 |
| Exercise compliance (% of attendance)5 | — | 92.6 ± 5.5 | 90.0 ± 8.7 |
| Total exercise volume (kcal/wk) | — | 751 ± 210 | 646 ± 200 |
CR, calorie restriction; HR, heart rate.
No significant difference among groups prescribed exercise was the final exercise goal; actual exercise was calculated after 6-wk ramp-up.
Mean ± SD (all such values).
All exercise data are calculated without the 5 women who completed the study and stayed on the prescribed diet but discontinued exercising; percentage of attendance with these 5 women was 83.2 ± 27.6% and 87.7 ± 15.2% for the moderate-intensity and vigorous-intensity groups, respectively.
Of 53–58 possible days.
The average weight loss for all women was 12.1 ± 4.5 kg (13.4 ± 4.6%) and was not significantly different among groups (Table 3). The range of weight loss was 1.2–23.1 kg: 94% of the women lost ≥5 kg, and 85% of the women lost ≥7 kg. Both fat and lean mass decreased significantly with each intervention (P < 0.001), and the absolute decreases were similar among groups. However, when the amount of lean mass lost is expressed as a proportion of the total amount of weight lost, the CR-only group showed a larger relative lean mass loss than either exercise group (CR only: 36.2 ± 17.1%; CR + moderate-intensity: 27.7 ± 14.8%; CR + vigorous-intensity: 26.7 ± 12.1%; P = 0.029).
TABLE 3.
Effects of study interventions on changes in body composition, fat distribution, and aerobic fitness1
| CR only (n = 29) | CR + moderate-intensity (n = 36) | CR + vigorous-intensity (n = 30) | P2 | |
| ΔBody weight (kg) | −11.8 ± 4.1 | −12.2 ± 4.5 | −12.3 ± 4.9 | 0.92 |
| ΔTotal fat mass (kg) | −7.4 ± 2.8 | −8.2 ± 3.2 | −8.5 ± 3.8 | 0.45 |
| ΔTotal lean mass (kg) | −4.1 ± 1.9 | −3.4 ± 2.0 | −3.3 ± 1.7 | 0.17 |
| ΔPercentage of body fat (%) | −3.3 ± 2.0 | −4.1 ± 2.2 | −4.3 ± 2.2 | 0.16 |
ΔRelative  O2max (mL · kg bw−1 · min−1) O2max (mL · kg bw−1 · min−1) |
2.0 ± 2.6 | 2.5 ± 2.6 | 4.1 ± 3.73 | 0.03 |
ΔAbsolute  O2max (mL/min) O2max (mL/min) |
−71 ± 255 | −8 ± 213 | 68 ± 252 | 0.10 |
| ΔWaist circumference (cm) | −8.9 ± 3.9 | −9.3 ± 5.0 | −9.9 ± 4.6 | 0.67 |
| ΔHip circumference (cm) | −8.5 ± 4.6 | −10.1 ± 4.6 | −9.4 ± 3.8 | 0.35 |
| ΔWHR | −0.016 ± 0.039 | −0.011 ± 0.044 | −0.020 ± 0.038 | 0.68 |
| ΔAbdominal visceral fat (cm3) | −612 ± 338 | −591 ± 340 | −630 ± 298 | 0.90 |
| ΔAbdominal subcutaneous fat (cm3) | −1051 ± 1023 | −1426 ± 764 | −1007 ± 1136 | 0.18 |
| ΔVSF ratio | −0.03 ± 0.08 | −0.02 ± 0.10 | −0.06 ± 0.09 | 0.34 |
All values are means ± SDs. CR, calorie restriction;  O2max, maximal oxygen uptake; bw, body weight; WHR, waist-hip ratio; VSF, visceral-subcutaneous fat.
O2max, maximal oxygen uptake; bw, body weight; WHR, waist-hip ratio; VSF, visceral-subcutaneous fat.
Decreases within each group for all body composition and fat distribution variables were significant, P < 0.0001 for all, except WHR and VSF, P = 0.003 (paired t test). Increases within each group for relative  O2max were significant, P < 0.05; changes within group for absolute
O2max were significant, P < 0.05; changes within group for absolute  O2max were not significant.
O2max were not significant.
Significantly different from the CR-only and CR + moderate-intensity groups, P < 0.05 (one-factor ANOVA).
 O2max expressed per kilogram of body weight increased significantly (P < 0.05; Table 3) within each group by 9.6 ± 11.2%, 12.7 ± 12.7%, and 24.2 ± 27.6% for the CR-only, CR + moderate-intensity, and CR + vigorous-intensity groups, respectively (Table 3). The mean change in relative
O2max expressed per kilogram of body weight increased significantly (P < 0.05; Table 3) within each group by 9.6 ± 11.2%, 12.7 ± 12.7%, and 24.2 ± 27.6% for the CR-only, CR + moderate-intensity, and CR + vigorous-intensity groups, respectively (Table 3). The mean change in relative  O2max was larger in the CR + vigorous-intensity group than in either of the other groups (P < 0.05 for both comparisons). The increase in relative
O2max was larger in the CR + vigorous-intensity group than in either of the other groups (P < 0.05 for both comparisons). The increase in relative  O2max in the CR-only and CR + moderate-intensity groups was likely because of the decrease in body weight, because there were no significant within-group changes for absolute
O2max in the CR-only and CR + moderate-intensity groups was likely because of the decrease in body weight, because there were no significant within-group changes for absolute  O2max.
O2max.
All 3 groups showed significant and similar decreases in waist and hip girths (P < 0.001), as well as waist-hip ratio (P = 0.003; Table 3). Likewise, the primary outcome of abdominal visceral fat volume decreased similarly among groups (P < 0.001), as did the abdominal subcutaneous fat volume (P < 0.001) and the ratio of visceral to subcutaneous abdominal fat (P = 0.003). The volume of visceral fat decreased by 25.5 ± 10.5%, 27.2 ± 12.3%, and 25.2 ± 11.0% in the CR-only, CR + moderate-intensity, and CR + vigorous-intensity groups, respectively.
Baseline values and changes in lipoprotein lipids, fasting glucose and insulin, and 2-h glucose and insulin areas during the oral-glucose-tolerance test are shown in Table 4. All variables were similar at baseline across treatment groups. There were no differences among groups in the magnitude of change for any of these variables.
TABLE 4.
Metabolic risk factor variables at baseline and changes with intervention1
| CR only (n = 29) | CR + moderate-intensity (n = 36) | CR + vigorous-intensity (n = 30) | |
| HDL cholesterol (mg/dL) | |||
| Baseline | 52.2 ± 13.1 | 54.9 ± 13.0 | 53.6 ± 10.6 |
| After intervention | 48.9 ± 10.1 | 53.2 ± 9.3 | 51.1 ± 9.1 |
| Change from baseline | −3.3 ± 6.1 | −1.7 ± 8.6 | −2.5 ± 6.9 |
| Triglycerides (mg/dL) | |||
| Baseline | 134.8 ± 67.4 | 127.6 ± 51.1 | 130.7 ± 46.6 |
| After intervention | 108.4 ± 51.3 | 102.4 ± 35.9 | 112.0 ± 34.7 |
| Change from baseline | −26.4 ± 47.8 | −24.8 ± 37.8 | −18.7 ± 34.9 |
| LDL cholesterol (mg/dL) | |||
| Baseline | 125.8 ± 34.0 | 116.8 ± 21.1 | 134.2 ± 30.6 |
| After intervention | 116.7 ± 31.7 | 110.9 ± 23.0 | 130.8 ± 27.9 |
| Change from baseline | −9.2 ± 21.5 | −6.1 ± 14.9 | −3.3 ± 18.2 |
| Fasting glucose (mg/dL) | |||
| Baseline | 98.8 ± 10.1 | 94.7 ± 7.7 | 97.1 ± 15.5 |
| After intervention | 92.0 ± 8.3 | 90.5 ± 8.0 | 92.0 ± 7.9 |
| Change from baseline | −6.8 ± 8.9 | −4.3 ± 7.9 | −5.1 ± 11.1 |
| Fasting insulin (μIU/mL) | |||
| Baseline | 11.9 ± 5.6 | 9.8 ± 4.5 | 11.7 ± 8.4 |
| After intervention | 7.2 ± 4.4 | 6.8 ± 3.5 | 8.8 ± 5.5 |
| Change from baseline | −4.6 ± 3.9 | −3.0 ± 3.3 | −2.9 ± 5.9 |
| Glucose area (mg/dL · 2 h)2 | |||
| Baseline | 17,730 ± 3857 | 16,116 ± 2972 | 17,909 ± 3893 |
| After intervention | 16,084 ± 2541 | 14,878 ± 2098 | 16,231 ± 3537 |
| Change from baseline | −1646 ± 3460 | −1238 ± 2329 | −1677 ± 2208 |
| Insulin area (μIU/mL · 2 h)3 | |||
| Baseline | 9038 ± 4469 | 9534 ± 4949 | 9529 ± 6376 |
| After intervention | 6313 ± 3046 | 7055 ± 3363 | 8094 ± 4476 |
| Change from baseline | −2172 ± 2474 | −2479 ± 3475 | −904 ± 3536 |
All values are means ± SDs. CR, calorie restriction. No significant differences were observed among groups at baseline, or in the magnitude of change, for any variable (one-factor ANOVA).
n = 24 CR only; n = 32 CR + moderate-intensity; n = 29 CR + vigorous-intensity.
n = 21 CR only; n = 31 CR + moderate-intensity; n = 26 CR + vigorous-intensity.
Data from all 95 women who completed the study were combined, and regression analyses were used to determine the independent predictors of the overall mean changes in abdominal visceral fat volume, HDL-cholesterol and triglyceride concentrations, and glucose tolerance. Simple correlation analyses showed that decreases in abdominal visceral fat were strongly inversely related to the amount of visceral fat at baseline (r = −0.63, P < 0.001) and directly related to the amount of weight lost (r = 0.61, P < 0.001). In addition, changes in visceral fat were inversely related to increases in relative  O2max (r = −0.28, P < 0.01). White women lost more visceral fat than black women (white = −703 ± 306 cm3, black = −396 ± 297 cm3; P < 0.001), and more visceral fat was lost with older age (r = −0.23, P < 0.05).
O2max (r = −0.28, P < 0.01). White women lost more visceral fat than black women (white = −703 ± 306 cm3, black = −396 ± 297 cm3; P < 0.001), and more visceral fat was lost with older age (r = −0.23, P < 0.05).
No bivariate correlations were observed between changes in glucose tolerance (2-h glucose area), HDL-cholesterol or triglyceride concentrations, and age or changes in  O2max in these women. Changes in glucose tolerance correlated inversely with the baseline glucose area (r = −0.69, P < 0.001) and with the amount of weight (r = 0.26, P < 0.05) and visceral fat (r = 0.28, P < 0.05) lost but did not differ by race. Changes in HDL-cholesterol concentrations were inversely related to baseline HDL (r = −0.48, P < 0.001), but not to race, or to changes in the amount of weight or visceral fat lost (all P > 0.10). Changes in triglyceride concentrations were also inversely related to baseline triglycerides (r = −0.61, P < 0.001) and were directly related to the amount of weight (r = 0.27; P < 0.01) and visceral fat (r = 0.29, P < 0.01) lost. In addition, triglyceride concentrations decreased more in white (−30.4 ± 42.7 mg/dL) than in black (−7.8 ± 27.1 mg/dL) women (P < 0.01).
O2max in these women. Changes in glucose tolerance correlated inversely with the baseline glucose area (r = −0.69, P < 0.001) and with the amount of weight (r = 0.26, P < 0.05) and visceral fat (r = 0.28, P < 0.05) lost but did not differ by race. Changes in HDL-cholesterol concentrations were inversely related to baseline HDL (r = −0.48, P < 0.001), but not to race, or to changes in the amount of weight or visceral fat lost (all P > 0.10). Changes in triglyceride concentrations were also inversely related to baseline triglycerides (r = −0.61, P < 0.001) and were directly related to the amount of weight (r = 0.27; P < 0.01) and visceral fat (r = 0.29, P < 0.01) lost. In addition, triglyceride concentrations decreased more in white (−30.4 ± 42.7 mg/dL) than in black (−7.8 ± 27.1 mg/dL) women (P < 0.01).
The independent predictors of postintervention values of our primary (visceral fat) and secondary (glucose tolerance, HDL cholesterol, triglycerides) study outcomes are shown in Table 5. Stepwise linear regression was used to model each outcome, with the baseline value of that outcome, change in body weight (and change in visceral fat for the metabolic variables), change in  O2max, race, and treatment group as factors in the model. The results showed that baseline visceral fat volume, amount of weight loss, and race were the only independent predictors of visceral fat at follow-up, and these factors accounted for 94% of the variation in the postintervention amount of abdominal visceral fat. After accounting for the initial values of each of the metabolic outcomes, only the amount of weight loss—and not changes in abdominal visceral fat volume, changes in
O2max, race, and treatment group as factors in the model. The results showed that baseline visceral fat volume, amount of weight loss, and race were the only independent predictors of visceral fat at follow-up, and these factors accounted for 94% of the variation in the postintervention amount of abdominal visceral fat. After accounting for the initial values of each of the metabolic outcomes, only the amount of weight loss—and not changes in abdominal visceral fat volume, changes in  O2max, or race—contributed to the variance in glucose tolerance and HDL-cholesterol and triglyceride concentrations at follow-up.
O2max, or race—contributed to the variance in glucose tolerance and HDL-cholesterol and triglyceride concentrations at follow-up.
TABLE 5.
Independent predictors of postintervention values of primary (visceral fat) and secondary (glucose tolerance, HDL cholesterol, triglycerides) study outcomes1
| Predictor | Regression coefficient | SE | Cumulative R2 | P for independent predictor |
| Abdominal visceral fat (cm3) | ||||
| Initial visceral fat | −0.80 | 0.02 | 0.87 | <0.0001 |
| ΔBody weight | 36.8 | 4.4 | 0.93 | <0.0001 |
| Race | 98.2 | 46.5 | 0.94 | <0.05 |
| Glucose area (mg/dL · 2 h) | ||||
| Initial glucose area | −0.54 | 0.06 | 0.48 | <0.0001 |
| ΔBody weight | 124 | 59 | 0.51 | <0.05 |
| Triglycerides (mg/dL) | ||||
| Initial triglycerides | −0.53 | 0.06 | 0.47 | <0.0001 |
| ΔBody weight | 2.26 | 0.70 | 0.53 | <0.01 |
| Initial HDL cholesterol (mg/dL) | −0.63 | 0.05 | 0.67 | <0.0001 |
n = 95. Stepwise linear regression was used to model each outcome, with the baseline value of that outcome, change in body weight (and change in visceral fat for the metabolic variables), change in maximal oxygen uptake, race, and treatment group as factors in the model. Variables included were only those that showed a statistically significant independent association with the dependent variable.
DISCUSSION
Debate continues among health professionals about whether low- or moderate-intensity aerobic exercise is as beneficial as more-vigorous exercise for reducing abdominal obesity and maximizing improvements in CVD risk factors. Our results confirm that, with a similar amount of total weight loss, there is not a preferential loss of abdominal fat when either moderate- or vigorous-intensity aerobic exercise is performed during calorie restriction. All 3 groups reduced abdominal visceral fat by ≈25%. These results are in line with those showing that, when the energy deficit is matched, fat loss from the abdominal region is not enhanced by addition of aerobic exercise to calorie restriction in younger persons (33). However, we did observe that changes in visceral fat were inversely related to increases in aerobic fitness, and addition of exercise did result in less lean mass loss.
Our results also show that addition of aerobic exercise of either intensity to the diet did not enhance improvements in the measured CVD risk factors in response to an equal amount of weight loss. Because most of these risk factors improved to a similar degree in each group, the effects of the weight loss itself appears to have “trumped” any potential benefits of the exercise. This conclusion is in line with other data showing that, when compared directly, weight loss has greater benefit for improving CVD risk than exercise in the absence of weight loss (34–36), but that CVD risk profiles improve similarly with weight loss induced either by increasing energy expenditure with exercise or decreasing energy intake by diet (37, 38). In addition, adding exercise to a weight-loss program does not always improve CVD risk beyond the weight loss alone (39–42). Moreover, improvements in glucose and lipid CVD risk factors with most prior exercise studies are more closely related to reductions in body weight, or total or abdominal fat or both, rather than with changes in aerobic fitness (36, 41, 43–51). Our results are consistent with those studies because changes in the measured risk factors were not related to changes in  O2max, but improvements in glucose tolerance and triglyceride concentrations were related to total weight loss. However, systemic inflammation, an important CVD risk factor, was not measured in this study, and it may be that there are effects of exercise intensity in the context of fat loss on biomarkers of inflammation.
O2max, but improvements in glucose tolerance and triglyceride concentrations were related to total weight loss. However, systemic inflammation, an important CVD risk factor, was not measured in this study, and it may be that there are effects of exercise intensity in the context of fat loss on biomarkers of inflammation.
Few prior studies examined the effects (on any outcome) of either intensity, duration, or frequency of exercise independent of their contribution to total exercise energy expenditure. Because vigorous-intensity exercise performed for a specific duration and frequency results in greater energy expenditure than low- or moderate-intensity exercise, differences in total expenditure need to be controlled to definitely determine any effects of exercise training intensity per se on changes in body composition and fat distribution. Existing data show that regular exercise performed at a higher intensity or duration or both results in greater loss of abdominal fat, even in the absence of measurable loss of total weight (10, 13–15, 52, 53); however, these findings can likely be attributed to differences in total energy expenditure of the exercise. In addition, even when compared with lower-intensity exercise of an equal energy deficit, vigorous-intensity exercise favors negative energy balance because of a greater postexercise energy expenditure but less postexercise energy intake (54, 55). However, over the course of 20 wk, and in the setting of an even larger energy deficit induced by calorie restriction, our results did not show an effect of exercise intensity per se on location of fat loss.
Current practice guidelines suggest inclusion of physical activity for 30 min/d on most days of the week as part of an overall obesity treatment program (7–9), yet the ideal intensity of exercise necessary to maximize potential benefit is not clear. The findings from the present clinical trial showing no added benefit of eucaloric vigorous-intensity aerobic exercise on the outcome of abdominal fat loss has important ramifications for optimizing and refining public health recommendations for treatment of abdominal obesity. Women in the vigorous-intensity group exercised, on average, at the equivalent of a fast walk with an incline, or a slow jog, compared with women in the moderate-intensity group who walked at a slow pace with no incline. Because obese persons tend to prefer, and are more likely to engage in, low- or moderate-intensity (eg, slow walking) physical activity (56, 57), it may be impractical and unnecessary to prescribe vigorous-intensity aerobic exercise as part of a weight-loss therapy with the primary goal of treating abdominal obesity. However, we did observe other health benefits of performing exercise during calorie restriction. Specifically, there was a dose-response effect of exercise intensity on maximal aerobic capacity, and women who increased aerobic capacity more experienced greater loss of abdominal visceral fat. In addition, our study confirms prior data showing a benefit of performing exercise during calorie restriction for attenuating the loss of lean mass (39, 58–60). Furthermore, there may be other benefits of performing aerobic exercise during energy restriction that were not assessed in the present study. For example, some data point to better long-term maintenance of weight loss when exercise is added to a dietary weight-loss intervention (61, 62).
The present study has several strengths, including the highly controlled interventions, which allowed us to examine the independent effects of 2 levels of aerobic exercise intensity on several clinically important health outcomes, without the confounding influence of differential exercise energy expenditure or differential weight loss. In addition, our study was conducted in abdominally obese, postmenopausal women—a population at high metabolic risk of CVD. However, there are several caveats to keep in mind when interpreting and translating our findings. Importantly, because the diet portion of the intervention was designed to eliminate group differences in total weight loss (eg, the 2 exercise groups consumed more calories), our results are only applicable in this context. We have shown that there is no “preferential” loss of abdominal fat with addition of exercise to a short-term period of calorie restriction, yet, in an uncontrolled setting, performing aerobic exercise while dieting could result in a larger negative energy deficit and therefore greater total and abdominal fat loss than dieting alone (60, 63, 64). Also, the intervention was of relatively short duration in comparison to some weight-loss programs, and results may differ if the exercise and calorie restriction were extended to a year or more. The findings of this study should have important ramifications for refining current strategies for treatment of abdominal obesity and will contribute to knowledge about whether there is any advantage to performing vigorous-intensity exercise for treatment of obesity in abdominally obese, postmenopausal women.
Acknowledgments
We thank the women who volunteered for this study, as well as the study coordinators, dietitians, nurses, exercise physiologists, and other research staff of the Wake Forest University General Clinical Research Center and the Geriatric Research Center.
The authors' responsibilities were as follows—BJN: was responsible for study concept and design; XW, TY, MFL, JD, LE, LL, and JJC: acquired the data; BJN, XW, TY, LE, and MJB: analyzed and interpreted data; BJN and XW: drafted the manuscript; MFL, JD, LE, MJB, LL, and JJC: were responsible for critical revision of the manuscript for important intellectual content; BJN and XW: performed statistical analysis; BJN: obtained funding; and BJN, XW, TY, MFL, and JD: supervised the study. None of the authors had a potential, real or perceived, conflict of interest.
REFERENCES
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006;295:1549–55 [DOI] [PubMed] [Google Scholar]
- 2.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond) 2008;32:949–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lovejoy JC. The menopause and obesity. Prim Care 2003;30:317–25 [DOI] [PubMed] [Google Scholar]
- 4.Haffner SM. Abdominal adiposity and cardiometabolic risk: do we have all the answers? Am J Med 2007;120(suppl):S10–6 [DOI] [PubMed] [Google Scholar]
- 5.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–7 [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Obes Res 1998;6(suppl):51S–209S [PubMed] [Google Scholar]
- 7.Lau DC, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary]. CMAJ 2007;176(suppl):S1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein S, Burke LE, Bray GA, et al. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation 2004;110:2952–67 [DOI] [PubMed] [Google Scholar]
- 9.Jakicic JM, Clark K, Coleman E, et al. American College of Sports Medicine position stand: appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 2001;33:2145–56 [DOI] [PubMed] [Google Scholar]
- 10.Ohkawara K, Tanaka S, Miyachi M, Ishikawa-Takata K, Tabata I. A dose-response relation between aerobic exercise and visceral fat reduction: systematic review of clinical trials. Int J Obes (Lond) 2007;31:1786–97 [DOI] [PubMed] [Google Scholar]
- 11.McTiernan A, Sorensen B, Irwin ML, et al. Exercise effect on weight and body fat in men and women. Obesity (Silver Spring) 2007;15:1496–512 [DOI] [PubMed] [Google Scholar]
- 12.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA 2007;297:2081–91 [DOI] [PubMed] [Google Scholar]
- 13.Slentz CA, Aiken LB, Houmard JA, et al. Inactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol 2005;99:1613–8 [DOI] [PubMed] [Google Scholar]
- 14.Irwin ML, Yasui Y, Ulrich CM, et al. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. JAMA 2003;289:323–30 [DOI] [PubMed] [Google Scholar]
- 15.Ross R, Janssen I. Physical activity, total and regional obesity: dose-response considerations. Med Sci Sports Exerc 2001;33(suppl):S521–7 [DOI] [PubMed] [Google Scholar]
- 16.Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol 2006;97:141–7 [DOI] [PubMed] [Google Scholar]
- 17.Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA 2002;288:1994–2000 [DOI] [PubMed] [Google Scholar]
- 18.Manson JE, Hu FB, Rich-Edwards JW, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med 1999;341:650–8 [DOI] [PubMed] [Google Scholar]
- 19.Mensink GB, Heerstrass DW, Neppelenbroek SE, Schuit AJ, Bellach BM. Intensity, duration, and frequency of physical activity and coronary risk factors. Med Sci Sports Exerc 1997;29:1192–8 [DOI] [PubMed] [Google Scholar]
- 20.Tremblay A, Despres J, Leblanc C, et al. Effect of intensity of physical activity on body fatness and fat distribution. Am J Clin Nutr 1990;51:153–7 [DOI] [PubMed] [Google Scholar]
- 21.DiPietro L, Dziura J, Yeckel CW, Neufer PD. Exercise and improved insulin sensitivity in older women: evidence of the enduring benefits of higher intensity training. J Appl Physiol 2006;100:142–9 [DOI] [PubMed] [Google Scholar]
- 22.O'Donovan G, Owen A, Bird SR, et al. Changes in cardiorespiratory fitness and coronary heart disease risk factors following 24 wk of moderate- or high-intensity exercise of equal energy cost. J Appl Physiol 2005;98:1619–25 [DOI] [PubMed] [Google Scholar]
- 23.Asikainen TM, Miilunpalo S, Kukkonen-Harjula K, et al. Walking trials in postmenopausal women: effect of low doses of exercise and exercise fractionization on coronary risk factors. Scand J Med Sci Sports 2003;13:284–92 [DOI] [PubMed] [Google Scholar]
- 24.Kraus WE, Houmard JA, Duscha BD, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med 2002;347:1483–92 [DOI] [PubMed] [Google Scholar]
- 25.Bopp MJ, Houston DK, Lenchik L, Easter L, Kritchevsky SB, Nicklas BJ. Lean mass loss is associated with low protein intake during dietary-induced weight loss in postmenopausal women. J Am Diet Assoc 2008;108:1216–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Lyles MF, You T, Berry MJ, Rejeski WJ, Nicklas BJ. Weight regain is related to decreases in physical activity during weight loss. Med Sci Sports Exerc 2008;40:1781–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.You T, Yang R, Lyles MF, Gong D, Nicklas BJ. Abdominal adipose tissue cytokine gene expression: Relationship to obesity and metabolic risk factors. Am J Physiol Endocrinol Metab 2005;288:E741–7 [DOI] [PubMed] [Google Scholar]
- 28.You T, Murphy KM, Lyles MF, Demons JL, Lenchik L, Nicklas BJ. Addition of aerobic exercise to dietary weight loss preferentially reduces abdominal adipocyte size. Int J Obes (Lond) 2006;30:1211–6 [DOI] [PubMed] [Google Scholar]
- 29.Bruce RA, Horstein TR. Exercise stress testing in evaluation of patients with ischemic heart disease. Prog Cardiovasc Dis 1969;11:371–90 [DOI] [PubMed] [Google Scholar]
- 30.Karvonen MJ, Kentala E, Mustala O. The effects of training heart rate: a longitudinal study. Ann Med Exp Biol Fenn 1957;35:307–15 [PubMed] [Google Scholar]
- 31.Myers J, Buchanan N, Smith D, et al. Individualized ramp treadmill. Observations on a new protocol. Chest 1992;101:236S–41S [PubMed] [Google Scholar]
- 32.Tai MM. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes Care 1994;17:152–4 [DOI] [PubMed] [Google Scholar]
- 33.Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab 2007;92:865–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katzel LI, Bleecker ER, Colman EG, Rogus EM, Sorkin JD, Goldberg AP. Effects of weight loss vs aerobic exercise training on risk factors for coronary disease in healthy, obese, middle-aged and older men. JAMA 1995;274:1915–21 [DOI] [PubMed] [Google Scholar]
- 35.Dengel DR, Pratley RE, Hagberg JM, Rogus EM, Goldberg AP. Distinct effects of aerobic exercise training and weight loss on glucose homeostasis in obese sedentary men. J Appl Physiol 1996;81:318–25 [DOI] [PubMed] [Google Scholar]
- 36.Dengel DR, Galecki AT, Hagberg JM, Pratley RE. The independent and combined effects of weight loss and aerobic exercise on blood pressure and oral glucose tolerance in older men. Am J Hypertens 1998;11:1405–12 [DOI] [PubMed] [Google Scholar]
- 37.Fontana L, Villareal DT, Weiss EP, et al. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab 2007;293:E197–202 [DOI] [PubMed] [Google Scholar]
- 38.Weiss EP, Racette SB, Villareal DT, et al. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr 2006;84:1033–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Svendsen OL, Hassager C, Christiansen C. Effect of an energy-restrictive diet, with or without exercise, on lean tissue mass, resting metabolic rate, cardiovascular risk factors, and bone in overweight postmenopausal women. Am J Med 1993;95:131–40 [DOI] [PubMed] [Google Scholar]
- 40.Janssen I, Fortier A, Hudson R, Ross R. Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care 2002;25:431–8 [DOI] [PubMed] [Google Scholar]
- 41.Nieman DC, Brock DW, Butterworth D, Utter AC, Nieman CC. Reducing diet and/or exercise training decreases the lipid and lipoprotein risk factors of moderately obese women. J Am Coll Nutr 2002;21:344–50 [DOI] [PubMed] [Google Scholar]
- 42.Weinstock RS, Dai H, Wadden TA. Diet and exercise in the treatment of obesity: effects of 3 interventions on insulin resistance. Arch Intern Med 1998;158:2477–83 [DOI] [PubMed] [Google Scholar]
- 43.O'Leary VB, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol 2006;100:1584–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicklas BJ, Dennis KE, Berman DM, Sorkin J, Ryan AS, Goldberg AP. Lifestyle intervention of hypocaloric dieting and walking reduces abdominal obesity and improves coronary heart disease risk factors in obese, postmenopausal, African-American and Caucasian women. J Gerontol A Biol Sci Med Sci 2003;58:181–9 [DOI] [PubMed] [Google Scholar]
- 45.Katzmarzyk PT, Leon AS, Rankinen T, et al. Changes in blood lipids consequent to aerobic exercise training related to changes in body fatness and aerobic fitness. Metabolism 2001;50:841–8 [DOI] [PubMed] [Google Scholar]
- 46.Pratley RE, Hagberg JM, Dengel DR, Rogus EM, Muller DC, Goldberg AP. Aerobic exercise training-induced reductions in abdominal fat and glucose-stimulated insulin responses in middle-aged and older men. J Am Geriatr Soc 2000;48:1055–61 [DOI] [PubMed] [Google Scholar]
- 47.Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men: a randomized, controlled trial. Ann Intern Med 2000;133:92–103 [DOI] [PubMed] [Google Scholar]
- 48.Katzel LI, Bleecker ER, Rogus EM, Goldberg AP. Sequential effects of aerobic exercise training and weight loss on risk factors for coronary disease in healthy, obese middle-aged and older men. Metabolism 1997;46:1441–7 [DOI] [PubMed] [Google Scholar]
- 49.Poirier P, Catellier C, Tremblay A, Nadeau A. Role of body fat loss in the exercise-induced improvement of the plasma lipid profile in non-insulin-dependent diabetes mellitus. Metabolism 1996;45:1383–7 [DOI] [PubMed] [Google Scholar]
- 50.Lamarche B, Despres JP, Pouliot MC, et al. Is body fat loss a determinant factor in the improvement of carbohydrate and lipid metabolism following aerobic exercise training in obese women? Metabolism 1992;41:1249–56 [DOI] [PubMed] [Google Scholar]
- 51.Despres JP, Pouliot MC, Moorjani S, et al. Loss of abdominal fat and metabolic response to exercise training in obese women. Am J Physiol 1991;261:E159–67 [DOI] [PubMed] [Google Scholar]
- 52.Kay SJ, Fiatarone Singh MA. The influence of physical activity on abdominal fat: a systematic review of the literature. Obes Rev 2006;7:183–200 [DOI] [PubMed] [Google Scholar]
- 53.Ross R, Janssen I, Dawson J, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res 2004;12:789–98 [DOI] [PubMed] [Google Scholar]
- 54.Yoshioka M, Doucet E, St-Pierre S, et al. Impact of high-intensity exercise on energy expenditure, lipid oxidation and body fatness. Int J Obes Relat Metab Disord 2001;25:332–9 [DOI] [PubMed] [Google Scholar]
- 55.Imbeault P, Saint-Pierre S, Almeras N, Tremblay A. Acute effects of exercise on energy intake and feeding behaviour. Br J Nutr 1997;77:511–21 [DOI] [PubMed] [Google Scholar]
- 56.King AC, Haskell WL, Young DR, Oka RK, Stefanick ML. Long-term effects of varying intensities and formats of physical activity on participation rates, fitness, and lipoproteins in men and women aged 50 to 65 years. Circulation 1991;91:2596–604 [DOI] [PubMed] [Google Scholar]
- 57.Lasco RA, Curry RH, Dickson VJ, Powers J, Menes S, Merritt RK. Participation rates, weight loss, and blood pressure changes among obese women in a nutrition-exercise program. Public Health Rep 1989;104:640–6 [PMC free article] [PubMed] [Google Scholar]
- 58.Hansen D, Dendale P, Berger J, van Loon LJ, Meeusen R. The effects of exercise training on fat-mass loss in obese patients during energy intake restriction. Sports Med 2007;37:31–46 [DOI] [PubMed] [Google Scholar]
- 59.Ross R, Rissanen J, Pedwell H, Clifford J, Shragge P. Influence of diet and exercise on skeletal muscle and visceral adipose tissue in men. J Appl Physiol 1996;81:2445–55 [DOI] [PubMed] [Google Scholar]
- 60.Ross R, Pedwell H, Rissanen J. Effects of energy restriction and exercise on skeletal muscle and adipose tissue in women as measured by magnetic resonance imaging. Am J Clin Nutr 1995;61:1179–85 [DOI] [PubMed] [Google Scholar]
- 61.Curioni CC, Lourenco PM. Long-term weight loss after diet and exercise: a systematic review. Int J Obes (Lond) 2005;29:1168–74 [DOI] [PubMed] [Google Scholar]
- 62.Miller WC, Koceja DM, Hamilton EJ. A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes Relat Metab Disord 1997;21:941–7 [DOI] [PubMed] [Google Scholar]
- 63.Okura T, Nakata Y, Lee DJ, Ohkawara K, Tanaka K. Effects of aerobic exercise and obesity phenotype on abdominal fat reduction in response to weight loss. Int J Obes (Lond) 2005;29:1259–66 [DOI] [PubMed] [Google Scholar]
- 64.Ross R, Rissanen J. Mobilization of visceral and subcutaneous adipose tissue in response to energy restriction and exercise. Am J Clin Nutr 1994;60:695–703 [DOI] [PubMed] [Google Scholar]